Abstract
Nuclear transfer (NT) is an inefficient but invaluable tool of the biotechnology industry. This study looks at abnormalities associated with NT porcine embryos at days 10, 12, and 14.
Methods
4 experimental groups were examined: non-pregnant animals, in vivo pregnant animals, NT recipients, and manipulation control recipients (MC). Maternal blood samples were collected until euthanasia (d10, 12, or 14) at which time embryos were collected and preserved. Serum samples were assayed for progesterone and insulin-like growth factor-1. All embryos were evaluated for embryonic disc diameter and gross morphology; they were then histologically evaluated for nucleoli density and mitotic figure index.
Results
Day 12 (P ≤ 0.03) and Day 14 (P ≤ 0.01) NT embryos had increased numbers of nucleoli and Day 14 NT embryos had an increased (P ≤ 0.03) mitotic index when compared to in vivo and MC embryos. In vivo produced embryos had larger (P ≤ 0.01) disk diameters at day 12 when compared to both MC and NT embryos. At day 14, the disk diameters of the MC embryos was statistically identical to those for the in vivo embryos and both of these groups had larger disk diameters than the NT embryos In vivo produced day 14 embryos were morphologically more advanced (P ≤ 0.01) than day 14 NT and MC counterparts.
Conclusions
Nuclear transfer embryos develop at a slower rate than their in vivo produced counterparts. The increase in nucleoli and mitotic index of NT embryos suggest the cell cycle may be affected or the NT embryos are employing other means to compensate for slow development. The techniques used during nuclear transfer also appear to compromise embryo development to a limited extent.
Introduction
During the course of a natural porcine pregnancy there are several time periods when embryonic losses occur. Of particular interest is the period of maternal recognition of pregnancy (MRP), which is thought to be due to conceptus-derived estrogens (Geisert et al., 1990) produced around days 11-14 of gestation as the embryo begins a morphological transformation from a spherical to a filamentous form. Estrogen production by the conceptus, as measured by maternal serum estrone sulfate, peaks at day 12 before decreasing on day 14. Furthermore, estrone sulfate concentrations tend to be higher in pregnant animals compared to those during a non-pregnant estrous cycle (Geisert et al., 1990). The synthesis and secretion of estrogen by the conceptus has previously been shown to cause the release of secretory vesicles from the epithelial cells of the uterine glands. These secretions, which include proteins, calcium, growth factors, and prostaglandins, provide the rapidly developing embryos with an environment conducive to normal growth (Davis and Blair, 1993; Geisert et al., 1982; Ka et al., 2001; Simmen et al., 1990).
A typical day 10 embryo can range in size from 3 to l0 mm across with initial visualization of an embryonic disk occurring at this time (Patten, 1948). On day 11, the spherical embryo will begin to migrate through the uterine lumen (Waite and Day, 1967). By day 12, a rapid trophoblastic elongation occurs in the developing embryo, wherein the conceptus becomes tubular in form, being anywhere from 10 to 50 mm in length. Once the embryo reaches day 14, it has become filamentous in form and can reach over 1000 mm in length. The entire process of elongation occurs within 12-24 hours (Geisert et al., 1982).
Due to the high failure rate of nuclear transfer (NT) pregnancies very early in gestation it has been hypothesized that NT derived embryos have difficulty transitioning through the critical points of elongation and successfully producing a signal for MRP. Because of the high incidence of embryonic loss during a nuclear transfer pregnancy, cloning is a relatively inefficient process. However, it remains an invaluable tool for the biotechnical industry as it is the only method currently available for the production of gene-ablated livestock,.
The goal of this study was to assess the developmental competence of normal, MC and NT-derived conceptuses at days 10, 12, and 14. Physical characteristics were evaluated such as morphology and embryonic disk diameter in order to map proper development, and to use histological indicators of abnormality such as nucleoli number and mitotic figure density to appraise biologic potential of the conceptuses.
Materials and Methods
Animals
Forty-eight gilts were randomly assigned to four embryo treatment groups (n=12): 1) In Vivo 2) Nuclear Transfer (NT) 3) Manipulation Controls (MC) and 4) Non-Pregnant (NP) [used as a negative control for those experiments not involving embryos]. Animals were heat checked every 12 hours for signs of estrus. When heat was first detected the animal was considered to be at day 0 of her estrous cycle. Embryo transfers were performed upon treatment groups receiving NT embryo and MC embryos as described (Betthauser et al., 2000; Lai et al., 2002). Gilts within the in vivo treatment group were bred by artificial insemination 12 hours after initial detection of heat.
Tissue Collection and Histology
Gilts were catheterized via the jugular vein on day 1 of the estrous cycle according to their assigned experimental group as previously described (Fudge et al., 2002). Blood samples were collected daily from day 5 to day of sacrifice. The blood was allowed to clot, and then centrifuged for 30 minutes at 4°C, 1500 g. The serum was then frozen and stored at -20°C. Gilts were euthanized at day 10, 12, or 14 at which time any embryos were recovered from the uterine horns by flushing them with phosphate buffered saline (PBS). Uterine horn sections and/or embryos were collected and preserved in 10% neutral buffered formaldehyde. Collected tissues were subsequently dehydrated through a series of ethanol washes and stored in 70% ethanol.
Hormone Assays
Serum samples were measured for progesterone (P4) using Coat-A-Count® progesterone kits (Diagnostics Products Corporation, Los Angeles, CA). The intraassay and interassay coefficients of variation were ≤10%, respectively. Serum samples were measured for IGF-1 as previously described (Lamberson et al., 1995). The intraassay and interassay coefficients of variation were ≤10%, respectively.
Production of Nuclear Transfer Embryos
Recipient oocytes were matured in TCM 199 (supplemented with 0.1% polyvinyl alcohol, cysteine (0.1 mg/ml), epidermal growth factor (10 ng/ml), 0.91 mM Na-pyruvate, 3.05 mM D-glucose, follicle-stimulating hormone (0.5 μg/ml), luteinizing hormone (0.5 μg/ml), penicillin (75 μg/ml), and streptomycin (50 μg/ml) (Lai et al., 2002)) for 48 hours after collection from the ovary. The matured oocytes were then vortexed in hyaluronidase for 4 minutes and rinsed twice in TL-HEPES to strip away cumulus cells. The oocytes were placed in culture medium (NCSU-23) containing 5 μg/mL cytochalasin B, a cytoskeletal inhibitor. Using the polar body as an indicator of the position of the metaphase plate, the polar body and a small amount of cytoplasm containing the metaphase chromosomes, were then aspirated from the oocyte by using a microinjection pipette (Prather et al., 1999). Using the same micropipette, a fibroblast cell was placed within the perivitelline space of the recipient oocyte in close contact with the plasma membrane. Reconstructed oocyte/fibroblasts were then placed in 0.3 M mannitol, 1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM Hepes (Kuhholzer et al., 2001) and fusion/activation was performed by using a BTX Electro-Cell Manipulator 200 (BTX, San Diego, CA) at 2 pulses of 1.2 kV/cm for 30 msec (direct current) (Lai et al., 2002). Presumptive embryos were then cultured overnight in NCSU-23 supplemented with 0.4% BSA (Abeydeera et al., 1998).
In Vitro Production of Manipulation Control Embryos
Matured oocytes were stripped of cumulus as above. Oocytes were then washed three times and co-incubated with boar sperm for 6 hrs in 50 μL drops of modified Tris-buffered medium with 1mM caffeine and 0.1% BSA. Post-fertilization, the embryos' zona pellucida was pierced with a microinjection pipette and a small amount of cytoplasm removed being careful to avoid the polar body and cytoplasm directly surrounding it. Presumptive embryos were then cultured overnight in NCSU 23 medium supplemented with 0.4% BSA before being transferred to a recipient (Abeydeera et al., 1998).
Microscopy
Embryos recovered from the gilts were initially evaluated for morphology and were ranked as one of 4 stages: spherical, ovoid, tubular, and filamentous. After being imbedded in paraffin, uterine and embryonic tissues were sectioned, placed on slides and stained with hematoxylin and eosin. Uterine gland density was measured by visually counting uterine glands present in 3 random locations of a uterine section under 10× power. Embryonic disk measurements were made by using a dissection scope and a micrometer. Nucleoli density was evaluated by visually counting the nucleoli in 10 nuclei in 5 random views within a single embryo at 100× power (Figure 1). The percentage of mitotic figures was evaluated by counting the number of figures within a view divided by the number of cells per view. This was done for 5 random views within a single embryo at 100× power (Figure 2).
Figure 1.

Nucleoli (black arrows) within the trophoblast of A) a day 14 in vivo embryo and B) a day 14 NT embryo. Scale bar: 0.02 mm.
Figure 2.

Mitotic figures (black arrows) within the trophoblast of A) a day 14 in vivo embryo and B) a day 14 NT embryo. Scale bar: 0.02 mm.
Statistics
Data collected from uterine gland density, embryo nucleoli density, embryo mitotic figure percentage, and embryonic disk diameter was analyzed by ANOVA using the mixed model in the Statistical Analysis System program to determine treatment differences. Results are expressed as mean ± SEM. A probability of P ≤ 0.05 was considered to be statistically significant.
Data obtained from embryo morphology was analyzed by the chi-square test using the general linear model in the Statistical Analysis System program. A probability of P ≤ 0.05 was considered to be statistically significant.
Data collected from progesterone, estrone sulfate, and IGF-I found in the maternal blood serum was analyzed by ANOVA using the mixed model in the Statistical Analysis System program to verify treatment differences. Results are expressed as mean ± SEM. A probability of P ≤ 0.05 was considered to be statistically significant.
Results
Embryo Nucleoli Density
When embryos within the same treatment groups were compared with each other, the in vivo-produced and MC embryos maintained the same number of nucleoli per cell through days 10-14 of gestation. The NT embryos also had similar numbers of nucleoli per cell on day 10, but had significant increases in nucleoli per cell by day 12 (P ≤ 0.003) and day 14 (P ≤ 0.002) of development (Figure 3).
Figure 3.
Nucleoli density by treatment group (a,b P ≤ 0.05 within treatment group).
When compared to embryos of the same day but different treatment, the embryos produced by NT had significantly increased numbers of nucleoli per trophoblast cell by day 12 (P ≤ 0.03) and day 14 (P ≤ 0.01) when compared to embryos produced by any other method (Figure 4).
Figure 4.
Nucleoli density by day of development. (a,b P ≤ 0.05 within day).
Nuclear transfer day 12 and NT day 14 embryos were not found to be significantly different from each other either within day or treatment.
Embryo Mitotic Figure Index
In vivo-produced embryos exhibited no significant change in mitotic figure density over time, however, day 14 MC and day 14 NT embryos showed significant increases (P ≤ 0.009 and P ≤ 0.008, respectively) when compared to early embryos within the same treatment group (Figure 5).
Figure 5.
Mitotic index by treatment group. (a,b P ≤ 0.05 within treatment group).
The day 14 embryos produced by NT had a significantly increased (P ≤ 0.03) number of mitotic figures per trophoblast cell population when compared to day 14 in vivo-produced embryos. Day 14 MC embryos were found to have no significant differences when compared to either day 14 in vivo-produced or NT embryos (Figure 6).
Figure 6.
Mitotic index by day of development. (a,b P ≤ 0.05 within day).
Embryonic Disk Diameter
As in vivo-produced embryos developed from day 10 to day 14 there were significant increases in disk diameter between days 10 to 12 (P ≤ 0.003) and days 12 to 14 (P ≤ 0.013). In comparison, NT embryos showed no significant changes in embryonic disk growth as gestation progressed. MC embryos only showed significant embryonic disk growth between days 12 to 14 (P ≤ 0.008) (Figure 7).
Figure 7.
Embryonic disk diameter by treatment group. (a,b P ≤ 0.05 within treatment group).
When compared to embryos of different treatment groups but at the same developmental stage, day 12 in vivo-produced embryos had significantly increased (P ≤ 0.02) embryonic disk compared to MC and NT embryos, but by day 14, only NT embryos had significantly smaller (P ≤ 0.0002) embryonic disk size when compared to in vivo-produced embryos (Figure 8).
Figure 8.
Embryonic disk diameter by day of development. (a,b P ≤ 0.05 within day).
Embryo Morphology
Embryo morphology was determined on 146 embryos. Data analysis showed a difference in morphology between treatments (P ≤ 0.01) on day 14, but not at other days. In vivo produced day 14 embryos were 6.41 times more likely to be in a more advanced stage of development than their NT and MC counterparts (Figure 9). There were no significant differences between any of the treatments at day 12. All embryos recovered at day 10 were morphologically spherical and were not analyzed.
Figure 9.
Percentage of embryo morphology by treatment group. Difference in developmental stage found at day 14 (P ≤ 0.01).
Maternal Serum IGF-I
There were no significant differences in IGF-I levels between day, treatment, or treatment by day in any of the four groups.
Maternal Serum Progesterone and Uterine Gland Density
Maternal serum progesterone levels and uterine gland density were used as controls for determining gilt suitability as recipients and to assist in limiting the contribution of environmental variation. There were no significant differences for either parameter in the recipients of all four treatment groups.
Incidental Findings
Histologically, other abnormalities were observed between in vivo and nuclear transfer embryos but were not submitted to statistical analysis as they did not fit within the experimental model. Within certain of the NT embryos there were cytoplasmic and peri-nuclear vacuoles present as well as abnormally enlarged cells and “layering” of trophoblast cells where only single cell layers should be present (Figure 10).
Figure 10.
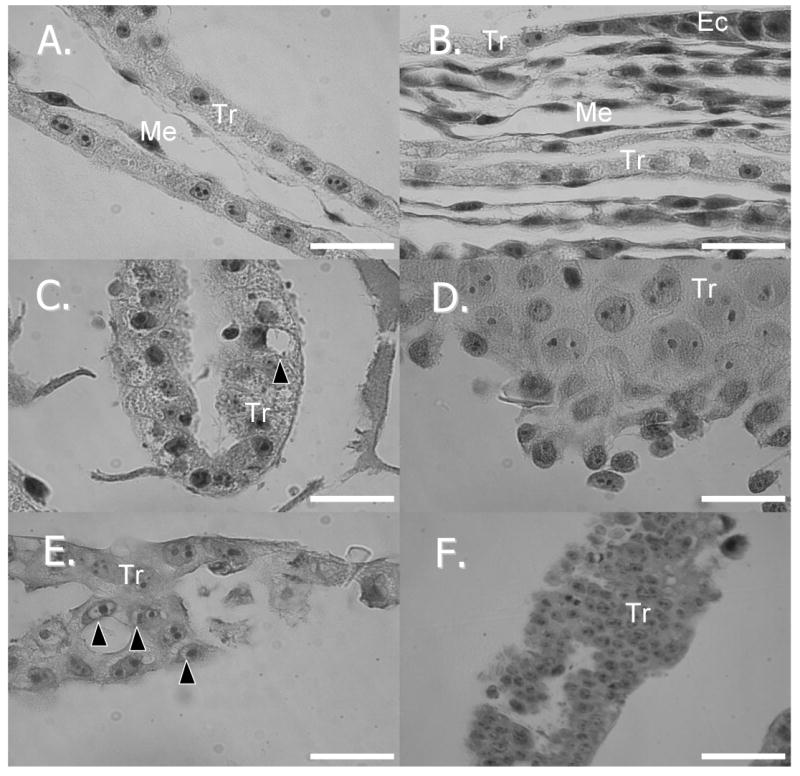
Histologic sections of embryos A.) day 10 in vivo produced embryo (100×) B). day 14 in vivo produced embryo (100×) C.) day 10 nuclear transfer embryo (100×) with cytoplasmic vacuole (black arrow) D.) day 14 nuclear transfer embryo (100×) with enlarged cells E.) day 14 nuclear transfer embryo (100×) with perinucleolar vacuoles (black arrows) F.) day 14 nuclear transfer embryo (40×) with abnormal “layering” of cells. Scale bar: A-E = 0.02 mm; F = 0.05 mm. Tr=trophoblast, Me=mesoderm, Ec=ectoderm
Discussion
The main objective of the study was to evaluate porcine nuclear transfer embryos for potential abnormalities that could contribute to the high mortality rate of these embryos during early pregnancy. It was predicted that the period of elongation, a phase in which the porcine embryo undergoes extreme morphological changes as well as increased steroid secretion, would be a point of failure in the development of many porcine embryos.
As a result of this study, it was determined that most nuclear transfer embryos were unable to attain a filamentous form as they approached day 14 - a time at which elongation should be nearly complete. These embryos also had increasingly smaller embryonic disks when compared to normal embryos, another sign of their developmental retardation. An inability to develop morphologically could also be indicative of abnormalities in other areas, such as steroid production, thereby contributing to a lack of maternal recognition of pregnancy, resulting in subsequent termination of the pregnancy. It is also possible that the presence of a small number of advanced embryos could be inducing, via the secretion of estrogen, changes in the uterine environment that are required for the more advanced embryos to develop. As Pope (1988) demonstrated, this situation could create an environment that is incompatible with the needs of less developed embryos, resulting in their mortality. An inability to maintain the minimum number of embryos required for pregnancy would cause the recipient to exhibit luteolysis and initiate a return to estrus.
Specific histological abnormalities were also noted, with nuclear transfer embryos having increased numbers of nucleoli within their trophoblast as they approached day 14 of gestation. The re-formation of nucleoli in the early embryo is integral to the initiation of embryonic protein production as the nucleoli are the sites of ribosomal RNA synthesis (Hyttel et al., 2000; Pederson, 1998). Under normal conditions, the nucleoli disassemble during the early M-phase of the oocyte cell cycle (which is then arrested at the meiosis II stage awaiting fertilization (Senger, 2003)), and are reformed during the third cell cycle, or at the four-cell stage, after fertilization (Dundr et al., 2000; Hyttel et al., 2000). By the time the embryo reaches the blastocyst stage at day 6, its nuclei contain fully functional nucleoli that are comparable to somatic cell nucleoli (Hyttel et al., 2000). During the course of the cell cycle in a somatic cell, the nucleolus is disassembled during mitosis and re-assembled during telophase (Olson et al., 2000). The increased number of nucleoli does not necessarily indicate increased levels of gene transcription, but more likely indicates erroneous re-programming of the embryonic genome and possible production of inaccurate gene transcripts directing the cell down an abnormal developmental pathway. Some of the sections that had multiple nucleoli were deparaffinized and subjected to transmission electron microscopy (data not shown). While the quality of these sections was not ideal, it was easy to see that the nucleoli had reticulated and had a granular surface. Thus it appears that these multiple nucleoli had a morphology that is similar to normally transcribing nucleoli.
Mitotic figures, condensed DNA, are present in any dividing cell population and are indicators of mitosis that appear during the metaphase period. The increase in the mitotic index may indicate a potential problem with control of the cell cycle resulting from improper re-programming of the re-constructed nucleus. There are reports of a mutant mouse line with the inability to transition from metaphase to anaphase as a result of a mutation in the anaphase-promoting complex component APC10/DOC1 (Pravtcheva and Wise, 2001) leading to a higher than normal mitotic index (Magnuson and Epstein, 1984). Yet another mouse mutation affecting Omcg-1, a gene coding for a nuclear zinc finger protein, exhibits a high mitotic index as well as the inability to develop past the pre-implantation stage (Artus et al., 2005). There are numerous possibilities as to the reasons why this abnormality is present. Based on these examples it appears that the inability of a cell to exit mitosis, as the increase in the mitotic index might suggest, may also be contributing to the notably slower development seen in NT embryos.
Another possibility is that the nuclear transfer embryos are attempting to augment their development by altering cell division, in this instance increasing it. This phenomenon has been seen before in the production of mouse aggregation chimeras. The addition of blastomeres to an already developing mouse embryo does not produce a larger than normal fetus, but through a decreased rate of cell division, a normal sized embryo is obtained (Buehr and McLaren, 1974; Lewis and Rossant, 1982). A reversal of this event can be seen in the production of multiple embryos by splitting a single embryo. This time an increase in cell division results in the development of a normal sized embryo (Rands, 1985). Comparisons to embryos further in development will be necessary to understand the mechanisms that nuclear transfer embryos utilize to adjust their cell numbers.
Conclusions
Nuclear transfer embryos appear to develop at a slower rate than their in vivo counterparts as demonstrated by their less advanced morphology and decreased embryonic disk size as gestation progresses. Due to the increase in nucleoli and mitotic figures within the trophoblast cell population of NT embryos, it would seem that the cell cycle is either adversely affected, possibly by incomplete re-programming of that portion of the embryonic genome, or the embryo itself is attempting to compensate for earlier, inadequate growth. The mechanical perturbations caused by the nuclear transfer technique itself also appear to retard development but not to the extent that the actual cloning process does as MC embryos often appear to re-cover some of the developmental ground they lost as they approach day 14. It would appear from these results that increasing the accuracy of the re-programming events would contribute to increasing the survival rate of NT embryos; however the exact means by which to accomplish this are still unknown and require further study.
Acknowledgments
The authors would like to acknowledge funding from the NIH National Center for Research Resources (R01 RR013438), the F.B. Miller Fund, and Food for the 21st Century.
Literature Cited
- Abeydeera LR, Wang WH, Prather RS, Day BN. Maturation in vitro of pig oocytes in protein-free culture media: Fertilization and subsequent embryo development in vitro. Biol Reprod. 1998;58:1316–1320. doi: 10.1095/biolreprod58.5.1316. [DOI] [PubMed] [Google Scholar]
- Artus J, Vandormael-Pournin S, Frodin M, Nacerddine K, Babinet C, Cohen-Tannoudji M. Impaired mitotic progression and preimplantation lethality in mice lacking omcg1, a new evolutionarily conserved nuclear protein. Mol Cell Biol. 2005;25:6289–6302. doi: 10.1128/MCB.25.14.6289-6302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Thompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000;18:1055–1059. doi: 10.1038/80242. [DOI] [PubMed] [Google Scholar]
- Buehr M, McLaren A. Size regulation in chimaeric mouse embryos. J Embryol Exp Morphol. 1974;31:229–234. [PubMed] [Google Scholar]
- Davis DL, Blair RM. Studies of uterine secretions and products of primary cultures of endometrial cells in pigs. J Reprod Fertil Suppl. 1993;48:143–155. [PubMed] [Google Scholar]
- Dundr M, Misteli T, Olson MO. The dynamics of postmitotic reassembly of the nucleolus. J Cell Biol. 2000;150:433–446. doi: 10.1083/jcb.150.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge M, Coleman RE, Parker SB. A minimally invasive percutaneous technique for jugular vein catheterization in pigs. Contemp Top Lab Anim Sci. 2002;41:38–42. [PubMed] [Google Scholar]
- Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982;27:925–939. doi: 10.1095/biolreprod27.4.925. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Zavy MT, Moffatt RJ, Blair RM, Yellin T. Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl. 1990;40:293–305. [PubMed] [Google Scholar]
- Hyttel P, Laurincik J, Rosenkranz C, Rath D, Niemann H, Ochs RL, Schellander K. Nucleolar proteins and ultrastructure in preimplantation porcine embryos developed in vivo. Biol Reprod. 2000;63:1848–1856. doi: 10.1095/biolreprod63.6.1848. [DOI] [PubMed] [Google Scholar]
- Ka H, Jaeger LA, Johnson GA, Spencer TE, Bazer FW. Keratinocyte growth factor is up-regulated by estrogen in the porcine uterine endometrium and functions in trophectoderm cell proliferation and differentiation. Endocrinology. 2001;142:2303–2310. doi: 10.1210/endo.142.6.8194. [DOI] [PubMed] [Google Scholar]
- Kuhholzer B, Hawley RJ, Lai L, Kolber-Simonds D, Prather RS. Clonal lines of transgenic fibroblast cells derived from the same fetus result in different development when used for nuclear transfer in pigs. Biol Reprod. 2001;64:1695–1698. doi: 10.1095/biolreprod64.6.1695. [DOI] [PubMed] [Google Scholar]
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Lamberson WR, Safranski TJ, Bates RO, Keisler DH, Matteri RL. Relationships of serum insulin-like growth factor i concentrations to growth, composition, and reproductive traits of swine. J Anim Sci. 1995;73:3241–3245. doi: 10.2527/1995.73113241x. [DOI] [PubMed] [Google Scholar]
- Lewis NE, Rossant J. Mechanism of size regulation in mouse embryo aggregates. J Embryol Exp Morphol. 1982;72:169–181. [PubMed] [Google Scholar]
- Magnuson T, Epstein CJ. Oligosyndactyly: A lethal mutation in the mouse that results in mitotic arrest very early in development. Cell. 1984;38:823–833. doi: 10.1016/0092-8674(84)90277-0. [DOI] [PubMed] [Google Scholar]
- Olson MO, Dundr M, Szebeni A. The nucleolus: An old factory with unexpected capabilities. Trends Cell Biol. 2000;10:189–196. doi: 10.1016/s0962-8924(00)01738-4. [DOI] [PubMed] [Google Scholar]
- Patten BM. Embryology of the pig. Maple Tree Press; York, PA: 1948. [Google Scholar]
- Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather RS, Tao T, Machaty Z. Development of the techniques for nuclear transfer in pigs. Theriogenology. 1999;51:487–498. doi: 10.1016/S0093-691X(98)00242-8. [DOI] [PubMed] [Google Scholar]
- Pravtcheva DD, Wise TL. Disruption of apc10/doc1 in three alleles of oligosyndactylism. Genomics. 2001;72:78–87. doi: 10.1006/geno.2001.6474. [DOI] [PubMed] [Google Scholar]
- Rands GF. Cell allocation in half- and quadruple-sized preimplantation mouse embryos. J Exp Zool. 1985;236:67–70. doi: 10.1002/jez.1402360110. [DOI] [PubMed] [Google Scholar]
- Senger PL. Pathways to pregnancy and parturition. 2nd. Current Conceptions; Pullman, WA: 2003. [Google Scholar]
- Simmen RC, Simmen FA, Hofig A, Farmer SJ, Bazer FW. Hormonal regulation of insulin-like growth factor gene expression in pig uterus. Endocrinology. 1990;127:2166–2174. doi: 10.1210/endo-127-5-2166. [DOI] [PubMed] [Google Scholar]
- Waite AB, Day BN. Intrauterine migration following unilateral fertilization in gilts. J Anim Sci. 1967;26:790–791. doi: 10.2527/jas1967.264790x. [DOI] [PubMed] [Google Scholar]









