Abstract
Key words: granuloma, lung disease, tumor necrosis factor alpha inhibitor
Case presentation
A 28-year-old Hispanic female was transferred to New York Presbyterian Hospital for pneumonia, unresponsive to antibiotics. She had been in her usual state of health until the preceding summer, when she developed progressive shortness of breath and a cough. Her cough was initially nonproductive, but she eventually developed blood-tinged green sputum, pleuritic chest pain, and persistent fevers and chills.
The patient was admitted to an outside hospital in the fall, but left against medical advice and was readmitted 2 months later. An admission arterial blood gas demonstrated a pH of 7.50, PaCO2 of 43 mm Hg and PaO2 of 68 mm Hg on 28% inspired oxygen. A chest film revealed bilateral lower lobe infiltrates and effusions, and a chest computed tomography (CT) scan showed diffuse bilateral airspace disease. Over the course of her admission, the patient was treated with multiple antibiotics including vancomycin, levofloxacin, meropenem, imipenem, linezolid, and amikacin, without significant response. She was ultimately transferred to our institution for further diagnostic workup and treatment.
Review of systems upon transfer was notable for a 20-lb weight loss over 2 months, intermittent nausea and vomiting, and mild frontal headaches. She also described bilateral eye pain and redness occurring over the summer, which had resolved spontaneously. The patient had no history of alopecia, rash, oral ulcers, photosensitivity, myalgias, parasthesias, focal weakness, or Raynaud's symptoms. She had occasional arthralgias, but no morning stiffness.
Her medical history was remarkable for morbid obesity, chronic anemia with sickle cell and thalassemia traits, cholelithiasis, and recurrent upper respiratory infections occurring two to three times per year. She had undergone three previous Caesarian sections and had no history of spontaneous pregnancy loss. The patient was single with two living children and studying to be a medical assistant. She had no history of alcohol, tobacco, or illicit drug use, no pets, and no sick contacts. Her last travel was to Puerto Rico at age 11. A tuberculosis purified protein derivative (PPD) skin test yielded negative results 5 years prior to admission, and a test for human immunodeficiency virus (HIV) was also negative.
Her family history was remarkable for parents with diabetes and hypertension, a father with alcohol abuse, and a sister with latent tuberculosis, who reportedly completed treatment with isoniazid. She had no known family history of pulmonary or connective tissue disease, and no known drug allergies. Upon transfer to our institution, her medications were imipenem, linezolid, amikacin, prevacid, lopressor, albuterol and atrovent via nebulizer, subcutaneous heparin, and insulin on a sliding scale.
Physical examination revealed an obese Hispanic female in moderate respiratory distress. She was febrile (39.6°C) with a heart rate of 106 beats/min and blood pressure of 127/85 mm Hg. She was tachypneic with a respiratory rate of 20 breaths/min and an oxygen saturation of 96% on a 40% face mask. Her cardiopulmonary exam was notable for a grade II/VI systolic murmur and scattered coarse rales throughout both lung fields. Her abdomen was obese but benign, her extremities were without edema and had strong pulses, her skin was without rashes, and her joints exhibited full range of motion without warmth, synovitis, or effusion. Her neurologic exam was unremarkable and formal ophthalmological evaluation did not reveal any eye disease.
Initial laboratory evaluation was notable for a white blood cell count of 19.1 × 109/L, with 81% neutrophils and 12.6% lymphocytes. Hemoglobin was 8.2 g/dL and platelet count was 463 × 109/L. Metabolic profile was remarkable for normal renal and hepatic function, lactate dehydrogenase (LDH) of 298 U/L (normal 80–225 U/L), and albumin of 2.7 g/dL. Prothrombin time was elevated at 14.4 s (international normalized ratio of 1.44) and partial thromboplastin time was normal. Urinalysis showed trace protein, small leukocyte esterase, and no cells, and an arterial blood gas on room air demonstrated a pH of 7.46, PaCO2 of 46 mm Hg and PaO2 of 69 mm Hg.
Multiple serial blood cultures were without growth. A repeat PPD was negative and sputum for acid-fast bacteria was negative on three occasions by polymerase chain reaction and culture. All fungal and pneumocystis studies were negative, as was a Legionella urinary antigen. Serologic and genomic studies for influenza, herpesvirus, cytomegalovirus and adenovirus were negative as were tests for Bartonella, Tularia and Coxiella.
Additional testing revealed a sedimentation rate of 57 mm/h (normal <27 mm/h) and C-reactive protein of 26.5 mg/dL (normal 0.30–0.80 mg/dL). Antinuclear antibody titer was 1:40 with a speckled pattern, and antidouble-stranded DNA and extractable nuclear antigen antibodies were not detected. Complement levels C3 and C4 were elevated at 282 mg/dL (normal 85–193 mg/dL) and 52.3 mg/dL (normal 12–36 mg/dL), respectively. Rheumatoid factor (RF) was elevated at 309 IU/mL (normal <20 IU/mL). Cryoglobulins, antineutrophil cytoplasmic antibodies, and glomerular basement membrane antibodies were negative. Angiotension converting enzyme level was normal. Staclot lupus anticoagulant assay was positive, but dilute Russell Viper Venom time and anticardiolipin antibodies were negative.
Echocardiography revealed an ejection fraction of 65%, a thickened pericardium, and a small pericardial effusion. Pulmonary function testing revealed severe restrictive disease (forced vital capacity 23% of predicted, forced expiratory volume in one second 27% of predicted) and hypoxia (room air oxygen saturation of 72%). Multiple imaging studies were obtained and the patient underwent a video-assisted thoracoscopy with lung biopsy.
Radiological findings
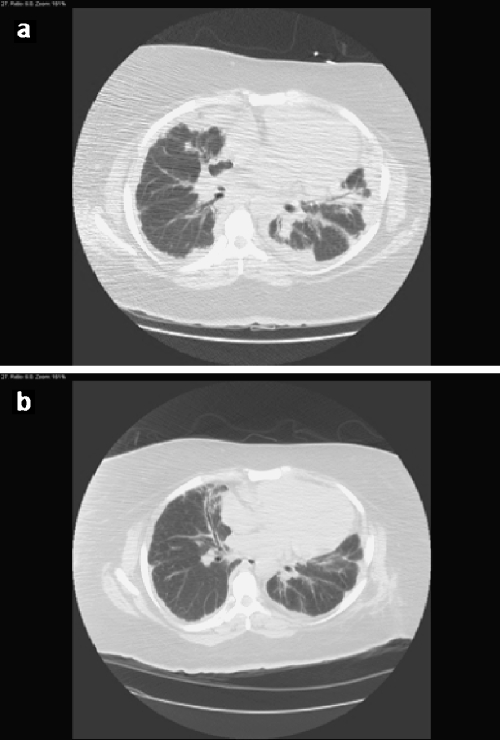
This patient underwent several thoracic imaging studies over her prolonged hospitalization. Her initial chest CT scan upon transfer to our institution demonstrated patchy consolidation involving all lobes of the lung in a largely peribronchial distribution, most prominent at the lung bases, and some ground glass opacification. There were small bilateral pleural effusions, and no thoracic lymphadenopathy (Fig. 1a). Given her clinical situation and in the absence of documented infection, this radiographic presentation was suggestive of a cryptogenic organizing pneumonia.
Fig 1.
Representative chest CT images (a) before and (b) 10 days after treatment with oral steroids. Before therapy, there is scattered peribronchial consolidation and no thoracic lymphadenopathy. After steroid treatment, there is a notable improvement in this consolidation
A chest CT scan obtained approximately 1 month into her admission demonstrated a more focal process radiating out from the hila, but there was still a predominantly consolidative process in both lungs at multiple sites. Given the clinical absence of an infectious etiology, this picture could suggest a nonspecific interstitial pneumonitis. However, a specific diagnosis could not be made based on radiographic findings.
Pathological findings
Review of this patient's open lung biopsy revealed morphologic diagnoses of acute and organizing granulomatous pneumonia and acute fibrinous pleuritis.
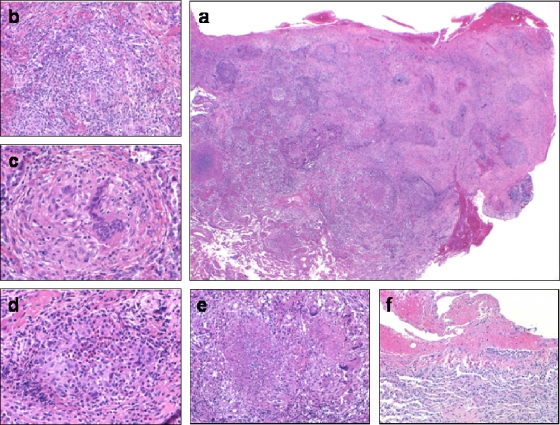
Histologic examination demonstrated consolidated pulmonary parenchyma littered with round to irregularly shaped nodules (Fig. 2). Small airways and airspaces were filled with fibrin and inflammatory cells including neutrophils, lymphocytes, and plasma cells. Fibromyxoid connective tissue plugs also filled airspaces. At higher magnification, the nodules represented peribronchiolar and alveolar septal compact granulomas, seen as collections of epithelioid histiocytes admixed with benign lymphocytes. These granulomas exhibited varying degrees of central necrosis ranging from scattered neutrophils and eosinophils to obvious coagulative necrosis with cellular debris. Overlying visceral pleura featured fibrinous exudates and reactive mesothelial cells. No vasculitis, including capillaritis, was seen. There was no viral cytopathic effect, and special stains for fungi and acid-fast organisms were negative.
Fig 2.
Patient's open lung biopsy prior to treatment. (a) Low-power magnification showing largely consolidated lung tissue with irregular nodularity. High-power magnifications demonstrating (b) air spaces filled with fibrin and inflammatory cells, (c) a compact nonnecrotizing granuloma, (d) a granuloma with central neutrophils and eosinophils in the early phase of necrosis, (e) a granuloma with central coagulative necrosis, and (f) overlying pleura showing acute fibrinous pleuritis
Granulomatous diseases of the lung represent the morphologic expression of many systemic and pulmonary pathologic processes (Table 1). A granuloma is a discrete avascular collection of epithelioid histiocytes, and the presence of only one or two epithelioid histiocytes is required for a diagnosis. The most common causes of pulmonary granulomatous lung disease include infections, immunologic processes including hypersensitivity pneumonitis, and drug effect. Sarcoidosis is by far the most common systemic disorder producing granulomatous disease in the lungs. Connective tissue disorders and vasculitis syndromes are rare in the general population, but pulmonary granulomatous manifestations are seen in a large proportion of such individuals. The pathogenesis of granuloma formation differs for different disease entities.
Table 1.
Broad differential diagnosis of granulomatous lung disease
| Infection |
| Bacterial |
| Fungal |
| Parasitic |
| Pneumoconiosis |
| Aluminum |
| Beryllium |
| Cobalt |
| Hypersensitivity pneumonitis |
| Drug effect |
| Malignancy |
| Lymphoma |
| Lung cancer |
| Eosinophilic pneumonia |
| Aspiration pneumonia |
| Intravenous talcosis |
| Sarcoidosis |
| Connective tissue disordersa |
| Vasculitis syndromes |
| Wegener's granulomatosis |
| Churg–Strauss syndrome |
| Immunodeficiency states |
| Common variable immunodeficiency |
| Chronic granulomatous disease |
| Incidental with no clinical importance |
aFurther detailed in Table 3.
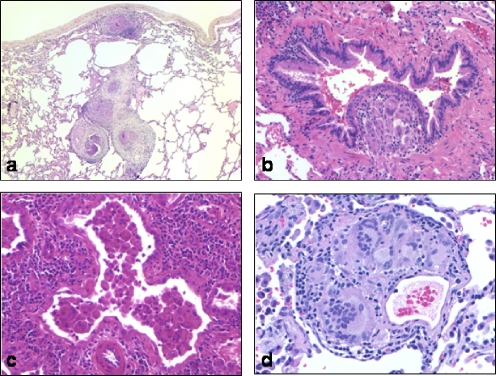
The surgical pathologist's approach to lung biopsies with granulomatous disease requires evaluation of the distribution of granulomas, the type of granulomas, and other findings in the lung tissue. An appreciation of granuloma distribution is of great help in narrowing the differential diagnosis (Fig. 3). Granulomas that track along pulmonary lymphatic channels in the visceral pleura, interlobular septa, and within the bronchovascular bundles suggest a diagnosis of sarcoidosis, or less commonly pneumoconiosis, whereas airway-centered granulomas may represent hypersensitivity pneumonitis. Alveolar septal and airspace involvement are nonspecific, although these so-called “parenchymal granulomas” are often noted in individuals with Wegener's granulomatosis and other vasculitis syndromes. Granulomatous vasculitis may be seen in lung infections and systemic disorders including sarcoidosis, as well as with intravenous injection of pulverized illicit drugs bound with talc. One may suppose that bronchial, bronchiolar, and alveolar duct-centered granulomas would indicate an infectious etiology, but most infections demonstrate randomly distributed lesions. Occasional minute nonnecrotizing granulomas may be an incidental finding.
Fig 3.
Representative distribution patterns of granulomas in the lung. (a) Lymphangitic distribution of granulomas in pulmonary sarcoidosis. (b) Clusters of histiocyte-filled alveolar spaces and alveolar septae expanded with lymphocytes in cobalt pneumoconiosis. (c) Perivascular granuloma from intravenous talcosis. (d) Granuloma expanding the bronchiolar submucosa and constricting the lumen in a case of hypersensitivity pneumonitis
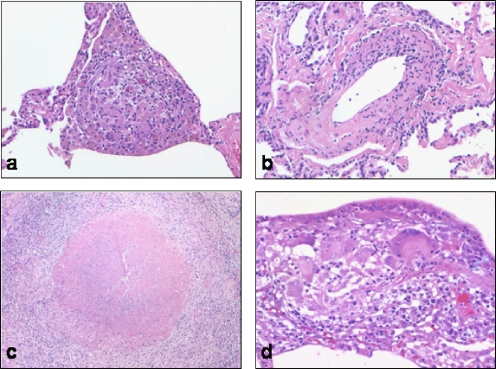
Although an infectious etiology should be considered in all cases of granulomatous pneumonitis, suspicion of infection is greater for necrotizing lesions. Even when confronted with the classic histomorphology of Wegener's granulomatosis, this diagnosis can only be suggested after a thorough exclusion of an infection. Albeit rare, rheumatoid nodules manifest as necrotizing granulomas in the lung. However, these rare lesions feature a “picket fence” or linear arrange of epithelioid histiocytes, but few multinucleated giant cells. Other qualitative differences may suggest particular diagnoses (Fig. 4). For example, compact tightly formed granulomas suggest sarcoidosis, whereas irregular collections of histiocytes raise the possibility of hypersensitivity pneumonitis.
Fig 4.
Quality of granulomas in the lung. (a) Tight collections of epithelioid histiocytes expanding the alveolar interstitium in chronic Mycobacterium avium intracellulare infection. (b) A subtle granulomatous vasculitis in systemic lupus erythematosus-related pneumonitis. (c) Granuloma with central necrosis suggesting mycobacterial infection; Mycobacterium tuberculosis was cultured from the tissue sample. (d) Loose collection of peribronchiolar epithelioid histiocytes in bagassosis (sugar cane picker's lung)
Surrounding lung pathology or significant lack thereof also guides the pathologist toward a diagnosis. Small nonnecrotizing granulomas in the presence of a malignant lymphoma are considered an immune response to the tumor, eosinophils may suggest drug effect, and a fibrinous pneumonia and/or pleuritis, as in the current case, suggest an infectious or systemic etiology.
The morphologic findings in this patient's biopsy narrowed the working differential diagnosis (Table 2). Evidence of a pneumoconiosis, hypersensitivity pneumonitis, or drug effect was not seen, given the presence of necrotizing granulomas in this specimen. Drugs that may promote granulomatous disease—such as methotrexate, etanercept, and various medications used in the treatment of inflammatory bowel disease—would also characteristically produce only nonnecrotizing granulomas. Malignancy was not seen, and sarcoidosis was excluded given the localization of granulomas to the interstitium and air spaces. Although necrotizing granulomas may be seen with Wegener's granulomatosis or Churg–Strauss syndrome in the setting of eosinophils, these diagnoses require the triad of granulomatous disease, necrosis, and vasculitis. This patient's biopsy lacked fibrinoid necrosis of vessels.
Table 2.
Summary of key histopathologic features of patient's lung biopsy
| Distribution of granulomas | Involve many anatomic compartments |
| Quality of granulomas | Compact and loose; necrotizing and nonnecrotizing |
| Quantity of granulomas | Abundant |
| Accompanying findings | Acute and organizing pneumonia |
| Scattered eosinophils | |
| Acute fibrinous pleuritis | |
| Pertinent negatives | No vasculitis |
| No malignancy | |
| No viral cytopathic effect |
This histopathology essentially narrowed the differential diagnosis to infection or rheumatologic disease. Infectious etiologies for granulomatous pneumonitis include mycobacteria, fungi, protozoa including pneumocystis, bacteria, viruses, and parasites, as well as atypical bacterial infections with organisms such as Actinomyces, Nocardia, and Tularia. Viral infections, such as herpes simplex and cytomegalovirus, and parasites including Toxoplasma can also cause granulomatous pneumonitis in the immunocompromised host. However, an extensive clinical infectious workup, as well as several tissue blocks analyzed for microorganisms, did not reveal anything definite in this patient.
Granulomatous lung disease is a well-recognized feature of connective tissue disorders, such as rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, and inflammatory bowel disease (Table 3). An underlying connective tissue disorder in this patient could not be excluded on the basis of the lung pathology alone. Chronic granulomatous disease and common variable immunodeficiency, as underlying disease states predisposing to chronic granulomatous pneumonitis, could not be excluded by histomorphology alone.
Table 3.
Lung pathology findings in connective tissue disorders
| RA | SLE | SSc | PM/DM | SS | MCTD | AS | IBD | PC | |
|---|---|---|---|---|---|---|---|---|---|
| Pleuritis | + | + | + | + | + | + | + | ||
| Diffuse alveolar damage | + | + | + | + | + | ||||
| Usual interstitial pneumonia | + | + | + | + | + | + | |||
| Organizing pneumonia | + | + | + | + | + | + | + | ||
| Granulomatous pneumonia | + | + | + | + | |||||
| Lymphoid infiltrates | + | + | + | ||||||
| Rheumatoid nodules | + | + | |||||||
| Diffuse hemorrhage | + | + | + | + | + | + | |||
| Eosinophilic pneumonia | + | + | + | ||||||
| Vasculitis | + | + | + | + | + | + | + | ||
| Bronchiolitis | + | + | + | + | + | + | + |
RA = rheumatoid arthritis, SLE = systemic lupus erythematosus, SSc = scleroderma, PM = polymyositis, DM = dermatomyositis, MCTD = mixed connective tissue disease, AS = ankylosing spondylitis, IBD = inflammatory bowel disease, PC = polychondritis.
In summary, this patient had an acute and organizing fibrinous pneumonia with necrotizing and nonnecrotizing granulomas, probably as a result of infection or perhaps a connective tissue disorder.
Further hospital course and discussion
The patient remained hospitalized for approximately 2 months. After infection was convincingly excluded, she began treatment with oral prednisone at 40 mg twice daily. In this setting, she showed progressive clinical improvement, with a notable decrease in her oxygen requirement and increase in activity tolerance. She went from being predominantly bedbound to ambulatory in the hallway with oxygen by nasal canula. A chest CT scan obtained 10 days following treatment with steroids demonstrated significant improvement in the diffuse bilateral patchy peribronchial consolidations and ground glass opacities, with some residual subpleural consolidation (Fig. 1b). The patient was discharged to a rehabilitation facility, but was subsequently lost to follow-up. She was variably compliant with medications, and was readmitted to our hospital 2 months later in respiratory failure.
Upon readmission, all blood cultures were again negative. Repeat chest CT scan revealed worsening bilateral patchy consolidation, most confluent in the right lower lobe. A repeat bronchoscopy demonstrated a normal tracheobronchial tree and cytology consistent with acute and chronic inflammation with atypical glandular cells. Stains for acid-fast bacteria, fungi, and pneumocystis were negative. A transbronchial biopsy demonstrated chronic inflammation in the absence of granulomatous inflammation, with all stains negative for organisms. Rheumatoid factor was 96 IU/mL and anticyclic citrullinated peptide antibodies were absent. Antinuclear antibody titer remained at 1:40 in a speckled pattern.
Given a history of recurrent upper respiratory infections since childhood, a workup for immune deficiency syndromes that may produce granulomatous disease was pursued. Common variable immunodeficiency is a primary antibody deficiency syndrome characterized by low serum immunoglobulin levels and an inability to produce specific antibodies [1]. To the contrary, this patient had elevated total IgA, IgE, IgG, and IgG subtype levels, and IgM levels within the normal range. Complement deficiency was not evident, as all complement components and a CH50 were elevated. Furthermore, there was no evidence for chronic granulomatous disease, which comprises a family of genetic disorders characterized by various defects in the ability of phagocytes to generate reactive oxygen intermediates, leading to frequent severe infections [2]. A nitroblue tetrazolium assay, which assesses the ability of neutrophils to reduce nitroblue tetrazolium to formazan in the setting of a respiratory burst, was normal in this patient.
The patient's clinical diagnosis at this point was that of a noninfectious granulomatous lung disease, possibly secondary to an underlying connective tissue disorder. In this context, her elevated RF was of interest. In addition to rheumatoid arthritis, there are numerous infectious and noninfectious disorders, including pulmonary processes, which can associate with an elevated RF [3]. In some patients, lung disease may be the initial presentation of a subsequent connective tissue disorder. For instance, in patients with rheumatoid arthritis and interstitial lung disease, pulmonary involvement may precede systemic disease in up to 20% of patients [4].
The patient was treated with intravenous methylprednisolone (32 mg, twice daily) and broad-spectrum antibiotics, with marginal clinical improvement. She had a mixed radiographic response, with decreased ground glass opacities in the right lung, but an interval increase in ground glass opacification of the left apex.
Infliximab was ultimately added as a steroid-sparing agent, given its usefulness in the treatment of other granulomatous diseases such as Crohn's disease and sarcoidosis [5–12]. The patient was treated with two doses of infliximab at 5 mg/kg over 2 weeks. She experienced an impressive improvement following her first dose, with an increased exercise tolerance and decreased oxygen requirement. By the time of her discharge 1 month later, the patient had an oxygen saturation of 99% on 3 L of oxygen by nasal canula and was able to ambulate short distances without supplemental oxygen. She was discharged on oral prednisone (60 mg daily). Repeat laboratory studies revealed an RF of 36.7 IU/mL, absent anticyclic citrullinated peptide antibodies, antinuclear antibodies, antidouble stranded DNA antibodies, extractable nuclear antigen antibodies, anticardiolipin antibodies, and a negative lupus anticoagulant. Complement C3 and C4 levels were within normal range. She received a third dose of infliximab as an outpatient, but was subsequently lost to both pulmonary and rheumatologic follow-up.
The use of tumor necrosis factor (TNF) inhibitors to treat noninfectious granulomatous disease is a rational choice from a scientific perspective. TNF has been shown to play a critical role in granuloma formation in experimental models using TNF-deficient mice and neutralizing anti-TNF antibodies [13]. In sarcoidosis, TNF is released from alveolar macrophages and is important for the induction and maintenance of granulomas [5, 13]. The successful use of infliximab in refractory sarcoidosis was reported by two groups in 2001 [7, 12], and infliximab has since been used successfully in the treatment of various manifestations of sarcoidosis, including pulmonary disease [6, 8, 10, 11]. Infliximab was also effective in improving hepatic and pulmonary lesions in a case of metastatic Crohn's disease [9]. More recently, treatment with high-dose infliximab was associated with the resolution of severe pulmonary and hepatic granulomatous disease in a patient with underlying common variable immunodeficiency [1].
In contrast, etanercept has not demonstrated efficacy in the treatment of various granulomatous diseases. This was originally observed for moderate-to-severe Crohn's disease, in which etanercept was no more effective than placebo in a randomized, double-blind, placebo-controlled trial [14]. In addition, a prospective open-label study of etanercept for the treatment of pulmonary sarcoidosis was terminated early because of excessive treatment failures [15]. Furthermore, in a multicenter, randomized, placebo-controlled trial, etanercept was ineffective in the maintenance of remission in patients with Wegener's granulomatosis [16]. Unlike infliximab, which is a chimeric monoclonal anti-TNF antibody that binds to soluble and membrane-bound TNF, etanercept is a soluble TNF receptor–immunoglobulin fusion construct that binds to soluble TNF and lymphotoxin-α [17]. The mechanistic differences between these two TNF inhibitors may be one explanation for the observed superiority of infliximab in the treatment of granulomatous diseases [5]. Of note, a recent case report describes the successful treatment of refractory pulmonary sarcoidosis with the humanized anti-TNF monoclonal antibody adalimumab [18].
In conclusion, the differential diagnosis and treatment of noninfectious granulomatous disease of the lung can be extremely broad and challenging. Given our present understanding of granuloma formation and the accumulating clinical experience with specific TNF antagonists, these agents are likely to play an increasingly important role in the management of granulomatous diseases.
Acknowledgments
The authors would like to thank Drs. Gordon Gamsu and Thomas King for their presentations and helpful discussions regarding this case, and Dr. C. Ronald MacKenzie for critical review of the manuscript.
Footnotes
A Clinical Pathology Conference Held by Hospital for Special Surgery and Weill Medical College of Cornell University.
References
- 1.Thatayatikom A, Thatayatikom S, White, AJ (2005) Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immun 95:293–300 [DOI] [PubMed]
- 2.Lukela M, DeGuzman D, Weinberger S, et al. (2005) Clinical problem-solving. Unfashionably late. N Engl J Med 352:64–69 [DOI] [PubMed]
- 3.Shmerling RH, Delbanco TL (1991) The rheumatoid factor: an analysis of clinical utility. Am J Med 91:528–534 [DOI] [PubMed]
- 4.du Bois RM, Wells AU (2003) The lung in rheumatic diseases. In: Hochberg MC, Silman AJ, Smolen JS, et al. (eds) Rheumatology, 3rd edn. Mosby, New York, pp 315–323
- 5.Keystone EC (2004) The utility of tumour necrosis factor blockade in orphan diseases. Ann Rheum Dis 63(Suppl 2):ii79–ii83 [DOI] [PMC free article] [PubMed]
- 6.Baughman RP (2004) Pulmonary sarcoidosis. Clin Chest Med 25:521–530 [DOI] [PubMed]
- 7.Baughman RP, Lower EE (2001) Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 18:70–74 [PubMed]
- 8.Doty JD, Mazur JE, Judson MA (2005) Treatment of sarcoidosis with infliximab. Chest 127:1064–1071 [DOI] [PubMed]
- 9.Gill KR, Mahadevan U (2005) Infliximab for the treatment of metastatic hepatic and pulmonary Crohn's disease. Inflamm Bowel Dis 11:210–212 [DOI] [PubMed]
- 10.Roberts SD, Wilkes DS, Burgett RA, et al. (2003) Refractory sarcoidosis responding to infliximab. Chest 124:2028–2031 [DOI] [PubMed]
- 11.Ulbricht KU, Stoll M, Bierwirth J, et al. (2003) Successful tumor necrosis factor alpha blockade treatment in therapy-resistant sarcoidosis. Arthritis Rheum 48:3542–3543 [DOI] [PubMed]
- 12.Yee AM, Pochapin MB (2001) Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med 135:27–31 [DOI] [PubMed]
- 13.Moller DR (2003) Treatment of sarcoidosis—from a basic science point of view. J Intern Med 253:31–40 [DOI] [PubMed]
- 14.Sandborn WJ, Hanauer SB, Katz S, et al. (2001) Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121:1088–1094 [DOI] [PubMed]
- 15.Utz JP, Limper AH, Kalra S, et al. (2003) Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest 124:177–185 [DOI] [PubMed]
- 16.The WGET Research Group (2005) Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med 352:351–361 [DOI] [PubMed]
- 17.Khanna D, McMahon M, Furst DE (2004) Safety of tumour necrosis factor-alpha antagonists. Drug Safety 27:307–324 [DOI] [PubMed]
- 18.Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, et al. (2005) Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol 1–2 [DOI] [PubMed]