Abstract
Background
Neonatal hypothyroidism has been associated in animal models with maternal exposure to several environmental contaminants; however, evidence for such an association in humans is inconsistent. We evaluated whether maternal exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a persistent and widespread toxic environmental contaminant, is associated with modified neonatal thyroid function in a large, highly exposed population in Seveso, Italy.
Methods and Findings
Between 1994 and 2005, in individuals exposed to TCDD after the 1976 Seveso accident we conducted: (i) a residence-based population study on 1,014 children born to the 1,772 women of reproductive age in the most contaminated zones (A, very high contamination; B, high contamination), and 1,772 age-matched women from the surrounding noncontaminated area (reference); (ii) a biomarker study on 51 mother–child pairs for whom recent maternal plasma dioxin measurements were available. Neonatal blood thyroid-stimulating hormone (b-TSH) was measured on all children. We performed crude and multivariate analyses adjusting for gender, birth weight, birth order, maternal age, hospital, and type of delivery. Mean neonatal b-TSH was 0.98 μU/ml (95% confidence interval [CI] 0.90–1.08) in the reference area (n = 533), 1.35 μU/ml (95% CI 1.22–1.49) in zone B (n = 425), and 1.66 μU/ml (95% CI 1.19–2.31) in zone A (n = 56) (p < 0.001). The proportion of children with b-TSH > 5 μU/ml was 2.8% in the reference area, 4.9% in zone B, and 16.1% in zone A (p < 0.001). Neonatal b-TSH was correlated with current maternal plasma TCDD (n = 51, β = 0.47, p < 0.001) and plasma toxic equivalents of coplanar dioxin-like compounds (n = 51, β = 0.45, p = 0.005).
Conclusions
Our data indicate that environmental contaminants such as dioxins have a long-lasting capability to modify neonatal thyroid function after the initial exposure.
Andrea Baccarelli and colleagues show that maternal exposure to a dioxin following the industrial accident in Seveso, Italy in 1976 is associated with modified neonatal thyroid function even many years later.
Editors' Summary
Background.
The thyroid, a butterfly-shaped gland in the neck, controls the speed at which the human body converts food into the energy and chemicals needed for life. In healthy people, the thyroid makes and releases two hormones (chemical messengers that travel around the body and regulate the activity of specific cells) called thyroxine (T4) and triiodothyronine (T3). The release of T4 and T3 is controlled by thyroid secreting hormone (TSH), which is made by the pituitary gland in response to electrical messages from the brain. If the thyroid stops making enough T4 and T3, a condition called hypothyroidism (an underactive thyroid) develops. Adults with hypothyroidism put on weight, feel the cold, and are often tired; children with hypothyroidism may also have poor growth and mental development. Because even a small reduction in thyroid hormone levels increases TSH production by the pituitary, hypothyroidism is often diagnosed by measuring the amount of TSH in the blood; it is treated with daily doses of the synthetic thyroid hormone levothyroxine.
Why Was This Study Done?
Although hypothyroidism is most common in ageing women, newborn babies sometimes have hypothyroidism. If untreated, “neonatal” hyperthyroidism can cause severe mental and physical retardation so, in many countries, blood TSH levels are measured soon after birth. That way, levothyroxine treatment can be started before thyroid hormone deficiency permanently damages the baby's developing body and brain. But what causes neonatal hypothyroidism? Animal experiments (and some but not all studies in people) suggest that maternal exposure to toxic chemicals called dioxins may be one cause. Dioxins are byproducts of waste incineration that persist in the environment and that accumulate in people. In this study, the researchers investigate whether exposure to dioxin (this name refers to the most toxic of the dioxins—2,3,7,8-Tetrachlorodibenzo-p-dioxin) affects neonatal thyroid function by studying children born near Seveso, Italy between 1994 and 2005. An accident at a chemical factory in 1976 heavily contaminated the region around this town with dioxin and, even now, the local people have high amounts of dioxin in their bodies.
What Did the Researchers Do and Find?
The researchers identified 1,772 women of child-bearing age who were living very near the Seveso factory (the most highly contaminated area, zone A) or slightly further away where the contamination was less but still high (zone B) at the time of the accident or soon after. As controls, they selected 1,772 women living in the surrounding, noncontaminated (reference) area. Altogether, these women had 1,014 babies between 1994 and 2005. The babies born to the mothers living in the reference area had lower neonatal blood TSH levels on average than the babies born to mothers living in zone A; zone B babies had intermediate TSH levels. Zone A babies were 6.6. times more likely to have a TSH level of more than 5 μU/ml than the reference area babies (the threshold TSH level for further investigations is 10 μU/ml; the average TSH level among the reference area babies was 0.98 μU/ml). The researchers also examined the relationship between neonatal TSH measurements and maternal dioxin measurements at delivery (extrapolated from measurements made between 1992 and 1998) in 51 mother–baby pairs. Neonatal TSH levels were highest in the babies whose mothers had the highest blood dioxin levels.
What Do These Findings Mean?
These findings suggest that maternal dioxin exposure has a long-lasting, deleterious effect on neonatal thyroid function. Because the long-term progress of the children in this study was not examined, it is not known whether the increases in neonatal TSH measurements associated with dioxin exposure caused any developmental problems. However, in regions where there is a mild iodine deficiency (the only environmental exposure consistently associated with reduced human neonatal thyroid function), TSH levels are increased to a similar extent and there is evidence of reduced intellectual and physical development. Future investigations on the progress of this group of children should show whether the long-term legacy of the Seveso accident (and of the high environmental levels of dioxin elsewhere) includes any effects on children's growth and development.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050161.
The MedlinePlus encyclopedia provides information about hypothyroidism and neonatal hypothyroidism; MedlinePlus provides links to additional information on thyroid diseases (in English and Spanish)
The UK National Health Service Direct health encyclopedia provides information on hypothyroidism
The Nemours Foundation's KidsHealth site has information written for children about thyroid disorders
Toxtown, an interactive site from the US National Library of Science, provides information on environmental health concerns including exposure to dioxins (in English and Spanish)
More information about dioxins is provided by the US Environmental Protection Agency and by the US Food and Drug Administration
Wikipedia has a page on the Seveso disaster (note: Wikipedia is a free online encyclopedia that anyone can edit; available in several languages)
Introduction
Variations in neonatal thyroid function evaluated at birth through blood thyroid-stimulating hormone (b-TSH) are associated with changes in iodine availability and maternal intake [1]. According to the World Health Organization (WHO), the percentage of newborns with b-TSH > 5 μU/ml should be less than 3% in iodine-replete populations [1]. Aside from iodine deficiency, no environmental exposure has been conclusively associated with reduced neonatal thyroid function in humans [2–4].
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a ubiquitous low-level contaminant of the environment, is the most toxic compound of a class of toxicologically related environmental chemicals, including other dioxins, polychlorinated biphenyls (PCBs), furans, brominated compounds, polycyclic aromatic hydrocarbons, and organochlorine pesticides [5,6]. High exposures to dioxin have occurred in chemical workers, Vietnam Agent Orange veteran sprayers, accidental rice oil contamination, and recent isolated poisonings such as those of the current Ukrainian president and of five coworkers, two of whom had particularly elevated exposures, in Vienna, Austria [6]. In animal models, maternal exposure to TCDD induces elevated b-TSH and neonatal primary hypothyroidism [2,7–10], as TCDD and other related compounds have been shown to accelerate thyroid hormone clearance by increasing metabolic enzyme activity and competing with plasma binding proteins [11–13].
In July 1976, an industrial accident caused the exposure to high TCDD doses of a large population living in a residential area in Seveso, Italy [14]. TCDD has an extremely long half life, particularly in women (∼10 y) [15,16], and was still elevated in plasma samples collected from exposed women 20 y after the accident [17,18]. Starting in 1994, we conducted two related investigations on the Seveso population to determine whether maternal exposure to TCDD, as well as elevated current plasma dioxin levels, modified neonatal thyroid function.
Material and Methods
Study Population
After the Seveso accident, three zones of decreasing contamination (A, B, and R) were delimited based on TCDD soil concentrations. TCDD concentrations measured in soil samples shortly after the accident were between 15.5–580.4 μg/m3 in zone A, 1.7–4.3 μg/m3 in zone B, and 0.9–1.4 μg/m3 in zone R [19]. A cohort including all individuals living in these three zones (804 in A, 5,941 in B, 38,624 in R) and in the surrounding noncontaminated area (reference, 232,740 individuals) was established for follow-up studies [20]. Measurements performed on plasma samples collected from individuals within 1–2 y after the accident showed median TCDD levels of 447 parts per trillion (ppt) in zone A (n = 296), 94 ppt in zone B (n = 80), and 48 ppt in zone R (n = 48) [21]. A later campaign performed in 1993–1994 still found elevated plasma levels in the exposed population, particularly among women, who had geometric means of plasma TCDD equal to 60.5 ppt in zone A individuals (n = 7), 17.6 ppt in zone B individuals (n = 51), and 6.1 ppt in individuals from the reference area (n = 52) [17].
Seveso is located in Lombardy, a region in northern Italy with a population of 9.2 million where thyroid function is tested in all newborns by b-TSH measurements. Blood samples for b-TSH screening are taken 72 h after birth from a heel prick directly onto filter paper using a standardized collection protocol, and shipped to the Milan Central Neonatal Screening Laboratory where b-TSH determination is performed by fluorometric immunoassay using the AutoDELFIA automatic immunoassay system (PerkinElmer). Since January 1, 1994, all b-TSH data have been recorded in the Neonatal Screening Registry, which also includes information on date and hospital of birth, weight at birth, and place of residence. The two investigations described below received approval from the institutional review board of the Ospedale Maggiore Policlinico, Mangiagalli, Regina Elena Foundation, Milan, Italy.
Residence-Based Population Study
We selected from the Seveso cohort all the 1,772 women from the highly contaminated areas (A and B) who were: (i) residents of zone A (n = 186) or B (n = 1,231) at the time of the accident (July 10, 1976), or potentially exposed to TCDD, because they moved into zone A (n = 27) or B (n = 328) between July 10, 1976 and December 31, 1979; (ii) of fertile age (i.e., date of birth after December 31, 1947); (iii) alive on January 1, 1994. We randomly sampled 1,772 nonexposed women from the eligible (ii and iii above) female participants (n = 55,576) of the cohort established from the population of the reference area. Nonexposed women were frequency-matched to the exposed women by year of birth and residence in the reference area on the date of the accident (i above). We contacted 472 population registry offices (PROs) of the towns of residence of the women to identify all children born to the study participants. PRO personal records were traced for 1,761 (99.4%) of the 1,772 women from the contaminated areas (A and B) and 1,762 of the 1,772 women (99.4%) from the reference area. Because b-TSH measurements for our study were obtained from the Lombardy Neonatal Screening Registry, we excluded all children born outside the Lombardy region (n = 156; 13.3% of the 1,170 children traced). After such exclusion, we identified 1,014 singletons (56 from zone A, 425 from zone B, 533 from reference) born between January 1, 1994 and June 30, 2005 to 42 women from zone A, 327 women from zone B, and 403 women from the reference area. For all births, we obtained information on the type of delivery (vaginal, cesarean) through the regional registration system of hospital discharges. In a case-control study on chloracne conducted between 1993–1998 [22], most of the participants with elevated (> 0 ppt) TCDD levels were from zones A and B, whereas only a minority of zone R individuals exhibited elevated TCDD. In planning the residence-based population study, we hypothesized that contrasting newborns from zones A and B to the reference area would have provided us with the most efficient design to detect potential effects of the exposure on neonatal TSH. Thus, the zone R population was not included in the study.
Study Based on Plasma Dioxin Measurements
We conducted a second investigation on the children born to the 109 women of fertile age (date of birth after 31 December 1947) who were part of the Seveso Chloracne Study [22]. The original population sample included 211 male and female healthy participants representative of the Seveso population and 101 individuals who had developed chloracne, the skin disorder associated with TCDD toxicity [22]. All participants gave written informed consent. Between 1 January 1994 and 30 June 2005, 51 children (12 from zone A, ten from zone B, 20 from zone R, and nine from Reference) were born to 38 of the 109 women; the remaining 71 women did not give birth in the study period. All children from zones A and B were also part of the residence-based population study (which included all zone A and B women), while none from zone R and reference had been sampled in the residence-based population study. Dioxin measurements were performed at the Centers for Disease Control (Atlanta, Georgia, United States of America) using a high-resolution gas chromatography/high-resolution mass spectrometry analysis [23] on plasma samples collected between December 1992 and September 1998. Specifically, 24 congeners were measured, including TCDD and six additional dibenzo-p-dioxins (PCDDs), ten dibenzofurans (PCDFs), and four coplanar PCBs. Starting from 1996, 36 non-coplanar PCBs, including six mono-ortho congeners, were added to the panel of the congeners we tested for. Non-coplanar PCBs were thus measured on a subset of 37 of the 51 mother–child pairs. Results are reported in ppt, lipid adjusted. For women with concentrations > 10 ppt, plasma TCDD were extrapolated to the date of delivery with a first-order pharmacokinetic model [24,25], using the elimination rate estimated in Seveso (equivalent to 9.8 y half-life for women) [15].
Toxic equivalent concentrations (TEQs) were defined for a mixture of dioxin-like compounds as the product of the concentration of each congener multiplied by its specific toxic equivalency factor (TEF) [26]. Maternal mean TCDD levels were 18.9 ppt (n = 51, range 1.4–309.5). Mean plasma TEQs were 44.8 ppt (n = 51, range 11.6–330.4) for PCDDs, PCDFs, and coplanar PCBs; and 1.8 ppt (n = 37, range 0.6–4.2) for non-coplanar PCBs. Total mean TEQs, including the sum of PCDDs, PCDFs, coplanar PCBs, and non-coplanar PCBs, were 41.8 ppt (n = 37, range 12.2–334.5). Although total TEQs also included TEQs from PCDDs, PCDFs, and coplanar PCBs, mean total TEQs were lower than TEQs from PCDDs, PCDFs, and coplanar PCBs. Measurement of non-coplanar PCBs, which were used to compute total TEQs, started later during the study (from 1996). Thus, total TEQs are available only on a subset of participants who have lower dioxin levels likely because of later sampling and longer time from the accident.
Statistical Analysis
b-TSH levels were log-transformed to approximate normal distribution. Consequently, geometric b-TSH means and 95% confidence intervals (CIs) are shown. Graphical distributions of b-TSH were plotted using the Epanechnikov kernel function to obtain density estimates. In descriptive analyses, we used the Fisher's exact test to evaluate associations of the study participant's general characteristics with the zone of residence, and the Student t-test for associations with b-TSH. We calculated correlations with b-TSH and tests for trend using linear regression analysis. Unconditional logistic regression was used to estimate relative odds of elevated b-TSH levels (> 5 μU/ml). In multivariate analyses, regression models included gender, birth weight, birth order of the newborn, and maternal age at delivery, hospital, and type of delivery as independent variables. Correlation between siblings was accounted for by using generalized estimating equations in all models. However, in analysis testing differences for groups that included a very small number of observations, generalized estimating equation may produce unreliable results. In such instances, we used either the Fisher's exact test (for categorical outcomes) or Wilcoxon (Mann-Whitney) nonparametric test (for continuous outcomes), as indicated in the text. We performed tests for trend across contamination zones by scoring the areas using the logarithm of the geometric means of plasma TCDD (60.5 ppt in zone A, 17.6 ppt in zone B, and 6.1 ppt in the reference area) measured in female participants in a previous investigations conducted between 1993–1995 [17]. In the residence-based population study, results did not show major differences after excluding 66 children (6.5% of the total sample size) born to mothers who had moved into the study areas after the date of the accident. Results including all newborns are reported throughout the paper. In the study based on plasma dioxin, information on additional possible confounders, including maternal body mass index (BMI), smoking habits, alcohol consumption, and neonatal age in hours at b-TSH measurement, was available. In multivariate models, inclusion or exclusion of these variables did not modify statistical significance. To confirm the results of linear models, we used Spearman's rank-correlation statistics. In both studies, statistical significance was not modified by adding to the models indicator variables for year of birth to adjust for trends through the study period. All tests were two-sided. All analyses were performed in Stata 9.0 (Stata Corporation).
Results
Residence-Based Population Study
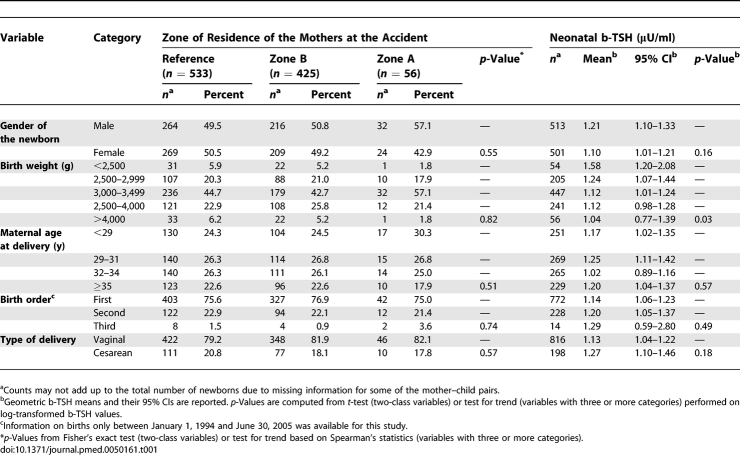
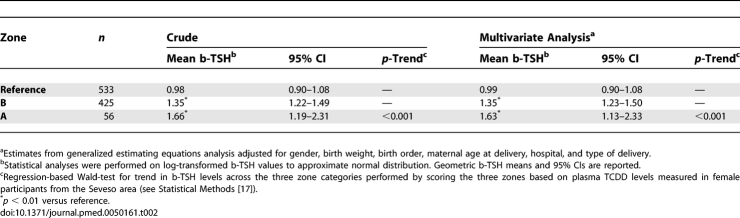
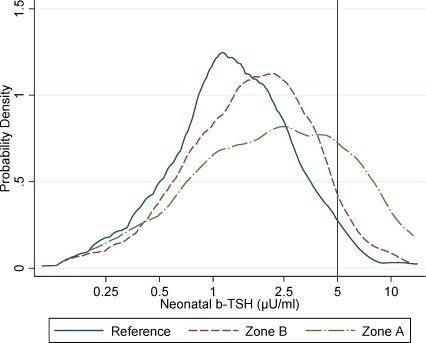
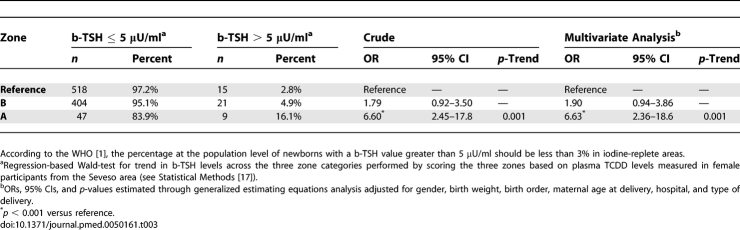
The characteristics of the newborns included in the residence-based population study were similar across the three contamination zones (Table 1). Neonatal b-TSH values ranged between 0.2 and 14.0 μU/ml. Mean neonatal b-TSH decreased with increasing birth weight (p = 0.03), consistent with previous observations on other populations [27], and showed moderate, nonsignificant variations in association with birth order and type of delivery (Table 1). Mean neonatal b-TSH levels were significantly higher in the populations who lived in the TCDD-contaminated zones at the time of the accident (Table 2). Mean b-TSH was 0.98 μU/ml (95% CI 0.90–1.08) in the reference population, 1.35 μU/ml (95% CI 1.22–1.49) in zone B, and 1.66 μU/ml (95% CI 1.19–2.31) in zone A, the most contaminated area (p < 0.001 for trend across zones). Distributions of b-TSH by contamination zones are shown in Figure 1. The proportion of newborns with b-TSH > 5 μU/ml (Table 3) was equal to 2.8% in the reference area, 4.9% in zone B, and 16.1% in zone A (p < 0.001). Compared to the reference area, the relative odds of elevated b-TSH increased through contamination zones, with odds ratio (OR) = 1.79 (95% CI 0.92–3.50) for zone B and OR = 6.60 (95% CI 2.45–17.8) for zone A (p = 0.002 for trend across zones).
Table 1.
Characteristics, Dioxin Contamination Zones, and Neonatal b-TSH Levels of the Mother–Child Pairs Included in the 1994–2005 Seveso Population-Based Study
Table 2.
Neonatal b-TSH Levels in Children Born between 1994 and 2005 to Women from Zone A (the Zone Most Contaminated after the Seveso Accident), Zone B, and the Surrounding Noncontaminated Area (Reference)
Figure 1. Distribution of Neonatal b-TSH by Dioxin Contamination Zone.
Neonatal b-TSH distribution for children born between 1994 and 2005 to women from zone A (n = 54), the zone most contaminated by TCDD after the Seveso accident; zone B (n = 425); and the surrounding noncontaminated reference area (n = 533). The graph shows kernel density estimates by zones.
*According to the WHO [1], the percentage at the population level of newborns with a b-TSH value greater than 5 μU/ml should be less than 3% in iodine-replete areas.
Table 3.
Frequency and Relative Odds of Elevated Neonatal b-TSH Levels (> 5 μU/ml) in Children Born between 1994 and 2005 to Women from Zone A (the Zone Most Contaminated after the Seveso Accident), Zone B, and the Surrounding Noncontaminated Area (Reference)
Eight of the newborns in our study (three from reference [0.6%], four from zone B [0.9%], and one from zone A [1.8%]) had b-TSH levels > 10 μU/ml, which is set as the recall threshold for further laboratory and clinical investigations in Lombardy Region. Two of them (one from zone B [0.2%] and one from zone A [1.8%]) had b-TSH > 10 μU/ml twice in recall tests and were eventually diagnosed with congenital primary hypothyroidism (p = 0.049, Fisher's exact test). The remaining five children all had b-TSH < 5 μU/ml at the first recall and did not undergo further testing.
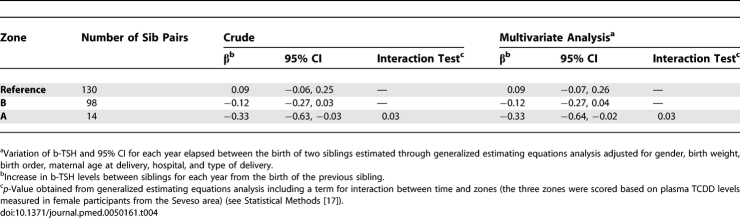
In this study, 228 women had more than one child during the study period. We calculated the difference in b-TSH between the first and the second child (228 pairs), and between the third and the second child (14 pairs). Neonatal b-TSH decreased with time between one birth and the next (Table 4) in the contaminated zones (A and B), while no decrease was found in the reference population (p = 0.03 for the interaction with zones).
Table 4.
Variations in Neonatal b-TSH between Siblings born between 1994 and 2005 to Women from Zone A, the Zone Most Contaminated after the Seveso Accident, Zone B, and the Surrounding Noncontaminated Area (Reference)
All results in the residence-based population study were similar after adjustment for gender, birth weight, birth order, maternal age at delivery, hospital, and type of delivery (Tables 2–4).
Study Based on Plasma Dioxin Measurements
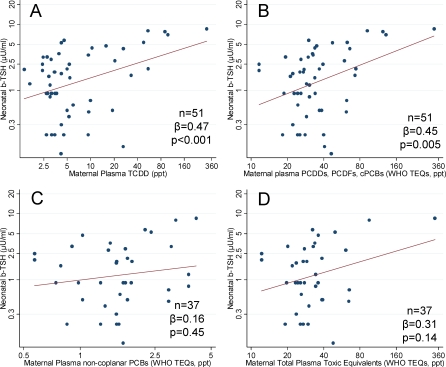
Maternal TCDD levels estimated at the date of delivery were positively associated with neonatal b-TSH (n = 51, standardized regression coefficient [β] = 0.47, p < 0.001, Figure 2A). When also other dioxin congeners were considered, a similar correlation was found with plasma TEQs for PCDDs, PCDFs, and coplanar PCBs (n = 51, β = 0.45, p = 0.005, Figure 2B), but not with non-coplanar PCBs (n = 37, β = 0.16, p = 0.45, Figure 2C). Multivariate regression models adjusting for gender, birth weight, birth order, maternal age at delivery, hospital, and type of delivery confirmed the association of neonatal b-TSH with plasma TCDD (β = 0.75, p < 0.001), PCDDs, PCDFs, and coplanar PCBs (β = 0.68, p < 0.001), and the lack of significant correlation with non-coplanar PCBs (β = 0.24, p = 0.46). When the sum of all total TEQs from the measured compounds was considered, the correlation with neonatal b-TSH levels was not significant in the crude analysis (n = 37, β = 0.31, p = 0.14, Figure 2D). However, in the multivariate analysis the correlation was significant (β = 0.65, p < 0.001).
Figure 2. Plasma Dioxin Levels and Neonatal b-TSH.
Neonatal b-TSH and maternal plasma levels of dioxin-related compounds estimated at the date of delivery for (A) TCDD; (B) PCDDs, PCDFs, coplanar PCB TEQs; (C) non-coplanar PCB TEQs; and (D) total TEQs (i.e., the sum of TEQs from all measured congeners, including PCDDs, PCDFs, coplanar PCBs, and non-coplanar PCBs).
Number of mother–child pairs (n), standardized regression coefficients (β), and p-values (p) from models using generalized estimating equations are reported.
We performed regression diagnostics and sensitivity analyses to evaluate the role of influential data points in our analyses [28]. We identified influential points using either of the following two criteria: (i) largest Cook's distance (highest 5%); (ii) strongest impact on the TSH-exposure slope (highest 5% difference). Including or removing single influential points did not modify the strength of the positive association between maternal plasma levels of dioxins and neonatal TSH levels, which always remained statistically significant at the 0.05 level. All positive associations were dependent on the presence in the analyses of participants with very high plasma TCDD level (> 50 ppt, n = 5). When the analysis was restricted to individuals with TCDD ≤ 50 ppt, none of the correlations described above was statistically significant. However, when the nonparametric Spearman's rank correlation coefficients (rs) were used, the associations of b-TSH with plasma TCDD (rs = 0.28, p = 0.04), and TEQs for PCDDs, PCDFs, and coplanar PCBs (rs = 0.33, p = 0.02) were significant.
When newborns were divided by contamination zones, correlations with neonatal b-TSH were highest in zone A for both plasma TCDD (rs = 0.70, p = 0.01), and PCDD, PCDFs, and coplanar PCBs (rs = 0.82, p = 0.001), whereas in the reference area no association was found with either plasma TCDD (rs = −0.25; p = 0.51), or PCDD, PCDFs, and coplanar PCBs (rs = 0.11, p = 0.78).
The analyses described above were based on plasma TCDD levels that, for women with plasma TCDD > 10 ppt, were extrapolated to the date of delivery using a first-order pharmacokinetic model. Using the measured TCDD concentrations in place of the extrapolated levels affected the results only marginally. In particular, neonatal b-TSH levels exhibited significant associations in multivariable models with plasma TCDD (β = 0.68, p = 0.002); plasma TEQs for PCDDs, PCDFs, and coplanar PCBs (β = 0.60, p = 0.004); and sum of all total TEQs (β = 0.65, p = 0.001).
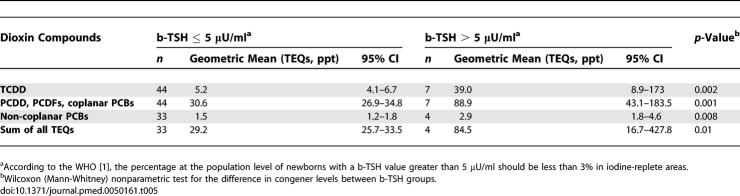
As shown in Table 5, plasma dioxin levels were significantly higher in newborns with b-TSH > 5 μU/ml. Plasma TCDD was 5.2 ppt (95% CI 4.1–6.7) in newborns with b-TSH ≤ 5 μU/ml and 39.0 ppt (95% CI 8.9–173) in those with b-TSH > 5 μU/ml (p = 0.005). Plasma TEQs for PCDDs, PCDFs, and coplanar PCBs were 30.6 ppt (95% CI 26.9–34.8) in newborns with b-TSH ≤ 5 μU/ml and 88.9 ppt (95% CI 43.1–183.5) in those with b-TSH > 5 μU/ml (p = 0.002). Also, in the group with b-TSH <5 μU/ml non-coplanar PCBs levels (1.5 ppt, 95% CI 1.2–1.8) and the sum of all TEQs (29.2 ppt, 95% CI 25.3–33.5) were significantly different from the levels found in the in the group with b-TSH ≥ 5 μU/ml (2.9 ppt, 95% CI 1.8–4.6, p = 0.003 for non-coplanar PCBs; 84.5 ppt, 95% CI 16.7–427.8, p = 0.01 for the sum of all TEQs).
Table 5.
Plasma Levels of Dioxin Compounds by b-TSH Levels (≤ 0.5 μU/ml or > 5 μU/ml)
Discussion
Neonatal b-TSH, which is used in most countries to screen for congenital hypothyroidism, is considered a sensitive marker of subclinical primary hypothyroidism and a suitable index of the presence of factors causing thyroid enlargement and potential alterations in function [1,29,30]. Our results from the Seveso population showed that newborns of mothers with high body burdens of TCDD, resulting from accidental dioxin exposure occurring approximately 20–30 y earlier, had higher neonatal b-TSH concentrations compared to newborns of nonexposed women.
In our residence-based population study, we observed a shift in the distribution of b-TSH toward higher levels in the exposed groups, thus suggesting that dioxin exposure may produce effects that are detectable at the population level. Mean b-TSH levels increased through the contamination zones, with proportions of b-TSH > 5 μU/ml in the highly contaminated areas (zones A and B) equivalent to those associated with mild iodine deficiency (3%–19.9% according to the WHO) [1]. Epidemiological studies conducted in areas with mild to moderate iodine deficiency have demonstrated, even in the absence of clinical hypothyroidism, abnormalities in psychoneuromotor and intellectual development, including impairment of visual-motor performances, motor skills, perceptual and neuromotor abilities, as well as reduced development and intellectual quotients (IQs) [29,31]. Postnatal cognitive and motor alterations have also been described in children with perinatal exposure to dioxin-related compounds [10,32–36]. At the individual level, only eight of the newborns in our study had b-TSH levels > 10 μU/ml, which is commonly set as the recall threshold for further laboratory and clinical investigations for congenital hypothyroidism. After further testing, two children from the contaminated areas and none from the reference were diagnosed with primary hypothyroidism. Our residence-based population study did not include women from the zone R area, which was an area with low-level and patchy contamination, representing a circular strip between the highly contaminated zones (A and B) and the surrounding reference area [20]. Additional research is warranted to determine whether neonatal thyroid function has been altered by dioxin exposure in the zone R population.
Plasma dioxin has been shown in Seveso and elsewhere to decrease exponentially with time in individuals with high body burdens, while it is nearly constant in individuals with background exposure [24,25]. In our residence-based population study, the analysis of changes in neonatal b-TSH between siblings from the contaminated zones showed that b-TSH was higher in the first child and tended to decrease with time in subsequent children. No time-related decrease in b-TSH was seen in the reference zone. This finding provides indirect evidence for a decrease of dioxin effects on neonatal b-TSH in conjunction with the time-related elimination of dioxin described in Seveso [15].
Our analysis based on dioxin plasma measurements confirmed the results of the residence-based population study and permitted directly confirming the presence of a positive correlation of b-TSH levels with current plasma TCDD estimated at birth, as well as with TEQs of dioxin-like compounds. Persistence of elevated TCDD levels more than 20 y after the accident and the relative strength of the associations suggest that TCDD was the main factor driving the relation between dioxin plasma concentrations and b-TSH. Exposure in the Seveso accident was predominantly to TCDD [14], and the associations we observed with neonatal b-TSH may reflect differences in exposure dose, as well as differential susceptibility in the infants to dioxin effects.
Our results, showing higher b-TSH levels in TCDD-exposed individuals, are consistent with animal investigations indicating that maternal TCDD exposure induces elevated b-TSH and neonatal primary hypothyroidism [2,7,8,10]. Previous investigations in humans, which have measured thyroid function and TCDD exposure in mother–child pairs from the general population, produced inconsistent results [2]. Initial reports from the Netherlands suggested that infants born to mothers with PCDD, PCDF, and PCB concentrations on the higher side of the population range had higher plasma TSH levels [37,38]. Koopman-Esseboom et al. [37] evaluated thyroid function on 78 breast-fed children at 2 wk and 3 mo of age. Levels of 22 individual PCDD, PCDF, and PCB congeners, measured in human milk samples, were within background levels and correlated with higher infant plasma TSH levels measured in the second week and third month after birth. In a subsequent study, Pluim et al. [38] performed thyroid function tests in 38 breast-fed infants at birth and at 1 and 11 wk of age and found that infants breast-fed with milk containing TEQ levels above the median of the study group had higher mean plasma TSH at 11 wk of age, relative to infants below the TEQ median, while TSH levels were not different between the two groups at birth and 1 wk after birth.
After these two initial reports, a series of studies have been conducted that have not confirmed the association between dioxin exposure and thyroid function alterations [39–42]. The largest of these studies, which was conducted in Japan, showed no association of serum TSH, total T4, total T3, and free T4, measured on 337 breast-fed children at 1 y of age, with breast milk TEQ background levels from 41 PCDD, PCDF, and PCB congeners measured in maternal breast milk 30 d after birth [40]. More recently, a study conducted on a sample of 118 children from the general population of central Taiwan found in female newborns, but not in males, a negative correlation between cord b-TSH levels and TEQ levels from 17 PCDDs and PCDFs, and 12 PCBs measured in placental tissue [43]. A recent study that used the CALUX assay as a functional measure of dioxin activity on cord blood samples from 198 newborns observed a negative association with cord blood free T3 and T4, but not with TSH [44]. Several studies have investigated the association of dioxin-like and nondioxin-like PCBs on neonatal thyroid function [45–49], reporting associations of either the sum of PCBs or individual congeners with decreased free T4 [45,46], and increased TSH [47,48], though between-study consistency was limited. Discrepancies among studies may reflect random variability in investigations with relatively small sample size, likely to have insufficient statistical power to detect potentially subtle effects from low dioxin and PCB levels [2]. Our study has the advantage of being based on a unique population of women exposed to high levels of TCDD. Our residence-based population study included over 1,000 newborns whose thyroid function was evaluated after birth using standardized procedures for blood sample collection, handling, and shipment. b-TSH measurements were performed at a single laboratory using the same method throughout the study period. Our study based on plasma dioxin measurements, which was conducted on a smaller sample of individuals only partially included in the residence-based population study, allowed for the evaluation of the dose-effect relationship with different metrics of dioxin concentrations. Because b-TSH levels were measured 72 h after birth in our study, mother–child dioxin transfer through colostrum [50] might have contributed to the correlation in our study between dioxin and b-TSH levels. Because we did not have information on breast-feeding before b-TSH measurement, the relative contribution of dioxins from colostrum could not be assessed in our study.
In all our analyses, we adjusted for potential determinants of b-TSH or TCDD levels, including gender, birth weight, birth order of the newborn, maternal age at delivery, hospital, and type of delivery. Information on other determinants of neonatal b-TSH levels, including maternal iodine intake, was not available. However, there is no indication that exposed and unexposed women had differences in iodine intake in the study period. The exposed and referent populations all lived in a relatively small geographical area at the time of the accident [14]. Changes of residence immediately after the accident and later migrations determined geographical distributions across the Lombardy region that are very similar for the exposed and reference populations [14,20,51]. All available indicators have shown close comparability in terms of social, educational, cultural, and occupational characteristics, as well as of access to health-care services, including primary care physicians, obstetrics and gynecology specialists, and hospital care [14,20,52]. In addition, by adjusting for hospital of birth in multivariable models, we also controlled in our analyses for geographical location, thus reducing the likelihood that systematic environmental differences in iodine concentrations between exposed and reference populations might have biased the results.
Our findings from the Seveso population indicate that maternal exposure to persistent environmental contaminants such as TCDD produces effects on neonatal thyroid function that may occur far apart in time from the initial exposure. To clarify the clinical significance of our findings, further investigation on developmental outcomes after maternal dioxin exposure is warranted.
Supporting Information
(22 KB DOC)
(36 KB DOC)
(21 KB DOC)
Abbreviations
- b-TSH
blood thyroid-stimulating hormone
- CI
confidence interval
- OR
odds ratio
- PCB
polychlorinated biphenyl
- PCDD
dibenzo-p-dioxin
- PCDF
dibenzofuran
- ppt
parts per trillion
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TEQ
toxic equivalent
- WHO
World Health Organization
Footnotes
Author contributions. AB, CC, DC, and PAB designed the study. SMG and PG organized and performed the data collection. MTL, DGP, ACP, and PAB designed and established the Seveso Choracne Study. MB performed data analyses under AB's and DC's supervision. AB and SMG wrote the manuscript. AB, SMG, CC, MTL, MB, DC, PG, DGP, ACP, and PAB contributed to the data interpretation and critical revision of the manuscript.
Funding: This work was supported by the following Research Grants: Italian Ministry of University and Scientific Research (MIUR) Internationalization Program 2004–2006/97-C, and CARIPLO Foundation and Lombardy Region Research Contracts numbers UniMi 8614/2006 and UniMi 9167/2007. The study sponsors had no role in the study design; collection, analysis, and interpretation of data; writing of the paper; and decision to submit it for publication.
Competing Interests: The authors have declared that no competing interests exist.
References
- World Health Organization. Indicators for assessing iodine deficiency disorders and their control through salt iodization. Geneva: World Health Organization; 1994. WHO/NUT/94.6 WHO/NUT/94.6. [Google Scholar]
- Giacomini SM, Hou L, Bertazzi PA, Baccarelli A. Dioxin effects on neonatal and infant thyroid function: routes of perinatal exposure, mechanisms of action and evidence from epidemiology studies. Int Arch Occup Environ Health. 2006;79:396–404. doi: 10.1007/s00420-005-0049-4. [DOI] [PubMed] [Google Scholar]
- Porterfield SP. Thyroidal dysfunction and environmental chemicals–potential impact on brain development. Environ Health Perspect. 2000;108(Suppl 3):433–438. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Research Council. Health implications of perchlorate ingestion. Washington (D.C.): The National Academies Press; 2005. [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101:419–428. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Capen CC. Mechanisms of chemical injury of thyroid gland. Prog Clin Biol Res. 1994;387:173–191. [PubMed] [Google Scholar]
- Birnbaum LS, Tuomisto J. Noncarcinogenic effects of TCDD in animals. Food Addit Contam. 2000;17:275–288. doi: 10.1080/026520300283351. [DOI] [PubMed] [Google Scholar]
- Kakeyama M, Tohyama C. Developmental neurotoxicity of dioxin and its related compounds. Ind Health. 2003;41:215–230. doi: 10.2486/indhealth.41.215. [DOI] [PubMed] [Google Scholar]
- ten Tusscher GW, Koppe JG. Perinatal dioxin exposure and later effects–a review. Chemosphere. 2004;54:1329–1336. doi: 10.1016/S0045-6535(03)00254-6. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Miyabara Y, Fujii-Kuriyama Y, Tohyama C. Altered thyroxin and retinoid metabolic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin in aryl hydrocarbon receptor-null mice. Arch Toxicol. 2005;79:260–267. doi: 10.1007/s00204-004-0626-4. [DOI] [PubMed] [Google Scholar]
- McKinney JD, Chae K, Oatley SJ, Blake CC. Molecular interactions of toxic chlorinated dibenzo-p-dioxins and dibenzofurans with thyroxine binding prealbumin. J Med Chem. 1985;28:375–381. doi: 10.1021/jm00381a018. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Pesatori AC, Bertazzi PA. Occupational and environmental agents as endocrine disruptors: experimental and human evidence. J Endocrinol Invest. 2000;23:771–781. doi: 10.1007/BF03345069. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Di Domenico A. Health consequences of the Seveso, Italy, accident. In: Schecter A, Gasiewicz TA, editors. Dioxins and health. New York: Wiley-Interscience; 2003. pp. 827–853. 2nd edition. [Google Scholar]
- Michalek JE, Pirkle JL, Needham LL, Patterson DG, Jr., Caudill SP, et al. Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso adults and veterans of operation Ranch Hand. J Expo Anal Environ Epidemiol. 2002;12:44–53. doi: 10.1038/sj.jea.7500201. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, et al. Handling of dioxin measurement data in the presence of nondetectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere. 2005;60:898–906. doi: 10.1016/j.chemosphere.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Landi MT, Consonni D, Patterson DG, Jr., Needham LL, Lucier G, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environ Health Perspect. 1998;106:273–277. doi: 10.1289/ehp.98106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Needham LL, Lucier G, Mocarelli P, Bertazzi PA, et al. Concentrations of dioxin 20 years after Seveso. Lancet. 1997;349:1811. doi: 10.1016/s0140-6736(97)24025-0. [DOI] [PubMed] [Google Scholar]
- di Domenico A, Silano V, Viviano G, Zapponi G. Accidental release of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at Seveso, Italy. VI. TCDD levels in atmospheric particles. Ecotoxicol Environ Saf. 1980;4:346–356. doi: 10.1016/0147-6513(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- Needham LL, Gerthoux PM, Patterson DG, Jr., Brambilla P, Turner WE, et al. Serum dioxin levels in Seveso, Italy, population in 1976. Teratog Carcinog Mutagen. 1997;17:225–240. [PubMed] [Google Scholar]
- Baccarelli A, Pesatori AC, Consonni D, Mocarelli P, Patterson DG, Jr., et al. Health status and plasma dioxin levels in chloracne cases 20 years after the Seveso, Italy accident. Br J Dermatol. 2005;152:459–465. doi: 10.1111/j.1365-2133.2005.06444.x. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Hampton L, Lapeza CR, Jr., Belser WT, Green V, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59:2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Pirkle JL, Caudill SP, Tripathi RC, Patterson DG, Jr., et al. Pharmacokinetics of TCDD in veterans of Operation Ranch Hand: 10-year follow-up. J Toxicol Environ Health. 1996;47:209–220. doi: 10.1080/009841096161744. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Tripathi RC. Pharmacokinetics of TCDD in veterans of Operation Ranch Hand: 15-year follow-up. J Toxicol Environ Health A. 1999;57:369–378. doi: 10.1080/009841099157584. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi, Seth A, Sekhri T, Agarwal A. Effect of perinatal factors on cord blood thyroid stimulating hormone levels. J Pediatr Endocrinol Metab. 2007;20:59–64. doi: 10.1515/jpem.2007.20.1.59. [DOI] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. New York: John Wiley; 1980. [Google Scholar]
- Delange F. Iodine deficiency in Europe and its consequences: an update. Eur J Nucl Med Mol Imaging. 2002;29(Suppl 2):S404–S416. doi: 10.1007/s00259-002-0812-7. [DOI] [PubMed] [Google Scholar]
- Carta Sorcini M, Diodato A, Fazzini C, Sabini G, Carta S, et al. Influence of environmental iodine deficiency on neonatal thyroid screening results. J Endocrinol Invest. 1988;11:309–312. doi: 10.1007/BF03350156. [DOI] [PubMed] [Google Scholar]
- Aghini Lombardi FA, Pinchera A, Antonangeli L, Rago T, Chiovato L, et al. Mild iodine deficiency during fetal/neonatal life and neuropsychological impairment in Tuscany. J Endocrinol Invest. 1995;18:57–62. doi: 10.1007/BF03349700. [DOI] [PubMed] [Google Scholar]
- Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health. 1994;84:415–421. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18:415–424. [PubMed] [Google Scholar]
- Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140:48–56. doi: 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, Van der Paauw CG, Tuinstra LG, et al. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants' mental and psychomotor development. Pediatrics. 1996;97:700–706. [PubMed] [Google Scholar]
- Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, Lutkeschipholt IJ, Van der Paauw CG, et al. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994;36:468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Pluim HJ, de Vijlder JJ, Olie K, Kok JH, Vulsma T, et al. Effects of pre- and postnatal exposure to chlorinated dioxins and furans on human neonatal thyroid hormone concentrations. Environ Health Perspect. 1993;101:504–508. doi: 10.1289/ehp.93101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama J, Okamura K, Iida T, Hirakawa H, Matsueda T, et al. Postnatal exposure to chlorinated dioxins and related chemicals on thyroid hormone status in Japanese breast-fed infants. Chemosphere. 1998;37:1789–1793. doi: 10.1016/s0045-6535(98)00244-6. [DOI] [PubMed] [Google Scholar]
- Matsuura N, Uchiyama T, Tada H, Nakamura Y, Kondo N, et al. Effects of dioxins and polychlorinated biphenyls (PCBs) on thyroid function in infants born in Japan–the second report from research on environmental health. Chemosphere. 2001;45:1167–1171. doi: 10.1016/s0045-6535(01)00050-9. [DOI] [PubMed] [Google Scholar]
- Nagayama J, Tsuji H, Lida T, Nakagawa R, Matsueda T, et al. Effect of lactational exposure to organochlorine pesticides, PCBs and dioxins on immune response and thyroid hormone systems in Japanese male and female infants. Organohalogen Compounds. 2004;66:3217–3223. [Google Scholar]
- Wilhelm M, Wittsiepe J, Lemm F, Ranft U, Kramer U, et al. The Duisburg birth cohort study: influence of the prenatal exposure to PCDD/Fs and dioxin-like PCBs on thyroid hormone status in newborns and neurodevelopment of infants until the age of 24 months. Mutat Res. 2007. In press. [DOI] [PubMed]
- Wang SL, Su PH, Jong SB, Guo YL, Chou WL, et al. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environ Health Perspect. 2005;113:1645–1650. doi: 10.1289/ehp.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maervoet J, Vermeir G, Covaci A, Van Larebeke N, Koppen G, et al. Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ Health Perspect. 2007;115:1780–1786. doi: 10.1289/ehp.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Gladen BC, Patterson DG, Jr., Rogan WJ. Polychlorinated biphenyl (PCB) exposure in relation to thyroid hormone levels in neonates. Epidemiology. 2000;11:249–254. doi: 10.1097/00001648-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Pentachlorophenol and hydroxylated polychlorinated biphenyl metabolites in umbilical cord plasma of neonates from coastal populations in Quebec. Environ Health Perspect. 2002;110:411–417. doi: 10.1289/ehp.02110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Pedrerol M, Ribas-Fito N, Torrent M, Carrizo D, Garcia-Esteban R, et al. Thyroid disruption at birth due to prenatal exposure to beta-hexachlorocyclohexane. Environ Int. 2008. In press. [DOI] [PubMed]
- Chevrier J, Eskenazi B, Bradman A, Fenster L, Barr DB. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ Health Perspect. 2007;115:1490–1496. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, et al. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res. 2007;105:240–246. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Yu Z, Palkovicova L, Drobna B, Petrik J, Kocan A, et al. Comparison of organochlorine compound concentrations in colostrum and mature milk. Chemosphere. 2007;66:1012–1018. doi: 10.1016/j.chemosphere.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Pesatori AC. Dioxin contamination in Seveso: the social tragedy and the scientific challenge. Med Lav. 1995;86:111–124. [PubMed] [Google Scholar]
- Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Sanarico M, et al. Mortality in an area contaminated by TCDD following an industrial incident. Med Lav. 1989;80:316–329. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(22 KB DOC)
(36 KB DOC)
(21 KB DOC)