Abstract
It is well established that sexually dimorphic neural regions are organized by steroid hormones during development. In many species, neonatal males are exposed to more testosterone than their female littermates, and ultimately it is the estradiol, produced by aromatization of testosterone, that affects sexual differentiation. However, the androgen receptor also plays an important role in the masculinization of brain and behavior. Here we tested the hypothesis that sexually dimorphic social and odor preference behaviors can be differentiated by a nonaromatizable androgen during development by treating female mice on the day of birth (PN0) with dihydrotestosterone (DHT). Control mice received a single vehicle injection on PN0. Adults were gonadectomized, treated with estradiol, and tested for social behaviors. In contrast with control females, females treated on PN0 with DHT, like male controls, exhibited a preference for female-soiled vs. male-soiled bedding, a preference to investigate a female vs. a male and reduced c-Fos-immunoreactivity (ir) in several neural areas after exposure to male-soiled bedding. However, females treated with DHT on PN0 had normal female-typical sexual behavior. The number of calbindin-ir cells in the preoptic area is sexually dimorphic (males more than females), but females given DHT on PN0 had intermediate numbers of calbindin-ir neurons, not significantly different from control males or females. Our data demonstrate that organization of social and olfactory preferences in mice can be affected by perinatal DHT and lends support to the role of androgen receptor in organization of sexual differentiation of brain and behaviors.
GONADAL STEROIDS PLAY a central role in the organization and differentiation of many sexually dimorphic behaviors (1,2). Male rodents are exposed to testosterone (T) during the final few days of gestation and on the day of birth (3,4,5). In humans, male fetuses produce elevated amounts of T during the end of the first trimester, at birth, and male babies experience high T levels for several months after birth (6,7,8). Much of the action of T occurs after its local aromatization to estradiol (E2) (9,10,11,12). Genetically engineered mice with mutations in the estrogen receptor (ER)-α gene (ERαKO) or the aromatase enzyme (ArKO) display many sexual, social, and olfactory behavior deficits (13,14,15,16,17,18,19). Yet male sexual behaviors and social odor preferences are not permanently impaired in ERαKO males and can be displayed after treatment with a dopamine agonist (20). Likewise, preferences for female-soiled bedding are displayed by adult aromatase enzyme knockout (ArKO) male mice after treatment with E2 (5,18,19). Habituation/dishabituation odor discrimination tasks conducted with wild-type (WT) and ArKO mice confirm that ArKO mice can make fine olfactory discriminations, but their motivation to do so may be reduced (21). In food-motivated discrimination tasks, WT males and ArKO mice of both sexes demonstrate the ability to discriminate male from female odorants (17). Taken together the role for E2 in neonatal organization of social olfactory discriminations is unclear.
However, androgen metabolites also act via the androgen receptor (AR), which is expressed in areas that undergo sexual differentiation including the medial amygdala, arcuate nucleus, ventromedial hypothalamus, anteroventral periventricular nucleus, and the bed nucleus of the stria terminalis (22,23,24,25,26). In addition it is well established that AR is essential in the determination of genital morphology and its corresponding innervation (27,28,29). Finally, in olfactory discrimination tasks, male mice with a mutation of their single copy of the AR gene (XTfmY) exhibit female-typical preference behaviors. Unlike WT males they do not preferentially investigate females in a Y maze nor do they prefer to spend time sniffing female-soiled bedding in a three-choice test (30).
Neural regions, which are part of the pheromone detection pathway and associated with social behaviors including the medial preoptic area (mPOA), bed nucleus of the stria terminalis (BNST), and the medial amygdala (31,32,33,34), are sexually dimorphic in mice and rats. In male mice, including ERαKO males, exposure to pheromones from female-soiled bedding elevates fos-immunoreactivity (ir) in mPOA (16). Exposure to male-soiled bedding has no effect on fos-ir in males but enhances fos-ir in the female mouse BNST and mPOA (35). Interestingly, this effect is retained in the mPOA of ArKO female mice (36) as well as the BNST and mPOA of (XTfmY) males (30).
The best-studied sexually dimorphic nucleus in the hypothalamus is the sexually dimorphic nucleus of the mPOA. There are some data to suggest that this nucleus is specifically involved in partner preference in several species (37,38,39,40). Unlike other rodents, many strains of mice (including C57BL/6J) do not exhibit a Nissl-defined sexually dimorphic nucleus of the mPOA (41,42), making it difficult to determine the relationship between early steroid exposure and putative morphological changes in this area. However, a few biomarkers for sexually dimorphic neuronal populations in the mPOA have been described in both mice and rats; one of these is calbindin D28k (43,44).
In this set of experiments, we tested the hypothesis that sexual differentiation of social preference behaviors involves AR activation during development. We also assessed fos-ir responses to pheromones in male-soiled bedding and calbindin-ir cell numbers in adult brains in relation to developmental activation of AR to determine whether morphological and functional changes in relevant hypothalamic areas in this species are also dependent, at least in part, on the action of nonaromatized androgens.
Materials and Methods
Animals
All mice were in a C57BL/6J background strain. Animals used in these studies were weaned at 20–21 d of age, group housed until they underwent gonadectomy (between 50 and 60 d of age), and individually housed afterward for the rest of the experiments. They were kept on 12-h light, 12-h dark cycle (lights off at 1200 h eastern daylight time), with food (mouse/rat sterilizable diet 7012 Harlan Teklad, Madison, WI) and water provided ad libitum. Animal care, testing, and surgery were conducted in accordance with the University of Virginia Animal Care and Use Committee guidelines.
Experiment 1: effect of dihydrotestosterone (DHT) on day of birth (PN0) on adult olfactory preferences
Female pups that received either DHT (100 μg per 0.01 ml sesame oil; Sigma, St. Louis, MO; n = 12) or vehicle (n = 8) on the day of birth (PN0) as well as vehicle-treated males (n = 10) were tested for olfactory preference in adulthood. In each experiment pups in each litter received the same hormone or vehicle treatments. In this experiment, each group was composed of animals from four different litters (in total 12 litters were used). Before the olfactory preference test, the subjects were gonadectomized under general anesthesia (20 mg ketamine and 2 mg/ml xylazine; 0.1 ml per 25 g body weight). At the time of gonadectomy, all mice received a sc E2 implant (5 mm SILASTIC brand silicon tube 1.02 inner diameter × 2.16 outer diameter; Dow Corning, Midland, MI) filled with 17β-estradiol and cholesterol (1:1; Sigma, St. Louis, MO). This procedure was used to equalize levels of circulating hormones in adult males and females. Thus, when behavioral differences were noted, they could be attributed to developmental differences in hormone exposure, independent from differences in hormones at the time of testing.
All mice were tested for olfactory preference as described previously (30). Briefly, one at a time, each mouse was placed in a clear Plexiglas box (18 × 30 cm) with three circular acrylic containers (height 6 cm, diameter 7 cm) placed equidistant from each other and filled with clean bedding for 10 min to habituate to the testing environment. Both habituation and experimental tests took place during the lights-off phase of the light-dark cycle, under red light illumination. After habituation, the mice were exposed simultaneously to three different bedding choices: male-soiled, female-soiled, and clean bedding. Bedding from males was obtained by combining the bedding from four different cages in which sexually experienced males had been individually housed. Bedding from estrous females was obtained from cages housing four gonadectomized females primed with estradiol benzoate (EB; 0.5 μg in 0.05 μl sesame oil) 48 h before the test. The time spent actively sniffing the contents of each container was recorded for a total time of 10 min. Between tests the containers were emptied, cleaned with 10% EtOH, filled with new bedding, and shuffled to ensure the observer was blind to their specific content.
Experiment 2: effect of DHT on PN0 on adult partner preferences
Female pups were treated on PN0 with DHT (same dose as in experiment 1, n = 13) or vehicle (sesame oil, n = 11), and male pups were treated on PN0 with vehicle (n = 10). We used DHT-treated females from three litters, and three other litters were used to generate the female controls; males were taken from seven litters in this study. Before being tested for partner preferences, in adulthood, all subjects were gonadectomized and treated with a sc E2 implant. After recovery from surgery, all subjects received social exposure to both male and female conspecifics during 3 consecutive days (20). The stimulus mice for the partner preference tests were a gonad-intact C57BL/6J adult male and an ovariectomized female bearing an EB implant (sc, 50 μg 17β-estradiol benzoate in 0.03 ml sesame oil in a SILASTIC brand implant 1.96 mm inner diameter × 3.18 mm outer diameter). The stimulus animals were placed at the end of each of the short arms and separated from the rest of the maze by a wire mesh. All tests were performed during the dark phase of the light-dark cycle under red light. The subjects were placed at the end of the long arm at the beginning of each test and allowed to freely explore the maze during 20 min. For each subject, time spent actively exploring the areas on each arm within 10 cm from the wire mesh separating the stimulus animals from the rest of the maze was recorded, including olfactory exploration of the barrier itself; and the maze was carefully cleaned with a 10% EtOH solution between tests.
Experiment 3: effect of DHT on PN0 on adult lordosis behavior
Mice treated neonatally (PN0) with either DHT (100 μg/0.01 ml sesame oil) or vehicle (DHT treated females, n = 11 from four litters; vehicle treated females, n = 9 from three litters; vehicle treated males, n = 6 from three litters) were tested as adults for female sexual behavior. The subjects were gonadectomized, and 1 wk to 10 d later, they were injected sc with EB (0.5 μg per 0.05 ml sesame oil); 48 h later they received a sc injection of progesterone (1 mg per 0.06 ml sesame oil). Behavioral tests started 3–4 h after the progesterone injection, during the lights-off phase of the light-dark cycle and under red light. Sexually experienced C57BL/6J and DBA/2J males were used as testing partners. Each test began with the introduction of the stud male into the home cage of the subject and was terminated after the subject received 20 mounts (defined as both forepaws on the hind region) or an ejaculation from the stud male, whichever occurred first (45). For each individual mount, whether the experimental subject exhibited lordosis was recorded (defined as the position in which all four paws are grounded, the hind region is elevated off the floor, and the back is slightly arched). The lordosis quotient (LQ; lordosis number/number of mounts) was scored for each mouse on each test. Each subject was given a series of six consecutive tests, with 4–6 d between the tests.
Experiment 4: effect of E2 on PN0–2 on adult olfactory preferences
Based on previous work showing that daily E2 treatment on days PN0–2 blocked expression of lordosis behavior in adult females (46), a second cohort of newborns received this same treatment (E2 0.1 μg per 0.01 ml sesame oil; Sigma), resulting in the following three groups: E2-treated females (n = 10 from three litters), vehicle-treated females (n = 7 from four litters), and vehicle-treated males (n = 10 from five litters). As adults, before olfactory preference testing, all subjects were gonadectomized and treated with a sc E2 implant and then tested for olfactory preferences as described in experiment 1.
Experiment 5: Fos responses to male-soiled bedding
A few days after completion of their final behavior tests, subjects were used to assess neural activation of c-Fos in response to soiled bedding. Brains from 14 animals that were used in experiment 1 and all of the animals from experiments 2 and 4 were included in the c-Fos response study (total, n = 48). The number of subjects in each group was: DHT-treated on PN0 females, clean bedding, n = 6; male-soiled bedding, n = 8; vehicle-treated on PN0 females, clean bedding, n = 8; male-soiled bedding, n = 10; vehicle-treated on PN0 males, clean bedding, n = 6; male-soiled bedding, n = 10; E2-treated on PN0–2 females, clean bedding, n = 4; male-soiled bedding, n = 6; vehicle-treated on PN0–2 females, clean bedding, n = 2; male-soiled bedding, n = 5; vehicle-treated on PN0–2 males, clean bedding, n = 4; male-soiled bedding, n = 6.
During the light phase of the light-dark cycle (1000–1200 h), the mice were placed individually into plastic cages containing either clean or male-soiled bedding. In the latter case, the cages had previously housed a single, gonad-intact adult male from the C57BL/6J strain for 4 consecutive days. After 90 min, the experimental subjects were anesthetized using halothane inhalant and killed by decapitation; brains were quickly removed, fixed in 5% acrolein overnight, cryoprotected in 0.2 m Tris-buffered saline containing 30% sucrose, frozen, and sliced in 30-μm coronal sections using a cryostat. Sections were collected in antifreeze and stored at −20 C.
Experiment 6: effect of DHT on PN0 on calbindin expression in the mPOA
After completion of the behavioral protocol for female sexual behavior, subjects from experiment 3 were given no further hormonal treatment for 2 wk, at the end of which they were killed by an overdose of euthasol and perfused with heparinized saline followed by 4% paraformaldehyde. Brains were collected and postfixed for 2 h at 4 C using the same fixative, cryoprotected in 0.2 m Tris-buffered saline containing 30% sucrose, frozen, and sliced in 30-μm coronal sections using a cryostat. Sections were collected in antifreeze and stored at −20 C until processing. All except two of the brains (total, n = 24) were harvested to examine calbindin-ir in the mPOA; the number of experimental subjects per group were: DHT-treated females, n = 10; vehicle-treated females, n = 9; vehicle-treated males, n = 5.
Immunocytochemistry
Sections were processed to identify c-Fos or calbindin D28k-ir neurons, following established methods (30,44). In brief, sections were rinsed, treated with 0.3% H2O2 and 1% NaBH4, and then incubated overnight in c-Fos antiserum (rabbit polyclonal sc-52 1:5000; Santa Cruz Biotechnology, Santa Cruz, CA) or in anticalbindin primary antiserum (rabbit polyclonal D-28K, 1:2500; Chemicon International, Temecula, CA). After incubation in secondary antiserum (biotinylated goat antirabbit IgG, 1:500; Vector Laboratories, Burlingame, CA) followed by a second incubation in avidin-biotin complex (1:1000, Vectastain Elite; Vector Laboratories), ir cells were revealed with a nickel-intensified diaminobenzidine solution (Sigma) activated by 0.1% H2O2.
Image analysis
Sections were mounted on gel-coated glass slides coded to make the observer blind to the sex and treatment of each subject and analyzed with a BX60 microscope (Olympus, Tokyo, Japan) fitted with a Photometrics CoolSNAP charge-coupled device video camera (Photometrics, Tucson, AZ). Sections were identified using the shape of the lateral and third ventricles, anterior commissure, and optic tract as landmarks (47). Counting fos-ir neurons was performed in the mPOA, the medial amygdala (MePD), and both posteromedial and posterointermediate subdivisions of the medial BNST in sections (corresponding to Figs. 30 and 33, respectively, in Ref. 47). In the sections containing calbindin-ir, counting was performed in the upper section of the caudal preoptic area, in the boundary between this region and the anterior hypothalamus (Fig. 32 in Ref. 47). Our image analysis software (MetaMorph version 4.5; Molecular Devices, West Chester, PA) counts immunoreactive cells selected based on an average pixel size. All images were captured at a ×100 magnification. In the case of fos-ir, only the immunoreactive cells within a circular area of 250 μm of diameter were considered; for the mPOA a small region was used (a circular area of 200 μm of diameter). In all cases, the circular counting area was superimposed to the captured image of the relevant section from a randomly selected experimental subject exhibiting robust levels of expression for each particular staining. Once in position, the distances to relevant histological landmarks (third ventricle, anterior commissure, stria medullaris, stria terminalis, and/or internal capsule were used according to the identity of the region) were recorded and used to place the counting in an identical position for every subject.
Statistical analysis
Data from the olfactory preference for soiled bedding tests was analyzed with two consecutive one-way ANOVAs. First, the time spent sniffing the clean bedding was compared across the three groups. Next the time investigating male-soiled bedding was subtracted from the time investigating female-soiled bedding, and this difference score was used in the analysis. In both cases, experimental group was considered the independent factor. Partner preference data were analyzed using two one-way ANOVAs. First, the total time investigating either of the Y-maze arms was compared among the three groups, and then the time spent by each subject investigating the arm containing the stimulus male was subtracted from the time spent with the stimulus female. The resulting variable was analyzed using a second one-way ANOVA with experimental group as the independent factor. Data from the female sexual behavior tests were analyzed using a repeated-measures ANOVA with experimental group and trial number as the independent factors.
Data on c-Fos responses to bedding corresponding to the first cohort of mice that received either DHT or vehicle on PN0 were analyzed with a two-way ANOVA with experimental group and bedding type as the independent factors. Due to the low number of experimental subjects in the second study, with PN0–2-treated mice, data from those exposed to clean bedding (control) were analyzed by a one-way ANOVA with the three groups as the only factor. Because there were no differences in numbers of fos-ir cells among the three groups of animals exposed to clean bedding before brain collection, we pooled all these data into a single group and used this as the composite control. The number of calbindin-expressing neurons in the mPOA was compared using a one-way ANOVA with three experimental groups as the single factor.
Bonferroni’s tests, which correct for errors due to multiple comparisons, were used in all cases for pair-wise planned comparisons.
Results
Experiment 1: DHT treatment on PN0 affects olfactory preference for soiled bedding in adult females
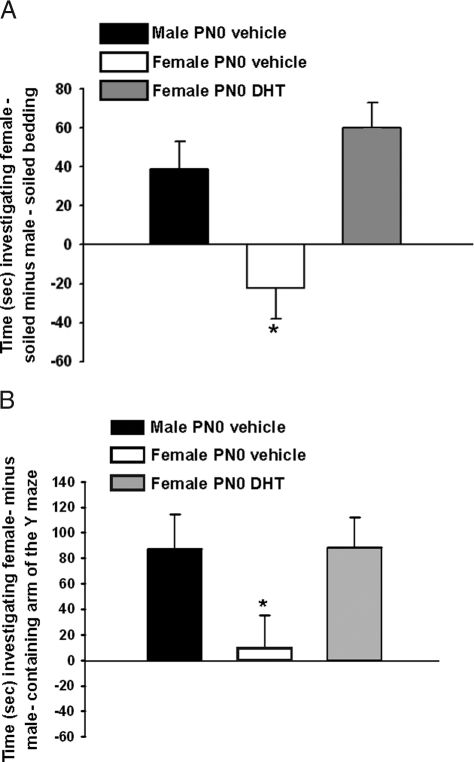
Significant differences between groups were identified in olfactory preference [F(2, 29) = 8.43, P < 0.01]. Both vehicle-treated males and PN0 DHT-treated females exhibited a preference to investigate female-soiled bedding, but vehicle-treated females displayed no preferences (P < 0.05; Fig. 1A). No differences between the groups were detected in the amount of time spent sniffing clean bedding [F(2, 29) = 2.74].
Figure 1.
A, Mean (±sem) time spent sniffing soiled bedding from hormone-primed females minus time spent sniffing male-soiled bedding. Groups included mice treated on PN0 with vehicle, males (n = 10), and females (n = 8), and females treated with DHT (n = 12). At the time of testing, all animals were gonadectomized and treated with an E2 implant to provide equivalent levels of activational hormone. B, Mean (±sem) time in seconds spent investigating the Y maze arm containing a hormone-primed stimulus female, minus time spent in the arm containing a stimulus male. Groups contained mice treated on PN0 with either vehicle (males, n =10; females, n =11) or females treated with DHT (n = 13). At the time of testing, all animals were gonadectomized and treated with an E2 implant to provide equivalent levels of activational hormone. *, Significantly different from both other groups (P < 0.05).
Experiment 2: DHT treatment on PN0 affects partner preference in adult females
We also noted a significant effect of the PN0 treatment on partner preference [F(2, 32) = 5.53, P < 0.01]. Both the DHT-treated females and the vehicle-treated males exhibited a clear preference to spend time in the arm of the Y maze containing the stimulus female, whereas vehicle-treated females showed no preference (P < 0.05; Fig. 1B). The total amount of time spent investigating both of the arms combined was not significantly different between the experimental groups [F(2, 32) = 1.90].
Experiment 3: neonatal treatment with DHT does not impair lordosis behavior
When the values for the LQ were analyzed, a significant effect of the treatment group [F(2, 154) = 12.55, P < 0.001], trial [F(5, 151) = 31.26, P < 0.0001], and a significant interaction between the two factors [F(10, 146) = 3.26, P < 0.001; Fig. 2) were identified. Overall, DHT- and vehicle-treated females displayed significantly higher LQ values than vehicle-treated males, and the values increased over trials in all experimental groups (P < 0.05). Vehicle-treated males exhibited significantly lower LQ values, compared with the other two groups on every trial except the first (P < 0.05). There were no differences between DHT- and vehicle-treated females on any of the six trials.
Figure 2.
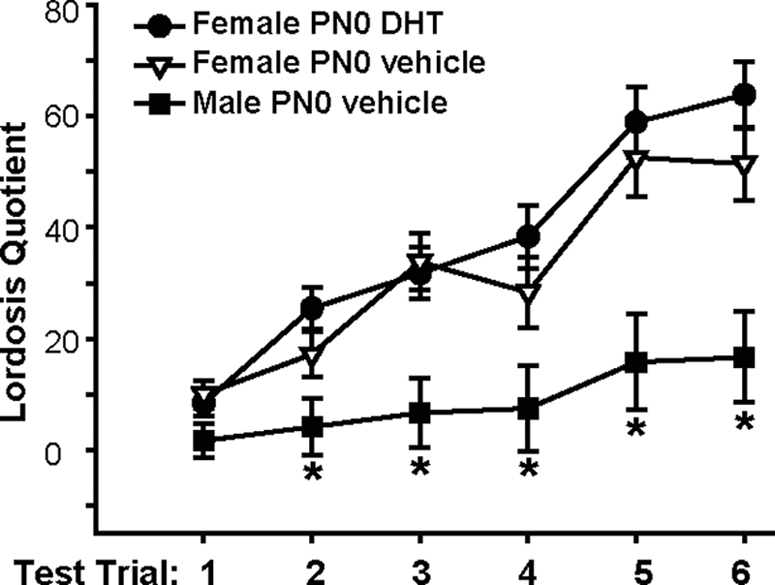
Mean (±sem) LQ exhibited by female mice treated on PN0 with either DHT (•, n = 11) or vehicle (▿, n = 9). Males given vehicle on PN0 were also tested (▪, n = 6). Adults were gonadectomized and after priming with EB and progesterone given a series of six sexual behavior tests. *, Significantly lower lordosis was exhibited by males on trials 2–6, as compared with both groups of females (P < 0.05).
Experiment 4: E2 treatment on PN0–2 has no effect on adult female olfactory preferences
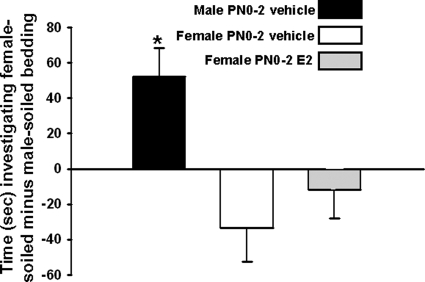
Significant differences between groups were identified in our measures of olfactory preference [F(2, 26) = 6.78; P < 0.01]. Neonatally vehicle-treated males preferred to investigate female-soiled bedding, whereas neither vehicle- nor E2-treated females had an olfactory preference (P < 0.05; Fig. 3). No differences between groups were detected in the amount of time spent sniffing clean bedding [F(2, 26) = 0.84].
Figure 3.
Mean (±sem) time spent sniffing soiled bedding from hormone-primed females minus time spent sniffing male-soiled bedding. Groups were composed of males treated on PN0–2 with vehicle (n = 10), females that received vehicle (n = 7), and females that received E2 on PN0–2 (n = 10). As adults, animals were gonadectomized and treated with an E2 implant to provide equivalent levels of activational hormone. *, Significantly different from the other two groups (P < 0.05).
Experiment 5: neonatal treatment with DHT, but not E2, blocks the c-Fos response to male-soiled bedding in the female forebrain
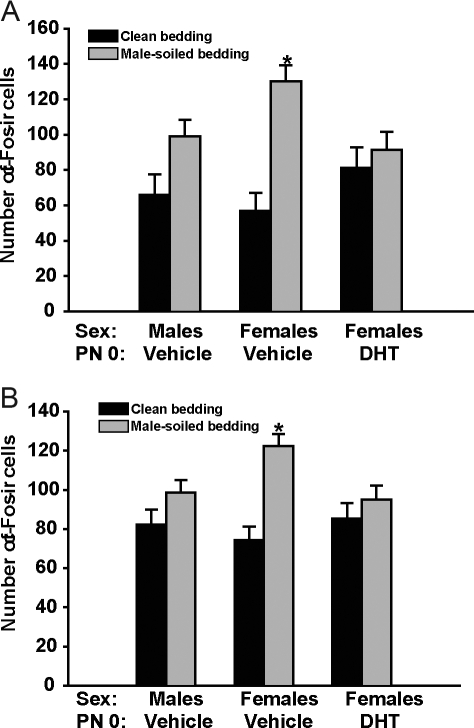
In groups that received treatment with DHT or vehicle on PN0, we noted a significant effect of bedding type [F(1, 47) = 25.52, P < 0.001] and a significant interaction between neonatal treatment and bedding [F(2, 47) = 5.69, P < 0.01] on the number of fos-ir neurons present in the mPOA (Fig. 4A). A similar pattern of response was observed in the BNST, with a significant effect of bedding type [F(1, 45) = 17.11, P < 0.001] and a significant interaction between neonatal treatment and bedding [F(2, 47) = 3.90, P < 0.05; Fig. 4B). In the MePD, we noted an effect of bedding [F(1, 46) = 27.77, P < 0.0001] and neonatal treatment [F(2, 46) = 4.49, P < 0.02) but no interaction [F(2, 46) = 0.80; Table 1]. Post hoc analysis showed that the number of fos-ir neurons in the mPOA and BNST was significantly higher in the neonatally vehicle-treated females exposed to male-soiled bedding, compared with their control group exposed to clean bedding (P < 0.05). No significant differences related to bedding type exposure were identified in the other two experimental groups.
Figure 4.
Mean (±sem) number of Fos-ir cells present in the mPOA (A) and the BNST (B) after exposure to either clean (black histograms) or male-soiled bedding (gray histograms). Males (n = 6 in clean bedding and n = 10 in male-soiled bedding) and females (n = 8 in clean bedding and n = 10 in male-soiled bedding) were treated with vehicle on PN0. Another group of females was treated on PN0 with DHT (n = 6 in clean bedding and n = 8 in male-soiled bedding). All mice were gonadectomized and treated with an E2 implant as adults to provide equivalent levels of activational hormone. *, Significantly different from PN0 vehicle-treated females exposed to clean bedding (P ≤ 0.05).
Table 1.
Mean ±sem (n per group) Fos-ir cells counted in the medial amygdala after mice resided in clean or male-soiled bedding
| Groupsa/bedding typeb | Females injected on PN0 with DHT | Females injected on PN0 with vehicle | Males injected on PN0 with vehicle |
|---|---|---|---|
| Clean | 105.7 ± 20.7 (6) | 165.5 ± 15.0 (8) | 131.5 ± 17.4 (6) |
| Male soiled | 178.7 ± 20.1 (7) | 210.6 ± 6.1 (10) | 214.8 ± 15.4 (9) |
Significant group effect: females with DHT less than females with vehicle, P < 0.05.
Significant bedding effect: animals in clean less than mice in male-soiled bedding, P < 0.05.
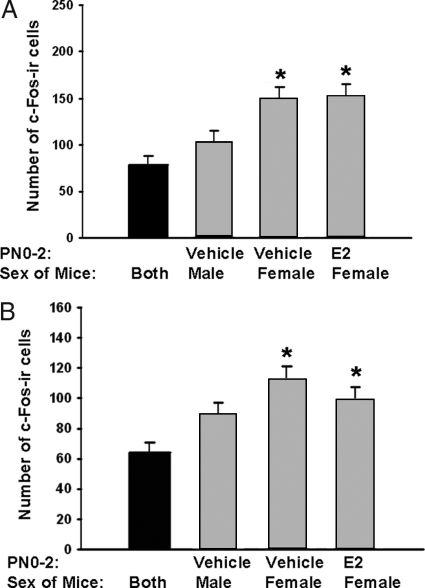
In the other c-Fos study, using adults treated on PN0–2, with E2 or vehicle, we pooled the control groups (exposed to clean bedding before the animals were killed) because of the small number of subjects. First we conducted a one-way ANOVA on the control data by neonatal treatment and sex and found that neither effected numbers of fos-ir cells when animals were placed in clean bedding before brain collection [for the mPOA [F(2, 9) = 0.53] and the BNST [F(2, 9) = 0.30]]. Thus, the data from all mice exposed to clean bedding were pooled into a single group for subsequent comparisons. When the data were analyzed in this manner, we had four groups; mice exposed to clean bedding, neonatally E2-treated female mice exposed to male-soiled bedding, control females, and control males both exposed to male-soiled bedding. Significant differences were identified between the groups in the mPOA [F(3, 26) = 9.90, P < 0.001, Fig. 5A] and BNST [F(3, 26) = 7.76; P < 0.001, Fig. 5B]. Post hoc analysis showed that for both regions, the numbers of fos-ir cells in brains of females treated neonatally with either E2 or vehicle were significantly higher than those counted in brains of mice exposed to clean bedding (P < 0.05).
Figure 5.
Mean (±sem) number of Fos-ir cells present in the mPOA (A) and BNST (B) after exposure to either clean (black histograms) or male-soiled (gray histograms) bedding. Mice were treated on PN0–2 with either E2 or vehicle. The control group (black histograms) exposed to clean bedding had subjects from all three neonatal/sex conditions (n = 10). Adults exposed to male-soiled bedding included three groups: males treated with neonatal vehicle (n = 6), females similarly treated with vehicle (n = 5), and females given E2 from PN0–2 (n = 6). At the time of testing, all adults were gonadectomized and treated with an E2 implant to provide equivalent levels of activational hormone. *, Significantly different from the controls exposed to clean bedding (P < 0.05).
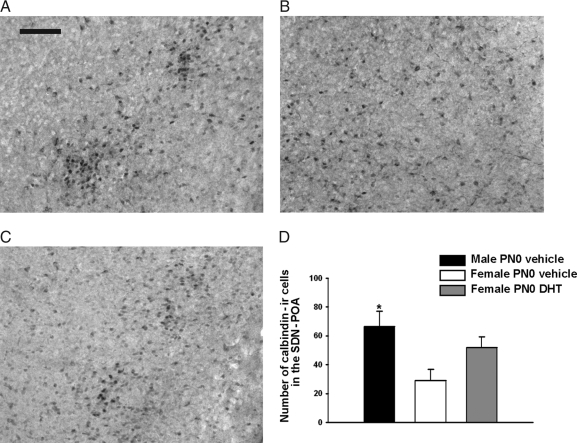
Experiment 6: neonatal DHT increases the number of calbindin-ir neurons in the adult female mPOA
A significant difference in the number of calbindin-ir neurons in the SDN-mPOA was identified [F(2, 23) = 4.51, P < 0.05; Fig. 6]. The expected sex difference was found; PN0 vehicle-treated males had more calbindin-ir cells than the control females (P < 0.05). Cell numbers in brains of PN0 DHT-treated females were intermediate between the control males and females and did not differ significantly from either of those groups.
Figure 6.
A–C, Representative photomicrographs of calbindin D28K-ir cells in adult mouse brains. These cells are observed in the boundary between the anterior preoptic area and the anterior hypothalamus in each experimental group. A, A male treated on PN0 with vehicle. B, An identically treated female. C, The calbindin-ir in a female that received DHT on PN0. Scale bar, 100 μm. D, The mean (±sem) numbers of calbindin-ir cells present in the mPOA. In this study we used brains from five control males, nine brains from vehicle-treated control females, and 10 females treated with DHT on PN0. At the time the animals were killed, all adults were gonadectomized and had been without hormone treatment for at least 2 wk. *, Significantly different from the vehicle-treated female group (P < 0.05).
Discussion
Our results demonstrate that AR activation during neonatal development dramatically affects adult social preference behaviors. Neonatal DHT given on the day of birth inhibits neural c-Fos responses to male-soiled bedding in the mPOA and BNST in adult females. Estradiol administered during the same developmental period, in a regimen that inhibits adult lordosis behavior (46), failed to modify the preference of females for male-soiled bedding, or their pattern of neural activation in the mPOA and BNST in response to male soiled-bedding. In addition, a single injection of DHT on PN0 increased numbers of calbindin-ir neurons in the female mPOA. Whereas this DHT treatment did not completely masculinize the numbers of calbindin-ir, neurons in the mPOA-treated females were not significantly different from either male or female controls. These results suggest that androgens, acting specifically through the AR during a precise perinatal period, provide a signal that triggers the sexual differentiation of this set of traits in mice. Follow-up studies with small interfering RNA for AR and/or administration of specific androgen antagonists are needed to clarify the role of AR.
In contrast to the effects on olfactory and partner preferences, neonatally DHT-treated females exhibited normal sexual receptivity after appropriate hormonal priming. This is in agreement with data from Tfm (XTfmY) rats (46,48,49,50) and Tfm and ARKO mice (51,52), showing that males with an AR mutation do not exhibit female-like receptivity including lordosis. Differentiation of lordosis behavior is not influenced by neonatal AR activation, but instead androgens act to inhibit adult lordosis in males after aromatization, selectively at the ERβ (45,46). As first articulated more than 25 yr ago (53), neural circuits regulating different aspects of reproductive behavior may be differentially affected by different gonadal steroids acting on different receptors. In mice, we hypothesize that neonatal concentrations of T normally present in the developing male activates AR directly and leads to the differentiation of partner and olfactory preferences and c-Fos responses to conspecifics, whereas at the same time, after aromatization to E2, ERβ activation blocks differentiation of the adult lordosis response. Recently it has been shown that a DHT metabolite, 3β-diol, can bind to ERβ (54). However, because ERβKO males have normal olfactory preferences (45), we believe it is unlikely that DHT affects the behaviors measured here via the ERβ.
The differentiation of the neural pathways that underlie sexually dimorphic behaviors has been studied primarily in rats (55,56,57,58,59). Importantly, discovery of proteins and pathways downstream from the steroid receptors is underway. For example, in rats prostaglandin-E2 is downstream of the E2 signal that masculinizes adult sexual behaviors but does not play a role in defeminization (57). The kisspeptin/G protein-coupled receptor (GPR)-54 system is essential for puberty in humans, and in rodents it is sexually dimorphic (60,61,62,63,64). Adult knockout male and female GPR54 mice receiving appropriate hormone treatments can express sex-typical mating behaviors. However, male GPR54KO mice do not exhibit normal social preferences for female partners in a choice test, and in addition, several sexually dimorphic brain structures are more female-like than male-like (62). Thus, in mice GPR54 may act downstream of AR or ERα to affect sexual differentiation.
Although Tfm mice and rats, and more recently AR knockout mice, have been useful for the identification of specific roles of the AR in sexual differentiation (22,23,52,65,66,67), the model has some limitations that need to be considered. Specifically, adult Tfm rats exhibit reduced levels of aromatase activity in the hypothalamus (9,68), which is consistent with the evidence linking androgens with the regulation of aromatase expression and activity in rodents (69,70,71,72,73). In addition, AR affects the release of gonadotropins in response to steroid feedback (74,75,76,77,78), and thus, a nonfunctional AR could affect the amount of T the brain is exposed to during the neonatal period. In the ARKO mouse, T levels on the day after birth are not different between WT males and those with the AR knockout (52); however, this sampling time point is past the critical period in mice (3). In androgen-insensitive human infants, the postnatal surge of gonadotropins and elevation of testosterone normally observed during the first 3 months of life are compromised (7). Thus, an AR mutation may impact the prenatal T surge as well as the aromatization of androgens to estrogens. Our present data confirm and extend findings from Tfm mice (30) and strongly support the hypothesis that AR activation, during development, normally contributes to the sexual differentiation of these traits.
Much of the data supporting a role for steroid hormones in the sexual differentiation of the central nervous system have been from studies of morphological differences (22,24,25,26,79). The mPOA has been particularly well studied and contains one of the first sex differences established in brain (80). This hypothalamic area has also been repeatedly associated with the ability of the individual to exhibit normal mating behavior (37,38,81,82,83). Interestingly, the display of normal partner social preference in both male rats and ferrets has been shown to be affected by lesions in the mPOA (33,34,38,84). Calbindin-ir in the mPOA is significantly more abundant in males than females, and Tfm male mice have an intermediate number of calbindin-ir neurons (44). In rats, studies using manipulation of neonatal hormones suggest that the sexual dimorphism is ER dependent because treatment with DHT alone had no effect on calbindin-ir in females, whereas both T and E2 produced an increase in calbindin-ir cells (43). The results presented here are in agreement with data from Tfm mice; AR appears to play a role in the sexual differentiation of this population of cells in mice, and thus, it is possible that AR-dependent reorganization of the neural circuits in this area may be required for the display of male-typical olfactory and partner preferences. However, DHT treatment in this study did not completely masculinize the female calbindin neurons in the mPOA. The reasons for this might be methodological; the dose and/or timing of the DHT may not have been optimal. Or, more interestingly, the calbindin-ir neurons observed in the area may represent several subpopulations that are responsive to different hormonal signals during development.
In summary, our results demonstrate that nonaromatized androgens administered neonatally are capable of masculinizing several sexually dimorphic behaviors that involve processing of olfactory cues derived from conspecifics in mice. On the other hand, PN0 DHT-treated female mice exhibit normal behavioral receptivity, which confirms previous studies specifically pointing to an exclusive role of aromatized derivates of testosterone in the differentiation of that trait. The results nevertheless suggest that in mice sex differences are driven by both estrogens and AR activation.
Acknowledgments
We thank Aileen Wills for excellent technical assistance and Dr. Jin Ho Park for comments on the manuscript and assistance with the figures.
Footnotes
This work was supported by National Institutes of Health Grant R01 MH57759.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: AR, Androgen receptor; ArKO, aromatase enzyme knockout; BNST, bed nucleus of the stria terminalis; DHT, dihydrotestosterone; E2, estradiol; EB, estradiol benzoate; ER, estrogen receptor; ERαKO, genetically engineered mice with mutations in the ERα gene; GPR, G protein-coupled receptor; ir, immunoreactivity; LQ, lordosis quotient; mPOA, medial preoptic area; PN0, day of birth; T, testosterone; WT, wild type.
References
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Celotti F, Motta M 2004 Sexual differentiation of the brain: role of testosterone and its active metabolites. J Endocrinol Invest 27:120–127 [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J 1992 The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131 [DOI] [PubMed] [Google Scholar]
- Vomachka AJ, Lisk RD 1986 Androgen and estradiol levels in plasma and amniotic fluid of late gestational male and female hamsters: uterine position effects. Horm Behav 20:181–193 [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, Groeneveld JO, Post PE, Ooms MP 1983 Concentrations of testosterone and androsterone in peripheral and umbilical venous plasma of fetal rats. J Reprod Fertil 68:171–175 [DOI] [PubMed] [Google Scholar]
- Corbier P, Dehennin L, Castanier M, Mebazaa A, Edwards DA, Roffi J 1990 Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab 71:1344–1348 [DOI] [PubMed] [Google Scholar]
- Bouvattier C, Carel JC, Lecointre C, David A, Sultan C, Bertrand AM, Morel Y, Chaussain JL 2002 Postnatal changes of T, LH, and FSH in 46,XY infants with mutations in the AR gene. J Clin Endocrinol Metab 87:29–32 [DOI] [PubMed] [Google Scholar]
- Prince FP 2001 The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol 168:213–216 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA 1987 Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology 121:2205–2210 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Schlenker EH, Pfaff DW 1993 Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology 133:433–439 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC 1977 Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav 9:249–263 [DOI] [PubMed] [Google Scholar]
- Baum MJ 2003 Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand J Psychol 44:213–220 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithes O, Korach KS, Pfaff DW 2000 Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO). Proc Natl Acad Sci USA 97:14737–14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB 1997 Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 31:232–243 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ 1997 Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor α gene. Horm Behav 32:176–183 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF 2000 Oestrogen receptor α is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J Neuroendocrinol 12:103–110 [DOI] [PubMed] [Google Scholar]
- Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J 2006 Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Horm Behav 49:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J 2002 Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav 42:158–171 [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J 2004 Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav 46:1–10 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF 2000 Dopamine activates masculine sexual behavior independent of the estrogen receptor α. J Neurosci 20:4248–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierman S, Tirelli E, Douhard Q, Baum MJ, Bakker J 2006 Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behav Brain Res 174:64–69 [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM 2007 Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav 51:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Morris JA, Breedlove SM, Jordan CL 2007 Effects of the testicular feminization mutation (tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett 419:168–171 [DOI] [PubMed] [Google Scholar]
- Ciofi P, Lapirot OC, Tramu G 2007 An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience 146:630–642 [DOI] [PubMed] [Google Scholar]
- Lund TD, Salyer DL, Fleming DE, Lephart ED 2000 Pre- or postnatal testosterone and flutamide effects on sexually dimorphic nuclei of the rat hypothalamus. Brain Res Dev Brain Res 120:261–266 [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM 2005 Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol 487:217–226 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1981 Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res 225:297–307 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP 1982 Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res 237:173–181 [DOI] [PubMed] [Google Scholar]
- Grisham W, Casto JM, Kashon ML, Ward IL, Ward OB 1992 Prenatal flutamide alters sexually dimorphic nuclei in the spinal cord of male rats. Brain Res 578:69–74 [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF 2007 Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci 25:2182–2190 [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB 1980 Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science 210:557–560 [DOI] [PubMed] [Google Scholar]
- Lehman MN, Powers JB, Winans SS 1983 Stria terminalis lesions alter the temporal pattern of copulatory behavior in the male golden hamster. Behav Brain Res 8:109–128 [DOI] [PubMed] [Google Scholar]
- Paredes RG, Tzschentke T, Nakach N 1998 Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res 813:1–8 [DOI] [PubMed] [Google Scholar]
- Cherry JA, Baum MJ 1990 Effects of lesions of a sexually dimorphic nucleus in the preoptic/anterior hypothalamic area on the expression of androgen- and estrogen-dependent sexual behaviors in male ferrets. Brain Res 522:191–203 [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ 1999 Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol 39:249–263 [PubMed] [Google Scholar]
- Lau YE, Cherry JA, Baum MJ, Mani SK 2003 Induction of Fos in the accessory olfactory system by male odors persists in female mice with a null mutation of the aromatase (cyp19) gene. Brain Res Bull 60:143–150 [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK 1994 SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav 56:535–541 [DOI] [PubMed] [Google Scholar]
- Kindon HA, Baum MJ, Paredes RJ 1996 Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm Behav 30:514–527 [DOI] [PubMed] [Google Scholar]
- Baum MJ 2006 Mammalian animal models of psychosexual differentiation: when is ‘translation’ to the human situation possible? Horm Behav 50:579–588 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Schrunk JM, Stormshak F 2004 Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav 83:233–245 [DOI] [PubMed] [Google Scholar]
- Henderson RG, Brown AE, Tobet SA 1999 Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol 41:252–266 [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA 2005 Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res 157:34–41 [DOI] [PubMed] [Google Scholar]
- Sickel MJ, McCarthy MM 2000 Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol 12:397–402 [DOI] [PubMed] [Google Scholar]
- Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S 2007 Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol 67:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF 2005 A previously uncharacterized role for estrogen receptor β: defeminization of male brain and behavior. Proc Natl Acad Sci USA 102:4608–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF 2006 Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience 138:921–928 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G 1997 The mouse brain in stereotaxic coordinates. New York: Academic Press [Google Scholar]
- Olsen KL 1979 Androgen-insensitive rats are defeminised by their testes. Nature 279:238–239 [DOI] [PubMed] [Google Scholar]
- Olsen KL, Whalen RE 1981 Hormonal control of the development of sexual behavior in androgen-insensitive (tfm) rats. Physiol Behav 27:883–886 [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Goldman AS, Steinbeck HF, Neumann F 1976 Is feminine differentiation of the brain hormonally determined? Experientia 32:650–651 [DOI] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV 1974 TfM mutation and masculinization versus feminization of the mouse central nervous system. Cell 3:235–242 [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S 2004 Brain masculinization requires androgen receptor function. Proc Natl Acad Sci USA 101:1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RE 1974 Gonadal hormones and the developing brain. Adv Behav Biol 8:67–79 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L 2007 An alternate pathway for androgen regulation of brain function: activation of estrogen receptor β by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM 2007 Glutamate AMPA/kainate receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol 67:304–315 [DOI] [PubMed] [Google Scholar]
- Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM 2007 Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology 148:3391–3401 [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM 2005 Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav 48:512–521 [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM 2007 Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol 19:302–308 [DOI] [PubMed] [Google Scholar]
- Davis AM, Ward SC, Selmanoff M, Herbison AE, McCarthy MM 1999 Developmental sex differences in amino acid neurotransmitter levels in hypothalamic and limbic areas of rat brain. Neuroscience 90:1471–1482 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Kuohung W 2005 KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty. Endocrine 26:277–284 [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M 2006 GPR54 and kisspeptin in reproduction. Hum Reprod Update 12:631–639 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF 2007 The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1983 Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci 3:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Pellis SM, Watson NV 2006 Play fighting in androgen-insensitive tfm rats: evidence that androgen receptors are necessary for the development of adult playful attack and defense. Dev Psychobiol 48:111–120 [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV 2005 Spatial memory performance in androgen insensitive male rats. Physiol Behav 85:135–141 [DOI] [PubMed] [Google Scholar]
- Rosenfeld JM, Daley JD, Ohno S, YoungLai EV 1977 Central aromatization of testosterone in testicular feminized mice. Experientia 33:1392–1393 [DOI] [PubMed] [Google Scholar]
- Paden CM, Roselli CE 1987 Modulation of aromatase activity by testosterone in transplants of fetal rat hypothalamus-preoptic area. Dev Brain Res 33:127–133 [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA 1997 Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol 61:365–374 [PubMed] [Google Scholar]
- Roselli CE, Klosterman SA 1998 Sexual differentiation of aromatase activity in the rat brain: effects of perinatal steroid exposure. Endocrinology 139:3193–3201 [DOI] [PubMed] [Google Scholar]
- Connolly PB, Roselli CE, Resko JA 1990 Aromatase activity in adult guinea pig brain is androgen dependent. Biol Reprod 43:698–703 [DOI] [PubMed] [Google Scholar]
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE 1994 Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135:395–401 [DOI] [PubMed] [Google Scholar]
- Connolly PB, Resko JA 1994 Prenatal testosterone differentiates brain regions controlling gonadotropin release in guinea pigs. Biol Reprod 51:125–130 [DOI] [PubMed] [Google Scholar]
- Masek KS, Wood RI, Foster DL 1999 Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology 140:3459–3466 [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Foster DL, Padmanabhan V 2002 Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav 31:35–41 [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Grindrod JA, Taylor JA, Unsworth WP 2003 Sexually differentiated regulation of GnRH release by gonadal steroid hormones in sheep. Reprod Suppl 61:299–310 [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Koizumi K, Ohta Y, Hashi M, Fujii Y, Ohbo N, Saika O, Szuki H, Saito K, Suzuki K 2005 Evaluation of general behavior, memory, learning performance, and brain sexual differentiation in F1 offspring males of rats treated with flutamide during late gestation. J Toxicol Sci 30:249–259 [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM 1978 Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res 148:333–346 [DOI] [PubMed] [Google Scholar]
- Anderson RH, Fleming DE, Rhees RW, Kinghorn E 1986 Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res 370:1–10 [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Wallen K, Edwards DA 1986 Preoptic lesions increase the display of lordosis by male rats. Brain Res 370:21–28 [DOI] [PubMed] [Google Scholar]
- Rhees RW, Al-Saleh HN, Kinghorn EW, Fleming DE, Lephart ED 1999 Relationship between sexual behavior and sexually dimorphic structures in the anterior hypothalamus in control and prenatally stressed male rats. Brain Res Bull 50:193–199 [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Waters P, Zhou H, Baum MJ 2007 Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav 90:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]