Abstract
Sciatic nerve injury and dysfunction is not an uncommon cause of lower extremity symptoms in a musculoskeletal practice. We present the case of a man who presented with lower extremity weakness, pain, and cramps, and was initially diagnosed at an outside institution with bilateral S1 radiculopathies and recommended for spine surgery. He came to us for a second opinion. Electrodiagnostic testing revealed an isolated sciatic neuropathy and the patient was referred for imaging, which showed a sciatic nerve sheath tumor. Review of the literature on sciatic neuropathies shows that there can be many possible etiologies of sciatic nerve dysfunction, but that hip arthroplasty continues to be the leading risk factor. Sciatic nerve tumors are not commonly described in the literature and their definitive management remains unclear.
Key words: Sciatic nerve, Lumbar radiculopathy, Electrodiagnostics, Electromyography, Nerve tumors
Case discussion
The subject is a 52-year-old man with a 16-year history of mild, intermittent low back pain who presented with a chief complaint of pain, cramps, and weakness in the left lower extremity. He complained of a 2-month history of persistent pain in the upper thigh–lower buttock region with pain radiating to the left leg, calf, and toes. His pain ranged from 6 to 9 on a 10-point scale, with worse pain when lying down, flexing, walking uphill, and bending. Pain lessened with rest and short periods of stretching. He denied any specific complaint of back pain, numbness, and changes in bowel or bladder function. He denied any constitutional symptoms.
The patient had his first episode of back pain 16 years earlier, at which time he responded to a treatment of acupuncture and stretching. He had occasional recurrent symptoms but did well until 4 years ago, when he began to experience pain predominantly down the right lower limb and sciatica. He underwent a course of physical therapy and trigger point injections and his symptoms resolved. He was then relatively well until the current episode.
The patient underwent electrodiagnostic testing several weeks after the onset of his presenting symptoms. As per written reports presented by the patient, abnormal spontaneous activity was detected in isolation in the right and left gastrocnemius muscles. There was no involvement of any of the proximal muscles including the paraspinal muscles. The patient was diagnosed with bilateral S1 radiculopathies. A recent magnetic resonance imaging (MRI) showed mild disk bulges and disk degeneration most pronounced at the L4/5 level, without significant foraminal or canal compromise. This study, in comparison to a study performed 4 years earlier during the patient's prior episode of right-sided sciatic complaints, showed no significant changes.
Except for this main complaint, the patient's medical history was significant only for hypertension and partial amputation of the left thumb secondary to trauma. The patient had no medication allergies and was taking p.r.n. narcotic pain medications, antihypertensive pain medication, and Neurontin for symptom control of paresthesias. His family history was significant for cancer. He was a married nonsmoker who did not drink alcohol. The patient's symptoms limited his work to several hours of desk work a day.
During physical examination, the patient ambulated with a nonantalgic gait. All dural tension signs were negative. Lumbar flexion and extension were minimally restricted and without end range pain. Muscle bulk was symmetric in the upper extremities and asymmetric in the lower extremities with significant left calf atrophy, and with no fasciculations. Overall tone was normal. Strength was measured on MRC scale as 4/5 in the left ankle dorsiflexors, 4+/5 in the left hamstrings, and 4+/5 in the left hip abductors with normal strength in all other muscle groups tested in the bilateral upper and lower extremities. Deep tendon reflexes were 2+/4 in bilateral quadriceps, 2+/4 in the right Achilles, and 1+/4 in the left Achilles. Deep tendon reflexes were 2+/4 in the left upper extremity and 1+/4 in the right upper extremity. Sensation was diminished to pinprick along the lateral aspect of the left foot, the fifth toe, and the left heel. Vibratory sensation with a 256-MHz tuning fork appeared to be normal. Position sense was normal. Tibial pulses were 2+ bilaterally, and capillary refill was normal. There was no peripheral edema.
After initial evaluation and examination, the patient was referred for electrodiagnostic testing with an initial diagnosis of left S1 radiculitis vs sciatic neuropathy. Electrodiagnostic testing was performed 1 week after initial examination. Nerve conduction studies showed an absent left sural sensory response, normal right sural, and bilateral superficial peroneal sensory amplitudes. The left peroneal and tibial motor amplitude were normal. The left peroneal and tibial F waves were normal. Needle electromyographic examination showed abnormal spontaneous activity in the form of positive sharp waves and fibrillation potentials with normal motor unit configuration and reduced recruitment in muscles corresponding to the left sciatic nerve, including the left long head of the biceps femoris, extensor digitorum brevis, tibialis anterior, extensor hallucis longus, first dorsal interossei (foot intrinsics), and medial gastrocnemius. The left gluteus maximus, tensor fascia lata, and paraspinal muscles were normal. Electromyographic examination of muscles in the right lower extremity showed normal results.
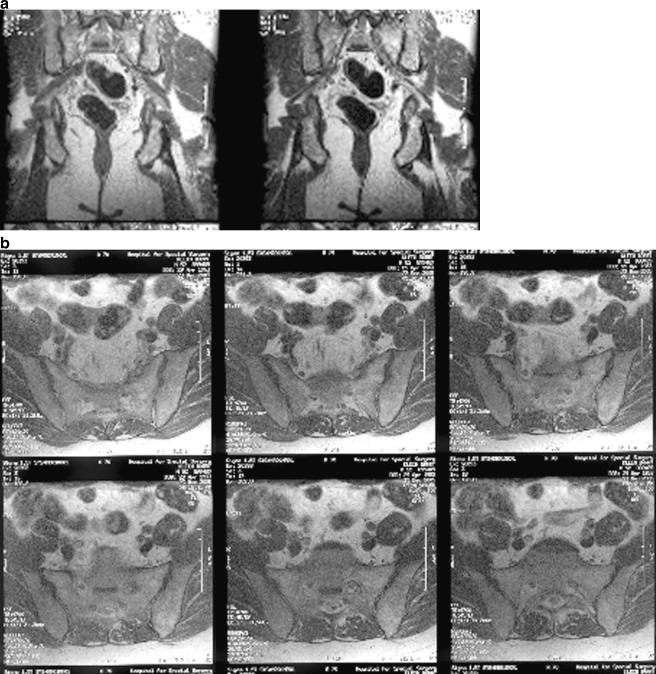
Electrodiagnostic impression was of a left sciatic neuropathy. The patient was referred for a noncontrast MRI of the pelvis with attention to the left sciatic nerve. In these images, a 2.8-cm intermediate focus of abnormal signal in the region of the left S1 neural foramen and around the nerve as it exits from the sacrum was observed (Fig. 1). The nerve adjacent to this was thickened with lobulation anteriorly, high signal adjacent to the bone, and high signal extending through the distal course of the left sciatic nerve, without distal thickening. Further MRI of the pelvis with contrast was obtained and this showed a 2.4-cm area of enhancement and thickening of the left S1 nerve with nodular areas of enhancement on the posterior aspect (Fig. 2). These findings were deemed to suggest the presence of a nonaggressive nerve sheath tumor although the pattern of enhancement given the two locations was not entirely typical.
Fig 1.
MRI pelvis, non-contrast: (a) coronal and (b) axial images of the pelvis show a 2.8-cm intermediate focus of abnormal signal probably representing a mass associated with a thickened left S1 nerve as it courses out of the foramen. Sciatic nerve shows high signal extending through its distal course, but no distal thickening
Fig 2.
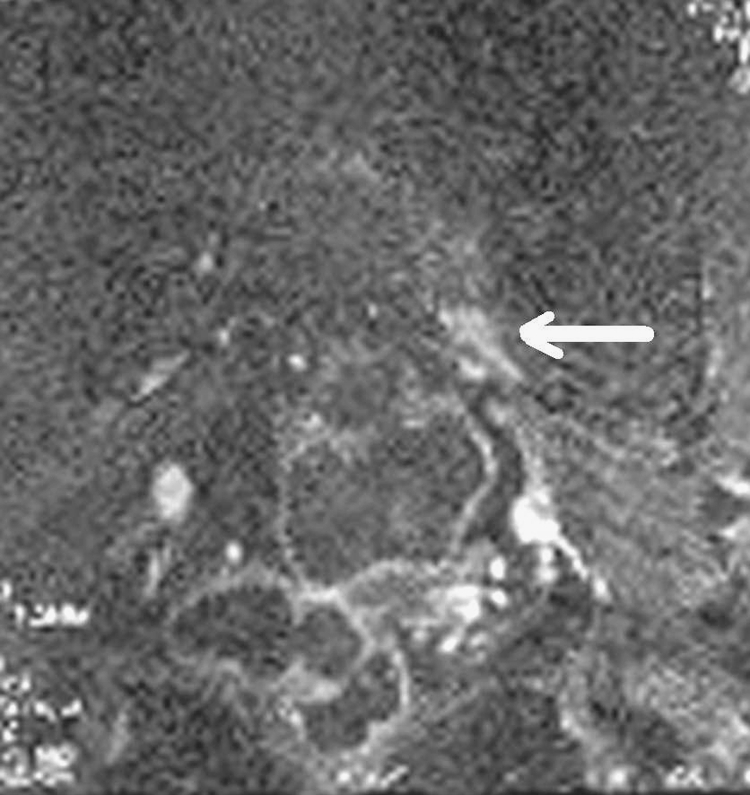
MRI pelvis with contrast: coronal fat-sat image of the pelvis shows posterior and anterior enhancement of the left S1 nerve and left sciatic nerve (white arrow) suggestive of a nonaggressive nerve sheath tumor
Consultations with surgical pathologists and orthopedics were conducted to discuss management options for this patient. The patient's symptoms remained stable during this time. Upon review of the case and presentation in multidisciplinary grand rounds, it was felt that the tumor was most likely a benign neural sheath tumor. Surgical approach to this nerve sheath tumor involved significant risk to adjacent structures, and tissue diagnosis was deferred. Conservative management was advocated with repeat electrodiagnostic examination at 3 months showing no worsening of nerve function, stable gait, and stable neurologic deficits. The patient would be monitored every 4–5 months and intervention planned if functional deficits or lower extremity pain worsens.
Etiologies of sciatic neuropathy
The sciatic nerve derives its nerve fibers from the L4, L5, S1, S2, and S3 nerve roots. The sciatic nerve arises from the lumbosacral plexus and is composed of two distinct trunks: the lateral (peroneal division) and the medial (tibial division). The peroneal and the tibial divisions lie next to each other to form the sciatic nerve. The divisions physically separate from each other at the midthigh to the distal thigh to form the common peroneal and tibial nerves. Occasionally, the divisions separate at the proximal thigh [1].
Sciatic neuropathy can be the result of any focal lesion of the nerve in the hip or thigh, distal to the lumbosacral plexus but proximal to the separation of the nerve into its distal branches. The lesion can involve demyelinative injury, axonal injury, mixed axonal and demyelinative injury, or partial or complete nerve discontinuity [2]. Etiologies of sciatic neuropathy can include traumatic, compressive, ischemic, neoplastic, or idiopathic etiologies. Traumatic injuries can include injury to the sciatic nerve in association with femur fracture, hip dislocation or fracture, laceration, gunshot wound, or posterior thigh compartment syndrome [1, 3, 4, 24–26]. Compressive injuries can include compression from compartment syndrome, hematoma, hamstring injuries, fibrous bands, persistent sciatic artery, or controversially, from piriformis syndrome [6, 7, 26–35]. Reported injuries relating to the perioperative period include injury from ischemia and positioning during cardiac surgery, lithotomy position, vaginal delivery, prolonged sitting as in some craniotomy or other neurosurgical operative positions, or nerve injury from a variety of causes during and after hip arthroplasty [1, 14, 15, 36–41]. In a review of reported cases of sciatic neuropathy in the English literature from 1966 until 1997, 33% (167/492) of reported cases were attributable to or related to hip surgery and represented the most often reported cause of sciatic neuropathy [3]. Other commonly reported causes included injury relating to intragluteal injection (28%), hip fracture or dislocation (10%), external compression (8%), and benign or malignant tumors (8%) [3, 19].
Yuen and Onley [5] examined the clinical features of 73 consecutive patients referred for electrodiagnostic evaluation of sciatic neuropathy. Etiologies of these 73 cases included hip arthroplasty (21.9%), acute external compression (13.7%), infarction (9.6%), gunshot wound (9.6%), hip fracture/dislocation (9.6%), femur fracture (4.1%), contusion (4.1%), and uncertain causes (16.4%). Moderate or better recovery, defined as improvement to MRC grade 2/5 strength or improvement of two grades on the MRC scale, occurred in most patients (30% by 1 year, 50% by 2 years, and 75% by 3 years) [5]. An initial absence of paralysis of muscles controlling ankle dorsiflexion and plantar flexion was a significant (p < 0.05) predictor of earlier or better clinical recovery.
Severe, traumatic, or refractory sciatic neuropathy that does not show improvement over time may be referred for surgical exploration. In a retrospective series of 353 surgically treated traumatic buttock and thigh level sciatic nerve lesions from 1968 until 1999, Kim et al [4] found an equal proportion of buttock- and thigh-level injuries. Of these, gunshot wounds represented 22% of all injuries; intramuscular intragluteal injections of analgesic and antiemetic drugs or antibiotics, steroids, or local anesthetic agents represented 18% (all buttock level injuries); whereas stretch injury associated with hip and femoral fractures and dislocations accounted for 17% of all injuries. In this series, hip arthroplasty accounted for less than 5% of the cases. The relatively decreased incidence of hip arthroplasty cases in this surgical series may be related to the higher prevalence of gunshot wounds and intragluteal injections in earlier decades, increasing prevalence of hip arthroplasty in recent years, and—if we assume equal referral patterns of lesions due to hip arthroplasty and other causes of sciatic neuropathy—perhaps fewer refractory cases of sciatic neuropathy after hip arthroplasty compared with other traumatic etiologies.
Differential diagnosis of sciatic neuropathy
Sciatic neuropathy may present with motor and sensory deficits that may mimic other neuropathies and radiculopathies in the lower extremity. A complete sciatic nerve lesion, proximal to the innervation to the hamstrings, can present with sciatic distribution pain and paresthesias, difficulty with knee flexion, and a flail foot with loss of dorsiflexion, plantarflexion, invertors, and evertors [4]. Sensory loss in a complete lesion involves the posterior thigh, lower lateral leg, and the entire foot [4]. These findings may also occur with lumbosacral plexus lesions or polyradiculopathy. Sciatic injury that spares the hamstrings will allow the patient to extend, lock, and flex the leg, but assistance from orthotics is often required for functional gait because of loss of dorsiflexion and impaired plantarflexion [4]. Sensory and motor deficits in more distal injuries will be more focal and may mimic tibial or peroneal mononeuropathies or L5 and S1 radiculopathies. In these cases, careful examination of muscles in the buttocks innervated by the L5 and S1 nerve roots, but not innervated by the sciatic nerve, can help differentiate between diagnoses. When physical examination and clinical history are not conclusive, electrodiagnostic evaluation may help localize the nerve injury. The utility of emerging radiographical techniques such as MR neurography and visualization of denervated muscles by gadolinium-enhanced MRI has yet to be established in studies of sufficient numbers of patients [8, 9].
In the general population, sciatic neuropathy as a cause of lower extremity weakness or sensory deficit is considerably less common than other etiologies. In a retrospective series of 303 consecutive patients evaluated for footdrop over an 18-month period in one electrodiagnostic laboratory, etiologies of footdrop consisted of 31% central nervous system causes and 68% peripheral nervous system causes [10]. Among the peripheral nervous system causes, the most important subgroups included common peroneal nerve lesions (30.6%), L5 radiculopathies (19.7%), and polyneuropathies (18.3%). Pure sciatic neuropathies were, as expected, an uncommon diagnosis in routine electrodiagnostic studies. Separately, Van Langenhove et al also examined cases of sciatic nerve neuropathy seen over a 7-year period in one electrodiagnostic laboratory. Three fourths of sciatic neuropathies were attributable to traumatic causes, and the majority of cases involved the peroneal division of the sciatic nerve [10].
It is generally accepted that the peroneal division of the sciatic nerve is more commonly and often more severely affected than the tibial division in sciatic nerve injury. Reasons for susceptibility of the peroneal division to injury have been discussed in the literature [1–4, 10–12]. Anatomic reasons for susceptibility of the peroneal division in traumatic and compressive injuries include its superficial location relative to the tibial division in the hip and proximal thigh, and its fewer and larger fascicles and less supportive endoneurium and perineurium relative to the tibial division. The peroneal division also has a smaller blood supply compared with the tibial division. In addition, the peroneal division is securely fixed at both the sciatic notch and the fibular neck, making it more susceptible to stretch injuries. The tibial division is only fixed at the sciatic notch, and not as tightly secured distally. The tibial division primarily innervates two large, bulky muscle groups—the hamstrings and the gastrocnemius–soleus complex—which are thought to need less reinnervation after injury to produce functional muscle contraction [4]. In contrast, the peroneal division innervates the long, relatively thin extensor muscles of the anterior compartment of the leg, requiring coordinated nerve input for effective muscle contraction to produce functional dorsiflexion during gait [4]. Coordinated nerve input may be less likely to occur through reinnervation after nerve injury.
Because the peroneal division of the sciatic nerve is more commonly and severely affected than the tibial division, sciatic neuropathy can be difficult to distinguish from a peroneal neuropathy [1]. This difficulty is especially evident when nerve conduction abnormalities of the tibial or the sural nerve or electromyographic findings in the short head of the biceps (the only muscle proximal to the knee that is innervated by the peroneal division) are mild.
Electrodiagnostic evaluation of sciatic neuropathy
When clinical history and physical examination are inconclusive, electrodiagnostic evaluation can help establish a diagnosis of sciatic neuropathy and differentiate it from other, more common nerve pathologies. In suspected cases of sciatic neuropathy, electrodiagnosis can also help establish the severity and the chronicity of the lesion. In our case, careful electrodiagnostic examination was essential in differentiating our findings for our patient from the incorrect referring diagnosis of bilateral S1 radiculopathies.
In general, sensory nerve conduction studies are expected to be abnormal in sciatic neuropathy or sacral plexopathy and normal in root lesions or radiculopathy. The superficial peroneal sensory and sural sensory amplitudes will often be reduced. Motor amplitudes may be reduced in severe cases of both radiculopathies and plexopathies, but all distal conduction velocities will be normal. If the peroneal division is more severely affected, sensory amplitudes may be close to normal in the sural nerve. In cases of suspected lumbar plexopathy, saphenous nerve conduction studies may be used to investigate L4 nerve root or lumbar plexus involvement. Needle examination in a study of peroneal division sciatic neuropathy should include a study of the short head of the biceps femoris to differentiate sciatic neuropathy from peroneal mononeuropathy. Needle findings in sciatic neuropathy may include only subtle findings in tibial-innervated muscle, depending on the extent of involvement of the tibial division. In cases of suspected radiculopathy, the paraspinal muscles and gluteal muscles innervated by the involved nerve root are usually involved. These muscles are typically spared with a sciatic neuropathy although a very proximal lesion involving the lumbosacral plexus may affect the proximal gluteal muscles. Chronicity of nerve injury may be determined by examination of the amplitude, duration, and configuration of the motor unit.
Yuen et al [13] studied the electrodiagnostic features of sciatic neuropathy in 100 consecutive patients seen in their electrodiagnosis laboratory. Ninety-three percent showed evidence of significant axonal loss and 7% of patients had evidence of solely demyelinative injury. The peroneal division was more severely affected than the tibial division in 64% of cases and equally affected in 38%. In only eight cases was the tibial division more severely affected. Seven of these eight cases were due to gunshot wounds or femur fracture. Ninety-two percent of cases demonstrated abnormal tibialis anterior electromyograms (EMGs). A recordable compound muscle action potential of the extensor digitorum brevis at initial examination was significantly associated (p < 0.025) with earlier or better recovery of nerve function [5].
Nerve palsy and hip arthroplasty
Despite the advances in surgical technique, hip arthroplasty remains a significant risk factor for sciatic neuropathy. The prevalence of nerve palsy associated with total hip replacement varies by institution but is generally low. Schmalzried et al [14] reviewed 32 studies from 1966 until 1996 including 34,335 hip arthroplasties, and found 359 reported cases of nerve palsy for an overall prevalence of 1%. Incidence of nerve injury reported varied from 0.08% to a high of 7.5% reported in series of revision cases. Twenty-eight of the studies reviewed by Schmalzried et al detailed which specific nerve was injured during arthroplasty. Of these, 52% of injuries occurred in peroneal nerves, 28% in sciatic nerves, and 13% in femoral nerves. Based on the data, the authors cited a threefold higher risk of nerve palsy in revision surgery and a twofold higher risk of nerve palsy in women.
A recent review by the Mayo Clinic [15] examined data from 27,004 primary total hip arthroplasties performed at the Mayo Clinic from 1970 until 2000. Forty-seven patients (0.17%) with documented postoperative motor nerve dysfunction were identified. Twenty-six of these nerve injuries were diagnosed within 24 h of surgery. The remaining cases were diagnosed 2–74 days postoperatively. Hip arthoplasties performed for posttraumatic arthritis or developmental hip dysplasia were almost four times more likely to have postoperative nerve palsy. The use of a posterior approach, lengthening of the extremity, or cementless femoral fixation was also associated with a significantly increased risk of postoperative motor nerve palsy. Thirty-six percent of patients with complete motor nerve palsy had complete recovery of their strength over an average of 21.1 months. The Mayo Clinic data represent the largest series and one of the lowest incidences of hip arthoplasty and associated nerve palsy reported in the literature. The low incidence of postoperative motor nerve palsy contrasts with work carried out by Weber et al [16] in 1976 and repeated by Weale et al [17] in 1996 showing an incidence of subclinical nerve injury of up to 70%. The difference may lie in the distinction between subclinical nerve injury that resolves transiently and improves with rehabilitation, and with documented nerve palsy that is less likely to recover.
Multiple etiologies for sciatic nerve injury in association with hip arthroplasty are cited in the literature. In Schmalzried et al's [14] meta-analysis, approximately half of the cases of perioperative nerve palsy were unexplained. Direct trauma, tension, and bleeding or hematomas accounted for 19%, 20%, and 11% of cases, respectively [14]. In the Mayo Clinic series, 45% (21/47) cases were of unknown etiology. Presumptive etiologies in the remaining cases were hematoma (17%), limb lengthening (17%), traction or retractors (15%), partial laceration (4%), and compressive dressing (2%). Other etiologies for nerve palsy, including sciatic nerve palsy, after hip arthroplasty have been proposed. These include direct nerve injury, injury from retractors, and postoperative hematoma [14, 15, 21, 29, 57]. Delayed-onset sciatic injury from methylmethacrylate [49], trochanteric wire [48], prosthetic dislocation [57], acetabular reinforcement rings [21, 47], and wear debris causing an inflammatory reaction and cystic masses compressing the sciatic nerve [18] have also been reported. A recent report suggests that intraoperative retraction of a piriformis muscle through which sciatic nerve fibers penetrate as an anatomic variant could cause what has before been viewed as idiopathic cases of postoperative sciatic neuropathy [22].
The risk factors for a sciatic nerve injury in patients undergoing hip athroplasty have not been established. They are probably multifactorial, but we believe that underlying spine pathology such as foraminal and/or spinal stenosis may make the sciatic nerve more susceptible to any manual traction.
Sciatic nerve tumors
Neoplastic involvement of the sciatic nerve has been reported in soft-tissue malignancies [50, 51], sarcoidosis [52], lymphoma [53, 54], and infiltrating intermuscular lipoma [46]. Primary tumors of the sciatic nerve include schwannomas, neurofibromatosis, neurolymphomatosis, and malignant neurofibrosarcomas [20, 23, 42–45, 55, 56]. Although many case reports appear in the literature on neoplastic involvement of the sciatic nerve, there are few published reports on long-term outcomes or comparisons of conservative and aggressive management options for primary nerve tumors.
A series from the Mayo Clinic documents 35 cases of neurogenic tumors of the sciatic nerve [21]. Early surgical therapy was advised including simple enucleation for neurilemomas, total or subtotal excision of neurofibromas with preservation of the nerve trunk, and disarticulation of the hip joint or hindquarter amputation in the highly malignant neurofibromas [21]. In another series from the University of Pennsylvania, six cases of schwannomatosis, multiple schwannomas in the peripheral or central nervous system without evidence of neurofibromatosis, some of which involved the sciatic nerve, were retrospectively reviewed. Surgery was indicated for symptomatic lesions, while asymptomatic lesions were advised to be followed conservatively [23].
Benzel et al [20] retrospectively described eight patients with sciatic nerve and sacral plexus tumors. These patients were divided into four groups: group I tumors were in a subgluteal or thigh location, group II tumors involved the neuroforamen, group III tumors were intrapelvic with extension into the thigh, and group IV tumors were intrapelvic without extension into the thigh. All four group I patients in Benzel et al's series underwent surgical debulking or excision of the tumor without sequelae. The authors recommend a surgical approach that preserves all but one of the nerve fascicles by sequentially peeling the nerve from the tumor in a bucket-handle fashion. Two cases of group II tumors each had extensive surgery to remove portions of the tumor both proximal and distal to the neuroforamen. The authors [20] note that “the surgical approach to the proximal neuroforamina is straightforward [but] the distal neuroforamina, a ‘no man's land,’ is nearly impossible to reach without an extensive retroperitoneal dissection with a less than optimal chance for cure” by excision alone. Benzel et al [20] conclude that a conservative nonoperative approach should be considered in asymptomatic and/or neurologically stable patients, and an operative approach should be considered when imaging and the tumor location make total surgical extirpation without sequelae possible.
Conclusion
Our patient, a 52-year-old man with a history of mild intermittent back pain, presented with significant lower extremity pain and weakness, out of character with his prior back pain exacerbations. Physical examination suggested both peroneal and tibial nerve involvement. Initial imaging was negative for any compressive or significant degenerative lesions in the lumbosacral spine. Electrodiagnostic studies led to the diagnosis of sciatic neuropathy. The key findings were an absent left sural sensory response and sparing of the lumbar paraspinal muscles, the tensor fascia lata, and gluteus maximus on EMG testing. In our patient, who had no history of typical risk factors for sciatic neuropathy such as trauma, hip arthroplasty, or intragluteal injection, neoplastic involvement of the sciatic nerve was suspected. Careful imaging of the sciatic nerve with magnetic resonance and limited MR athrography was essential to identify a likely nerve sheath tumor of the left sciatic nerve.
Under Benzel et al's scheme, our patient would be classified under group II because of the foraminal involvement of the S1 nerve root and sciatic nerve. We agree with Benzel et al that the surgical approach to this tumor involves extensive retroperitoneal dissection and associated morbidity. Any future surgical approach to this tumor would be prompted by increasing symptoms or neurologic deficits, and a surgery undertaken would be with the intent of radical resection.
References
- 1.Yuen EC, So YT (1999) Entrapment and other focal neuropathies: sciatic neuropathy. Neurol Clin 17(3):617–631 August [DOI] [PubMed]
- 2.Sunderland S (1951) A classification of peripheral nerve injuries producing loss of function. Brain 74:491–516 [DOI] [PubMed]
- 3.Plewnia C, Wallace C et al. (1999) Traumatic sciatic neuropathy: a novel cause, local experience and a review of the literature. J Trauma 47(5):986–999 November [DOI] [PubMed]
- 4.Kim DH, Murovic JA et al. (2004) Management and outcomes in 353 surgically treated sciatic nerve lesions. J Neurosurg 101:8–17 July [DOI] [PubMed]
- 5.Yuen EC, Onley RK et al. (1994) Sciatic neuropathy: clinical and prognostic features in 73 patients. Neurology 44(9):1669–1674 September [DOI] [PubMed]
- 6.Fishman LM, Schaefer MP (2003) The piriformis syndrome is underdiagnosed. Muscle Nerve 28(5):646–649 Nov [DOI] [PubMed]
- 7.Stewart JD (2003) The piriformis syndrome is overdiagnosed. Muscle Nerve 28(5):644–646 Nov [DOI] [PubMed]
- 8.Moore KR, Tsuruda JS, Dailey AT (2001) The value of MR neurography for evaluating extraspinal neuropathic leg pain: a pictorial essay. Am J Neuroradiol 22:786–794 April [PMC free article] [PubMed]
- 9.Bendszus M, Koltzenburg, M (2001) Visualization of denervated muscle by gadolinium-enhanced MRI. Neurology 57(9):1709–1711 Nov [DOI] [PubMed]
- 10.Van Langenhove M, Pollefliet A, Vanderstraeten G (1989) A retrospective electrodiagnostic evaluation of footdrop in 303 patients. Electromyogr Clin Neurophysiol 29(3):145–152 April [PubMed]
- 11.Sunderland S (1978) Sciatic, tibial and common peroneal nerve lesions. In Nerves and nerve injuries, 2nd edn. Churchill Livingstone, Edinburgh, pp 967–991
- 12.Sunderland S (1953) The relative susceptibility to injury of the medial and lateral popliteal divisions of the sciatic nerve. Br J Surg 41:300–302 [DOI] [PubMed]
- 13.Yuen EC, So YT, Onley RK (1995) The electrodiagnostic features of sciatic neuropathy in 100 patients. Muscle Nerve 18(4):414–420 April [DOI] [PubMed]
- 14.Schmalzried TP, Noordin S, Amstutz HC (1997) Update on nerve palsy associated with total hip replacement. Clin Orthop Relat Res 344:188–206 [DOI] [PubMed]
- 15.Farrell CM, Springer BD, Haidukewych GJ, Morrey BF (2005) Motor nerve palsy following primary total hip arthroplasty. J Bone Jt Surg-Am Vol 87(12):2619–2625 December [DOI] [PubMed]
- 16.Weber ER, Daube JR, Coventry MB (1976) Peripheral neuropathies associated with total hip arthroplasty. J Bone Jt Surg-Am Vol 58(1):66–69 January [PubMed]
- 17.Weale AE, Newman P, Ferguson IT, Bannister GC (1996) Nerve injury after posterior and direct lateral approaches for hip replacement. A clinical and electrophysiological study. J Bone Jt Surg-Br Vol 78(6):899–902 November [DOI] [PubMed]
- 18.Crawford JR, Van Rensburg L, Marx C (2003) Compression of the sciatic nerve by wear debris following total hip replacement: a report of three cases. J Bone Jt Surg-Br Vol 85(8):1178–1180 November [DOI] [PubMed]
- 19.Thomas JE, Piepgras DG, Scheithauser B, Onofrio BM, Shives TC (1983) Neurogenic tumors of the sciatic nerve: a clinicopathologic study of 35 cases. Mayo Clin Proc 58(10):640–647 Oct [PubMed]
- 20.Benzel EC, Morris DM, Fowler MR (1988) Nerve sheath tumors of the sciatic nerve and sacral plexus. J Surg Oncol 39:8–16 [DOI] [PubMed]
- 21.Bader R, Mittelmeier W et al. (2005) Pitfalls in the use of acetabular reinforcement rings in total hip revision. Arch Orthop Trauma Surg 125(8):558–563 October [DOI] [PubMed]
- 22.Sosna A, Pokorny D, Jahoda D (2005) Sciatic nerve palsy after total hip replacement. JBJS (Br) 87-B(8):1140–1141, August [DOI] [PubMed]
- 23.Huang JH, Simon SL, Nagpal S, Nelson PT, Zager EL (2004) Management of patients with schwannomatosis: report of six cases and review of the literature. Surg Neurol 62(4):353–361 October [DOI] [PubMed]
- 24.Tomaino MM (2002) Complete sciatic nerve palsy after open femur fracture: successful treatment with neurolysis 6 months after injury. Am J Orthop 31(10):585–588 October [PubMed]
- 25.Kline DG, Kim D, Midha R, Harsh C, Tiel R (1998) Management and results of sciatic nerve injuries: a 24-year experience. J Neurosurg 89(1):13–23 July [DOI] [PubMed]
- 26.Schmalzried TP, Eckardt JJ (1992) Spontaneous gluteal artery rupture resulting in compartment syndrome and sciatic neuropathy. Report of a case in Ehlers–Danlos syndrome. Clin Orthop Relat Res (275):253–257 Feb [PubMed]
- 27.Sheth D, Gutmann L, Blumenthal DT, Mullett M, Bodensteiner JB, Gutmann L (1994) Compressive sciatic neuropathy due to uterine abnormality. Muscle Nerve 17(12):1486–1488 Dec [DOI] [PubMed]
- 28.Butt AJ, McCarthy T, Kelly IP, Glynn T, McCoy G (2005) Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Jt Surg-Br Vol 87(11):1465–1467 Nov [DOI] [PubMed]
- 29.Austin MS, Klein GR, Sharkey PF, Hozack WJ, Rothman RH (2004) Late sciatic nerve palsy caused by hematoma after primary total hip arthroplasty. J Arthroplast 19(6):790–792 Sep [DOI] [PubMed]
- 30.Tani JC (1995) Fibrous band compression of the tibial nerve branch of the sciatic nerve. Am J Orthop 24(12):910–912 Dec [PubMed]
- 31.Ishida K, Imamaki M, Ishida A, Shimura H, Miyazaki M (2005) A ruptured aneurysm in persistent sciatic artery: a case report. J Vasc Surg 42(3):556–558 Sep [DOI] [PubMed]
- 32.Gasecki AP, Ebers GC, Vellet AD, Buchan A (1992) Sciatic neuropathy associated with persistent sciatic artery. Arch Neurol 49(9):967–968 Sep [DOI] [PubMed]
- 33.Hernesman SC, Hoch AZ, Vetter CS, Young CC (2003) Foot drop in a marathon runner from chronic complete hamstring tear. Clin J Sport Med 13(6):365–368 Nov [DOI] [PubMed]
- 34.Carmody C, Prietto C (1995) Entrapment of the sciatic nerve as a late sequela of injury to the hamstring muscles. A case report. J Bone Jt Surg-Am Vol 77(7):1100–1102 Jul [DOI] [PubMed]
- 35.Puranen J, Orava S (1988) The hamstring syndrome. A new diagnosis of gluteal sciatic pain. Am J Sports Med 16(5):517–521 Sep–Oct [DOI] [PubMed]
- 36.Poppi M, Giuliani G, Gambari PI, Acciarri N, Gaist G, Calbucci F (1989) A hazard of craniotomy in the sitting position: the posterior compartment syndrome of the thigh. Case report. J Neurosurg 71(4):618–619 Oct [DOI] [PubMed]
- 37.Gozal Y, Pomeranz S (1994) Sciatic nerve palsy as a complication after acoustic neurinoma resection in the sitting position. J Neurosurg Anesthesiol 6(1):40–42 Jan [DOI] [PubMed]
- 38.Dimachkie MM, Ohanian S, Groves MD, Vriesendorp FJ (2000) Peripheral nerve injury after brief lithotomy for transurethral collagen injection. Urology 56(4):669 October [DOI] [PubMed]
- 39.Romfh JH, Currier RD (1983) Sciatic neuropathy induced by the lithotomy position. Arch Neurol 40(2):127 Feb [DOI] [PubMed]
- 40.Roy S, Levine AB, Herbison GJ, Jacobs SR (2002) Intraoperative positioning during cesarean as a cause of sciatic neuropathy. Obstet Gynecol 99(4):652–653 Apr [DOI] [PubMed]
- 41.McManis PG (1994) Sciatic nerve lesions during cardiac surgery. Neurology 44(4):684–687 Apr [DOI] [PubMed]
- 42.Dakwar E, Teja S, Alleyne CH Jr (2004) Sciatic neurolymphomatosis. Neurology 63(9):1751 Nov 9 [DOI] [PubMed]
- 43.Maini A, Tripathi M, Shekar NC, Malhotra A (2003) Sciatic schwannoma of the thigh detected on bone scan: a case report. Clin Imaging 27(3):191–193 May–Jun [DOI] [PubMed]
- 44.Agarwal M, Shatma MC, Deol P, Mehta VS, Sarkar C (2002) Epithelioid sarcoma of the sciatic nerve perineural sheath: a mimic of nerve sheath tumor. Pathol Oncol Res 8(2):148–150 [DOI] [PubMed]
- 45.Yamamoto T, Maruyama S, Mizuno K (2001) Schwannomatosis of the sciatic nerve. Skelet Radiol 30(2):109–113 Feb [DOI] [PubMed]
- 46.Botwin KP, Shah CP, Zak PJ (2001) Sciatic neuropathy secondary to infiltrating intermuscular lipoma of the thigh. Am J Phys Med Rehabil 80(10):754–758 October [DOI] [PubMed]
- 47.Isiklar ZU, Lindsey RW, Tullos HS (1997) Sciatic neuropathy secondary to intrapelvic migration of an acetabular cup. A case report. J Bone Jt Surg-Am Vol 79(9):1395–1397 Sep [DOI] [PubMed]
- 48.Asnis SE, Hanley S, Shelton PD (1985) Sciatic neuropathy secondary to migration of trochanteric wire following total hip arthroplasty. Clin Orthop Relat Res (196):226–228 Jun [PubMed]
- 49.Oleksak M, Edge AJ (1992) Compression of the sciatic nerve by methylmethacrylate cement after total hip replacement. J Bone Jt Surg-Br Vol 74(5):729–730 Sep [DOI] [PubMed]
- 50.Brennan MF (2000) Soft tissue sarcoma involving the sciatic nerve. Br J Surg 87(8):983 Aug [DOI] [PubMed]
- 51.Melendez M, Brandt K, Evans GR (2001) Sciatic nerve reconstruction: limb preservation after sarcoma resection. Ann Plast Surg 46(4):375–381 Apr [DOI] [PubMed]
- 52.Dailey AT, Rondina MT, Townsend JJ, Shrieve DC, Baringer JR, Moore KR (2004) Sciatic nerve sarcoidosis: utility of magnetic resonance peripheral nerve imaging and treatment with radiation therapy. J Neurosurg 100(5):956–959 May [DOI] [PubMed]
- 53.Misdraji J, Ino Y, Louis DN, Rosenberg AE, Chiocca EA, Harris NL (2000) Primary lymphoma of peripheral nerve: report of four cases. Am J Surg Pathol 24(9):1257–1265 Sep [DOI] [PubMed]
- 54.Kanamori M, Matsui H, Yudoh K (1995) Solitary T-cell lymphoma of the sciatic nerve: case report. Neurosurgery 36(6):1203–1205 Jun [DOI] [PubMed]
- 55.Quinones-Hinojosa A, Friedlander RM, Boyer PJ, Batchelor TT, Chiocca EA (2000) Solitary sciatic nerve lymphoma as an initial manifestation of diffuse neurolymphomatosis. Case report and review of the literature. J Neurosurg 92(1):165–169 Jan [DOI] [PubMed]
- 56.Roncaroli F, Poppi M, Riccioni L, Frank F (1997) Primary non-Hodgkin's lymphoma of the sciatic nerve followed by localization in the central nervous system: case report and review of the literature. Neurosurgery 40(3):618–621; discussion 621–622 Mar [DOI] [PubMed]
- 57.Johanson NA, Pellicci PM, Tsairis P, Salvati EA (1983) Nerve injury in total hip arthroplasty. Clin Orthop Relat Res (179):214–222 Oct [PubMed]