Abstract
Nitration products of unsaturated fatty acids are formed via NO-dependent oxidative reactions and appear to be a new class of endogenous antiinflammatory mediators. Nitroalkene derivatives of nitrated linoleic acid (LNO2) and nitrated oleic acid (OA-NO2) alleviate inflammatory responses in macrophages, but the underlying mechanisms remain to be fully defined. Herein we report that LNO2 and OA-NO2 suppress proinflammatory signal transducer and activator of transcription (STAT) signaling in macrophages. In RAW264.7 cells, a murine macrophage cell line, LNO2 and OA-NO2 inhibited the lipopolysaccharide (LPS)-induced STAT1 phosphorylation and the STAT1-dependent transcriptional activity, thereby suppressing expression of its target gene such as iNOS and MCP-1. The nitroalkene-mediated inhibition of STAT1 activity was not affected by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (a NO scavenger), GW9662 (a peroxisome proliferator-activated receptor-γ-specific antagonist) or glutathione (an antioxidant), suggesting an underlying mechanism independent of NO, peroxisome proliferator-activated receptor-γ, or thio-nitralkylation. In contrast, LNO2 or OA-NO2 alone up-regulated both mRNA and protein levels of MAPK phosphatase 1 (MKP-1) and strongly augmented the LPS-induced MKP-1 protein expression. Knockdown of MKP-1 by MKP-1 small interfering RNA enhanced the LPS-induced STAT1 phosphorylation, suggesting that MKP-1 acts as a negative regulator for LPS-induced STAT signaling. In addition, the nitroalkene-mediated inhibitory effects on STAT1 phosphorylation, iNOS expression, and MCP-1 secretion were also largely attenuated by the MKP-1 small interfering RNA approach. Taken together, our data demonstrate that nitroalkenes inhibit proinflammatory STAT signaling through inducting MKP-1 in macrophages.
NITROALKENE PRODUCTS of unsaturated fatty acid nitration are formed via NO-dependent oxidative reactions (1,2,3). More than a footprint of NO-dependent redox reactions with lipids (1,4), these species represent a novel class of signaling molecules (5), because their fatty acid nature is related to prostaglandins, thromboxanes, leukotrienes, epoxyeicosatrienic acids (EETs), hydroxyeicosatetraenoic acids (HETEs), lipoxins, and resolvins that serve as lipid mediators or autacoids (6,7). This notion is supported by the observation that synthetic nitro derivatives of fatty acids trigger pluripotent cell signaling actions, including relaxation of phenylephrine-preconstricted rat aortic rings, inhibition of thrombin-induced Ca2+ elevation, and aggregation of human platelets as well as attenuation of human neutrophil superoxide generation, degranulation, and integrin expression (8,9,10). These cellular responses are mediated by both cGMP- and cAMP-dependent as well as independent mechanisms. Furthermore, both nitrated linoleic acid (LNO2) [positional isomers 10-nitro-9-cis,12-cis-octadecadienoic acid (C10) and 12-nitro-9-cis,12-cis-octadecadienoic acid (C12)] and nitrated oleic acid (OA-NO2) [9- and 10-nitro-9-cis-octadecenoic acid (C9 and C10)] activate peroxisome proliferator-activated receptor-γ (PPARγ) and induce adipogenesis (11,12). Finally, nitrated unsaturated fatty acids are present in healthy human blood (11,13).
LNO2 and OA-NO2 are electrophiles that can mediate reversible nitroalkylation reactions with glutathione (GSH) as well as the Cys and His residues of proteins such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) both in vitro and in vivo (14,15). These results suggest that nitroalkenes can mediate cell signaling reactions via covalent, thiol-reversible posttranslational modification of target molecules. Interestingly, LNO2 and OA-NO2 can inhibit inflammatory responses in many cell types including monocytes/macrophages and endothelial cells (16). Although repression of nuclear factor-κB activity of p65 by nitroalkylation contributes to the inflammatory inhibition of nitroalkenes (16), other potential underlying mechanisms remain to be fully defined.
Herein, we report that nitroalkenes suppress the activation of proinflammatory STAT signaling induced by lipopolysaccharide (LPS) in RAW264.7 macrophages. The inhibitory effect of nitroalkenes on STAT cascade is independent of PPARγ activation, NO release, or thiol-nitroalkylation but mediated by induction of MAPK phosphatase 1 (MKP-1). These results reveal a novel molecular mechanism contributing to the antiinflammatory signaling actions of fatty acid nitroalkene derivatives.
Materials and Methods
Cell culture and materials
RAW264.7 (American Type Culture Collection, Rockville, MD; catalog no. TIB-71) cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. The stable RAW264.7 clones with MKP-1 small interfering RNA (siRNA) or the empty vector pSR were generated as previously described (17). RAW264.7 cells were cultured in serum-free DMEM for 24 h to induce a quiescent status. LNO2 and OA-NO2 were synthesized and purified and quantitated as previously described (11,13). Rosiglitazone, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) and GW9662 were purchased from Cayman Chemical (Ann Arbor, MI). LPS, actinomycin D, GSH, 3-morpholinosydnonimine (SIN-1), and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (cPTIO) were purchased from Sigma Chemical Co. (St Louis, MO).
Transfection and signal transducer and activator of transcription (STAT) transcriptional activity assay
RAW264.7 cells were transfected with pISRE-TA-Luc that contains five copies of STAT1-binding interferon-γ stimulated response element (ISRE) enhancer elements, located upstream of minimal TA promoter-driven firefly luciferase reporter gene (CLONTECH, Palo Alto, CA) together with pRL-TK (Promega) for normalization of transfection efficiency using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). The STAT transcriptional activity was determined using a dual luciferase assay kit (Promega, Madison, WI) as previously reported (18).
Quantitative real-time PCR (Q-PCR)
Expression of inducible nitric oxide synthase (iNOS) and MPK-1 mRNAs was assessed by Q-PCR as described previously (16). Forward primer (5′-GAGGCCGCATGAGCTTGGTGTTT-3′) and reverse primer (5′-GGGGGTTGCATTTCGCTGTCTCC-3′) were used for PCR amplification of iNOS to yield a 511-bp product. Forward primer (5′-TGTGAAGCAGAGGCGGAGTA-3′) and reverse primer (5′-GGGGATGGAAACAGGGAAGT-3′) were used for PCR amplification of MKP-1 to yield a 178-bp product. Cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted control samples. Expression levels of iNOS or MKP-1 were normalized by concurrent measurement of GAPDH mRNA levels.
Chromatin immunoprecipitation (ChIP) assay
Chromatin from macrophages was fixed and immunoprecipitated using the EZ-ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) as recommended by the manufacturer. Sequences were identified for the mouse iNOS promoter (GenBank accession no. L09126). Purified chromatin was immunoprecipitated using 10 μg anti-STAT1 (Cell Signaling, Beverly, MA), 5 μl normal rabbit IgG (negative control), or anti-acetyl-histone H3 (positive control); eluted DNA fragments were purified to serve as templates. The input fraction corresponded to 5% of the chromatin solution before immunoprecipitation. The size of the sonicated DNA fragments subjected to immunoprecipitation was 0.5–1 kb as determined by ethidium bromide gel electrophoresis. After DNA purification, the presence of the selected DNA sequence was quantified using real-time PCR. Promoter-specific primers for mouse iNOS were forward primer 5′-GGGGGATTTTCCCTCTCTCTG-3′ and reverse primer 5′-GTGTTTGTATGTGTGGGTGAC-3′, which amplify 332-bp fragments flanking the ISRE sequences. To monitor the specificity for each assay, no observable DNA binding is found in normal IgG immunoprecipitates as background control, whereas a strong DNA binding is confirmed in anti-acetyl-histone H3 immunoprecipitates as positive control.
Immunoprecipitation and immunoblot analysis
Cells were cultured with serum-free DMEM for 24 h and followed various treatments as indicated. The cell lysates were subjected to immunoprecipitation and immunoblot as previously described (16) using antibodies of phospho-STAT1 (pSTAT1) (Tyr-701), pSTAT3 (Tyr-705), STAT1, and STAT3 (Cell Signaling Technology, Inc., Danvers, MA), MKP-1 and GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and iNOS, (BD Biosciences, San Jose, CA). For quantitation of the changes in STAT1 phosphorylation, the intensities of the bands representing STAT1 and pSTAT1 (Tyr-701) were measured by densitometry using an image scanner (EPSON GT-8000) and National Institutes of Health Image software.
Cytokine assay
Cytokine assay was performed as previously described (16). Briefly, RAW264.7 cells in culture medium containing fresh 1% delipidated fetal bovine serum (Cocalico Biologicals, Reamstown, PA) were treated as indicated. Medium was collected 18–20 h after treatment. The concentrations of mouse monocyte chemoattractant protein-1 (MCP-1) released from cells into medium were measured by ELISA kits using protocols supplied by the manufacturer (R&D Systems, Minneapolis, MN).
Statistics
Values are expressed as mean ± sd in the text and figures. The data were analyzed using ANOVA with the Newman-Keuls’ test unless specified. Values of P < 0.05 were considered to be statistically significant.
Results
Nitroalkene derivatives of LNO2 and OA-NO2 suppress proinflammatory STAT signaling
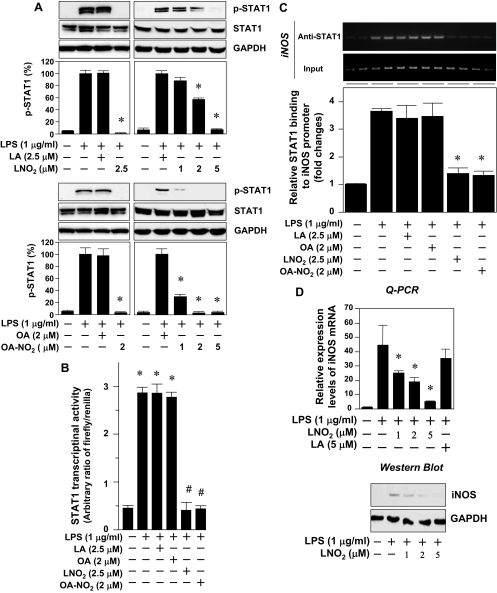
Although fatty acid nitroalkene derivatives of LNO2 and OA-NO2 are potent PPARγ ligands, they inhibit LPS-induced secretion of proinflammatory cytokines including IL-6, TNFα, and MCP-1 in macrophages independent of PPARγ activation (16). Interestingly, it has been demonstrated that several PPARγ ligands including 15d-PGJ2, rosiglitazone, ciglitazone, and GW1929 suppress the proinflammatory STAT cascade in macrophages and other cells in a PPARγ receptor-independent manner (19,20,21). Thus, we examined whether nitroalkene derivatives of LNO2 and OA-NO2 exert a similar inhibitory effect on proinflammatory STAT signaling in RAW264.7 macrophages. In quiescent RAW264.7 cells, LPS induced a weak phosphorylation of STAT3 (Tyr-705). In contrast, LPS potently activated STAT1. Phosphorylation of STAT1 (Tyr-701) was detectable at 30 min, peaked at about 4 h, and remained elevated for at least 6 h in RAW264.7 cells (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). LPS did not affect expression levels of STAT1 and STAT3 proteins (Fig. S1). The LPS-activated STAT1 phosphorylation was markedly inhibited by LNO2 and OA-NO2 in a dose-dependent manner, whereas LNO2 or OA-NO2 alone did not change either STAT1 phosphorylation or protein expression (Fig. 1A). Moreover, these nitroalkenes suppressed the STAT1-mediated reporter gene transcription induced by LPS (Fig. 1B). To further study whether the suppression of STAT1 phosphorylation and STAT1-driven transcriptional activity by nitroalkenes facilitates the resolution of LPS-induced inflammatory responses in macrophages, we examined the effect of nitroalkenes on the transcriptional control of iNOS expression in LPS-activated RAW264.7 cells. LPS significantly induced STAT1 binding to iNOS promoter at sites of STAT1 cis-acting elements (22,23) (Fig. 1C). Importantly, the LPS-induced chromatin binding activity of STAT1 (22) was dramatically suppressed by both LNO2 and OA-NO2 (Fig. 1C). Accordingly, the nitroalkene derivative of LNO2 dose-dependently inhibited the expression of iNOS at both mRNA and protein levels (Fig. 1D). OA-NO2 also down-regulated iNOS expression in the LPS-activated RAW264.7 cells (data not shown). In contrast, the native fatty acid precursors of LNO2 and OA-NO2, linoleic acid (LA) or oleic acid (OA) did not affect the LPS-induced STAT1 phosphorylation states, STAT1 transcriptional activity, and its target gene iNOS expression in RAW264.7 cells (Fig. 1). These data reveal that nitroalkene derivatives of LNO2 and OA-NO2 suppress proinflammatory STAT signaling in RAW264.7 macrophages. In addition, the close similarity between the LNO2- and OA-NO2-induced suppression of the STAT cascade suggests a common mechanism of action.
Figure 1.
LNO2 and OA-NO2 inhibit STAT signaling. A, Effect of LNO2 and OA-NO2 on LPS-induced STAT1 phosphorylation. Quiescent RAW264.7 cells were stimulated with LPS (1 μg/ml) for 2 h with or without LNO2, OA-NO2, LA, or OA as indicated. The cell lysates were subjected to Western blot analysis using antibodies to pSTAT1 (Tyr-701), STAT1, and GAPDH (as an internal control to determine protein loading). STAT1 activity (STAT1 phosphorylation) was quantified by densitometry and is expressed as the ratio of pSTAT1/STAT1 in the graphs. (Note that LPS-induced STAT1 activity was set as 100%.) Values are expressed as mean ± sd (n = 3). *, P < 0.05 vs. LPS alone. B, Effect of LNO2 and OA-NO2 on LPS-induced STAT1 transcriptional activity. Quiescent RAW264.7 cells transfected with pISRE-TA-Luc and pRL-TK were treated for 6 h with various stimuli as indicated. STAT1 transcriptional activity was measured as described in Materials and Methods. Values are expressed as mean ± sd (n = 6). *, P < 0.05 vs. LPS alone. C, Effect of LNO2 and OA-NO2 on the binding of STAT1 to iNOS promoter in response to LPS in vivo. Quiescent RAW264.7 cells were treated for 6 h with various stimuli as indicated, and then ChIP analysis for STAT1 bindings at the mouse iNOS promoter was measured as described in Materials and Methods. A representative result of PCR is shown in the upper panel, whereas the quantified result is shown in the lower panel. Input represents total iNOS promoter; anti-STAT1 indicates iNOS promoter specifically bound with STAT1; iNOS represents amount of iNOS promoter. Values are expressed as mean ± sd (n = 6). *, P < 0.01 vs. control (−); #, P < 0.01 vs. LPS alone. D, Effect of LNO2 on LPS-induced iNOS expression. Quiescent RAW264.7 cells were treated for 6 h with various stimuli as indicated, expression of iNOS mRNA was measured by Q-PCR (upper panel), and iNOS protein was detected by immunoblotting (lower panel). Results are representative of minimal three independent experiments.
Nitroalkene inhibits STAT phosphorylation independent of NO release and thiol-nitroalkylation
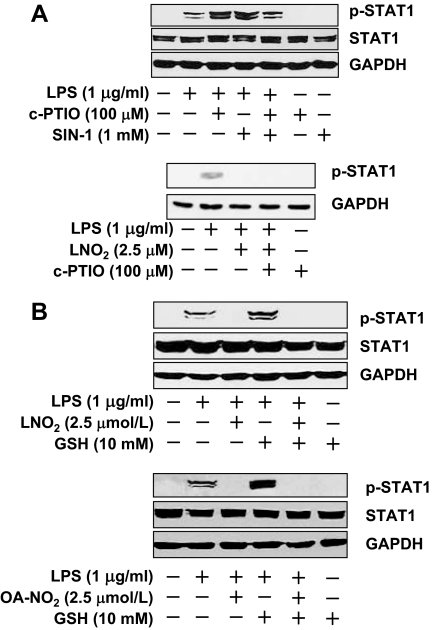
It has been reported that nitric oxide at micromolar levels inhibits STAT activity (24,25,26), suggesting that endogenous production of NO may act as a negative feedback regulator for the control of STAT signaling. Although we have observed that LNO2 is a weak NO donor capable of releasing NO in aqueous milieu, the partition coefficient of LNO2 is about 1500:1 (hydrophobic vs. aqueous compartments) (27). Thus, only one in 1500 molecules of LNO2 are expected to decay to yield NO in serum lipoprotein-containing media and cell models, yielding at the most femtomolar concentrations of NO. OA-NO2 is much more stable than LNO2 in aqueous milieu. It only minimally decays to release NO within the present experimental time frames. Nevertheless, it raises a question of whether the nitroalkene-mediated inhibition of STAT signaling is via NO release. To address this issue, we applied NO donor SIN-1 and NO scavenger cPTIO. Interestingly, coadministration of LPS with either cPTIO or SIN-1 exerted similar results; i.e. both scavenging NO and exogenous releasing NO enhanced the LPS-induced STAT1 phosphorylation (Fig. 2A). The efficacy of cPTIO was confirmed by the fact that cPTIO was able to reverse the SIN-1-mediated enhancement of STAT1 phosphorylation in the LPS-activated RAW264.7 cells (Fig. 2A). Although cPTIO substantially abolished the stimulatory effect of SIN-1 on LPS-induced STAT1 activation, it had little effect on the attenuation of STAT1 phosphorylation in response to LNO2 (Fig. 2A). Thus, release of NO from nitroalkene cannot explain the inhibitory effect of nitroalkenes on STAT phosphorylation.
Figure 2.
Effect of cPTIO and GSH on LNO2- and OA-NO2-mediated inhibition of STAT phosphorylation. A, Role of exogenous NO release on LPS-induced STAT1 phosphorylation. Quiescent RAW264.7 cells were simultaneously treated with various stimuli for 2 h as indicated. The cell lysates were subjected to immunoblot analysis using antibodies to pSTAT1 (Tyr-701), STAT1, and GAPDH. Results are representative of at least three separate experiments. B, Effect of GSH quench on the inhibitory effect of LNO2 and OA-NO2 on LPS-induced STAT1 phosphorylation. Quiescent RAW264.7 cells were simultaneously treated with various stimuli for 2 h as indicated. Western blot was performed as described above. Results are representative of at least three separate experiments.
To address whether nitroalkene-mediated nitroalkylation is involved in the inhibition of STAT activity, we examined the effects of GSH on STAT signaling. GSH has been shown to conjugate with nitroalkenes directly, thus quenching the nitralkylation (15). Coadministration of LPS with excess GSH resulted in enhancement of the LPS-induced STAT1 phosphorylation, whereas GSH alone had no effect on STAT1 activity (Fig. 2B). However, the inhibitory effect of nitroalkenes on LPS-induced STAT1 was not affected by the addition of excess GSH (Fig. 2B), ruling out thio-nitroalkylation as an underlying mechanism.
Nitroalkenes inactivate STAT independent of PPARγ
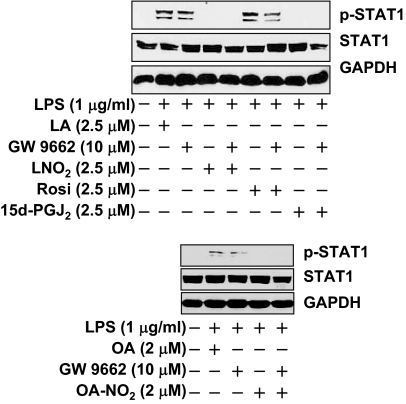
PPARγ is a receptor target of OA-NO2 and LNO2 (11,12). To determine whether PPARγ is involved in the inhibitory action of nitroalkenes on STAT signaling, we compared nitroalkenes with known PPARγ agonists or an antagonist, GW9662, on the regulation of LPS-induced STAT activity. Rosiglitazone is a high-affinity PPARγ ligand, whereas 15d-PGJ2 is a low-affinity PPARγ ligand. In RAW264.7 cells, the expression of PPARγ and the efficacy of GW9662 have been confirmed in our recent studies (12,16). In addition, we observed that the potency of OA-NO2 and LNO2 as PPARγ agonists, at 2.5 μm, is OA-NO2 is greater than LNO2, which is greater than or equal to rosiglitazone, which is greater than 15d-PGJ2 (16). Thus, the potential inhibitory effect of nitroalkenes and PPARγ agonists on STAT phosphorylation should parallel their potency as PPARγ agonists if this receptor was a putative mediator. However, LPS-induced phosphorylation of STAT1 was not inhibited by rosiglitazone but was significantly inhibited by 15d-PGJ2 (Fig. 3). In fact, the action of 15d-PGJ2 was comparable to the inhibitory effects of nitroalkenes (Fig. 3). Nevertheless, neither nitroalkene- nor 15d-PGJ2-induced inhibition of STAT1 phosphorylation was affected by GW9662 treatment (Fig. 3). These results support a notion that nitroalkenes inhibit the proinflammatory STAT signaling in macrophages via a PPARγ-independent mechanism.
Figure 3.
LNO2 and OA-NO2 inhibit LPS-induced STAT-1 phosphorylation independent of PPARγ. Quiescent RAW264.7 cells were treated with GW9662 (10 μm) for 2 h and then stimulated with the indicated agents for 20 min. The cell lysates were subjected to immunoblot analysis. Results are representative of at least three separate experiments.
Mechanism of nitroalkene-mediated termination of STAT signaling: a key role of MKP-1
Ligand and receptor binding triggers activation of Janus kinase (JAK) tyrosine kinases, leading to phosphorylation of specific tyrosine residues on the receptors and thereby resulting in docking sites for STAT proteins for subsequent phosphorylation by JAKs. Activation of the JAK/STAT cascade is regulated by at least two negative feedback mechanisms. First, both JAKs and STATs can be dephosphorylated by protein tyrosine phosphatase such as Src homology 2 containing protein tyrosine phosphatase 2 (SHP2), which is also rapidly recruited to the receptor complexes. Second, JAK/STAT signaling cascade can be attenuated due to the induction of the genes encoding two negative regulators of the JAK/STAT pathway: suppressors of cytokine signaling (SOCS) and protein inhibitor of activated STAT (PIAS) (28,29).
To gain insight into the inhibitory mechanism of nitroalkenes on the STAT pathway, we examined the effects of nitroalkenes on JAK2 activity. In RAW264.7 cells, LPS did not activate JAK2, whereas IL-6 induced rapid activation of the JAK2 (data not shown). Pharmacological inhibition of transcriptional activity by actinomycin D completely blocked the LPS-induced STAT1 phosphorylation but had little impact on the IL-6-induced JAK2 phosphorylation (data not shown). These results suggest that LPS or IL-6 activates the STAT cascade through different mechanisms in RAW264.7 cells. Clearly, LPS induces STAT activation through a process involving de novo gene transcription, whereas IL-6-induced STAT activation does not depend on de novo gene transcription. Then, we focused on nitroalkene-inducing genomic events that contribute to the suppression of LPS-triggered proinflammatory STAT signaling in RAW264.7 cells.
Induction of MKP-1 by nitroalkenes.
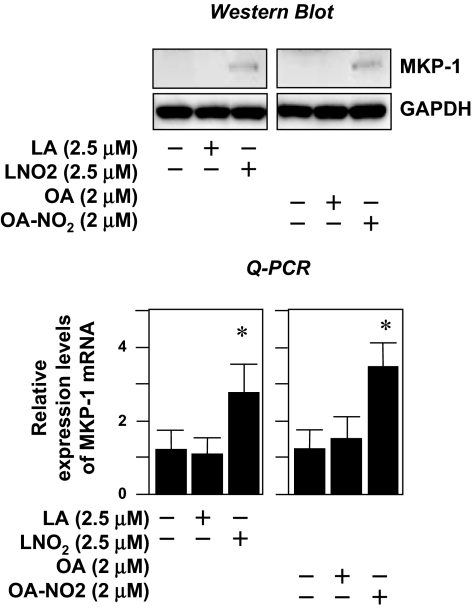
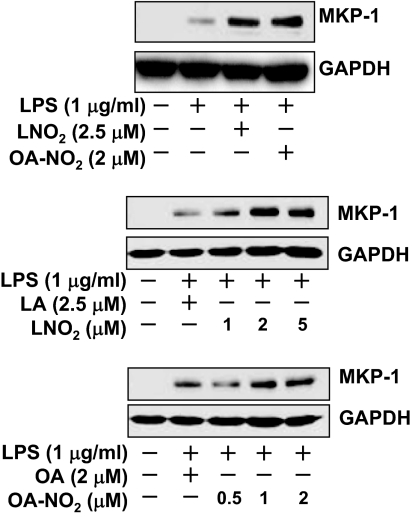
To identify the genes whose protein products mediate the suppression of STAT1 activity by nitroalkenes, we performed an oligo microarray analysis on RNA samples obtained from LNO2-stimulated human THP-1 macrophages using Aligent Whole Human Genome Oligo Microarray Kit (Aligent Technologies, Santa Clara, CA). We did not observe any effects of LNO2 on the expression of SOCS or PIAS genes (28,29). These results were confirmed by subsequent analysis with quantitative real-time PCR in both THP-1 and RAW264.7 macrophages (data not shown). Among the genes up-regulated by LNO2 in THP-1 macrophages is MKP-1, which encodes a dual-specificity phosphatase with substrate preference for p38 and c-Jun N-terminal kinase (JNK). We decided to focus on MKP-1 for the following reasons: 1) MKP-1 is up-regulated, and the induction of MKP-1 has been proposed to contribute to the attenuation of STAT1 activity in angiotensin II-activated vascular smooth muscle cells (30); 2) MKP-1 is a pivotal feedback regulator in the control of innate immune responses; and 3) MKP-1 plays a critical role in suppressing endotoxic shock, with critical downstream mediators remaining unexplored (31,32). We hypothesized that the induction of MKP-1 by nitroalkenes would serve as a negative feedback mechanism for the termination of proinflammatory STAT signaling in macrophages. Consistent with this hypothesis, we observed that LNO2 or OA-NO2 rather than LA or OA up-regulated MKP-1 expression at both mRNA and protein levels (Fig. 4). Moreover, these nitroalkenes also significantly enhanced LPS-induced MKP-1 protein expression in a dose-dependent manner in RAW264.7 cells (Fig. 5).
Figure 4.
LNO2 and OA-NO2 induce MKP-1 expression. Quiescent RAW264.7 cells were treated with LA, OA, LNO2, or OA-NO2 for 4 h or left unstimulated. A, The effects of nitroalkenes on MKP-1 protein expression. MKP-1 protein expression was assessed by immunoblot analysis (upper panels) using antibodies to MKP-1 and GAPDH. Results are representative of at least three separate experiments. B, The effects of nitroalkenes on mRNA expression of MKP-1. MKP-1 mRNA expression was assessed by Q-PCR (lower panels). Values are means ± sd (n = 6). *, P < 0.05 vs. control (−).
Figure 5.
LNO2 and OA-NO2 enhances LPS-induced MKP-1 expression. Quiescent RAW264.7 cells were treated with LPS in the presence of LNO2 or OA-NO2 for 2 h as indicated. The cell lysates were subjected to Western blot analysis with antibodies against MKP-1or GAPDH. Results are representative of three separate experiments.
Knockdown of MKP-1 blunts the inhibitory effects of nitroalkene on STAT signaling.
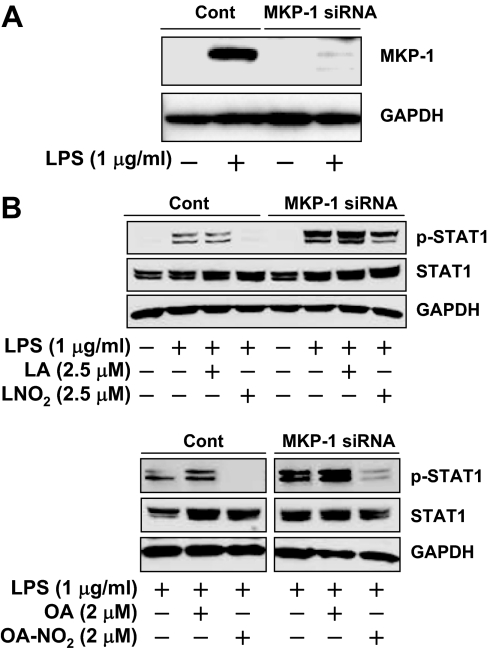
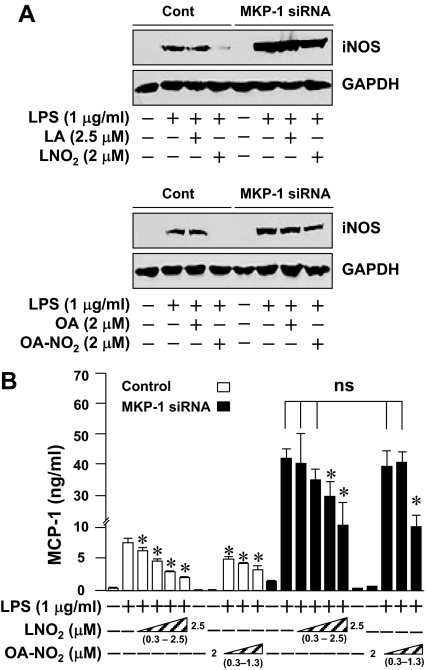
To establish a functional link between the nitroalkene-induced MKP-1 expression and the inhibition of STAT1 phosphorylation in LPS-activated macrophages, we applied an MKP-1 siRNA approach to knock down MKP-1 in RAW264.7 cells. Expression of MKP-1 siRNA in RAW264.7 cells significantly attenuated LPS-induced MKP-1 expression (Fig. 6A). Although knockdown of MKP-1 had little effect on the basal levels of STAT1 protein expression and STAT1 phosphorylation, it significantly enhanced and prolonged the STAT1 phosphorylation triggered by LPS. These results clearly indicate that MKP-1 is a negative feedback regulator for LPS-induced STAT signaling in macrophages (Fig. 6B). Moreover, the inhibitory effect of LNO2 and OA-NO2 on LPS-induced STAT1 phosphorylation was partially reversed by knocking down MKP-1 (Fig. 6B), suggesting that nitroalkenes suppress STAT signaling via induction of MKP-1. This hypothesis was further supported by the results that MKP-1 knockdown by siRNA substantially alleviated the inhibitory effect of nitroalkenes on the protein expression of two STAT1 target genes including iNOS (22,23) and MCP-1 (33) (Fig. 7). Of note, although nitroalkenes at the concentrations of 0.3–0.6 μm, which is within physiological range in human circulation (11,13), inhibited MCP-1 production in wild-type cells, they had no effect on MCP-1 production by cells whose MKP-1 expression was knocked down by MKP-1 siRNA (Fig. 7B). The potentiation of LPS-induced iNOS and MCP-1 expression by MKP-1 knockdown demonstrates the functional importance of MKP-1 as a negative regulator of STAT signaling in activated macrophages (Fig. 7).
Figure 6.
Effect of MKP-1 knockdown on LNO2- and OA-NO2-dependent dephosphorylation of STAT. A, Confirmation of the knockdown of MKP-1 expression in a RAW264.7 cell line expressing MKP-1 siRNA. Quiescent RAW264.7 cells stably transfected with either an empty vector (Cont) or a MKP-1 siRNA expression cassette were treated with LPS for 2 h, and MKP-1 expression was determined by Western blotting analysis. B, Effect of MKP-1 deficiency on nitroalkene-mediated suppression of STAT1 phosphorylation. Quiescent RAW264.7 cells either carrying a control empty vector (Cont) or expressing MKP-1 siRNA were treated with LPS for 2 h in the presence or absence of LNO2, OA-NO2, LA, or OA, as indicated. STAT1 phosphorylation was assessed as described in Materials and Methods. All results are representative of three separate experiments.
Figure 7.
Effect of MKP-1 knockdown on LNO2- and OA-NO2-mediated suppression of STAT target gene expression. A, Effect of MKP-1 deficiency on nitroalkene-mediated suppression of iNOS expression. Quiescent RAW264.7 cells carrying either a control empty vector (Cont) or stably expressing MKP-1 siRNA were treated for 6 h as indicated, and iNOS expression was determined by Western blot analysis. Results are representative of three separate experiments. B, Effect of MKP-1 deficiency on nitroalkene-mediated suppression of MCP-1 secretion. Quiescent RAW264.7 cells either carrying a control empty vector or stably expressing MKP-1 siRNA were treated with LPS in the presence or absence of LNO2 (0.3, 0.6, 1.3, or 2.5 μm) or OA-NO2 (0.3, 0.6, or 1.3 μm) for 16 h as indicated. MCP-1 secretion was assessed by ELISA. Values are means ± sd (n = 6). *, P < 0.05, compared with cells treated with LPS alone in the same group. *, P < 0.05 vs. LPS (+) alone in the same groups. ns, No significance.
Taken together, these results indicate that induction of MKP-1 is part of the feedback mechanism that governs the proinflammatory STAT cascade. Our results also demonstrate that the augmentation of MKP-1 expression by nitroalkenes contributes to termination of proinflammatory STAT signaling in macrophages.
Discussion
The STAT cascade plays a critical role in the regulation of host defense and inflammatory responses (34). For example, activation of STAT signaling contributes to LPS-induced endotoxin shock in mice (35). STAT3 signaling is essential for IL-6-mediated T cell infiltration in acute inflammation (36). Thus, components of the STAT signaling pathway are attractive molecular targets for treating various inflammatory diseases. Indeed, CP-690,550, a selective antagonist of JAK3 that is one of the upstream signaling molecules of the STAT cascade, shows remarkable efficacy in the prevention of graft rejection in primates (37). In the present study, we show that nitroalkene derivatives of LNO2 and OA-NO2 inhibit proinflammatory STAT signaling in macrophages. Previously, we have demonstrated that a class of nitrated unsaturated fatty acids exists in the circulation of healthy human subjects. The OA-NO2 concentration of blood is about 50% greater than that of LNO2, with the combined free and esterified blood levels of these two fatty acid derivatives exceeding 1 μm (11,13). Thus, local concentrations of the individual nitroalkenes could reach mircomolar range. Considering the concentrations of nitroalkenes used herein are within physiological ranges of healthy humans, it is conceivable that endogenous nitroalkenes could play a significant role in preventing abnormal activation of STAT signaling, thereby contributing to the maintenance of vascular homeostasis. Further characterization of nitro-fatty-acid-mediated signal transduction pathways will thus facilitate the development of rational therapeutic strategies for the treatment of inflammatory diseases.
From a mechanistic viewpoint, our findings raise a number of interesting issues that will be the subject of future investigations. First, the actions of nitroalkenes toward LPS-mediated MAPK signaling in macrophages may also contribute to the net antiinflammatory signaling actions of these fatty acid derivatives. For example MAPKs, including p38, ERK, and JNK, often act in concert with nuclear factor-κB to induce immune responses and inflammatory cytokine expression (38). In addition, MKP-1 deficiency leads to sustained activation of p38 and JNK associated with robust increases in the production of inflammatory cytokines in LPS-treated macrophages, emphasizing the inhibitory effect of MKP-1 in the control of proinflammatory p38 MAPK and JNK activities (31,32). Therefore, the strong augmentation of MKP-1 expression by nitroalkenes in LPS-activated macrophages might also mediate inactivation of p38 and JNK in addition to the inhibitory effect on the JAK/STAT pathway.
Another issue is to explore how nitroalkenes mediate induction of MKP-1. Although MKP-1 is a critical negative regulator for proinflammatory cytokine release via inactivating MAPK such as p38 and JNK as aforementioned, induction of MKP-1 is regulated by MAPK per se. It has been documented that phosphorylation of MKP-1 by ERK reduces ubiquitin-dependent degradation of the MKP-1 and thus enhances the stabilization of the phosphatase protein in CCL39 hamster fibroblasts (39). In contrast, Lin et al. (40) have reported that ERK triggers the degradation of MKP-1, and inhibiting the Pb(II)-induced ERK activation by PD 98059 greatly suppresses MKP-1 ubiquitination and degradation in Cl3 human lung cancer cells. In bone marrow macrophages, induction of MKP-1 by LPS is not affected when ERK activation is blocked by MAPK kinase inhibitor PD 98059 (41). However, by using U0126, another MAPK kinase inhibitor, Chen et al. (42) have demonstrated that protein levels of MKP-1 are controlled primarily by ERK at both transcriptional and posttranscriptional levels. Moreover, MKP-1 expression is also modulated by p38, likely at a posttranscriptional level, in LPS-activated RAW264.7 cells. Recently, the p38-mediated posttranscriptional control of MKP-1 expression has been demonstrated using a p38 RNA interference approach in primary culture of mouse peritoneal macrophages (43). Although these discrepancies remain to be genetically defined, it appears to be likely that a temporal dynamic interplay between MAPK and MKP-1 plays a crucial role in the control of immune balance and inflammatory responses (32). Therefore, it is intriguing whether nitroalkenes could temporally regulate the interaction between MAPK and MKP-1, thereby contributing to termination of proinflammatory responses in macrophages. The potential role of nitroalkenes in the regulation of MAPK activity and induction of MKP-1 deserves further investigation.
In summary, nitroalkenes are able to enhance induction of MKP-1 expression, leading to inactivation of the proinflammatory STAT cascade in macrophages. Our results provide new mechanistic insights into nitroalkene-mediated antiinflammatory actions in macrophages and support the notion that endogenous nitro-fatty acid derivatives serve as a novel class of antiinflammatory signaling mediators.
Footnotes
This work was supported by American Diabetes Association Grant JFA 7-05-JF-12 and the McKay Grant from University of Michigan Cardiovascular Center (T.C.), National Institutes of Health Grants HL68878, HL089544, and HL75397 (Y.E.C.) and HL58115 and HL64937 (B.A.F.). T.I. was supported by a postdoctoral fellowship (0720079Z) from the American Heart Association Greater Midwest. Y.E.C. is an established investigator of the American Heart Association (0840025N).
Disclosure Statement: All authors of this manuscript have nothing to declare.
First Published Online May 8, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; cPTIO, 2-(4- carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; 15d-PGJ2, 15-deoxy-Δ12,14-PGJ2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSH, glutathione; iNOS, inducible nitric oxide synthase; ISRE, interferon-γ stimulated response element; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LA, linoleic acid; LNO2 nitrated linoleic acid; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; OA, oleic acid; OA-NO2, nitrated oleic acid; MKP-1, MAPK phosphatase 1; PPARγ, peroxisome proliferator-activated receptor-γ; pSTAT1, phospho-STAT1; Q-PCR, quantitative real-time PCR; SIN-1, 3-morpholinosydnonimine; siRNA, small interfering RNA; STAT, signal transducer and activator of transcription.
References
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA 1994 Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269:26066–26075 [PubMed] [Google Scholar]
- Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA 1995 Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch Biochem Biophys 324:15–25 [DOI] [PubMed] [Google Scholar]
- O'Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA 1999 Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol 12:83–92 [DOI] [PubMed] [Google Scholar]
- Lima ES, Di Mascio P, Rubbo H, Abdalla DS 2002 Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry 41:10717–10722 [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B 2004 Nitrated lipids: a class of cell-signaling molecules. Proc Natl Acad Sci USA 101:11527–11528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Oliw E 2001 Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways. J Clin Invest 107:1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Langenbach R 2001 Why there are two cyclooxygenase isozymes. J Clin Invest 107:1491–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DG, Sweeney S, Bloodsworth A, White CR, Chumley PH, Krishna NR, Schopfer F, O'Donnell VB, Eiserich JP, Freeman BA 2002 Nitrolinoleate, a nitric oxide-derived mediator of cell function: synthesis, characterization, and vasomotor activity. Proc Natl Acad Sci USA 99:15941–15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O'Donnell VB 2002 Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem 277:5832–5840 [DOI] [PubMed] [Google Scholar]
- Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O'Donnell VB 2002 Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res 91:375–381 [DOI] [PubMed] [Google Scholar]
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA 2005 Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280:42464–42475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA 2005 Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proc Natl Acad Sci USA 102:2340–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PR, Schopfer FJ, Sweeney S, Freeman BA 2004 Red cell membrane and plasma linoleic acid nitration products: synthesis, clinical identification, and quantitation. Proc Natl Acad Sci USA 101:11577–11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA 2006 Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem 281:20450–20463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA 2007 Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem 282:31085–31093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE 2006 Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem 281:35686–35698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y 2004 The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem 279:54023–54031 [DOI] [PubMed] [Google Scholar]
- Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T 2007 Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol 293:H770–H776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Park SY, Joe EH, Jou I 2003 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem 278:14747–14752 [DOI] [PubMed] [Google Scholar]
- Chen CW, Chang YH, Tsi CJ, Lin WW 2003 Inhibition of IFN-γ-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor γ agonist, 15-deoxy-Δ12,14-prostaglandin J2, involves inhibition of the upstream Janus kinase/STAT1 signaling pathway. J Immunol 171:979–988 [DOI] [PubMed] [Google Scholar]
- Weber SM, Scarim AL, Corbett JA 2004 PPARγ is not required for the inhibitory actions of PGJ2 on cytokine signaling in pancreatic β-cells. Am J Physiol Endocrinol Metab 286:E329–E336 [DOI] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C 1993 Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med 177:1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt W, Kunz D, Hummel R, Pfeilschifter J 1996 Molecular cloning of the rat inducible nitric oxide synthase gene promoter. Biochem Biophys Res Commun 223:752–756 [DOI] [PubMed] [Google Scholar]
- Llovera M, Pearson JD, Moreno C, Riveros-Moreno V 2001 Impaired response to interferon-γ in activated macrophages due to tyrosine nitration of STAT1 by endogenous nitric oxide. Br J Pharmacol 132:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CW, Hsieh HJ, Chao YJ, Wang DL 2004 Interleukin-6-induced JAK2/STAT3 signaling pathway in endothelial cells is suppressed by hemodynamic flow. Am J Physiol Cell Physiol 287:C771–C780 [DOI] [PubMed] [Google Scholar]
- Villavicencio RT, Liu S, Kibbe MR, Williams DL, Ganster RW, Dyer KF, Tweardy DJ, Billiar TR, Pitt BR 2000 Induced nitric oxide inhibits IL-6-induced stat3 activation and type II acute phase mRNA expression. Shock 13:441–445 [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster Jr JR, Freeman BA 2005 Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem 280:19289–19297 [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F 2003 Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormald S, Hilton DJ 2004 Inhibitors of cytokine signal transduction. J Biol Chem 279:821–824 [DOI] [PubMed] [Google Scholar]
- Venema RC, Venema VJ, Eaton DC, Marrero MB 1998 Angiotensin II-induced tyrosine phosphorylation of signal transducers and activators of transcription 1 is regulated by Janus-activated kinase 2 and Fyn kinases and mitogen-activated protein kinase phosphatase 1. J Biol Chem 273:30795–30800 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y 2006 MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA 2006 Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 103:2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, Chaturvedi P, Han YL, Aras S, Li YS, Kolattukudy PE, Ping D, Boss JM, Ransohoff RM 1998 IFN-γ induction of the human monocyte chemoattractant protein (hMCP)-1 gene in astrocytoma cells: functional interaction between an IFN-γ-activated site and a GC-rich element. J Immunol 160:3908–3916 [PubMed] [Google Scholar]
- Pfitzner E, Kliem S, Baus D, Litterst CM 2004 The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des 10:2839–2850 [DOI] [PubMed] [Google Scholar]
- Kamezaki K, Shimoda K, Numata A, Matsuda T, Nakayama K, Harada M 2004 The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int Immunol 16:1173–1179 [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA 2005 IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA 102:9589–9594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Pesu M, Borie DC, Changelian PS 2004 A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov 3:555–564 [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA 2002 MAP kinases in the immune response. Annu Rev Immunol 20:55–72 [DOI] [PubMed] [Google Scholar]
- Brondello JM, Pouyssegur J, McKenzie FR 1999 Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286:2514–2517 [DOI] [PubMed] [Google Scholar]
- Lin YW, Chuang SM, Yang JL 2003 ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem 278:21534–21541 [DOI] [PubMed] [Google Scholar]
- Valledor AF, Xaus J, Comalada M, Soler C, Celada A 2000 Protein kinase Cε is required for the induction of mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 164:29–37 [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y 2002 Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 169:6408–6416 [DOI] [PubMed] [Google Scholar]
- Hu JH, Chen T, Zhuang ZH, Kong L, Yu MC, Liu Y, Zang JW, Ge BX 2007 Feedback control of MKP-1 expression by p38. Cell Signal 19:393–400 [DOI] [PubMed] [Google Scholar]