Abstract
The angiotensin converting enzyme inhibitor captopril prevents myosin-induced experimental autoimmune myocarditis. Captopril inhibits production of angiotensin II and increases bradykinin signaling, among other actions. To test whether captopril inhibits disease through blockade of angiotensin signaling, we tested the ability of losartan, an angiotensin II receptor blocker, to prevent myosin-induced myocarditis. A/J mice immunized with the heavy chain of cardiac myosin in complete Freund's adjuvant develop acute myocarditis by day 21 post immunization, consisting of severe focal inflammation, necrosis and fibrosis. Administration of losartan (250 mg/L in the drinking water) or captopril (75 mg/L in the drinking water) significantly reduced inflammation, necrosis, and fibrosis in myosin-immunized mice. The heart weights and the heart weight-to-body weight ratios were also significantly reduced in both treatment groups. However, whereas captopril reduced myosin-specific delayed-type hypersensitivity, losartan did not. Both captopril treated mice and losartan treated mice showed a decrease in myosin-specific autoantibody production. Because losartan treatment significantly reduced myocarditis, fibrosis and autoantibody production in EAM, it is likely that prevention of angiotensin II receptor stimulation is a major mechanism underlying the inhibition of myosin-induced myocarditis by captopril.
Keywords: myocarditis, angiotensin, autoimmunity, collagen, myosin
1. Introduction
Myocarditis, inflammation of the heart, is characterized by myocyte necrosis and degeneration with mononuclear cell infiltration in the presence or absence of fibrosis [1]. In the United States, approximately 2500 people are believed to develop myocarditis each year [2, 3], although the actual number is likely to be much higher [4, 5]. Causes of myocarditis vary widely and include infections with bacteria, viruses, parasites and fungi, exposure to drugs and toxins, and autoimmune diseases. Viral myocarditis is commonly caused by coxsackievirus B3 infection in North America and Europe, while parasite-induced myocarditis is commonly caused by the protozoan parasite Trypanosoma cruzi in Central and South America [6].
Treatments for myocarditis are directed toward reducing or eliminating the inciting agent when possible, and tailoring therapy toward the associated complications such as congestive heart failure, cardiogenic shock, dysrhythmias, and thromboembolism [7]. These complications are typically managed with sodium restriction, diuretics, digitalis, beta blockers and vasodilators [8]. The renin-angiotensin system is a key target of vasodilator therapy. Vasoconstriction induced by angiotensin II (Ang II) signaling of vascular smooth muscle cells can be inhibited by blockade of Ang II receptors or by prevention of the conversion of angiotensin I (Ang I) to Ang II by angiotensin converting enzyme (ACE). One commonly prescribed ACE inhibitor, captopril, has been used successfully to treat a wide variety of cardiovascular diseases, with few side effects.
Captopril acts primarily on the renin-angiotensin system. Normally, the inactive precursor peptide angiotensinogen is formed in the liver and converted to the inactive intermediate Ang I, a decapeptide, by renin, produced in the juxtaglomerular apparatus in the kidney. The two carboxyterminal amino acids are cleaved, converting Ang I to the active eight amino acid peptide Ang II by ACE, a metalloprotease. Ang II then binds to its target receptors, type 1 (AT1R) and type 2 (AT2R). AT1R has been better characterized and is responsible for many of the known actions of Ang II. The overall effects of Ang II signaling are to increase intracellular volume, peripheral vascular resistance, and blood pressure.
Captopril binds to ACE via its peptide-binding pocket and inhibits its activity. In particular, it inhibits the formation of Ang II from Ang I and prevents the degradation of bradykinin [9, 10]. By interfering with the renin-angiotensin system and bradykinin pathways, captopril reduces sytemic arterial pressure, peripheral vascular resistance and cardiac filling pressure, and increases cardiac output. Captopril is also an antiinflammatory agent, acting through the immunomodulatory actions of Ang II and the downstream effects of bradykinin. Among the many ACE inhibitors and Ang II receptor antagonists, captopril has been shown to modulate chemotaxis, motility, adhesion, differentiation, activation and cytokine and chemokine production of immune cells [10]. Together, the effects of captopril result in reduced inflammation and fibrosis, improvement of cardiac function and enhanced survival in heart failure patients. Captopril is effective at ameliorating many human cardiomyopathies [11, 12] although its direct effect in human myocarditis has not been addressed. The drug decreases inflammation, calcification and fibrosis in several models of infectious myocarditis including those caused by encephalomyocarditis virus [13-15], coxsackievirus B3 [16-18], and Trypanosoma cruzi [19].
In addition, various animal models of myocarditis and other cardiomyopathies have shown amelioration of disease with captopril treatment. An established model of experimental autoimmune myocarditis (EAM) is induced in susceptible strains of mice upon immunization with the α heavy chain of cardiac myosin [20]. EAM is histologically similar to human myocarditis, with myocyte swelling and necrosis accompanied by mononuclear cell infiltration and fibrosis. Studies have shown that EAM is a T cell mediated disease, involving both CD4+ and CD8+ subsets [21-25]. B cells are not vital for antigen presentation in EAM and autoantibodies are not necessary for the development of myocarditis [21, 26, 27]. The effects of captopril on experimental models of antigen-induced autoimmune myocarditis have only recently been addressed [28].
Both ACE inhibitors and Ang II receptor antagonists have been used to treat a variety of cardiovascular diseases, including hypertension and cardiomyopathy. However, there have been very few studies investigating the effects of Ang II type 1 receptor (AT1R) blockade in experimental myocarditis [15, 29-31], and even fewer on autoimmune myocarditis [32, 33]. To study the effect of AT1R antagonism on the development of autoimmune myocarditis, we compared the effects of the AT1R blocker losartan and the ACE inhibitor captopril on the development of myosin-induced autoimmune myocarditis. Losartan ameliorated cardiac inflammation, although not quite to the degree seen with captopril. While the decrease in myosin-specific antibody production was similar in mice treated with either drug, myosin-specific delayed-type hypersensitivity (DTH) was only reduced in the captopril-treated animals.
2. Methods
2.1. Experimental Animals
4-6 week old male A/J mice (Jackson Laboratories, Bar Harbor, ME) were housed under specific pathogen-free conditions. Mice were anesthetized by a single intraperitoneal injection of sodium pentobarbital (60 mg/kg) for each experimental manipulation. The use and care of mice were conducted in accordance with the guidelines of the Center for Comparative Medicine at Northwestern University.
2.2. Treatment Regimen
Some groups of mice were given captopril or losartan in their drinking water from initial myosin immunization to day of sacrifice. The drug solutions was changed twice per week and freshly prepared each time. Losartan tablets (Merck, Rahway, NJ) were crushed into a fine powder before use and captopril powder (the kind gift of Dr. Agostino Molteni) was used directly.
2.3. Serum ACE Activity
Serum ACE activity was determined by the spectrophotometric method of Groff et al. [34]. Briefly, the ACE-catalyzed hydrolysis of Hip-Gly-Gly was coupled to an indicator reaction, catalyzed by γ-glutamyltransferase (GGT). Gly-Gly is coupled to L-γ-glutamyl-3-carboxy-4-nitroanilide by GGT, producing a chromophore that is measured by absorbance at 410 nm over a 2 minute period in an Ultrospec 3000 UV/Visible Spectrophotometer (Pharmacia Biotech, Piscataway, NJ). ACE activity was determined by comparison of the absorbance change of each sample with that produced by defined Gly-Gly standards, and then normalized to a commercialized ACE standard (Sigma, St. Louis, MO).
2.4. Preparation of Myosin
Cardiac myosin heavy chains were purified according to the method of Shiverick et al. [35], with modifications as described [36]. Briefly, mouse hearts were minced and homogenized in 10 volumes of ice cold KCl buffer (0.3 M KCl, 0.15 M K2HPO4, 10 mM Na4P2O7, 1 mM MgCl2, pH 6.80). Myosin was extracted from the muscle homogenate by stirring at 4°C for 90 minutes. The suspension was centrifuged at 140,000 × g for 1 hour at 4°C and the decanted supernatant was diluted with 20 volumes of water and incubated at 4°C overnight to precipitate the myosin. The precipitate was collected by centrifugation at 12,000 × g for 30 minutes at 4°C and suspended in ice cold imidazole buffer (0.5 M KCl, 10 mM imidazole, 5 mM MgCl2, 5 mM Na2ATP, 2 mM DTT, pH 6.80). The solution was then centrifuged at 43,000 × g for 30 min at 4°C to remove actin. The myosin was precipitated in 8 volumes of ice cold water at 4°C overnight. The following day the precipitate was collected by centrifugation at 12,000 × g for 30 minutes at 4°C and the pellet was suspended in the imidazole buffer and centrifuged at 43,000 × g for 30 minutes at 4°C to remove residual actin. The supernatant was again precipitated overnight at 4°C in 6.5 volumes of ice cold water. The precipitate was then collected by centrifugation at 12,000 × g for 30 minutes at 4°C and suspended in 50 mM Na4P2O7, pH 6.8. Protein concentration was determined by comparing dilutions of the purified myosin solution with known concentrations of purified rabbit myosin heavy chain standards (Sigma, St. Louis, MO) by SDS-PAGE (REF). Total heart homogenate was prepared by washing A/J hearts with PBS, mincing hearts with a razor blade, and performing homogenization and lyophilization on hearts.
2.5. Immunizations
Mice were immunized with an emulsion of myosin (300 μg) in complete Freund's adjuvant (CFA), ovalbumin in CFA, or saline (PBS) in CFA, in a total volume of 0.1 ml. Three sites in the dorsal flank were given subcutaneous injections. Mice were given a second immunization of the same emulsion seven days later in an identical manner.
2.6. Histopathology
Hearts were removed, rinsed with PBS, and fixed for 24 hours in 10% buffered formalin. Fixed hearts were embedded in paraffin, sectioned, stained with hematoxylin-eosin or Masson's trichrome, and examined by light microscopy. Two sections were taken from each heart, one including both atria and the other both ventricles. Each section was examined for evidence of mononuclear and polynuclear cellular inflammation, necrosis and mineralization, and fibrosis and was assigned a histologic score and a fibrosis score between 0 (no involvement noted) to 4 (100% involvement), with 1, 2, 3 representing 25, 50, and 75% involvement of the histologic section [19] (see Fig. 1).
Fig. 1.

Histopathology scoring system. Cardiac sections from myosin-immunized mice were stained with hematoxylin and eosin and given inflammation scores of 0, 1, 2, 3, or 4 based on the indicted percentage of the tissue that was inflammation in the tissue section.
2.7. Serologic Analysis
Levels of cardiac myosin-specific IgG were analyzed by enzyme-linked immunosorbent assay (ELISA). Briefly, Maxisorp plates (Nalge Nunc, Rochester, NY) were coated overnight at 4°C with 100 μl of cardiac myosin (2.5 μg/ml) in PBS. The plates were washed with PBS containing 0.05% volume/volume Tween-20 and then blocked with 2% BSA and 5% normal goat serum. The plates were then incubated with twofold serial serum dilutions (1:10 initial dilution for 16 dilutions) for 2 hr at 37°C. After washing, peroxidase conjugated anti-mouse IgG (H+L) (0.25 μg/ml, KPL), was added for 1 hr at 37°C. The bound enzyme was detected with the 3,3′,5,5′-tetramethylbenzidine substrate (KPL), and quantitated by measurement of the A450 in a Kinetic MicroPlate Reader (Molecular Devices, Sunnyvale, CA). Endpoint dilution titers for total IgG were defined as the highest serum dilution that resulted in an absorbance value (OD450) of two standard deviations above the mean of a negative control (pooled sera from unimmunized mice) included in every plate.
2.8. Delayed-Type Hypersensitivity
Myosin-specific delayed-type hypersensitivity (DTH) was quantitated using a standard ear swelling assay. Pre-challenge ear thickness in pentobarbitol-anesthetized animals was measured with a Mitutoyo model 7326 engineer's micrometer (Mitutoyo MTI Corporation, Aurora, IL). Myosin antigen (10 μg in 0.15 M K2PO4, 0.01 Na4P2O7, 0.3 M KCl, pH 6.8) was injected intradermally into the dorsal surface of the ear using a 100 μl Hamilton syringe fitted with a 30 gauge needle. Bovine serum albumin was injected in the opposite ear as an injection control. After 24 hours, the net swelling of the injection control ear was subtracted from the net swelling of the myosin ear and expressed in units of 10-4 inch. Antigen-induced ear swelling was the result of mononuclear cell infiltration and exhibited typical DTH kinetics (i.e., minimal swelling at 4 hours, maximal swelling at 24-48 hours post-injection).
2.9. Statistical Analysis
The statistical significance of DTH, log transformed (base 2) antibody titers, and ACE activity were analyzed by one-way ANOVA followed by a 2-tailed t-test and post hoc Bonferroni analysis. Histologic scores and fibrosis scores were analyzed by the Mann-Whitney test. Histopathologic analysis was conducted on hearts of both expired and sacrificed mice unless otherwise stated. Values of p < 0.05 were considered significant. All values are expressed as mean ± SEM. The statistical significance of differences in disease incidence and histologic disease was determined by Pearson's χ2 test.
3. Results
3.1. Cardiac Hypertrophy
Mice were immunized with myosin, ovalbumin (Ova), or saline, and treated with captopril, losartan, or water. At 21 days post-immunization, heart weights (HW) and body weights (BW) were recorded and HW to BW ratios were calculated to assess cardiac hypertrophy (Table 1). However, among the myosin-immunized mice, the HW to BW ratio was only significantly lower in the captopril-treated and losartan-treated groups than in the group receiving no drug. There was also a significant reduction in HW of losartan-treated, myosin-immunized mice compared to untreated controls. Interestingly, captopril treatment, but not losartan treatment also led to a decrease in HW and BW even in mice immunized with Ova or saline, suggesting that this drug inhibits the growth of the animal generally.
Table 1. Heart Weight and Heart Weight to Body Weight Ratio are Both Reduced by Losartan and Captopril in Myosin-Immunized Mice.
| Immunization† | Treatment‡ | Body Weight (BW)
(g) |
Heart Weight (HW)
(g × 10-2) |
HW to BW Ratio
(× 10-3) |
|---|---|---|---|---|
| Myosin/CFA | Captopril (n = 18) | 18.1 ± 0.7* | 7.3 ± 0.3* | 4.0 ± 0.1* |
| Losartan (n = 19) | 20.9 ± 0.5 | 8.6 ± 0.3* | 4.2 ± 0.2* | |
| No drug (n = 20) | 20.1 ± 0.6 | 10.8 ± 0.5 | 5.5 ± 0.4 | |
| Ova/CFA | Captopril (n = 9) | 18.8 ± 1.0* | 7.5 ± 0.2* | 4.1 ± 0.1 |
| Losartan (n = 5) | 20.6 ± 0.9 | 8.7 ± 0.5 | 4.3 ± 0.3 | |
| No drug (n = 10) | 21.3 ± 0.5 | 8.4 ± 0.3 | 3.9 ± 0.1 | |
| PBS/CFA | Captopril (n = 9) | 17.8 ± 0.7* | 7.2 ± 0.2* | 4.1 ± 0.2 |
| Losartan (n = 5) | 21.9 ± 0.4 | 9.3 ± 0.7 | 4.2 ± 0.2 | |
| No drug (n = 9) | 21.9 ± 0.7 | 8.8 ± 0.3 | 4.1 ± 0.1 |
A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA).
Treatment with captopril, losartan, or water was started from day of immunization throughout the course of disease. Values shown are expressed as standard error.
p < 0.05, compared to the group receiving no drug.
3.2. Myocarditis
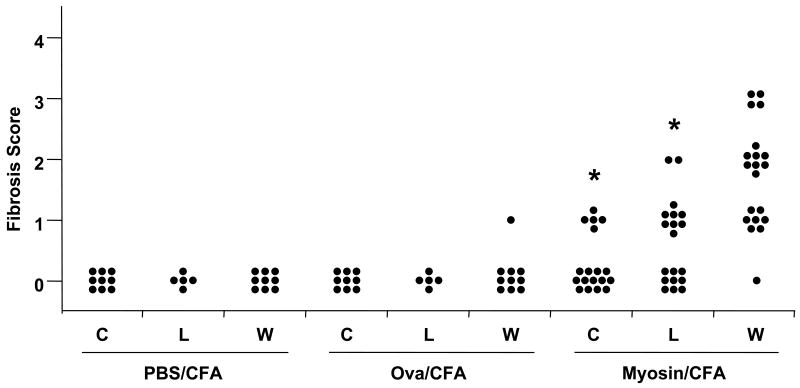
To investigate whether the gross reduction of hypertrophy corresponded to a difference in tissue inflammation, histopathologic analysis was performed on hearts removed from myosin- and saline-immunized mice at 21 days post-immunization (Fig. 1). The captopril-treated myosin-immunized mice had a significant reduction in the incidence and severity in myocarditis, consistent with previously published results [28] (Fig. 2). Disease incidence was reduced by 38%. Losartan also significantly reduced the incidence and severity of myocarditis in myosin-immunized mice, but not to the degree of the captopril treated group. Disease incidence was only reduced by 16%. Fibrosis was also significantly reduced in both the captopril and losartan-treated, myosin-immunized mice compared to control (Fig. 3). The control groups did not have any histologic findings of mononuclear cell infiltration, except for two mice in the captopril-treated, Ova-immunized group, and one mouse in the untreated Ova-immunized group. In our experience, an occasional naïve A/J mice spontaneously develops focal myocarditis. This finding is statistically insignificant.
Fig. 2.
Myocardial inflammation is reduced in both captopril-treated and losartan-treated mice immunized with myosin. A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA). Treatment with captopril (C), losartan (L), or water (W) was started from day of immunization throughout the course of disease. Mice were sacrificed on 21 d.p.i. and heart sections were stained for histologic scoring based on the scoring system shown in Fig. 1. *p < 0.001 compared to control (no treatment).
Fig. 3.
Myocardial fibrosis is reduced in both captopril-treated and losartan-treated mice immunized with myosin. A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA). Treatment with captopril (C), losartan (L), or water (W) was started from day of immunization throughout the course of disease. Mice were sacrificed on 21 d.p.i. and heart sections were stained with Masson's trichrome for fibrosis scoring based on the scoring system similar to that shown in Fig. 1 for mononuclear cell infiltration (inflammatory score). *p < 0.001 compared to control (no treatment).
3.3. Serum ACE Levels
The efficacy of captopril treatment was tested by assaying for serum ACE levels at 21 days post-immunization. Serum ACE increases in response to a decrease in Ang II, which occurs as a result of ACE inhibition by captopril [37]. As expected, captopril-treated mice had significantly higher levels of ACE than did untreated controls. However, mice treated with losartan did not show an increase in serum ACE levels (Table 2).
Table 2. Captopril, but not Losartan, Increases Serum ACE Activity.
| Immunization† | Treatment‡ (sample size) | ACE Activity (U/L) |
|---|---|---|
| Myosin/CFA | Captopril (18) | 259.6 ± 16.9* |
| Losartan (19) | 125.1 ± 4.1* | |
| No drug (20) | 109.7 ± 5.5 | |
| Ova/CFA | Captopril (9) | 272.9 ± 10.2* |
| Losartan (5) | 108.4 ± 5.4 | |
| No drug (10) | 106.0 ± 5.5 | |
| PBS/CFA | Captopril (9) | 265.3 ± 12.7* |
| Losartan (5) | 141.0 ± 7.1 | |
| No drug (9) | 133.0 ± 11.0 |
A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA).
Treatment with captopril, losartan, or water was started from day of immunization throughout the course of disease. Values shown are expressed +/- standard error of the mean.
p < 0.05, compared to the group receiving no drug.
3.4. Antigen-Specific Delayed-Type Hypersensitivity
DTH responses were investigated to help determine the role of T-cell mediated responses in reducing myocarditis in captopril-treated mice. Mice were challenged with the specific antigens Ova and myosin in Ova-immunized and myosin-immunized mice, respectively, on 20 days post-immunization. DTH responses were read on 21 days post-immunization. DTH response to myosin in captopril-treated myosin-immunized mice was significantly decreased, which is consistent with previous studies [28] (Fig. 4). Losartan-treated myosin-immunized mice challenged with myosin antigen did not have a reduction in DTH. Captopril-treated Ova-immunized mice challenged with Ova also showed a significant decrease in DTH response. Ova-immunized mice treated with losartan also did not have a significant decrease in DTH.
Fig. 4.
Losartan does not decrease myosin-specific delayed-type hypersensitivity (DTH) in myosin-immunized mice. A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA). Treatment with captopril (C), losartan (L), or water (W) was started from day of immunization throughout the course of disease. Mouse ears were challenged with 10 μg of myosin (myosin-immunized mice) or Ova (Ova-immunized mice) on day 20 post-immunization and net ear swelling was measured 24 hours later by subtracting the swelling of the contralateral ear, which was injected with 10 μg of BSA. Error bars represent standard error of the mean with sample size of at least 9 mice. * p < 0.05 compared to the group receiving water.
3.5. Antigen-Specific Antibody Production
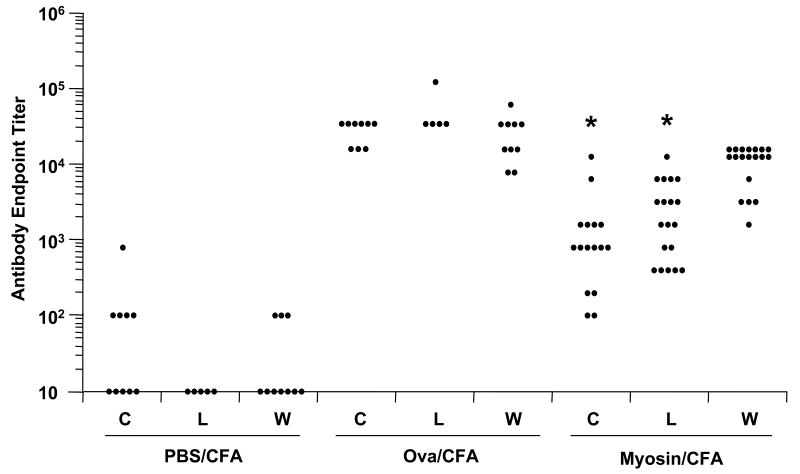
Antigen-specific antibody production was measured by ELISA to determine the effect of losartan on humoral autoimmunity. Humoral immunity to myosin or Ova are not significantly affected by captopril treatment [28]. In the current study, there was a statistically significant reduction in myosin autoantibodies in both captopril-treated and losartan-treated, myosin-immunized mice (Fig. 5). However, there was no difference in antibody production among the various Ova-immunized groups.
Fig. 5.
Myosin-specific IgG levels are decreased by both losartan and captopril. A/J mice were immunized with myosin in complete Freund's adjuvant (myosin/CFA), Ova in CFA (Ova/CFA), or saline in CFA (PBS/CFA). Treatment with captopril (C), losartan (L), or water (W) was started from day of immunization throughout the course of disease. Blood was obtained on day 21 post-immunization and serum was analyzed for antigen-specific IgG antibodies to myosin by ELISA. Serum from the Ova-immunized group was analyzed for antigen-specific IgG antibodies to Ova. *p < 0.05 compared to the group receiving water.
4. Discussion
The primary goal of this study was to compare the efficacies of ACE inhibition and Ang II receptor antagonism for the prevention of myosin-induced experimental autoimmune myocarditis. Among the various activities of captopril, ACE inhibition and its consequent reduction in Ang II receptor signaling seemed a probable mechanism by which the drug prevents myocarditis. We found that both captopril and losartan reduced myocarditis as shown by a reduction in cardiac hypertrophy and a reduction in the incidence and severity of cardiac inflammation and fibrosis. The reduction in HW and HW to BW ratio in myosin-immunized mice by both captopril and losartan was also observed in studies of encephalomyocarditis virus (EMCV) infection [14, 15, 29]. Losartan-treatment of EMCV-infected mice decreased left ventricular wall thickness and myofibrillar diameter, although there was no difference in inflammation [14]. This is likely due to the fact that EMCV infection has several pathogenetic mechanisms. The recent finding that olmesartan, another AT1R antagonist, reduced the HW to BW ratio and decreased myocarditis in a myosin-immunized rat model [32] further supports the notion that infectious and noninfectious myocarditis models are differentially modulable by these agents. Further, although captopril decreases myocarditis and DTH responses in experimental Chagas disease, HW and HW to BW ratio were not reduced [19]. The conclusion from the collective studies is that captopril and losartan decrease HW and HW to BW ratio in noninfectious models of autoimmune myocarditis but may have variable effects on these parameters in infectious disease models, depending on the existence of multiple mechanisms of tissue inflammation. Captopril reduced cell-mediated immunity as measured by a reduction of myosin DTH and Ova DTH in immunized mice, as observed previously [19, 28]. Interestingly, losartan did not reduce these DTH responses. This constitutes an important distinction between ACE inhibition and AT1R blockade and indicates that these two interventions have differential effects on cellular immunity. Our previous work demonstrated that captopril generally suppresses cell mediated immunity as evidenced by the reduction in myosin and Ova DTH [28]. However, even though the captopril-treated mice showed reduced DTH, splenocytes derived from these animals proliferated normally in response to culture ex vivo with antigen. This indicated that the reduction in cell mediated immunity was not due to a direct effect on T cell activation or effector function. Captopril may therefore reduce inflammation by inhibiting recruitment of T cells to the heart or by reducing local inflammatory processes.
The reduction in inflammation seen in the captopril-treated and losartan-treated, myosin-immunized mice could be directly due to the level of Ang II. A feedback loop operates at the level of ACE, in which a ACE inhibition leads to a decrease in Ang II, which stimulates ACE production and leads to increased levels of “ineffectual” serum ACE. As expected, ACE activity was increased in the captopril treated group to at least two times that of control groups, consistent with previously published results [28]. However, there was no increase in ACE activity in the losartan-treated group. This would suggest that Ang II levels in the losartan treated group are probably about the same as control and therefore ACE production is not induced.
Alteration of the immune response by captopril and losartan is not restricted to effects on cell-mediated immunity. Myosin-specific antibody responses were also reduced in both the captopril and losartan treated groups, although Ova-specific antibody responses in Ova-immunized mice were not. This finding has not been previously shown and contradicts previous work that showed no difference in myosin-specific antibody responses among various treatment groups [19, 28]. Others have shown a generalized decrease in antibody levels, but none of these decreases was antigen-specific [38, 39]. Two mechanisms might explain the reduction in the humoral response: (i) limitation of B cell responses via inhibition of cardiac myosin-specific B cell expansion, or (ii) limitation of secretion of cardiac myosin-specific IgG antibodies. Since there was a reduction in the myosin-specific antibody response but not in the Ova-specific antibody response, this might suggest that heart damage induced by the myosin-specific T cell response boosted the magnitude of the B cell response above that stimulated by immunization alone. If an overall decrease in the B cell response was due to a decrease in cell differentiation and migration, then Ova-specific antibody production would also have been reduced. The role of T cell-B cell interactions and the participation of specific cytokines were not examined in this study.
The exact mechanism(s) by which captopril reduces autoimmune myocarditis is not known. By blocking a step further downstream of ACE in the renin-angiotensin pathway, we hoped to elucidate the role of Ang II signal interference in the reduction of autoimmune myocarditis by captopril. Although Ang II receptor blockade clearly reduces myocarditis, the effects of captopril and losartan on all immunologic parameters are not superimposable. Ang II is involved in the chemotaxis and adhesion of monocytes and macrophages [10]. Therefore, Ang II may upregulate chemotactic factors important for attracting immune cells to the affected tissue and may induce expression of molecules that enable cells to adhere to endothelium. Ang II has also been shown to modulate the release of cytokines, including IL-6, IL-1β, IL-5, and TNF-α [10]. By blocking the Ang II receptors with losartan, chemotaxis and cytokine release would theoretically be decreased or inhibited and thus cause a decrease in myocarditis.
The other major function of captopril is to inhibit bradykinin degradation by ACE [40]. Not only does bradykinin promote vasodilatation, but also it has additional, multiple cardioprotective effects [41-43], including inhibition of fibrosis [44]. Thus, some of the differences in the reduction of inflammation by captopril and losartan may partly be due to captopril's additional effects on the kallikrein-kinin system. Other ACE inhibitors such as enalapril have also been shown to have a beneficial effect in many of these models [10]. There are, however, differences in effect of the various ACE inhibitors. The sulfhydryl group on captopril has the additional function of a free radical scavenger, reducing the amount of oxidative injury to the myocardium [45].
In conclusion, captopril and losartan are effective at reducing myosin-induced experimental autoimmune myocarditis, although losartan was somewhat less effective. The reduction in cardiac hypertrophy is likely due to a reduction in inflammation, myocyte necrosis, and consequent reparative fibrosis. DTH was reduced by captopril but not by losartan. Myosin-specific antibody levels are reduced with both captopril and losartan treatment, but again to a lesser degree with losartan. These collective results suggest that inhibition of Ang II receptor signaling is a major mechanism by which captopril prevents autoimmune myocarditis, although additional effects, including modulation of bradykinin degradation, are likely to participate as well. These results are consistent with those of additional studies demonstrating the beneficial effects of these agents on various cardiomyopathies [11, 12], infection-induced myocarditides [13-15], and experimental autoimmune diseases [36, 45].
Acknowledgments
This work was supported in part by grants from the United States Public Health Service. J.S. Leon was supported by a Predoctoral Fellowship from the American Heart Association. Captopril was the kind gift of Dr. Agostino Molteni (University of Missouri, Kansas City).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pisani B, Taylor DO, Mason JW. Inflammatory myocardial diseases and cardiomyopathies. Am J Med. 1997;102:459–469. doi: 10.1016/S0002-9343(97)00331-8. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl U, Schultheiss HP. Treatment of chronic myocarditis with corticosteroids. Eur Heart J. 1995;16 O:168–172. doi: 10.1093/eurheartj/16.suppl_o.168. [DOI] [PubMed] [Google Scholar]
- 5.Caforio AL, McKenna WJ. Recognition and optimum management of myocarditis. Drugs. 1996;52:515–525. doi: 10.2165/00003495-199652040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhoff LV. Chagas disease. American trypanosomiasis. Inf Dis Clin North Am. 1993;7:487–502. [PubMed] [Google Scholar]
- 7.Wenger NK, Abelmann WH, Roberts WC. In: Myocarditis in The Heart. Hurst JW, Schlant RC, editors. McGraw Hill; New York, NY: 1990. pp. 1256–1277. [Google Scholar]
- 8.Dec GW, DeSanctis RW. In: Cardiomyopathies, Chapter XIV in Scientific American Medicine. Dale DC, Federman DD, editors. Scientific American Inc.; New York, NY: 1998. pp. 1–21. [Google Scholar]
- 9.Migdalof BH, Antonaccio MJ, McKinstry DN, Singhvi SM, Lan SJ, Egli P, Kripalani KJ. Captopril: pharmacology, metabolism and disposition. Drug Metab Rev. 1984;15:841–869. doi: 10.3109/03602538409041080. [DOI] [PubMed] [Google Scholar]
- 10.Godsel LM, Leon JS, Engman DM. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists in experimental myocarditis. Curr Pharm Des. 2003;9:723–735. doi: 10.2174/1381612033455440. [DOI] [PubMed] [Google Scholar]
- 11.Plosker GL, McTavish D. Captopril. A review of its pharmacology and therapeutic efficacy after myocardial infarction and in ischaemic heart disease. Drugs Aging. 1995;7:226–253. doi: 10.2165/00002512-199507030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Di Bianco R. Captopril in the treatment of congestive heart failure. Herz. 1987;12 1:27–37. [PubMed] [Google Scholar]
- 13.Suzuki H, Matsumori A, Matoba Y, Kyu BS, Tanaka A, Fujita J, Sasayama S. Enhanced expression of superoxide dismutase messenger RNA in viral myocarditis. An SH-dependent reduction of its expression and myocardial injury. J Clin Invest. 1993;91:2727–2733. doi: 10.1172/JCI116513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda T, Araki M, Nakano M, Imai S, Suzuki T, Murata K, Kobayashi I. Chronic effect of losartan in a murine model of dilated cardiomyopathy: comparison with captopril. J Pharmacol Exp Ther. 1995;273:955–958. [PubMed] [Google Scholar]
- 15.Araki M, Kanda T, Imai S, Suzuki T, Murata K, Kobayashi I. Comparative effects of losartan, captopril, and enalapril on murine acute myocarditis due to encephalomyocarditis virus. J Cardiovasc Pharmacol. 1995;26:61–65. doi: 10.1097/00005344-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Takada H, Kishimoto C, Hiraoka Y, Kurokawa M, Shiraki K, Sasayama S. Captopril suppresses interstitial fibrin deposition in coxsackievirus B3 myocarditis. Am J Physiol. 1997;272:H211–219. doi: 10.1152/ajpheart.1997.272.1.H211. [DOI] [PubMed] [Google Scholar]
- 17.Rezkalla S, Kloner RA, Khatib G, Khatib R. Beneficial effects of captopril in acute coxsackievirus B3 murine myocarditis. Circulation. 1990;81:1039–1046. doi: 10.1161/01.cir.81.3.1039. [DOI] [PubMed] [Google Scholar]
- 18.Rezkalla S, Kloner RA, Khatib G, Khatib R. Effect of delayed captopril therapy on left ventricular mass and myonecrosis during acute coxsackievirus murine myocarditis. Am Heart J. 1990;120:1377–1381. doi: 10.1016/0002-8703(90)90251-r. [DOI] [PubMed] [Google Scholar]
- 19.Leon JS, Wang K, Engman DM. Captopril ameliorates myocarditis in acute experimental Chagas disease. Circulation. 2003;107:2264–2269. doi: 10.1161/01.CIR.0000062690.79456.D0. [DOI] [PubMed] [Google Scholar]
- 20.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 21.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- 22.Smith SC, Allen PM. The role of T cells in myosin-induced autoimmune myocarditis. Clin Immunol Immunopathol. 1993;68:100–106. doi: 10.1006/clin.1993.1103. [DOI] [PubMed] [Google Scholar]
- 23.Bachmaier K, Pummerer C, Shahinian A, Ionescu J, Neu N, Mak TW, Penninger JM. Induction of autoimmunity in the absence of CD28 costimulation. J Immunol. 1996;157:1752–1757. [PubMed] [Google Scholar]
- 24.Neu N, Pummerer C, Rieker T, Berger P. T cells in cardiac myosin-induced myocarditis. Clin Immunol Immunopathol. 1993;68:107–110. doi: 10.1006/clin.1993.1104. [DOI] [PubMed] [Google Scholar]
- 25.Penninger JM, Neu N, Timms E, Wallace VA, Koh DR, Kishihara K, Pummerer C, Mak TW. The induction of experimental autoimmune myocarditis in mice lacking CD4 or CD8 molecules. J Exp Med. 1993;178:1837–1842. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkiel S, Factor S, Diamond B. Autoimmune myocarditis does not require B cells for antigen presentation. J Immunol. 1999;163:5265–5268. [PubMed] [Google Scholar]
- 27.Neu N, Ploier B, Ofner C. Cardiac myosin-induced myocarditis: heart autoantibodies are not involved in the induction of the disease. J Immunol. 1990;145:4094–4100. [PubMed] [Google Scholar]
- 28.Godsel LM, Leon JS, Wang K, Fornek JL, Molteni A, Engman DM. Captopril prevents experimental autoimmune myocarditis. J Immunol. 2003;171:346–352. doi: 10.4049/jimmunol.171.1.346. [DOI] [PubMed] [Google Scholar]
- 29.Baba T, Kanda T, Kobayashi I. Reduction of cardiac endothelin-1 by angiotensin II type 1 receptor antagonist in viral myocarditis of mice. Life Sci. 2000;67:587–597. doi: 10.1016/s0024-3205(00)00654-8. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka A, Matsumori A, Wang W, Sasayama S. An angiotensin II receptor antagonist reduces myocardial damage in an animal model of myocarditis. Circulation. 1994;90:2051–2055. doi: 10.1161/01.cir.90.4.2051. [DOI] [PubMed] [Google Scholar]
- 31.Gardner PL, Mbuy GN, Knabb MT. Effects of the angiotensin II receptor antagonist losartan on herpes simplex virus-type 2 infection of cultured vero and cardiac neonatal myocytes. Life Sci. 1994;55:283–289. doi: 10.1016/0024-3205(94)00730-6. [DOI] [PubMed] [Google Scholar]
- 32.Nimata M, Kishimoto C, Yuan Z, Shioji K. Beneficial effects of olmesartan, a novel angiotensin II receptor type 1 antagonist, upon acute autoimmune myocarditis. Mol Cell Biochem. 2004;259:217–222. doi: 10.1023/b:mcbi.0000021379.82282.53. [DOI] [PubMed] [Google Scholar]
- 33.Tachikawa H, Kodama M, Hui L, Yoshida T, Hayashi M, Abe S, Kashimura T, Kato K, Hanawa H, Watanabe K, Nakazawa M, Aizawa Y. Angiotensin II type 1 receptor blocker, valsartan, prevented cardiac fibrosis in rat cardiomyopathy after autoimmune myocarditis. J Cardiovasc Pharmacol. 2003;41:S105–110. [PubMed] [Google Scholar]
- 34.Groff JL, Harp JB, DiGirolamo M. Simplified enzymatic assay of angiotensin-converting enzyme in serum. Clin Chem. 1993;39:400–404. [PubMed] [Google Scholar]
- 35.Shiverick KT, Thomas LL, Alpert NR. Purification of cardiac myosin: application to hypertrophied myocardium. Biochim Biophys Acta. 1975;393:124–133. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- 36.Leon JS, Godsel LM, Wang K, Engman DM. Cardiac myosin autoimmunity in acute Chagas heart disease. Infect Immun. 2001;69:5643–5649. doi: 10.1128/IAI.69.9.5643-5649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 38.Delfraissy JF, Galanaud P, Balavoine JF, Wallon C, Dormont J. Captopril and immune regulation. Kidney Int. 1984;25:925–929. doi: 10.1038/ki.1984.111. [DOI] [PubMed] [Google Scholar]
- 39.Tarkowski A, Carlsten H, Herlitz H, Westberg G. Differential effects of captopril and enalapril, two angiotensin converting enzyme inhibitors, on immune reactivity in experimental lupus disease. Agents Actions. 1990;31:96–101. doi: 10.1007/BF02003227. [DOI] [PubMed] [Google Scholar]
- 40.Gavras H, Gavras I. Cardioprotective potential of angiotensin converting enzyme inhibitors. J Hypertens. 1991;9:385–392. doi: 10.1097/00004872-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 42.Tschope C, Gohlke P, Zhu YZ, Linz W, Scholkens B, Unger T. Antihypertensive and cardioprotective effects after angiotensin-converting enzyme inhibition: role of kinins. J Card Fail. 1997;3:133–148. doi: 10.1016/s1071-9164(97)90047-6. [DOI] [PubMed] [Google Scholar]
- 43.Erdos EG, Marcic BM. Kinins, receptors, kininases and inhibitors -- where did they lead us? Biol Chem. 2001;382:43–47. doi: 10.1515/BC.2001.007. [DOI] [PubMed] [Google Scholar]
- 44.Fujii M, Wada A, Ohnishi M, Tsutamoto T, Matsumoto T, Yamamoto T, Takayama T, Dohke T, Isono T, Eguchi Y, Horie M. Endogenous bradykinin suppresses myocardial fibrosis through the cardiac-generated endothelin system under chronic angiotensin-converting enzyme inhibition in heart failure. J Cardiovasc Pharmacol. 2004;44:S346–S349. doi: 10.1097/01.fjc.0000166265.09550.7c. [DOI] [PubMed] [Google Scholar]
- 45.de Cavanagh EM, Fraga CG, Ferder L, Inserra F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am J Physiol. 1997;272:R514–518. doi: 10.1152/ajpregu.1997.272.2.R514. [DOI] [PubMed] [Google Scholar]