Abstract
The irreversible proteolytic mechanism by which protease-activated receptor-1 (PAR1), the G protein-coupled receptor (GPCR) for thrombin, is activated raises the question of how it is shut off. Like classic GPCRs, activated PAR1 is rapidly phosphorylated and internalized, but unlike classic GPCRs, which recycle, internalized PAR1 is sorted to lysosomes. A chimeric PAR1 bearing the substance P receptor’s cytoplasmic carboxyl tail sequestered and recycled like wild-type substance P receptor. In cells expressing this chimera, signaling in response to the PAR1-activating peptide SFLLRN ceased as expected upon removal of this agonist. Strikingly, however, when the chimera was activated proteolytically by thrombin, signaling persisted even after thrombin was removed. This persistent signaling was apparently due to “resignaling” by previously activated receptors that had internalized and recycled back to the cell surface. Thus the cytoplasmic carboxyl tail of PAR1 specifies an intracellular sorting pattern that is linked to its signaling properties. In striking contrast to most GPCRs, sorting of activated PAR1 to lysosomes rather than recycling is critical for terminating PAR1 signaling—a trafficking solution to a signaling problem.
Accurate desensitization and resensitization of signaling by G protein-coupled receptors (GPCRs) is critical for the regulation of a host of biological processes. Biochemical and genetic evidence demonstrates that GPCR desensitization is accomplished at least in part by the rapid phosphorylation of activated receptors by G protein-coupled receptor kinases. Phosphorylation of the agonist-occupied form of the receptor enhances its affinity for the cytosolic protein arrestin, and arrestin binding prevents receptor-G protein interaction thereby uncoupling the receptor from effectors of signaling. After this initial uncoupling, most activated GPCRs are internalized into endosomes where they dissociate from their ligands, become dephosphorylated, then return to the cell surface in a state capable of responding again to ligand (see refs. 1–3; reviewed in ref. 4). Thus for classic GPCRs that are activated by reversibly bound ligands, shut off occurs at the plasma membrane, and receptor trafficking is linked to resensitization of signaling.
Protease-activated receptor-1 (PAR1) is a GPCR for the serine protease thrombin. PAR1 mediates thrombin signaling in human platelets, endothelial cells, and fibroblasts and appears to play an important role in hemostasis and thrombosis, embryonic development and other processes (see refs. 5–7; reviewed in refs. 8 and 9). Unlike classic GPCRs, PAR1 is activated by an irreversible proteolytic mechanism. Thrombin binds to and cleaves PAR1’s amino-terminal exodomain thereby generating a new amino terminus that serves as a tethered ligand, binding intramolecularly to the body of the receptor to effect signaling (10–12). Synthetic peptides that mimic PAR1’s tethered ligand domain function as agonists for this receptor, activating it as a peptide hormone would activate a peptide receptor and independent of thrombin and receptor cleavage. In light of the reversible activation and recycling described above for classic GPCRs, the irreversibility of PAR1’s proteolytic activation mechanism begs the question of how desensitization is accomplished for an irreversibly activated receptor. An answer may be found in the unusual fate of activated PAR1.
In endothelial cells and fibroblasts, activated PAR1, like other activated GPCRs, becomes rapidly phosphorylated and uncoupled from signaling (13, 14). It also undergoes activation-triggered internalization (15–17). However, unlike classic GPCRs, which sequester and recycle, activated PAR1 is sorted largely to lysosomes (15, 18). Does this distinctive trafficking pattern provide a means for terminating PAR1 signaling? If PAR1 were to recycle like classic GPCRs, would this preclude termination of signaling? Toward answering such questions, we recently examined chimeras between PAR1 and the G protein-coupled receptor for substance P (SPR) to identify the domain(s) responsible for their distinct trafficking patterns (19). SPR, also known as the neurokinin-1 receptor, is a classic GPCR. It is activated reversibly by the peptide substance P, internalized, and recycled to the plasma membrane (20, 21). Our studies demonstrated that exchanging the cytoplasmic carboxyl tails of PAR1 and SPR switched their trafficking behaviors (19). Most remarkably, a chimeric PAR1 bearing SPR’s cytoplasmic carboxyl tail (P/S chimera) internalized upon activation but recycled back to the plasma membrane like the wild-type SPR.
The P/S chimera provided an opportunity to test the importance of lysosomal sorting for termination of PAR1 signaling. In cells expressing the P/S chimera, phosphoinositide hydrolysis in response to the PAR1-activating peptide SFLLRN ceased after removal of this agonist as expected. Strikingly, however, when activated proteolytically by thrombin, the P/S chimera continued signaling even after thrombin was removed. This persistent signaling appeared to be caused by “resignaling” by proteolytically activated receptors that internalized then returned to the cell surface with their tethered ligands still intact. Our findings strongly suggest that the sorting of activated PAR1 to lysosomes rather than its recycling is indeed critical for termination of PAR1 signaling.
MATERIALS AND METHODS
Antibodies and Reagents.
Polyclonal 1809 antibody was raised to a peptide representing the hirudin-like sequence in PAR1s amino-terminal exodomain (5). Horseradish peroxidase-conjugated goat anti-rabbit antibody was purchased from Bio-Rad. The PAR1 agonist peptide SFLLRN was synthesized as the carboxyl amide and purified by reverse phase HPLC liquid chromatography and human α-thrombin (α-Th) was obtained from Enzyme Research Laboratories. Leech hirudin, soybean trypsin inhibitor, and α-trypsin treated with tosylamido-2-phenylethyl chloromethyl ketone were obtained from Sigma.
cDNAs and Cell Lines.
A PAR1 cDNA containing a prolactin signal sequence followed by a FLAG epitope (DYKDDDD) was used for generating mutants (13). A chimeric PAR1 cDNA bearing SPR’s cytoplasmic carboxyl tail (P/S chimera) was generated as described (19). This was converted to the P/S* chimera by oligonucleotide-directed mutagenesis to insert a trypsin cleavage site into PAR1’s amino-terminal exodomain carboxyl tail to the native thrombin cleavage site and tethered ligand. The resulting amino acid sequence was GLTEYSKGR/SALLRLVSI where the inserted sequence is underlined and the peptide bond cleaved by trypsin is indicated (/). Mutations in all constructs were confirmed by dideoxy sequencing. cDNAs encoding wild-type and chimeric receptors were subcloned into the mammalian expression vector pBJ1 (provided by Mark Davis, Stanford University, Stanford, CA) for transfection into cells. Mouse lung fibroblasts derived from PAR1-deficient mice (7) were cotransfected with receptor expression vectors and a plasmid encoding a hygromycin resistance gene (provided by J. Michael Bishop, University of California, San Francisco); stable transfectants were selected in 250 μg/ml hygromycin B and screened by surface antibody binding.
Phosphoinositide Hydrolysis.
Cells were plated in 12-well dishes (Falcon) and were labeled overnight with 2 μCi/ml (1 Ci = 37 GBq) myo-[3H]inositol in DMEM containing 1 mg/ml BSA. Cells were washed with DMEM/BSA containing 20 mM LiCl and treated as described. The formation of inositol phosphates (IPs) was assayed as reported (22).
Cell Surface ELISA.
Cells were plated in 24-well dishes (Falcon) and treated as described in Fig. 4. After treatments cells were fixed with 4% PFA for 5 min at 4°C and washed twice with PBS. Cells were then incubated for 1 hr at 25°C with 1809 hirudin domain antisera (1:200) diluted in DMEM containing 10 mM Hepes (pH 7.4) and 1 mg/ml BSA followed by another 1 hr incubation at 25°C with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000); both antibodies were diluted in DMEM/Hepes/BSA. Cells were washed, incubated in 1-Step ABTS (Pierce) and after 20–30 min an aliquot was removed and the absorbance was read at 405 nm using a Molecular Devices microplate spectrophotometer.
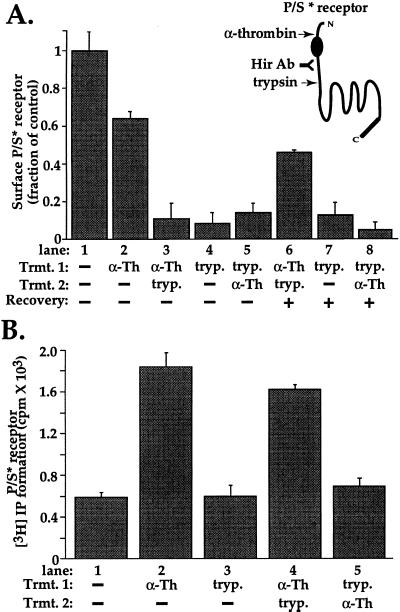
Figure 4.
Receptor recycling and “reactivation” of signaling by proteolytically activated P/S chimera. (A) Recovery of proteolytically activated P/S* receptor at the cell surface was measured in stably transfected mouse lung fibroblasts by cell surface ELISA by using the antibody to the receptor’s hirudin-like domain (Hir Ab). Cells were incubated for 5 min at 25°C in the absence (lane 1) or presence (lane 2) of 10 nM α-Th, then fixed and the amount of receptor remaining on the cell surface was measured. Note the decrease in surface expression after α-Th, consistent with internalization of some receptors. For lanes 3–5 cells, cells were incubated with either 10 nM α-Th for 5 min at 25°C or with 100 nM trypsin (tryp.) for 20 min at 4°C. Proteases were then removed and cells were washed with DMEM containing either 0.5 units/ml hirudin or 2 μg/ml soybean trypsin inhibitor, then exposed a second time to either α-Th or trypsin as indicated. Cells were then fixed and the amount of receptor on the cell surface was determined. Note that trypsin effectively removed the hirudin-like domain epitope from the cell surface. For lanes 6–8, cells were treated as in lanes 3–5, but were incubated for an additional 60 min in the absence of proteases before the amount of P/S* receptor on the cell surface was measured. Note that significant recovery was seen only in cells previously exposed to α-Th (lane 6). The data shown are mean ± SD (n = 3) specific binding of hirudin-domain antibody to the cell surface; nonspecific antibody binding measured in untransfected cells was subtracted from total binding for each condition. Data are expressed as a fraction of specific binding measured in untreated cells (lane 1). Similar results were obtained in four separate experiments. (B) Signaling by P/S* receptor was measured in stably transfected lung fibroblasts labeled with myo-[3H]inositol. For lanes 2 and 3, cells were incubated in the presence or absence of 10 nM α-Th for 5 min at 25°C or 100 nM trypsin (tryp.) for 20 min at 4°C, proteases were then removed as above. For lanes 4 and 5, cells were exposed to α-Th and trypsin sequentially as indicated. For all lanes, LiCl was added after removal of the proteases, and accumulated [3H]IPs were measured after an additional 60 min incubation at 25°C. The data shown are the mean values ± SD (n = 3); basal [3H]IP formation was 590 cpm/well (lane 1). This experiment is representative of three independent experiments. Note that exposure to trypsin before but not after thrombin prevented persistent signaling.
Receptor Phosphorylation.
Receptor phosphorylation was examined using a modification of a previously described procedure (14). Cells plated in six-well dishes (Falcon) were labeled with 250 μCi/ml [32P]orthophosphate (DuPont/NEN) in phosphate-free DMEM containing 1 mg/ml BSA for 3 hr at 37°C. Cells were then washed, incubated with agonists, and lysed in 1% Triton X-100 in 50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, 200 μM Na3VO4, and protease inhibitors (14). 1809 antibody was used for immunoprecipitations. Immunoprecipitates were resuspended in 2× SDS/gel loading buffer, resolved on SDS/9% polyacrylamide gel, and analyzed by autoradiography.
RESULTS
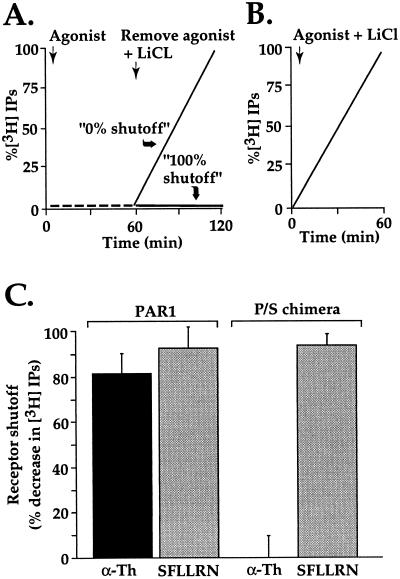
In the P/S chimera, we had created a protease-activated receptor that was demonstrated to sequester and recycle like a classic GPCR (19). By circumventing degradation in lysosomes after activation, this chimera provided an opportunity to determine the importance of lysosomal sorting instead of recycling for termination of PAR1 signaling. We therefore examined shutoff of phosphoinositide hydrolysis in fibroblasts derived from PAR1-deficient mice that had been stably transfected with either wild-type PAR1 or the P/S chimera. In this experimental paradigm cells labeled with myo-[3H]inositol were incubated with thrombin or PAR1 activating peptide SFLLRN in the absence of LiCl. Under these conditions, phosphoinositide hydrolysis is triggered by agonists but IPs are rapidly metabolized and do not accumulate (Fig. 1A, 0–60 min). After agonist removal, LiCl was added to allow accumulation of any IPs generated by ongoing phosphoinositide hydrolysis (Fig. 1A, 60–120 min). If receptor signaling had shut off completely after agonist removal, no IPs would accumulate (“100% shutoff”). If no shut off of signaling occurred after agonist removal, the rate of IP accumulation would not differ from that obtained when agonist and LiCl were added simultaneously (Fig. 1 A vs. B, “0% shutoff”). In cells expressing wild-type PAR1, phosphoinositide hydrolysis in response to either thrombin or agonist peptide SFLLRN was substantially shut off after removal of either agonist, ≈80% and 90%, respectively (Fig. 1C). Cells expressing the P/S chimera also showed substantial shutoff (≈90%) of phosphoinositide hydrolysis in response to SFLLRN after removal of this peptide agonist. Strikingly, however, when activated by thrombin, these same cells showed virtually no shutoff of phosphoinositide hydrolysis even after thrombin was removed (Fig. 1C). At face value, these data suggest that recycling is incompatible with termination of signaling by proteolytically activated PAR1.
Figure 1.
Persistent signaling by P/S chimera after activation by thrombin but not SFLLRN. (A) Schematic of experimental design. Cells labeled with myo-[3H]inositol were incubated with agonist in the absence of LiCl; under these conditions, phosphoinositide hydrolysis is stimulated but IPs do not accumulate. After 60 min, agonist was removed and LiCl was added to allow accumulation of IPs during a subsequent incubation period as a measure of persistent signaling in the absence of agonist. Results were normalized to the amount of IPs accumulated when agonist and LiCl were added simultaneously (B) and expressed as percent shutoff (i.e., zero shutoff = no difference in IP accumulation under conditions A vs. B.) (C) Phosphoinositide hydrolysis was measured in PAR1-deficient mouse lung fibroblasts stably expressing similar amounts of surface wild-type PAR1 or the P/S chimera. myo-[3H]Inositol labeled cells were incubated with 100 μM SFLLRN or 10 nM α-Th for 60 min at 25°C in the absence of LiCl. Agonist was removed and cells were washed three times with DMEM containing 0.5 units/ml hirudin (α-Th inhibitor). Cells were then incubated with DMEM containing 20 mM LiCl and 0.5 units/ml hirudin at 25°C for an additional 60 min, at which time accumulated [3H]IPs were measured. The data are the mean % shutoff (see above) ± SD (n = 3). In PAR1-expressing cells, α-Th and SFLLRN caused an initial 3- and 3.8-fold increase in phosphoinositide hydrolysis, respectively; whereas cells expressing P/S chimera showed an initial 13- and 16-fold increase in PI hydrolysis to α-Th and SFLLRN, respectively. Similar results were obtained in three separate experiments. Note remarkable failure of α-Th-activated P/S chimera to shut off.
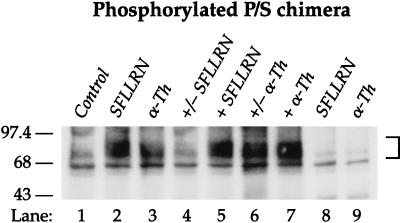
Receptor phosphorylation studies lend further support for this idea. Wild-type PAR1 is rapidly phosphorylated upon activation, an event thought to be important for acutely uncoupling the receptor from G protein and for its initial internalization (14, 17). Like wild-type PAR1, the P/S chimera was phosphorylated within 3 min of exposure to either SFLLRN or thrombin (Fig. 2, lanes 2 and 3). Phosphorylated P/S chimera became virtually undetectable within 30 min of removing SFLLRN (compare lanes 4 and 5). By contrast, phosphorylation of proteolytically-activated P/S chimera was easily detected 30 min after removal of thrombin (Fig. 2, lane 6 vs. 7). These data are consistent with the continued activation and phosphorylation of proteolytically activated receptors even after withdrawal of thrombin.
Figure 2.
Proteolytically activated P/S chimera is continually phosphorylated even after thrombin is removed. Cells labeled with [32P]orthophosphate were treated with media alone (Control) or with 100 μM SFLLRN or 10 nM thrombin (α-Th) for either 3 (lanes 2 and 3) or 30 min (lanes 5 and 7, “+”). For reversible phosphorylation studies cells were incubated with agonists for 3 min, agonists were removed, cells were washed three times with DMEM/BSA and then incubated in media alone up to 30 min (lane 4 and 6, “+/−”). DMEM/BSA wash solution was supplemented with 0.5 units/ml hirudin for thrombin treated cells. Cell lysates were prepared and receptors were immunoprecipitated as described in Materials and Methods. Receptor immnoprecipitates were resolved by SDS/PAGE and analyzed by autoradiography. No phosphorylated receptor was detected in immunoprecipitates from agonist treated untransfected mouse lung fibroblasts (see lanes 8 and 9). Similar findings were observed in P/S chimera-expressing Rat1 fibroblasts.
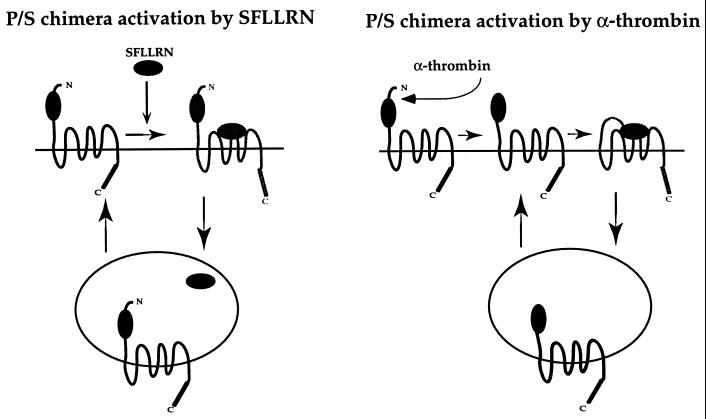
Taken together, these observations are consistent with the following model (Fig. 3): The P/S chimera behaves like a classic GPCR after activation by the PAR1 agonist peptide SFLLRN, i.e., it becomes phosphorylated, uncoupled from signaling, and internalized. Within an endosomal compartment, agonist peptide dissociates from the receptor, and the receptor then recycles back to the cell surface. When agonist is removed, cell surface receptors dissociate from agonist and enter their off state, and recycled receptors returning to the surface remain in their off state; signaling therefore ceases. By contrast, when the P/S chimera is activated irreversibly by thrombin, it follows the same path, but, because its tethered ligand is still present when the receptor reappears on the cell surface, it becomes “reactivated” and signals again whether or not thrombin is still present (Fig. 1C).
Figure 3.
Working model of reversible and irreversible activation of P/S chimera. When activated reversibly by agonist peptide SFLLRN, P/S chimera behaves like a classic GPCR. Activated P/S chimera is phosphorylated, internalized into an endosomal compartment where SFLLRN dissociates from receptor, then receptor recycles to the cell surface. When agonist is removed, receptors at the cell surface can enter their off state and receptors returning to the cell surface remain in their off state, thus signaling is effectively shutoff. When activated proteolytically by thrombin, P/S chimera follows the same trafficking route but because the tethered ligand remains present when receptor returns to the cell surface, P/S chimera becomes “reactivated” and signals again whether or not thrombin is present.
A key feature of this model is that persistent signaling by thrombin-activated P/S chimera is attributed to previously internalized receptors returning to the cell surface and “reactivating.” A possible alternative is that the P/S chimera shut off upon removal of SFLLRN simply as a result of the peptide agonist dissociating from the receptor and failed to shut off after thrombin activation because some receptors remained on the cell surface and continued to signal, perhaps due to defective uncoupling of the P/S chimera. Against this possibility is the observation that the P/S chimera was reversibly phosphorylated, internalized, and recycled after exposure to SFLLRN (Fig. 2 and data not shown). To test whether persistent signaling after thrombin activation of the P/S chimera was indeed due to receptors reappearing on the cell surface, we modified the P/S chimera such that its tethered ligand could be removed by trypsin (see Fig. 4A). When cells transfected with this mutant, designated P/S* receptor, were exposed briefly to thrombin (α-Th) at 25°C, an ≈30% decrease in cell surface amino-terminal exodomain epitope occurred, likely due to receptor internalization (lanes 1 and 2, Fig. 4A). Exposure to trypsin at 4°C largely removed cell surface amino-terminal exodomain epitope regardless of whether cells had been exposed to thrombin; at 4°C receptor endocytosis and recycling is inhibited (Fig. 4A, lanes 3–5). When proteases were removed and the cells incubated at 25°C for an additional 60-min period, a substantial fraction of the original cell surface level of amino-terminal exodomain epitope reappeared on the surface of cells that had been exposed to thrombin before trypsin, but not in cells exposed to trypsin alone or to trypsin before thrombin (Fig. 4A, lanes 6–8). These data suggest that thrombin triggered rapid internalization of the P/S* receptor, that trypsin treatment for 20 min at 4°C effectively cleaved the amino-terminal exodomain of P/S* receptor remaining at the cell surface, and that receptors previously internalized upon exposure to thrombin were protected from cleavage by trypsin and returned to the surface with their amino-terminal exodomains intact.
Persistent signaling by P/S* receptor appeared to be mediated by such recycled receptors. Cells expressing the P/S* receptor mutant showed persistent phosphoinositide hydrolysis after thrombin (α-Th) was removed (Fig. 4B). Exposure to trypsin at 4°C did not by itself activate signaling, but did prevent such signaling in response to subsequent exposure to thrombin. Strikingly, despite its effectiveness in cleaving cell surface receptors and preventing signaling to thrombin, exposure to trypsin at 4°C did not prevent persistent signaling after protease removal in cells that were exposed to thrombin before the trypsin treatment (Fig. 4B, lane 4). Such persistent signaling correlated with the return of receptors to the cell surface (Fig. 4A, lane 6). Moreover, exposure to trypsin at 25°C (as opposed to 4°C where receptor recycling is blocked) after initial receptor activation by thrombin did ablate persistent signaling (data not shown), suggesting that at 25°C the receptors responsible for such signaling at some point enter a compartment that is trypsin sensitive, most likely at the plasma membrane. Taken together, these observations suggest that proteolytically activated P/S* receptor enters a compartment that is trypsin-insensitive and that persistent signaling by P/S* receptor is mediated at least in part by such “protected” receptors recycling to the cell surface with tethered ligand intact and ready to reactivate the receptor (see Fig. 3).
DISCUSSION
In these studies we demonstrate that PAR1’s intracellular sorting pattern is linked to its signaling properties. To test whether recycling was incompatible with termination of signaling by an irreversibly activated receptor, we examined the signaling properties of the P/S chimera, a protease-activated receptor that internalizes but recycles back to the plasma membrane (19). These studies revealed a striking disparity in the behaviors of the P/S chimera depending upon the mode of activation. When activated reversibly by agonist peptide, the P/S chimera behaved like a classic GPCR and ceased signaling upon withdrawal of the agonist. However, when activated proteolytically by thrombin, signaling continued even after thrombin was removed. Additional studies suggested that such persistent signaling was due to resignaling by thrombin-activated receptors that internalized and recycled back to the plasma membrane with their tethered ligands intact. These observations suggest that lysosomal sorting of PAR1 is critical for termination of PAR1 signaling after proteolytic activation by its natural agonist thrombin. By contrast, the efficient shut off of the P/S chimera upon removal of its reversible peptide agonist SFLLRN emphasizes that agonist-receptor dissociation can be a rapid and efficient means of terminating receptor signaling—a means not normally available to PAR1.
Signaling and intracellular trafficking have been previously linked as a mechanism for resensitization of GPCRs, largely through studies of the β2-adrenergic receptor. β2-adrenergic receptors are phosphorylated upon activation, bind arrestin, and then internalize at least in part via clathrin-coated pits (23, 24). Within the endosomal compartment they dissociate from ligand, undergo dephosphorylation and recycle back to the plasma membrane in an “unactivated” state. A mutant β2-adrenergic receptor defective in sequestration had normal agonist-induced signaling and desensitization but failed to resensitize (1, 25). Agents that blocked sequestration and phosphatase activity impaired the ability of β2-adrenergic receptors to resensitize after agonist stimulation (1, 3). Our studies with the P/S chimera strongly support this model in which activated GPCRs can be internalized then return to the cell surface competent to signal again as a mechanism for resensitization. Moreover, these studies also provide an example of the converse. The apparent role for lysosomal sorting in terminating signaling by activated PAR1 provides an example in which GPCR signaling and trafficking are linked as a mechanism of receptor shut off and signal termination.
Gain-of-function mutations in GPCRs resulting in either constitutive activity or defective uncoupling can produce important effects in vivo (26–28) and underlie a number of diseases (29, 30). This study reveals a novel type of gain-of-function mutation in GPCRs, one that confers greater than wild-type signaling by altering receptor sorting. The magnitude and duration of signaling by this receptor greatly exceed that of the wild-type but it is still thrombin-dependent. It may thus be a useful tool for dissecting the roles of thrombin signaling in transgenic mouse models, where, for example, PAR1 plays an important but as yet unknown role in embryonic development (6). Whether such a mutant presages natural receptor mutations remains to be seen.
Acknowledgments
We thank Drs. Henry Bourne, Mark von Zastrow, and Harold S. Bernstein for critical review of this manuscript. This work was supported by HL44907 and HL59202 (S.R.C.). J.T. was supported by an American Heart Association Minority Scientist Career Development Award. S.H. was supported by a Howard Hughes Postdoctoral Fellowship.
ABBREVIATIONS
- GPCR
G protein-coupled receptor
- IP
inositol phosphate
- PAR1
protease-activated receptor-1
- SPR
substance P receptor
- α-Th
α-thrombin
References
- 1.Yu S S, Lefkowitz R J, Hausdorff W P. J Biol Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- 2.Krueger K M, Daaka Y, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Pippig S, Andexinger S, Lohse M J. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- 4.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–351. ; discussion 352–353. [PubMed] [Google Scholar]
- 5.Hung D T, Vu T-K H, Wheaton V I, Ishii K, Coughlin S R. J Clin Invest. 1992;89:1350–1353. doi: 10.1172/JCI115721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly A J, Ishihara H, Kahn M L, Farese R V, Coughlin S R. Nature (London) 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 7.Trejo J, Connolly A J, Coughlin S R. J Biol Chem. 1996;271:21536–21541. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- 8.Fenton J W, II. Semin Thromb Hemost. 1988;14:234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin S R. Trends Cardiovasc Med. 1994;4:77–83. doi: 10.1016/1050-1738(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 10.Vu T-K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 11.Vu T-K H, Wheaton V I, Hung D T, Coughlin S R. Nature (London) 1991;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Ishii M, Wang L, Ishii K, Coughlin S R. J Biol Chem. 1994;269:16041–16045. [PubMed] [Google Scholar]
- 13.Ishii K, Hein L, Kobilka B, Coughlin S R. J Biol Chem. 1993;268:9780–9786. [PubMed] [Google Scholar]
- 14.Ishii K, Chen J, Ishii M, Koch W J, Freedman N J, Lefkowitz R J, Coughlin S R. J Biol Chem. 1994;269:1125–1130. [PubMed] [Google Scholar]
- 15.Hein L, Ishii K, Coughlin S R, Kobilka B K. J Biol Chem. 1994;269:27719–27726. [PubMed] [Google Scholar]
- 16.Woolkalis M J, DeMelfi T J, Blanchard N, Hoxie J A, Brass L F. J Biol Chem. 1995;270:9868–9875. doi: 10.1074/jbc.270.17.9868. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro M J, Trejo J, Zeng D W, Coughlin S R. J Biol Chem. 1996;271:32874–32880. doi: 10.1074/jbc.271.51.32874. [DOI] [PubMed] [Google Scholar]
- 18.Hoxie J A, Ahuja M, Belmonte E, Pizarro S, Parton R, Brass L F. J Biol Chem. 1993;268:13756–13763. [PubMed] [Google Scholar]
- 19.Trejo, J. & Coughlin, S. R. (1998) J. Biol. Chem., in press.
- 20.Grady E F, Garland A M, Gamp P D, Lovett M, Payan D G, Bunnett N W. Mol Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantyh P W, DeMaster E, Malhotra A, Ghilardi J R, Rogers S D, Mantyh C R, Liu H, Basbaum A I, Vigna S R, Maggio J E, et al. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 22.Nanevicz T, Wang L, Chen M, Ishii M, Coughlin S R. J Biol Chem. 1996;271:702–706. doi: 10.1074/jbc.271.2.702. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson S S, Downey W R, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 24.Goodman O J, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 25.Barak L, Tiberi M, Freedman N, Kwatra M, Lefkowitz R, Caron M. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 26.Chen J, Makino C L, Peachey N S, Baylor D A, Simon M I. Science. 1995;267:374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- 27.Koch W J, Rockman H A, Samama P, Hamilton R A, Bond R A, Milano C A, Lefkowitz R J. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Dodd R L, Makino C L, Simon M I, Baylor D A, Chen J. Nature (London) 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- 29.Lefkowitz R J. Nature (London) 1993;365:603–604. doi: 10.1038/365603a0. [DOI] [PubMed] [Google Scholar]
- 30.Coughlin S R. Curr Opin Cell Biol. 1994;6(2):191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]