Abstract
We have previously shown that estrogen stimulates cell proliferation in both normal and transformed urothelial cells mainly through activation of the two primary estrogen receptors (ERs), ERα and ERβ. A growing body of evidence suggests that estrogen also initiates nongenomic effects that cannot be explained by activation of primary ERs. In the present study, we observed that urothelial cells express high amounts of GPR30, a G protein-coupled receptor recently identified as a candidate for membrane-associated estrogen binding. Membrane- impermeable bovine serum albumin-conjugated 17β-estradiol and the specific GPR30 agonist G-1 both inhibited urothelial cell proliferation in a concentration-dependent manner. Transient overexpression of GPR30 inhibited 17β-estradiol (E2)-induced cell proliferation. Decreased GPR30 expression caused by specific small interfering RNA increased E2-induced cell proliferation. These results indicate that membrane-associated inhibitory effects of E2 on cell proliferation correlate with abundance of GPR30. Although E2 induced a significant increase in caspase-3/7 activity, G-1 did not, suggesting that the GPR30-mediated inhibitory effect on cell proliferation was not caused by apoptosis. Furthermore, we found that G-1 failed to induce c-fos, c-jun, and cyclin D1 expression, and GPR30 overexpression abolished E2-induced c-fos, c-jun, and cyclin D1 expression. However, inactivation of GPR30 by small interfering RNA increased c-fos, c-jun, and cyclin D1 expression. These results suggest that GPR30-mediated inhibition of urothelial cell proliferation is the result of decreased cyclin D1 by down-regulation of activation protein-1 signaling.
THE EFFECTS OF estrogen on target cells have long been known to be mediated via binding to the two primary estrogen receptors (ERs), ERα and ERβ, that act as transcriptional factors (1). In addition to these well-characterized classical ERs, a growing body of functional, biochemical, and pharmacological evidence has led to the conclusion that some responses to estrogen are mediated by putative estrogen-binding proteins in the cytoplasm or located at the plasma membrane (2). These extranuclear or nongenomic signaling pathways are rapid and insensitive to inhibitors of transcription and translation (3). Several membrane-associated ERs have been described, including full-length (4) or truncated forms of ERα (5) and unknown proteins such as ER-X (6). The origin and function of membrane-associated ERs have not been well defined because of difficulties associated with isolating and characterizing these proteins. Recently, an orphan G protein-coupled receptor, GPR30, that is structurally unrelated to nuclear ERs, has been identified as a candidate for the estrogen-binding proteins that may mediate estrogen-induced nongenomic signaling (7,8). GPR30 is widely distributed in various tissues (9). However, thus far, the physiological activity of GPR30 is unclear (10).
Around one third of the genes in humans that are regulated by estrogen, including important physiological targets such as cyclin D1 and p21, do not contain ERE-like sequences. These genes typically are regulated via interactions with other transcription factors. For example, activation protein-1 (AP-1), a multi-protein complex composed of either two jun family protooncogenes or one jun and one fos family protein, integrates various mitogenic signals by binding to the AP-1 recognition site, also known as the phorbol myristate acetate-responsive element. Therefore, it is a major regulator of cell proliferation (11). Increased c-jun abundance has been correlated with muscle-invasive growth and expression of proliferating cell nuclear antigen in bladder transitional cell carcinoma (12). It has been suggested that GPR30 may mediate 17β-estradiol (E2)-induced c-fos up-regulation in MCF-7 and SKBR3 breast cancer cells (13), endometrial cancer cells (14), and thyroid cancer cells (15). Introduction of GPR30 cDNA into ER-negative low GPR30-expressing MDA-MB-231 cells enables ERK activation in response to E2 (16). Interestingly, GPR30-dependent ERK 1/2 activation may also be induced by an ER antagonist, ICI 182,780, but not by 17α-E2, suggesting that these estrogen effects are ER independent (17). Nevertheless, it has been observed that GPR30 plays a critical role in mediating progestin-induced ERK 1/2 inactivation and cell growth inhibition in MCF-7 cells (18).
Our previous studies demonstrated that E2 stimulates human urothelial cell proliferation through both ERα and ERβ (19,20). However, we also consistently observed that when the pure ER antagonist ICI 182,780 was combined with E2, it not only completely abolished increased cell proliferation or nerve growth factor production induced by E2, but also induced a further inhibition of these effects in urothelial cells. We also observed that at high concentrations (>10−7 m), E2-induced cell proliferation is lower than that observed at low concentrations (10−8 m). These phenomena cannot be explained completely by current models of estrogen signaling and suggest the existence of mechanisms other than classical genomic functions of estrogen. The recent identification of the first GPR30-selective ligand G-1 has provided new opportunities to investigate further the function of GPR30 in mediating various actions of estrogen (10,21). G-1 has stimulated cell proliferation through induction of the c-fos gene independently of the estrogen-responsive element in both BG-1 human ovarian cancer cells and SKBR3 human breast cancer cells (22). In the present study, we show that GPR30 expressed by urothelial cells fulfills the criteria for a membrane-associated ER. Contrary to results reported in ovarian cancer cells, using G-1 and specific small interfering RNAs (siRNAs) against GPR30, we demonstrate that GPR30 may mediate an inhibitory effect of estrogen on urothelial cell proliferation.
Materials and Methods
Cell culture and tissue specimens
All experimental protocols were reviewed and approved by the Health Sciences Human Subjects Committee of the University of Wisconsin. Normal human bladder urothelial cells (HBUCs) were primarily cultured from discarded bladder tissues obtained during surgeries performed at the University of Wisconsin Hospital and Clinics. Cells were plated on petri dishes coated with type I rat tail collagen in Ham’s F-12+ nutrient mixtures containing 2% fetal bovine serum (FBS) and used between passages 4 and 6. HeLa cells (CCL-2), a monkey kidney fibroblast cell line COS-7, and the human bladder tumor cell line T24 were purchased from the American Type Culture Collection (Manassas, VA). HeLa cells were maintained in MEM containing 10% FBS. COS-7 cells and T24 cells were maintained in DMEM containing 5% FBS. Cells were cultured in a humidified incubator at 37 C in 5% CO2. All media and supplements were purchased from Invitrogen Corp. (Carlsbad, CA). Phenol red-free medium and charcoal-stripped FBS (Sigma-Aldrich, St. Louis, MO) were used in all experiments.
Reagents, vector, and transfection
HEPES, threo-1,4-dimercapto-2,3-butanediol[dithiothreitol (DTT)], EDTA, and 1,3,5-estratriene-3,17β-diol (E2) were purchased from Sigma-Aldrich. 1,3,5(10)-estratriene-3,17 α-diol (17α-E2 or αE2), 1,3,5(10)-estratriene-3,17β-diol 17-hemisuccinate (E2H), and E2-17-hemisuccinate:BSA (E2B) were purchased from Steraloids (Newport, RI). ICI 182,780 was purchased from Tocris (Ellisville, MO). G-1 was provided by ChemDiv, Inc., (San Diego, CA), and 17β-[2,4,6,7-3H] E2 ([3H]E2), approximately 82.3 Ci/mmol, was purchased from GE Healthcare (Buckinghamshire, UK). Recombinant human (rh) epidermal growth factor (EGF) was purchased from MP Biomedicals (Solon, OH). Staurosporine (used to induce apoptosis) was purchased from Fisher Scientific (Hanover Park, IL). Ac-DEVD-CHO Caspase 3/7 inhibitor was purchased from BD PharMingen (Franklin Lakes, NJ). The pcDNA3-green fluorescent protein (GFP)-GPR30 vector has been previously described (7). Calcium phosphate-mediated transient transfection of pcDNA3-GFP-GPR30 and empty vectors into cells was performed 24 h after seeding cells using ProFection Mammalian Transfection Systems (Promega Corp., Madison, WI) according to manufacturer’s instructions.
Membrane preparation and solubilization
Protocols for membrane fractionations, solubilization, and receptor binding assay were provided by Dr. Peter Thomas [University of Texas-Austin, Austin, TX (8,23)] with few modifications for human urothelial cells. All the steps were performed at 4 C. Briefly, cells were collected in 25 mm HEPES, 10 mm NaCl, 1 mm dithioerythritol, and 1 mm EDTA (pH 7.6) containing protease inhibiters, and homogenized. The homogenate was centrifuged at 1,000 × g for 7 min to pellet the nuclear fractions, and the supernatant was centrifuged at 20,000 × g for 20 min to pellet the membrane fraction. Plasma membranes were further purified by centrifuging the membrane pellet with a sucrose pad (1.2 m sucrose) at 6,900 × g for 45 min. Membranes were then solubilized with 12 mm Triton X-100 in four volumes of 25 nm HEPES, 10 mm NaCl, 1 mm threo-1,4-dimercapto-2,3-butanediol, followed by removal of the detergent with SM-2 polystyrene adsorbents (2:1 vol-weight; Bio-Rad Laboratories, Inc., Hercules, CA) and subsequent removal of the adsorbents by filtration. Protein concentrations were determined using the BCA Protein Assay kit (Pierce, Rockford, IL).
Receptor binding assay
All steps of the binding assays were conducted at 4 C. The membrane preparations were diluted to 1.0 mg protein/ml in 25 mm HEPES, 10 mm NaCl, 1 mm dithioerythritol, and 1 mm EDTA (pH 7.6) immediately before binding assays. One hundred microliters of membrane preparation were used for each assay. For saturation analysis, total binding curves were generated by incubating one set of wells containing a range (0.5–10 nm) of [3H]E2 alone. Nonspecific binding was determined from a parallel set also containing 100-fold excess (50 nm–1 μm) unlabeled E2, as a competitor. For competitive binding assays, wells containing 5 nm [3H]E2 and the steroid competitors (concentration range 1 nm to 100 μm) were incubated with 100 μl membrane preparation. After 30-min incubation, the binding reactions were terminated by adding 50 μl 10% trichloroacetic acid and filtering the precipitated mixture over presoaked GF/B glass-fiber micro-filter plates (PerkinElmer, Wellesley, MA; pore size = 1 μm) to separate bound steroid from free steroid. The filter was then washed twice with wash buffer [25 mm HEPES, 10 mm NaCl, and 1 mm EDTA (pH 7.6)]. Micro-Scint 20 (30 μl) was added to each well, and radioactivity was counted with a TOPCount Microplate Scintillation Counter (Packard, Meriden, CT). Disintegrations per minute were calculated by count per minute/efficiency (∼20–30%). The displacement of [3H]E2 binding by steroid competitors was expressed as a percentage of the maximum specific binding of E2 (cpm = ∼2000–3000). Each assay point was run in quadruplicates, and the assays were repeated using at least four different batches of cultured cells for each test chemical. Linear and nonlinear regression analyses for all receptor binding assays and calculations of dissociation constant (Kd) and binding capacity were performed using GraphPad Prism for Windows (version 4.01; GraphPad Software, San Diego, CA).
RNA interference
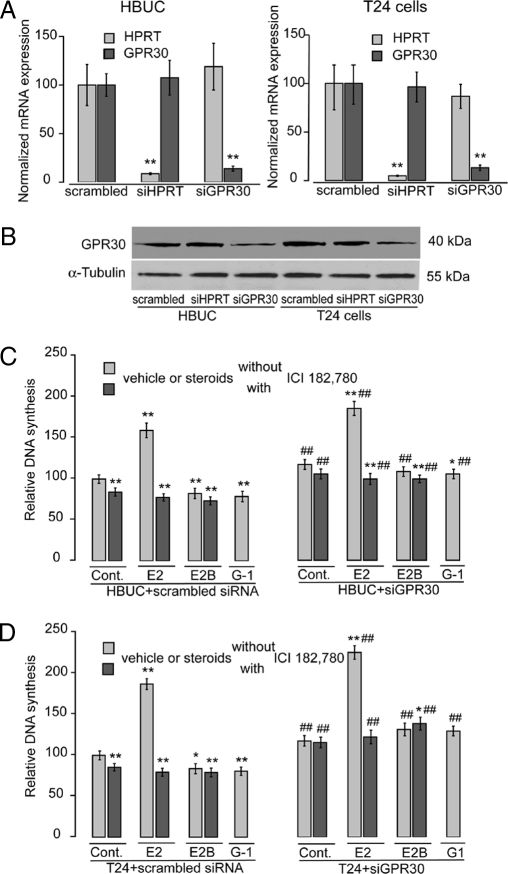
siRNA-Dicector-TriFECTa Kit with three Dicer-Substrate 27-mer (27-oligomer) duplexes targeting human GPR30 gene (HSC.RNAI.N001505.3) and control siRNAs were provide by Integrated DNA Technologies (Coralville, IA). The duplex (DX3) that had the most significant inhibitory effects (90%) on GPR30 mRNA expression but had no effect on hypoxanthine guanine phosphoribosyl transferase (HPRT)-S1 control mRNA in urothelial cells was used in the remainder of this study. The sense sequence was 5′-rCrCrUrGrUrGrCrUrArCrUrCrCrCrUrCrArUrUrGrUrCrCGG-3′, and the antisense sequence was 5′-rCrCrGrGrArCrArArUrGrArGrGrGrArGrUrArGrCrArCrArGrGrCrC-3′. Transfection of siRNA (10 nm) was performed using 5 μl/ml Oligofectamine (Invitrogen) when cells achieved 30–50% confluence. Control studies to evaluate the specificity of siRNAs were performed using scrambled control siRNA (Integrated DNA Technologies) under identical conditions. To quantify cell transfection efficiency, 10 nm fluorescent-labeled Cys DS, CAL Fluor Red 610 DS, or HPRT-S1 DS positive control duplexes (Integrated DNA Technologies) were cotransfected into cells, and nuclei were identified with Hoechst 33342 (Invitrogen) staining. More than 90% transfection efficiency and more than 90% inhibition of the positive control gene were reproducibly achieved in all experiments.
Total RNA isolation and PCR analysis
Total RNA was isolated from cultured cells using the QIAshredder and RNeasy Mini Kit (QIAGEN, Inc., Valencia, CA) following the manufacturer’s protocol. cDNA was synthesized from 0.5–1 μg RNA using the M-MLV Reverse Transcriptase kit (Promega). Quantitative real-time PCR was performed using SYBR Green Master Mix in a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA). Experiments were performed in duplicate for each data point, and abundance of human ribosomal protein S26 mRNA was determined as a reference gene. Primers for amplification of GPR30 were forward 5′-TCTAAACTGCGG TCAGATGTGGCT-3′ and reverse 5′-TGTGAAGAGTGCAAGGTGACCAGT-3′ or forward 5′-TCACCTTGCACTCTCACACAGAA-3′ and reverse 5′-TTCCTGGTCGACGGTGTCAGAAAT-3′, for c-fos were forward 5′-TGTCTGTGCTTCCCTTGATCTGA-3′ and reverse 5′-TGGATGATGCTGGGAACAGGAAGT-3′, for c-jun were forward 5′-AGATGAACTCTTTCTGGCCTGCCT-3′ and reverse 5′-AACACTGGGCAGGATACCCAAACA-3′, and for S26 were forward 5′-ACACTAGGAACGCATTTCCACCCT-3′ and reverse 5′-GGCTTCAAGAACGGCAACTCACTT-3′.
Immunoblotting
Whole cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce) containing protease inhibitors (Roche, Indianapolis, IN). The supernatant was collected by centrifugation at 10,000 × g for 10 min at 4 C, and protein content was measured using the BCA Protein Assay kit. Whole cell extracts or cellular membrane proteins (30 μg) were electrophoresed on 10% SDS-PAGE gels (Bio-Rad Laboratories) and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Antibodies used were rabbit antihuman GPR30 (1:5000, GTX78070; GeneTex Inc., San Antonio, TX), rabbit antihuman c-fos (1:1000, PC05T; Calbiochem, San Diego, CA), rabbit antihuman c-jun/AP-1 (1:1000, PC07; Calbiochem), mouse antihuman cyclin D1 (1:1000, HD11, sc-246; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse antihuman α-tubulin (1:5000, B-7, sc-5286; Santa Cruz Biotechnology), and diluted in Tris-buffered saline with Tween 20 containing 3% BSA. Membranes were blocked with SuperBlock (Pierce) for 3 h at room temperature and incubated with primary antibodies overnight at 4 C. They were then incubated at room temperature for 2 h in 1:10,000 horseradish peroxidase-conjugated antimouse or antirabbit antibodies (Santa Cruz Biotechnology) in Tris-buffered saline with Tween 20. Chemiluminescent signal was revealed with the enhanced chemiluminescence (ECL; Pierce) and detected by exposure to x-ray film. Band intensity was quantified with ImageJ (National Institutes of Health) and normalized to that of α-tubulin in the same sample. Experiments were repeated at least four times.
3H-thymidine incorporation assay
Cells were aliquoted into 96-well tissue culture plates at a density of about 1000 cells per well and serum starved for 24 h. After 10-h treatment, 0.5 μCi [methyl-3H]thymidine (GE Healthcare) was added to each well. After 6 h, DNA was precipitated with 10% trichloroacetic acid and washed with 70% ethanol. The acid-insoluble material was then dissolved with 0.5 m NaOH and collected onto UniFilter-96 GF/C plates (pore size = 1.2 μm; PerkinElmer). The plates were washed, and bound radioactivity was measured by scintillation counting as in receptor binding assays.
Apoptosis assay
Cellular apoptosis was determined by the Apo-ONE Homogeneous Caspase-3/7 Assay Kit (Promega). Briefly, cells were aliquoted into 96-well black tissue culture plates at a density of about 5000 cells per well. After 16-h treatment, 100 μl Apo-ONE Caspase-3/7 reagent was added to each well, and plates were incubated at room temperature for 2 h. Caspase-3/7 activities were measured using a Synergy HT fluorescence plate reader (BioTek Instruments, Inc., Winooski, VT) from the top of plates at 485 ± 20 nm excitation/530 ± 25 nm emission. The plate reader was operated by KC4 software (BioTek Instruments) with sensitivity set at 25.
Statistical analysis
All results are expressed as means ± sem and accompanied by the number of observations. Data were obtained from groups containing four to six replicates. The paired Student’s t test was used to compare self-matched samples. Comparisons among treatments over time or within groups and between multiple groups were performed by one-way or two-way ANOVA using SAS/STAT (SAS Institute Inc., Cary, NC). Fisher’s least significant difference and Tukey’s multiple tests were used to determine post hoc significance. A P value of less than 0.05 was considered statistically significant.
Results
GPR30 may mediate an inhibitory effect on urothelial cell proliferation
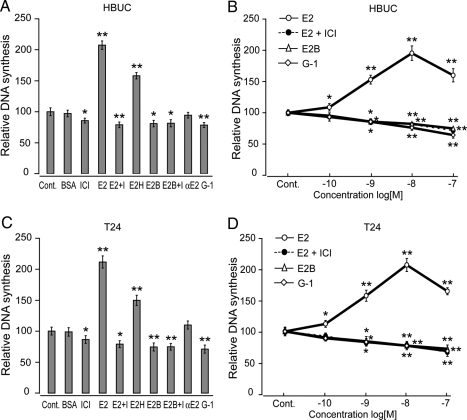
Consistent with our previous studies in both HBUCs and T24 cells, E2 (10−8 m) induced cell proliferation (increased 107.4 and 111.4%, respectively), and this effect was abolished by ICI 182,780 (10−7 m). E2H (10−8 m) also induced cell proliferation, and 17α-E2 (10−8 m) failed to induce cell proliferation. In both cell types, cell membrane-impermeable E2B (10−8 m, molar ratio E2:BSA = 12:1) or the specific GPR30 agonist G-1 (10−8 m) caused significant inhibition in DNA synthesis (Fig. 1, A and C). E2 (10−10 to 10−8 m) induced cell proliferation in a concentration-dependent manner. However, 10−7 m E2-induced cell proliferation was significantly lower than that of 10−8 m E2, suggesting that high concentrations of estrogen may have inhibitory effects on cell proliferation. E2B (10−9 to 10−7 m E2) failed to induce cell proliferation. Instead, concentration-dependent inhibition of cell proliferation was observed. G-1 (10−9 to 10−7 m) also had a significant inhibitory effect on cell proliferation in both HBUCs and T24 cells (Fig. 1, B and D). These observations suggest the presence of membrane-associated ER, in particular, the G protein-coupled receptor GPR30 may have an inhibitory effect on cell proliferation in urothelial cells.
Figure 1.
E2B or the GPR30 agonist G-1 inhibits human urothelial cell proliferation. A, HBUCs or T24 human bladder cancer cells (C) were treated with vehicle [0.1% (dimethylsulfoxide) plus 0.9% ethanol, control (Cont)], 0.1% BSA, 100 nm ICI 182,780 (ICI), 10−8 m E2 in the absence (E2) or presence [E2 plus I (ICI)] of 10−7 m ICI 182,780, 10−8 m E2H, 10−8 m E2B or presence (E2B plus I) of 10−7 m ICI 182,780, 10−8 m 17α-E2 (αE2), and 10−8 m GPR30 agonist G-1 for 16 h. B, HBUCs or T24 cells (D) were treated with 10−10 to 10−7 m E2 in the absence or presence of 10−7 m ICI 182,780, 10−10 to 10−7 m E2B, or 10−10 to 10−7 m G1 for 16 h. Cells were labeled with 0.5 μCi of [methyl-3H]thymidine to determine DNA synthesis. Relative increases in DNA synthesis were normalized to the average of vehicle-treated cells, which was arbitrarily set at 100. Each point represents the mean ± sem of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations performed in quadruplicate. *, P < 0.05; **, P < 0.01, compared with vehicle-treated cells.
Expression of GPR30 in human urothelial cells and its binding to estrogen
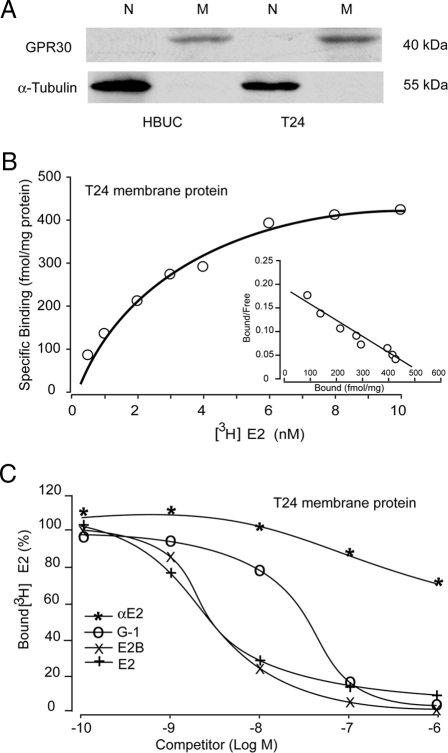
Using quantitative real-time PCR, we observed that normal primary bladder urothelial cells cultured from different donors, as well as immortalized urothelial cells and bladder tumor cell lines, all express relatively high levels of GPR30 mRNA (data not shown). Immunoblotting with a specific antibody against human GPR30 demonstrated the presence of high levels of protein (∼40 kDa) in enriched membrane proteins from HBUCs and T24 cells, but its expression within nuclear fractions from these cells was not detected (Fig. 2A). Nonlinear regression and Scatchard plotting of [3H]E2 binding to cell membrane preparations from T24 cells displayed the presence of a single, saturable, high-affinity estrogen binding site with Kd of 3.2 nm and maximal binding capacity (Bmax) of 577 fmol/mg protein (Fig. 2B). Competitive binding assays showed that E2, G-1, and E2B were all effective competitors. However, αE2 had very low affinity to these binding sites (Fig. 2C).
Figure 2.
Human urothelial cells express GPR30. A, Nuclear (N) and membrane (M) proteins from HBUCs and T24 cells were enriched. GPR30 protein was detected only in membrane fractions but not in nuclear fractions by immunoblotting using a specific antibody against human GPR30. α-Tubulin was detected only in nuclear fractions, but not in membrane fractions. Blots representative of four separate experiments are shown. B, Representative saturation curve and Scatchard plot (inset) of specific [3H]E2 binding to membrane protein preparations from T24 cells. C, Competition binding curves for [3H]E2 in the presence of E2, E2B, G1, or αE2 using membrane protein preparations from T24 cells. The maximum specific [3H]E2 binding was arbitrarily set at 100.
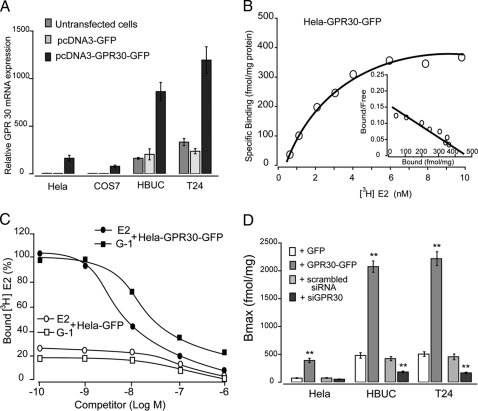
We induced overexpression of GPR30 in HeLa and COS-7 cells, as well as primary and tumor urothelial cells, by transient transfection with a GPR30-GFP fusion protein construct. Greater than 50% transfection efficiency revealed by fluorescence of GFP in transfected cells was consistently achieved. HeLa cells and COS-7 cells constitutively express a very low abundance of GPR30 mRNA, but HBUCs and T24 cells constitutively express a much greater amount of GPR30 mRNA, as shown by real-time PCR. Transfection with the vector that coded only for the GFP did not increase GPR30 expression. Transient overexpression with the GPR30-GFP fusion protein construct caused a 4- to 10-fold increase in GPR30 mRNA in these cells (Fig. 3A). Nonlinear regression and Scatchard plotting of [3H]E2 binding to cell membrane preparations from HeLa cells transfected with GPR30 displayed the presence of estrogen binding sites with similar binding affinities (Kd = 2.5 nm, Bmax = 450 fmol/mg protein (Fig. 3B). The membrane preparations from HeLa cells transfected with GFP had very low affinity to [3H]E2, and this binding was not affected by competition with E2 or G-1. In contrast, both E2 and G-1 specifically competed with [3H]E2 binding to membrane preparations from HeLa cells transfected with GPR30-GFP fusion vector (Fig. 3C). In plasma membrane preparations from HeLa, HBUCs, or T24 cells transfected with GPR30, higher Bmax (∼2000 fmol/mg protein for HBUCs and T24 cells) was detected than that in cells transfected with only GFP (Fig. 3D). In plasma membrane preparations from HBUCs or T24 cells pretreated with siRNA against GPR30, Bmax was significantly down-regulated compared with membranes from cells treated with scrambled siRNA. Although these data do not exclude the possible presence of classical ER associated with the cell membrane, they do suggest the presence of membrane-associated estrogen- binding sites that are structurally different than classical ERs, presumably GPR30, in urothelial cells.
Figure 3.
Overexpression of GPR30. A, GPR30 was overexpressed as a fusion protein with enhanced GFP in HeLa, COS-7, HBUCs, and T24 cells. Semiquantitative real-time PCR shows GPR30 mRNA in untransfected, pcDNA3-GFP-transfected, or pcDNA3-GPR30-GFP-transfected cells. Results are normalized to S26 ribosomal mRNA expression in the same sample. B, Representative saturation curve and Scatchard plot (inset) of specific [3H]E2 binding to membrane protein preparations from pcDNA3- GPR30-GFP-transfected HeLa cells. C, Competition binding curves for E2 and G-1 to membrane protein preparations from pcDNA3-GFP or pcDNA3-GPR30-GFP-transfected HeLa cells. The maximum specific [3H]E2 binding to the membrane protein preparations from pcDNA3-GFP-transfected HeLa cells was arbitrarily set at 100. D, The maximum specific [3H]E2 binding to membrane protein preparations from HeLa, HBUCs, or T24 cells transfected with vectors coding GFP or GPR30-GFP, scrambled siRNA, or siRNA against GPR30. Data represent the mean ± sem of three separate experiments. **, P < 0.01, compared with control (transfected with GFP or scrambled siRNA).
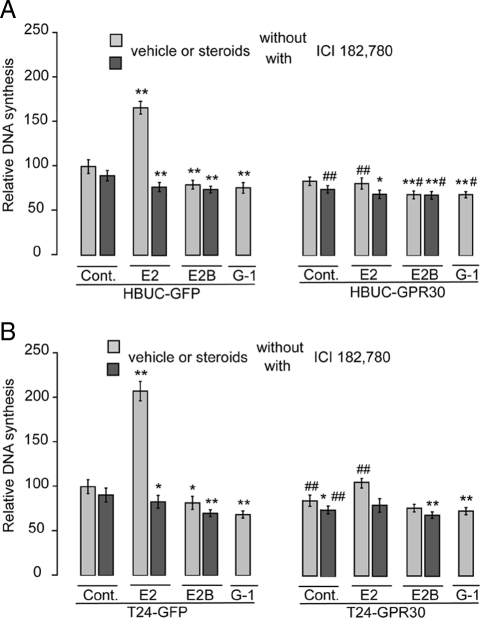
Overexpression of GPR30 in human urothelial cells inhibits urothelial cell proliferation
In HBUCs (Fig. 4A) and T24 (Fig. 4B) cells that express only GFP, E2 (10−8 m) induced significant increases in DNA synthesis as measured by the [3H]thymidine incorporation assay (66.2 and 107.5%, respectively). These effects were abolished by ICI 182,780 (10−7 m). E2B (10−8 m) reduced DNA synthesis in HBUCs and T24 cells by 20.4 and 18.3%, respectively. However, in GPR30-overexpressing HBUCs or T24 cells, E2 failed to induce cell proliferation. This response of GPR30-overexpressing urothelial cells to E2 treatment mimics the effects of membrane-impermeable E2B. In both control and GPR30-overexpressing HBUCs or T24 cells, G-1 caused significant decreases in DNA synthesis. These results suggest that GPR30 may mediate an inhibitory effect on urothelial cell proliferation.
Figure 4.
GPR30 overexpression inhibits E2-induced cell proliferation in urothelial cells. HBUCs (A) and T24 cells (B) were transfected with pcDNA3-GFP or pcDNA3-GPR30-GFP vectors and treated with 10−8 m E2 or E2B in the presence or absence of 100 nm ICI 182,780, or 10−8 m G1 alone. DNA synthesis was determined using the [methyl-3H]thymidine incorporation assay. Relative increases in DNA synthesis were normalized to the average of vehicle-treated cells, which was arbitrarily set at 100. Data represent the mean ± sem of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations performed in quadruplicate. *, P < 0.05; **, P < 0.01, compared with vehicle-treated cells. #, P < 0.05; ##, P < 0.01. GPR30-expressing cells compared with GFP-expressing cells. Cont, Control.
Inhibition of GPR30 expression by siRNA increases E2-induced urothelial cell proliferation
Greater than 90% transfection efficiency and perinuclear localization of siRNAs was confirmed by cotransfecting cells with fluorescence-labeled siRNAs and staining cells with Hoechst 33342. By introducing specific 27-mer (27-oligomer) siRNA duplexes against GPR30 into HBUCs or T24 cells, GPR30 expression was significantly inhibited as measured by real-time PCR (∼90%, Fig. 5A) or immunoblotting (∼60%, Fig. 5B), but siRNA against HPRT did not affect GPR30 expression nor was HPRT expression affected by siRNA against GPR30. Treatment of T24 cells with siRNA against GPR30 also caused an approximate 80% decrease in specific[3H]E2 binding to cell membrane preparations (data not shown). Inhibition of GPR30 expression by siRNA increased the proliferative response to E2 treatment relative to results in scrambled siRNA-transfected cells. Inhibition of GPR30 expression in HBUCs augmented cell proliferation in response to E2 (10−8 m) from 59.5–95.7% (Fig. 5C). Inhibition of GPR30 expression in T24 cells augmented E2-induced increased cell proliferation from 87.3–125.9% (Fig. 5D). E2B treatment decreased cell proliferation in both HBUCs and T24 cells, and this was reversed by down-regulation of GPR30. These data suggest that GPR30 is responsible for E2-induced inhibition of cell proliferation in urothelial cells.
Figure 5.
siRNA against GPR30 increases E2-induced cell proliferation in urothelial cells. HBUCs and T24 cells were transfected with 10−8 m scrambled siRNA, siRNA against GPR30, or HPRT-S1 positive control (Cont) siRNA. A, Semiquantitative real-time PCR for GPR30 and HPRT mRNA in scrambled-, siRNA against HPRT-, or siRNA against GPR30-transfected HBUCs or T24 cells. Results are normalized to S26 ribosomal mRNA expression in the same sample. **, P < 0.01, compared with scrambled siRNA-transfected cells. B, Representative immunoblotting for GPR30 in membrane proteins from scrambled-, siRNA against HPRT-, or siRNA against GPR30-transfected HBUCs or T24 cells. C, Transfected HBUCs or T24 cells (D) were treated with 10−8 m E2 or E2B in the presence or absence of 10−7 m ICI 182,780, or 10−8 m G1 alone. DNA synthesis was determined using the [3H]thymidine incorporation assay. Relative increases in DNA synthesis were normalized to the average of vehicle-treated cells, which was arbitrarily set at 100. Data represent the mean ± sem of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations performed in quadruplicate. *, P < 0.05; **, P < 0.01, compared with vehicle-treated cells. ##, P < 0.01, compared with scrambled siRNA-treated cells.
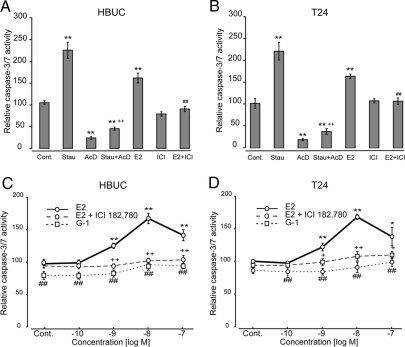
GPR30-mediated inhibition of proliferation of urothelial cells is independent of apoptosis
We next investigated whether the inhibitory effect of GPR30 on cell proliferation is associated with apoptosis. E2 (10−8 m) increased caspase-3/7 activity by 59.3 and 55.1% in HBUCs and T24 cells, respectively. These effects were abolished by ICI 182,780 (Fig. 6, A and B). Interestingly, E2 (10−9 to 10−8 m) induced caspase-3/7 activity in a concentration-dependent manner in both HBUCs and T24 cells, and this effect was abolished by ICI 182,780. G-1 (10−10 to 10−7 m) failed to increase caspase-3/7 activity (Fig. 6, C and D). These results suggest that E2-induced cell proliferation and apoptosis may be linked, and that GPR30-mediated E2-induced inhibition of cell proliferation is independent of apoptosis in urothelial cells.
Figure 6.
E2-induced cell apoptosis is independent of GPR30. HBUCs (A) or T24 cells (B) were treated with 10−8 m E2 in the presence or absence of 10−7 m ICI 182,780. Staurosporine (Stau) (10−7 m) was used as a positive control (Cont) to induce cell apoptosis, and Ac-DEVD-CHO (AcD) (10 μm) was used as a negative control. **, P < 0.01, compared with vehicle-treated cells. ++, P < 0.01, Staurosporine-treated cells vs. Staurosporine-treated cells in the presence of Ac-DEVD-CHO. ##, P < 0.01, cells in the presence of ICI 182,780 and E2 compared with results from cells treated with ICI 182,780 alone. HBUCs (C) or T24 cells (D) were treated with 10−10 to 10−7 m E2 in the presence or absence of 10−7 m ICI 182,780, or 10−10 to 10−7 m G-1 alone. Relative caspase-3/7 activity was calculated by setting the average fluorescence units of vehicle-treated cells in each group to 100 (corresponds to 600–1000 fluorescence units at 485 ± 20 nm excitation/530 ± 25 nm emission and sensitivity = 25). Data represent the mean ± sem of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations performed in quadruplicate. **, P < 0.01, compared with vehicle-treated cells. ++, P < 0.01, G-1-treated cells compared with E2-treated cells. ##, P < 0.01, cells in the absence of ICI 182,780 compared with cells in the presence of ICI 182,780.
The inhibitory effect of GPR30 on urothelial cell proliferation is mediated through blocking the c-fos/c-jun-cyclin D1 signaling pathway
To assess the capacity of E2, ICI 182,780, or G-1 to modulate receptor expression, HBUCs or T24 cells were treated with E2, E2B, or G-1 (all at 10−8 m), ICI 182,780 (10−7 m), or a combination of E2 and ICI 182,780 for 16 h, and expression of ERα, ERβ, and GPR30 was determined by immunoblotting. None of these treatments significantly affected receptor abundance (Fig. 7A). We have previously observed that E2 induces significant ERK1 and ERK2 phosphorylation within 5–30 min in urothelial cells, but ERK1/2 activation is not related to estrogen-induced cell proliferation (19). Overexpression of GPR30 in both HBUCs and T24 cells does not change ERK 1/2 phosphorylation induced by E2 (Fig. 7B). These results suggest that GPR30-mediated inhibition of cell proliferation is independent of receptor levels and the MAPK pathway.
Figure 7.
GPR30-mediated effects on cell growth are not dependent on receptor expression or ERK activation. A, HBUCs or T24 cells were treated with 10−8 m E2, E2B, G-1, 10−7 m ICI 182,780, or a combination of E2 and ICI 182,780 for 16 h. Whole cell lysates were blotted for human ERα, ERβ, and GPR30. Human α-tubulin was used as a loading control (Cont). Blots representative of four separate experiments are shown. B, HBUCs or T24 cells were transfected with pcDNA3-GFP or pcDNA3-GPR30 and then treated with 10−8 m E2 for up to 60 min. Whole cell lysates were blotted for phosphorylated (p)-ERK1/2 and ERK-1. Blots representative of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations are shown. E2+I, E2 + ICI 182,780.
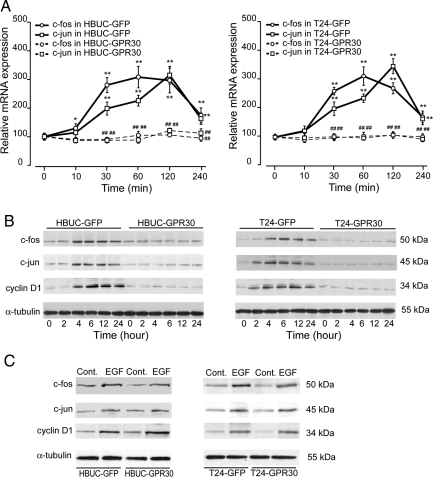
We then investigated the abundance of c-fos and c-jun mRNA and protein. These two proteins comprise the AP-1 transcription factor. E2 (10−8 m) treatment of HBUCs transfected with GFP only increased c-fos and c-jun mRNA by approximately 200 and 150%, respectively, within 30 min to 4-h treatment (Fig. 8A, left panel). However, E2 treatment of GPR30-overexpressing cells failed to induce increased c-fos and c-jun mRNA expression. Immunoblotting for c-fos and c-jun proteins showed that these proteins were significantly increased in response to E2 as early as 4 h and up to 24 h after treatment of control cells. In GPR30-overexpressing HBUCs, E2 failed to increase c-fos and c-jun protein expression (Fig. 8B, left panel). We have reported previously that cyclin D1, a protein that plays an important role in progression of urothelial cells through the G-1 phase of the cell cycle, is increased during estrogen-induced cell proliferation (20). In control HBUCs, cyclin D1 was induced after 4-h E2 treatment and remained relatively high for up to 24 h. In GPR30-overexpressing cells, E2 failed to induce cyclin D1 expression (Fig. 8B, left panel). Similar results were observed in T24 cells (Fig. 8, right panels). However, in both GPR30-overexpressing HBUCs and T24 cells, rhEGF is still capable of inducing c-fos, c-jun, or cyclin D1, suggesting that these effects of GPR30 are due to ligand activation. These results suggest that GPR30-mediated inhibition of cell proliferation is associated with blocking of cyclin D1 through the c-fos/c-jun signaling pathway in urothelial cells.
Figure 8.
GPR30 overexpression down-regulates expression of c-fos, c-jun, and cyclin D1. A, HBUCs or T24 cells were transfected with pcDNA3-EGFP or pcDNA3-GPR30 and treated with 10−8 m E2 for up to 4 h. Expression of c-fos or c-jun mRNA was determined by semiquantitative real-time PCR. Results are normalized to S26 ribosomal mRNA expression in the same sample. Data represent the mean ± sem of four different primary cultures or four separate T24 cell preparations. **, P < 0.01, compared with vehicle-treated cells. ##, P < 0.01, GPR30-expressing cells compared with GFP-expressing cells. B, Transfected HBUCs or T24 cells were treated with 10−8 m E2 for up to 24 h. Expression of c-fos, c-jun, or cyclin D1 proteins was determined by immunoblotting using specific antibodies. Blots representative of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations are shown. C, Transfected HBUCs or T24 cells were treated with 50 ng/ml rhEGF for 4 h. Expression of c-fos, c-jun, or cyclin D1 proteins was determined by immunoblotting. Blots representative of four independent experiments using different primary cultures for HBUCs or separate T24 cell preparations are shown. Cont, Control.
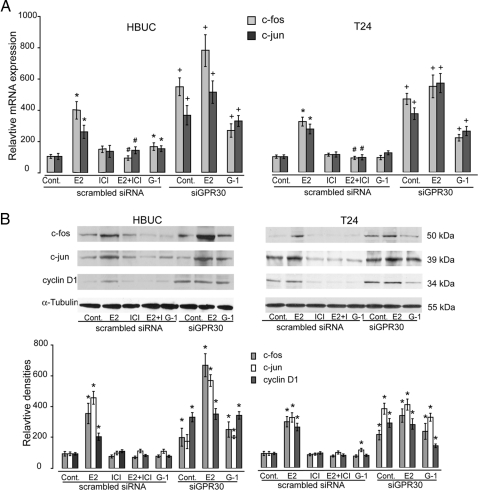
In HBUCs transfected with scrambled siRNA, E2 (10−8 m) significantly increased c-fos and c-jun mRNA expression by approximately 300 and 180%, respectively, within 1 h, and this effect was completely abolished by ICI 182,780. G-1 failed to induce c-fos and c-jun mRNA expression. However, in HBUCs transfected with siRNA against GPR30, c-fos and c-jun mRNA expression was significantly increased in E2-treated cells, as well as control cells. E2 and G-1 also induced significantly higher amounts of c-fos and c-jun in cells transfected with siRNA directly against GPR30 compared with that observed in scrambled siRNA-transfected cells (Fig. 9A, left panel). c-fos, c-jun, and cyclin D1 protein content was significantly increased by E2 within 6 h, and these effects were also completely abolished by ICI 182,780. In HBUCs transfected with siRNA against GPR30, c-fos, c-jun, and cyclin D1 protein was significantly increased in control, E2, or G-1 treated cells (Fig. 9B, left panel). Similar results were observed in T24 cells (Fig. 9, right panels). These results indicate that GPR30 mediates its inhibitory effects on cell proliferation through down-regulation of the c-fos/c-jun-cyclin D1 signaling pathway in urothelial cells.
Figure 9.
siRNA against GPR30 increases c-fos, c-jun, and cyclin D1. A, HBUCs or T24 cells were transfected with scrambled siRNA or siRNA against GPR30 and treated with 10−8 m E2, G-1, 10−7 m ICI 182,780, or a combination of E2 and ICI 182,780 for 4 h. Expression of c-fos or c-jun mRNA was determined by semiquantitative real-time PCR. Results are normalized to S26 ribosomal mRNA expression in the same sample. Data represent the mean ± sem of four separate cDNA preparations. *, P < 0.01, compared with vehicle-treated cells. #, P < 0.01, compared with cells treated with E2 alone. +, P < 0.01, cells treated with siRNA against GPR30 compared with cells treated with scrambled siRNA. B, HBUCs or T24 cells were transfected with scrambled siRNA or siRNA against GPR30 and treated with 10−8 m E2, G-1, 10−7 m ICI 182,780, or a combination of E2 and ICI 182,780 for 16 h. Expression of c-fos, c-jun, or cyclin D1 was determined by immunoblotting using specific antibodies. Blots representative of four separate experiments are shown. The average density of vehicle-treated scrambled siRNA-transfected cells was arbitrarily set at 100%. Data represent the mean ± sem of four separate experiments. *P < 0.01, compared with vehicle-treated scrambled siRNA-transfected cells. Cont, Control.
Discussion
Urothelium is the slowest growing epithelia in mammals under normal conditions. However, when stimulated appropriately, urothelium can undergo tremendous proliferation, particularly during carcinogenesis (24,25). Our previous data demonstrated that estrogen stimulates urothelial cell proliferation through both ERα and ERβ, and increased ERα expression may contribute to dysregulated cell proliferation in bladder cancer cells (20). In the present study, we demonstrated that human urothelial cells express GPR30, a G protein-coupled receptor with high-affinity specific binding to estrogen, and that GPR30 may mediate estrogen-induced inhibition of cell proliferation in these cells. To our knowledge, this is the first evidence correlating membrane-associated estrogen-binding sites that are structurally unrelated to classical ERs with inhibition of cell proliferation.
Investigations of nongenomic effects of estrogen have suggested that classical ERs or ER-like proteins are likely candidates for membrane-associated ERs mediating rapid estrogen actions in a variety of target cells (2). However, evidence supporting the involvement of novel proteins (many of which are associated with G proteins) unrelated to classical ERs in estrogen actions in several cell types also exists (26). Recent reports from two groups clearly link expression of GPR30 to estrogen-induced nongenomic effects (7,8). GPR30 has been widely distributed in neural, breast cancer, placental, heart, ovarian, prostate, hepatic, vascular epithelial, and lymphoid tissues (9). Previously, we found that primary human urothelial cells, immortalized urothelial cell lines, and bladder tumor cell lines all express high levels of GPR30 mRNA (unpublished data). In the present study, we demonstrated that the membrane protein fractions from these cells display high-affinity, saturable, displaceable, and specific binding of E2. These data suggest the presence of membrane-associated ERs in urothelial cells. More straightforward evidence was obtained from the studies with transfected HeLa and COS7 cells that express very low amounts of ERα, ERβ, and GPR30. Untransfected HeLa or COS7 cells showed negligible E2 binding in their membrane fractions. However, plasma membranes of cells transfected with GPR30 displayed specific E2 binding almost identical to that of urothelial cells. The steroid binding characteristics of this recombinant GPR30, like those of the wild-type receptor, fulfill the criteria for its designation as a membrane-associated ER. The Kd for E2 in all these preparations is similar to the affinities of other ERs (8,27).
We further characterized the effect of GPR30 on urothelial cell proliferation using a specific GPR30 agonist, overexpression of GPR30, and specific siRNA against GPR30. Stimulation by the specific GPR30 agonist G-1 and transient expression of GPR30 resulted in marked decreases in E2-induced cell proliferation of primary urothelial cells and bladder cancer cells. Transfection of these cells with specific siRNA against GPR30 enhanced E2-induced cell proliferation of these cells. Interestingly, we also observed that E2 stimulated increased activity of caspase-3/7, the enzymes that are crucial mediators of cellular apoptosis (28). It is not inconceivable that increased cell proliferation is associated with increased apoptosis. As somatic cells proliferate, cell-cycle progression is regulated by positive and negative signals, and a link between apoptosis and proliferation is suggested by studies that have demonstrated the presence of large numbers of dying cells in proliferating cell populations (29). Cell cycle regulators such as p53, RB, and E2F, and dominant oncogenes such as c-Myc have all participated in both progression of cells through the cell cycle and cellular apoptosis (30). Caspase-3/7 activity was not increased by G-1 treatment in the present study, suggesting that E2-induced cellular apoptosis is independent of GPR30 and that the growth inhibitory effect mediated by GPR30 is due to a decreased rate of cell proliferation.
GPR30 has been shown to mediate ERK1/ERK2 activity by regulating the amount of cAMP and through the activation of EGF receptor. Estrogen promoted ERK 1/2 phosphorylation via EGF receptor activation (17) and suppressed ERK 1/2 activation by simultaneously stimulating adenylyl cyclase (16). In our study, GPR30 overexpression did not affect the magnitude of E2-induced ERK 1/2 phosphorylation in urothelial cells. Our previous data showed that cyclin D1 expression during the G1 phase of the cell cycle is critical to urothelial cell proliferation (20). The cyclin D1 gene does not have an estrogen response element but rather has an AP-1 response element (31). The AP-1 transcription factor is formed of two prototypic transcription factors, c-fos and c-jun, and binds to specific sites within the regulatory region of target genes that stimulates cell-cycle progression. Thus, up-regulation of c-fos by extracellular stimuli could represent an early molecular sensor associated with relevant biological responses, including those involved in cell proliferation (32). It has been reported that GPR30 overexpression up-regulates c-fos expression (13,14). However, the concentration of E2 (1 μm) required to achieve this effect in those studies far exceeds physiological concentrations. In the current study, we observed that E2 treatment increased mRNA and protein for both c-fos and c-jun, and that this effect was significantly inhibited by GPR30 overexpression in urothelial cells. E2-induced cyclin D1 expression was also abolished when GPR30 was overexpressed in urothelial cells, suggesting that GPR30 may modulate the proliferative effect of E2 on urothelial cells.
Clinical and laboratory studies support the notion that estrogens are potent mitogens associated with cell proliferation, apoptosis, and progression of endocrine-related cancers (33). Although bladder cancer is considered to be primarily a disease of older, white males and relatively rare in women, the prevalence of bladder cancer in women is comparable to the number of women with cervical and ovarian cancer (34). Females are approximately twice as likely as males to die of the disease and survive 5 yr less than males. Molecular discrimination of classical ER has been proposed to be essential in modulating cell proliferation. However, the results of the present study suggest that GPR30 at least partially contributes to mediating an E2-dependent inhibitory effect on cell proliferation.
In conclusion, our data suggest a novel mechanism underlying estrogen-induced inhibition of cell proliferation in urothelial cells through membrane-associated G protein-coupled receptor signaling. GPR30 may be a target for therapeutic intervention when treating proliferative disorders or for countering the tumorigenic properties of estrogen. Future studies will address questions regarding whether and how GPR30 activation is related to cell cycle arrest. Differential regulation of the GPR30 receptor in various paracrine environments may also explain some of the conflicting effects of estrogen in various tissues. Further characterization of membrane-associated ERs, particularly GPR30, in the urothelium, as well as the subcellular distribution and modes of action in cell proliferation or apoptosis, is required for a better understanding of the complexity of estrogen-elicited signal transduction.
Footnotes
This study was supported by National Institutes of Health Grants R01 DK57258 and R01 DK066349 (to D.E.B.) and R01 CA 116662 (to E.R.P.).
Disclosure Statement: The authors have no potential conflicts of interest to declare.
First Published Online May 8, 2008
Abbreviations: AP-1, Activation protein-1; Bmax, maximal binding capacity; EGF, epidermal growth factor; E2, estradiol; E2H, 1,3,5(10)-estratriene-3,17β-diol 17-hemisuccinate; ER, estrogen receptor; FBS, fetal bovine serum; GFP, green fluorescent protein; E2B, E2-17-hemisuccinate:BSA; HBUC, human bladder urothelial cell; HPRT, hypoxanthine guanine phosphoribosyl transferase; rh, recombinant human; siRNA, small interfering RNA.
References
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA 1998 The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 19:253–286 [DOI] [PubMed] [Google Scholar]
- Song RX, Santen RJ 2006 Membrane initiated estrogen signaling in breast cancer. Biol Reprod 75:9–16 [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME 2002 Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol 90:3F–6F [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F 2000 Identification of a new isoform of the human estrogen receptor-α (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J 19:4688–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly Jr ES, Nethrapalli IS, Tinnikov AA 2002 ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ 1997 Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45:607–617 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ 2008 Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P 2000 Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- Skopelitou A, Hadjiyannakis M, Dimopoulos D, Kamina S, Krikoni O, Alexopoulou V, Rigas C, Agnantis NJ 1997 p53 and c-jun expression in urinary bladder transitional cell carcinoma: correlation with proliferating cell nuclear antigen (PCNA) histological grade and clinical stage. Eur Urol 31:464–471 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M 2006 17β-Estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol Pharmacol 70:1414–1423 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1460 [DOI] [PubMed] [Google Scholar]
- Ahola TM, Manninen T, Alkio N, Ylikomi T 2002 G protein-coupled receptor 30 is critical for a progestin-induced growth inhibition in MCF-7 breast cancer cells. Endocrinology 143:3376–3384 [DOI] [PubMed] [Google Scholar]
- Teng J, Wang ZY, Bjorling DE 2002 Estrogen-induced proliferation of urothelial cells is modulated by nerve growth factor. Am J Physiol Renal Physiol 282:F1075–F1083 [DOI] [PubMed] [Google Scholar]
- Teng J, Wang ZY, Jarrard DF, Bjorling DE 2008 Roles of estrogen receptor α and β in modulating urothelial cell proliferation. Endocr Relat Cancer 15: 351–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M 2007 G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Loomis AK, Thomas P 2000 Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol Reprod 62:995–1004 [DOI] [PubMed] [Google Scholar]
- Walker BE 1960 Renewal of cell populations in the female mouse. Am J Anat 107:95–105 [DOI] [PubMed] [Google Scholar]
- Cohen SM, Ellwein LB 1993 Use of cell proliferation data in modeling urinary bladder carcinogenesis. Environ Health Perspect 101(Suppl 5):111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P 2005 GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–367 [DOI] [PubMed] [Google Scholar]
- Verdier-Sevrain S, Yaar M, Cantatore J, Traish A, Gilchrest BA 2004 Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. FASEB J 18:1252–1254 [DOI] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS 2003 Mechanisms of caspase activation. Curr Opin Cell Biol 15:725–731 [DOI] [PubMed] [Google Scholar]
- Alenzi FQ 2004 Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci 61:99–102 [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC 1992 Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119–128 [DOI] [PubMed] [Google Scholar]
- Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price Jr RH, Pestell RG, Kushner PJ 2002 Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem 277:24353–24360 [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH 2005 Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol 19:362–378 [DOI] [PubMed] [Google Scholar]
- Sutherland RL, Prall OW, Watts CK, Musgrove EA 1998 Estrogen and progestin regulation of cell cycle progression. J Mammary Gland Biol Neoplasia 3:63–72 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ 2006 Cancer statistics, 2006. CA Cancer J Clin 56:106–130 [DOI] [PubMed] [Google Scholar]