Abstract
The effects of peripheral glucagon like peptide-1 receptor (GLP-1R) stimulation on feeding, gastric emptying, and energetic responses involve vagal transmission and central nervous system processing. Despite a lack of studies aimed at determining which central nervous system regions are critical for the GLP-1R response production, hypothalamic/forebrain processing is regarded as essential for these effects. Here the contribution of the caudal brainstem to the control of food intake, core temperature, heart rate, and gastric emptying responses generated by peripheral delivery of the GLP-1R agonist, exendin-4 (Ex-4), was assessed by comparing responses of chronic supracollicular decerebrate (CD) rats to those of pair-fed intact control rats. Responses driven by hindbrain intracerebroventricular (fourth icv) delivery of Ex-4 were also evaluated. Intraperitoneal Ex-4 (1.2 and 3.0 μg/kg) suppressed glucose intake in both CD rats (5.0 ± 1.2 and 4.4 ± 1.1 ml ingested) and controls (9.4 ± 1.5 and 7.7 ± 0.8 ml ingested), compared with intakes after vehicle injections (13.1 ± 2.5 and 13.2 ± 1.7 ml ingested, respectively). Hindbrain ventricular Ex-4 (0.3 μg) also suppressed food intake in CD rats (4.7 ± 0.6 ml ingested) and controls (11.0 ± 2.9 ml ingested), compared with vehicle intakes (9.3 ± 2.1 and 19.3 ± 4.3 ml ingested, respectively). Intraperitoneal Ex-4 (0.12, 1.2, 2.4 μg/kg) reduced gastric emptying rates in a dose-related manner similarly for both CD and control rats. Hypothermia followed ip and fourth icv Ex-4 in awake, behaving controls (0.6 and 1.0 C average suppression) and CD rats (1.5 and 2.5 C average suppression). Intraperitoneal Ex-4 triggered tachycardia in both control and CD rats. Results demonstrate that caudal brainstem processing is sufficient for mediating the suppression of intake, core temperature, and gastric emptying rates as well as tachycardia triggered by peripheral GLP-1R activation and also hindbrain-delivered ligand. Contrary to the literature, hypothalamic/forebrain processing and forebrain-caudal brainstem communication is not required for the observed responses.
GLUCAGON-LIKE-PEPTIDE 1 (GLP-1), released from L cells in the distal small intestine in response to nutrient entry into the gastrointestinal tract (1,2), is suggested to act on peripheral GLP-1 receptors (GLP-1R) (3,4). Peripheral GLP-1R ligand administration engages a set of physiological responses that include inhibition of gastric emptying (4,5), tachycardia (6,7,8,9,10), stimulation of glucose-dependent insulin secretion (11), and reduced food consumption (3,5,12,13). GLP-1 is also supplied by neurons of the caudal brainstem that project to GLP-1Rs distributed throughout the brain (14,15,16). It is interesting to note that stimulation of central GLP-1Rs results in many of the same responses, e.g. inhibition of food intake and increased insulin secretion, as are observed after peripheral ligand injection (17,18,19).
The effects of peripheral GLP-1R stimulation on feeding, gastric emptying, and energetic responses involve behavioral, sympathetic, and parasympathetic effector pathways that are downstream of central nervous system (CNS) processing (6,7,8,10,20). Vagal afferent transmission is suggested for the feeding and gastric emptying inhibition triggered by peripheral GLP-1R ligand injections because these responses have been reported to be blocked by vagotomy or vagal afferent damage with capsaicin treatment (3,4,21). Structures in the ascending visceral afferent pathway that includes nuclei of the caudal brainstem [nucleus tractus solitarius (NTS); parabrachial nucleus (PBN)], hypothalamus [lateral hypothalamus (LH); paraventricular nucleus (PVN)], and basal forebrain [bed nucleus of stria terminalis; central nucleus of the amygdala (CeA)] (22,23) may thereby play a role in mediating responses triggered by peripheral GLP-1R agonist treatment. Central GLP-1R ligand administration activates neurons (Fos-LI) in many of these same structures that show GLP-1-binding (15) and/or express GLP-1R mRNA (16). Moreover, many of the aforementioned nuclei in the visceral afferent pathway project to other GLP-1-binding and/or GLP-1R expressing structures involved in energy balance control [area postrema (AP); ventral tegmental area; arcuate nucleus (ARC); medial preoptic area] (see Refs. 15,16). A determination of which of these implicated structures are necessary for peripheral GLP-1-mediated response production has not been made. Nonetheless, it is often asserted that hypothalamic/forebrain processing is critical for mediating the effects of peripheral GLP-1R stimulation as well as central agonist delivery (3,17,18,24,25,26).
Experiments are described here that use complete supracollicular transection of the neuraxis to eliminate both the ascending forebrain projecting limb of the ascending visceral afferent pathway and the descending projections from the hypothalamus and basal forebrain to hindbrain and thereby block forebrain-caudal brainstem communication. This strategy is used to directly investigate the importance of hypothalamic/forebrain and caudal brainstem processing in the mediation of behavioral, sympathetic, and parasympathetic responses generated by peripheral GLP-1R agonist treatment and, in separate experiments, by hindbrain-delivered GLP-1R ligand.
Materials and Methods
Subjects and materials
Adult male Sprague Dawley rats (275–300 g; Charles River, Wilmington, MA) were housed in individual cages in a room maintained at 23 C with lights on (0800 h) for 12 h each day. Initially, all rats had ad libitum access to rodent chow (Rodent Chow 5001; Purina, St. Louis, MO) and water for 1 wk of adaptation. They were then tube fed a suspendable AIN 76A rodent diet (L1001, Research Diets, New Brunswick, NJ) during the light cycle in four evenly spaced meals. The volume of tube-fed meals was 9 ml, delivering a total of 79 kcal/d, which results in weight gain and provides adequate hydration (27). Rats were maintained on this feeding paradigm except as noted below during experimental testing.
The selective agonist to the GLP-1 receptor, exendin-4 (Ex-4; American Peptide Co., Sunnyvale, CA), a modified peptidase-resistant GLP-1 analog isolated from Heloderma suspectum venom, was selected as the ligand of choice to examine GLP-1R-mediated control of intraoral intake, gastric emptying, and energy expenditure because it is used both experimentally and clinically in the treatment of diabetes mellitus and obesity due to increased half-life (28,29,30,31,32).
Supracollicular decerebration, fourth intracerebroventricular (icv) cannulation, intraoral cannulation, and telemetric transponder surgery
For two separate sets of rats (n = 13–17/set), body weights were recorded for 1 wk, and then within each group, the animals were divided into two weight-matched neurological groups: controls (n = 7–8) and chronic supracollicular decerebrate (CD; n = 6–9). Supracollicular decerebration was performed in two hemitransection stages separated by at least 1 wk, as previously described (33). During the second hemitransection (or control anesthetization) surgery, control and CD rats were implanted with fourth icv cannula and either a telemetric transponder (HRC-4000, VitalView; Mini-Mitter, Bend, OR; described below) or intraoral cannula (PE-100) as previously described (34). Chronic indwelling fourth icv guide cannula (Plastics One, Roanoke, VA; 26 gauge) were implanted 2.0 mm above the fourth cerebral ventricle, under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam 2 mg/kg) at the following coordinates: 2.5 mm anterior to occipital suture, 4.5 mm ventral to dura, and on the midline. The cannula was cemented to four jeweler’s screws attached to the skull and closed with an obturator. For rats receiving transponders, a small midline abdominal incision was made below the diaphragm. The transponder was inserted into the abdominal cavity, with the leads tunneled under the skin and secured to the chest muscles with silk sutures. For animals receiving intraoral cannula, the cannula was placed just lateral to the first maxillary molar and led to emerge at the top of the head. CD rats do not eat or drink spontaneously, and therefore, an intraoral feeding paradigm was used to measure meal size in CD and control rats (for validity of method, see review in Ref. 35). The intended anatomical position of the fourth icv injection was evaluated 1 wk after recovery from surgery by measurement of the sympathetically mediated increase in plasma glucose 60 min after icv injection of 210 μg 5-thio-d-glucose in 2 μl of artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) (36). The presence of the response, at least a doubling of plasma glucose level after this treatment, confirmed cannula placement and served as an inclusion criteria for the experiments. After the second surgery, the rectal temperatures of CD rats were recorded at each tube feeding because the temperature of these rats is somewhat labile. When noted perturbations in core temperature (Tc) did not occur during experimental testing, adjustments were made by warming or cooling the epidermis. The completeness of the intended transection was verified histologically in all cases by sectioning the brain sagittally (40 μm) via microtome, staining the sections with cresyl violet, and confirming the completeness of the transection with light microscopic examination.
Comparative effects of peripheral and caudal brainstem delivered Ex-4 on intake in chronic decerebrate and neurologically intact rats
Chronic decerebrate rats do not forage, and therefore, assessment of their intake responses require the use of a method in which food is directly delivered to the oral cavity (37). Intraoral intake tests followed 2 wk of intraoral infusion habituation training. For both habituation and the experimental days, groups of rats were run, approximately 2 h after tube feeding, in individual hanging cages (18 × 25 × 36 cm). Five minutes before the start of intraoral nutrient infusions, rats received either an ip or fourth icv injection in a counterbalanced design. Fourth icv injections consisted of either Ex-4 (0.3 μg/μl) or aCSF, and ip injections consisted of either Ex-4 (1.2 or 3.0 μg/kg) or saline (dose selection based on Refs. 20,38). Ten percent glucose was infused orally at a rate of 0.8 ml/min with an infusion pump (Pump 44; Harvard Apparatus); that rate is within the ingestion rate of ad libitum-ingesting rats (39). Each rat’s infusion line was led through a computer-controlled miniature three-way solenoid valve either to a waste dish or directly into the oral cavity of the animal. Rats consumed the infused glucose solution until fluid was observed to drip from the mouth, at which time the infusion was stopped for 30 sec. The intake test was terminated and amount consumed calculated when a second rejection event was observed within the 60 sec of reinstated delivery.
Comparative effects of peripheral Ex-4 on liquid gastric emptying rates in chronic decerebrate and neurologically intact rats
After an overnight fast (15 h after last tube feeding), a separate group of CD (n = 9) and control (n = 8) rats received an ip injection containing either 0.9% NaCl or Ex-4 (0.12, 1.2, or 2.4 μg/kg; dose selection based on Ref. 21). Five minutes after drug administration, 5 ml of 0.9% NaCl containing 0.006% phenol-red was instilled into the rat’s stomach via an orally inserted 8-Fr polyethylene intragastric tube. Rats were immediately returned to their home cage. After a 5-min emptying period, the tube was reinserted into the stomach and the remaining gastric contents were withdrawn. The stomach was rinsed repeatedly with 0.9% NaCl until the withdrawn samples were void of any visible phenol indicator. Collected volume was measured, and the gastric contents were centrifuged at 3200 rpm for 10 min to remove any particulate matter. Gastric emptying was measured by dye-dilution spectrophotometry from absorption at 550 nm. Briefly, a 1.0-ml sample from the centrifuged gastric contents was buffered with 24.5 ml of 0.014 m Na3PO4.12H2O. The spectrophotometric absorbance of each buffered sample was compared with that of a 1.0-ml buffered sample from the originally instilled phenol red solution to determine the volume of the original test load remaining in the stomach at the end of the emptying period. Each drug treatment was bracketed by a control condition. These experimental techniques have been detailed previously (40,41,42).
Effects of peripheral and caudal brainstem delivered Ex-4 on energetic responses in chronic decerebrate and neurologically intact control rats
On experimental testing days of energetic response recording, rats previously used in the gastric emptying experiment received tube feedings before (before 0800 h) and after (after 1800 h) data collection. A minimum of 2 d separated each experimental day.
At 1100 h on testing days, rats received one of the three ip/fourth icv injection conditions that included: 1) ip Ex-4 (3 μg/kg) with fourth icv vehicle (1 μl of aCSF); 2) ip vehicle (0.9% NaCl) with fourth icv Ex-4 (0.3 μg/1 μl); 3) or combined ip/fourth icv vehicle injections (dose selection based on Ref. 7). All conditions were counterbalanced. Heart rate (HR), Tc, and spontaneous activity were recorded telemetrically beginning 1 h before injections and for a 7-h period after injections. HR was recorded every 30 sec; Tc and activity was recorded every 5 min. Spontaneous activity was recorded as cumulative spontaneous activity counts on an X-Y axis every 5 min (change in X-Y position is equal to 1 count). Data were collected in the rats’ home cages.
Data and statistical analyses
Data for each respective study were analyzed separately and expressed as mean ± sem. Intake data were analyzed separately for ip and fourth icv injections and were analyzed by two-way ANOVA, with drug treatment and neurological condition of the rat as the main variables. Gastric emptying data are expressed as the percentage of the 5-ml 0.9% NaCl load emptied after 5 min and were analyzed by two-way ANOVA, with drug treatment and neurological condition of the rat as the main variables. Data for heart rate, core temperature, and spontaneous activity reflect a 6.5 h average of collected data (after an initial 30 min postinjection recovery period) and were analyzed separately by two-way ANOVA with drug treatment and neurological condition as the main variables to examine differences between surgical groups and by one-way ANOVA to examine for any drug treatment effects within each surgical group. CD rats with an incomplete transection were removed from statistical analysis. Additionally, statistical outliers in the energy expenditure experiments were removed from further analysis. For all experiments, comparisons between treatment means were analyzed by post hoc pair-wise comparisons and Tukey’s honestly significant difference test with P < 0.05 considered statistically significant. Analyses were made using PC-SAS (version 8.02; SAS Institute, Cary, NC) mixed procedure and Statistica software (StatSoft, Tulsa, OK).
Results
Intake responses to peripheral and caudal brainstem delivered injection of Ex-4 in chronic decerebrate and neurologically intact control rats
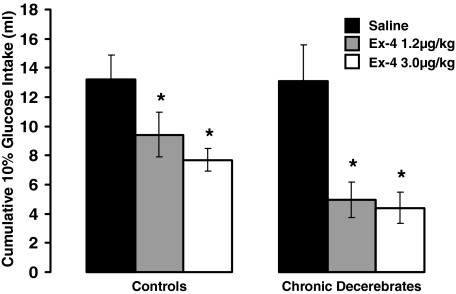
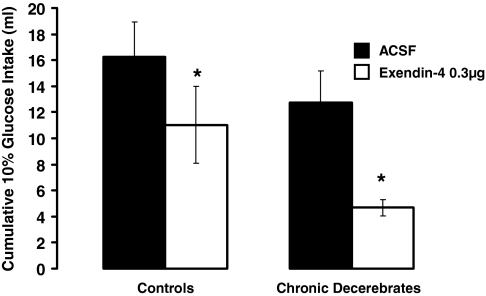
Ex-4 treatment delivered either ip or fourth icv, gave rise to an overall significant suppression of intake [IP: F(2,24) = 21.66, P < 0.0001 and fourth icv: F(1,11) = 15.48, P < 0.003] in both control and CD rats. For peripheral or fourth icv Ex-4 treatment, the intake suppression did not differ as a function of the neurological condition of the rat (CD vs. control), nor was there any significant interaction between drug treatment and neurological condition on glucose intakes, indicating no differences in the direction and magnitude of observed effects in CD and control groups. Figure 1 shows that ip Ex-4 at 1.2 and 3.0 μg/kg suppressed intraoral intake significantly (P < 0.05) in both groups, compared with respective vehicle intakes. There was no further statistical increase in the magnitude of intake suppression by the 3.0 μg/kg dose of ip Ex-4, compared with the 1.2 μg/kg dose in either neurological group. Figure 2 shows that fourth icv administration of Ex-4 (0.3 μg/μl) significantly suppressed intraoral intakes in both control and CD rats, compared with respective aCSF vehicle intakes (P < 0.05).
Figure 1.
Intraoral glucose (10%) intake (infused at 0.8 ml/min) for CD and control rats did not differ as a function of the neurological condition of the rat. Intraperitoneal Ex-4 at 1.2 and 3.0 μg/kg suppressed intake significantly in both control and CD rats, compared with respective vehicle intakes. The suppression of intake by peripheral Ex-4 did not differ as a function of the neurological condition of the rat (CD vs. control). *, P < 0.05 from respective vehicle.
Figure 2.
Intraoral glucose (10%) intake for CD and control rats did not differ as a function of the neurological condition of the rat. Fourth icv administration of Ex-4 (0.3 μg) significantly suppressed intakes in both control and CD rats, compared with respective aCSF vehicle intakes. The suppression of intake by hindbrain ventricular Ex-4 did not differ as a function of the neurological condition of the rat (CD vs. control). *, P < 0.05 from respective vehicle.
Effects of peripheral Ex-4 on liquid gastric emptying rates in chronic decerebrate and neurologically intact rats
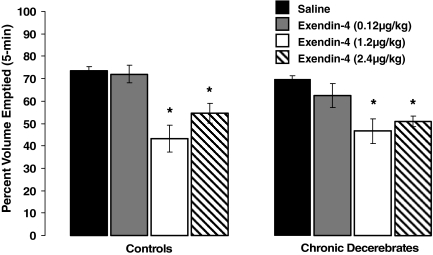
Ex-4 delivered ip suppressed gastric emptying in decerebrate and control rats, compared with the respective vehicle emptying rates [F(3,37) = 12.32, P < 0.05]. There was no significant main effect of the neurological condition of the rat (CD vs. control), nor was there any significant interaction between Ex-4 treatment and neurological condition on gastric emptying rates, indicating no differences in the direction and magnitude of observed effects in CD and control groups. The percent volume emptied in response to vehicle injections did not statistically differ between intact and decerebrate rats. The 5-min percent volume emptied was calculated and results are depicted in Fig. 3. For both control and CD rats, the percent volume emptied after the 0.12 μg/kg ip dose of Ex-4 was not significantly different from the percent volume emptied after respective vehicle administration. When the ip dose of Ex-4 was increased by a factor of 10 (1.2 μg/kg), the percent volume emptied for both neurological groups were significantly suppressed, compared with their respective vehicle-emptying rates. Increasing the dose of Ex-4 to 2.4 μg/kg did not further enhance the suppression of gastric emptying in either control or CD rats, similar to previous findings (4). Nonetheless, the percent volume emptied for both groups remained significantly suppressed, compared with respective vehicle-emptying rates.
Figure 3.
For control and CD rats, ip administration of Ex-4 (1.2 and 2.4 μg/kg) significantly suppressed 5 min gastric emptying of 0.9% saline, compared with vehicle in similar fashions. The gastric-emptying rates for the vehicle condition was not statistically different between control and CD rats, indicating that forebrain processing and forebrain-caudal brainstem communication is not necessary for the control of basal gastric emptying. *, P < 0.05 from respective vehicle.
Energetic responses to peripheral and caudal brainstem delivered injection of Ex-4 in chronic decerebrate and neurologically intact control rats
Tc (control, n = 5; CD, n = 7).
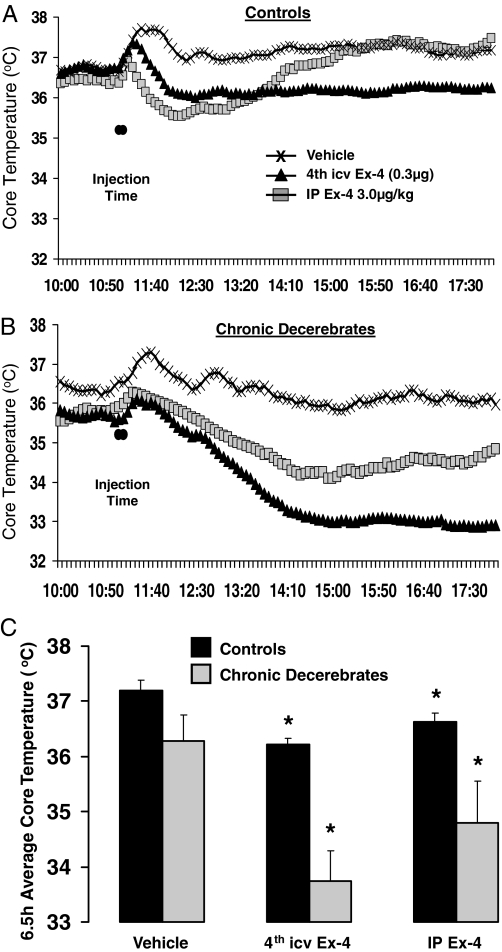
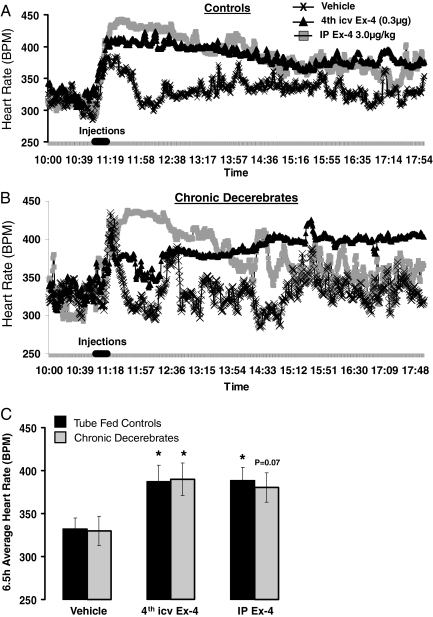
As shown in Fig. 4, Ex-4 administered peripherally (ip) and centrally (fourth ventricle) produced a large and long-lasting hypothermia in neurologically intact controls (ip: P < 0.05; fourth icv: P < 0.002, Fig. 4A) as well as in CDs (ip: P < 0.05; fourth icv: P < 0.002, Fig. 4B). Peripheral (ip) Ex-4 resulted in a significant 2 C change in Tc at nadir from vehicle baseline for both control and CD rats and an average decrease from vehicle baseline of 0.6 C for control and 1.5 C for CD rats. The response duration was approximately 4 h in intact rats. The response in CDs had a longer duration (persisting throughout the 7 h period of measurement). Caudal brainstem (fourth icv) Ex-4 delivery produced an approximately 1 C reduction in Tc at nadir, that persisted at that magnitude for the 7 h duration of measurement in neurologically intact rats. In CD rats, fourth icv Ex-4 produced a hypothermic response, with a change of approximately 3 C at nadir that lasted several hours after drug injection. The 6.5 h average hypothermic response from vehicle baseline after fourth icv Ex-4 was greater in CD rats (∼2.5 C) than neurological controls (∼1 C; Fig. 4C).
Figure 4.
Tc (C) in control and CD rats before and after injection of fourth icv Ex-4 (0.3 μg), ip Ex-4 (3.0 μg/kg), or vehicle. Across-rat averaged Tc throughout the 6.5-h period after injections in control (A) and CD (B) rats. The histograms (C) represent 6.5-h averages of Tc and show significant hypothermia after both fourth icv and ip Ex-4 administration for control and CD rats. *, P < 0.05 from respective vehicle Tc.
HR (control, n = 6; CD, n = 5).
Peripheral (ip) Ex-4 produced a robust and long-lasting tachycardia in neurologically intact controls (P < 0.05; Fig. 5A) and a trend to elevate HR in CDs (P = 0.07; Fig. 5B). The elevated HR response in both control and CD rats had a peak change from vehicle baseline of approximately 150 beats per minute (BPM) and a 6.5 h average increase of approximately 55 BPM. Caudal brainstem (fourth icv) Ex-4 produced an elevated HR in CDs (P < 0.05) and a similar tachycardia response in control rats (P = 0.05). The peak of the HR response was approximately 100 BPM for both control and decerebrate groups. The average change from vehicle baseline was approximately 50 BPM for both groups (Fig. 5C).
Figure 5.
HR (BPM) in control and CD rats before and after injection of fourth icv Ex-4 (0.3 μg), ip Ex-4 (3.0 μg/kg), or vehicle. Across-rat averaged HR throughout the 6.5-h period after injections in control (A) and CD (B) rats is shown. The histogram (C) represents 6.5-h averages of HR and shows elevated HR in response to fourth icv Ex-4 injections. After ip Ex-4, HR was significantly elevated in control rats, compared with HRs after vehicle injection, whereas CD rats showed a nonsignificant increase in HR after ip Ex-4. *, P < 0.05 from respective vehicle heart rate.
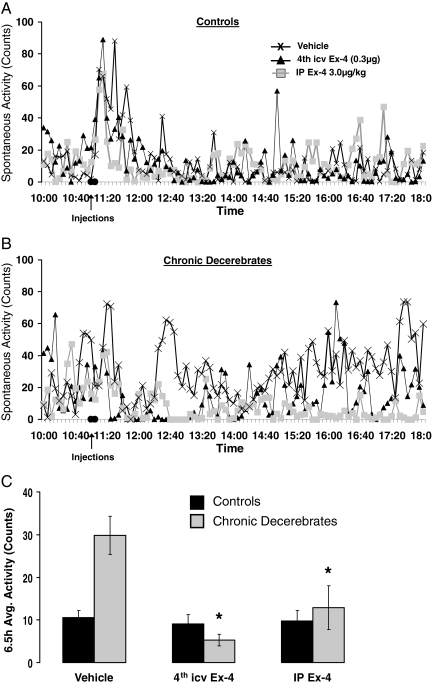
Activity (control, n = 6; CD, n = 6).
There was a significant interaction between drug treatment and neurological condition on activity (P < 0.0008). Post hoc tests revealed no effect of either peripheral or central Ex-4 on spontaneous activity in control rats (Fig. 6, A and C). However, CD rats showed a significant suppression of activity after fourth icv Ex-4 (P < 0.002, ∼75% suppression), as well as IP Ex-4 (P < 0.02, ∼60% suppression; Fig. 6, B and C). This group difference appears to result from the elevated activity of the CD rats under vehicle baseline conditions, compared with control (P < 0.003).
Figure 6.
Spontaneous activity (counts) in control (A) and CD (B) rats before and after injection of fourth icv Ex-4 (0.3 μg), ip Ex-4 (3.0 μg/kg), or vehicle. The histogram (C) represents 6.5-h averages for spontaneous activity counts. Control rats showed no significant alteration in spontaneous activity after Ex-4 administration. Activity counts after vehicle injection for CD rats were significantly greater than for control rats. This difference in vehicle values rather than differences for the ip and fourth icv Ex-4 conditions between CD and control rats contributes to the group differences observed. *, P < 0.05 from respective vehicle activity counts.
Discussion
To assess the importance of central processing from hypothalamic-forebrain and caudal brainstem sources to the inhibition of food intake and gastric emptying as well as the tachycardia and hypothermia driven by vagally transmitted peripheral or caudal brainstem directed central delivery of a GLP-1R agonist, responses of supracollicular decerebrate rats were compared with those of pair-fed neurologically intact control rats. Overall, the magnitude and duration of responses observed in CD and control rats were comparable. Peripheral administration of Ex-4 suppressed food intake and reduced gastric emptying and Tc in both control and CD rats. Tachycardia was seen in controls and CD rats in response to peripheral Ex-4 administration. Hindbrain ventricular central delivery of Ex-4 also produced similar intake, emptying, and thermal responses in CD and neurotologically intact controls. Tachycardia was observed after fourth icv injection of Ex-4 in control rats, whereas for CD rats, the trend was not significant. These data provide clear support for the hypothesis that central processing restricted to the caudal brainstem is sufficient for the generation of responses triggered by exogenous peripheral GLP-1R stimulation and also by central GLP-1R ligand delivered to the caudal brainstem. Additional work is required to identify the site(s) of action for endogenous peripheral and central GLP-1 that contributes to the control of energy balance responses.
Hypothalamic processing has been hypothesized to mediate the profile of responses evoked by peripheral GLP-1R ligand delivery (3,24,43). Peripheral administration of GLP-1R agonists induces central neuronal activation, as indicated by Fos-like immunoreactivity (3,7,21,43) and magnetic resonance imaging (24), in the PVN and in some reports, the ARC and ventral medial hypothalamus nuclei. Abbott et al. (3) examined the neural mediation of the anorexic effect of peripheral GLP-1R stimulation by interrupting connections between the caudal brainstem and hypothalamus with midbrain knife cuts. The authors concluded that hypothalamic processing is required for GLP-1R-induced anorexia because rats with knife cuts (histological details not provided) did not show this response. By contrast, here we show that CD rats lacking all neural communication between the caudal brainstem and hypothalamus (histologically confirmed complete transections) showed not only the same suppression of intake to peripheral GLP-1R agonist as that observed in neurologically intact control rats but also other physiological responses that were comparable with intact controls. The current results thereby show that neuronal activation of PVN, ARC or other hypothalamic neurons by peripheral GLP-1R treatment observed in intact rats (3,7,21,43) is not required for intake suppression or gastric emptying reduction. Roles for hypothalamic/forebrain processing (ARC, PVN, LH, and CeA neurons) in combination with autonomic and neuroendocrine processing from medullary catecholamine neurons (A5, rostral ventrolateral medulla, caudal ventral lateral medulla, and PBN) are hypothesized for the mediation of the cardiac and energetic effects of peripheral GLP-1R agonists (7,44). The current results for cardiac and energetic responses, like those for intake and gastric emptying responses, bolster the conclusion that forebrain/hypothalamic processing and hypothalamic-caudal brainstem communication are not required for peripheral GLP-1R stimulated responses.
Current findings show that the caudal brainstem is sufficient to mediate suppression of food intake and core body temperature elicited by central, caudal brainstem-delivered exogenous Ex-4 and that hypothalamic/forebrain processing is not necessary for these centrally evoked responses. At the same time, however, it is known that intake inhibition is observed after ventral forebrain application of GLP-1R ligands. Whereas it is difficult to ascribe the sites mediating forebrain ventricular GLP-1R agonist delivery because ligand is accessible to both forebrain and caudal brainstem GLP-1Rs, ventral forebrain parenchymal application of GLP-1R ligands does elicit behavioral responses, and this result supports a direct role for forebrain GLP-1R-driven effects (45,46). Neither our data nor other central agonist delivery studies address the sites of central mediation of responses resulting from endogenous activation of central GLP-1Rs. Whereas proglucagon-expressing neurons are located in the NTS of the caudal brainstem, these neurons project to both caudal brainstem and forebrain nuclear targets (47). Further investigations are certainly warranted to examine the role of caudal brainstem and ventral forebrain GLP-1 in the central mediation of responses resulting from endogenous central GLP-1 activation. For hindbrain-directed central delivery of exogenous agonist by contrast, the findings are clear. Responses induced by exogenous hindbrain application of GLP-1R agonists do not require forebrain processing or caudal brainstem-forebrain communication.
The specific caudal brainstem nuclei and efferent output pathways involved in mediating the gastric emptying and energy intake/expenditure effects by peripheral GLP-1R ligand administration are still under investigation. Rinaman et al. (48) showed monosynaptic vagovagal input-output circuitry in the NTS and dorsal motor nucleus of the vagus, providing support for the sufficiency of caudal brainstem processing in the control of gastric emptying (for review, see Ref. 49). Indeed, Fos-Li and electrophysiological recordings indicate that peripheral administration of Ex-4 activates NTS and dorsal motor nucleus of the vagus neurons (7,50,51). Other caudal brainstem nuclei have been implicated in mediating the intake suppressive effects of gastrointestinal satiation signals including, but not limited to, the PBN, AP, and ventrolateral medulla and all nuclei of the visceral afferent pathway (52,53,54). Caudal brainstem neurons including NTS, RVLM, and medullary raphe (raphe magnus and raphe pallidus) contribute to sympathetic outflows that control energy expenditure and are considered sympathetic premotor neurons by some (55,56,57,58). Recent experiments show that when surgically isolated from the forebrain, caudal brainstem neurons process and integrate energy status signals and issue efferent commands to sympathetic nervous system outflows producing compensatory adjustments in energy expenditure driven by food deprivation (27) or cold temperature exposure (59).
As noted in the introductory text, peripheral and central GLP-1R stimulation leads to a comparable set of intake, gastric emptying, and energetic responses. The broad distribution of GLP-1R in the CNS (16), and the comparable pattern of responses observed with hindbrain vs. forebrain-ventricular and forebrain-parenchymal GLP-1R agonist injections, indicates that the functional effects of central GLP-1R stimulation are not localized to one brain region but are distributed in nature (7,17,18). At the same time, however, a common assertion in the neural analysis of the GLP-1 contribution to energy balance control is that hypothalamic-forebrain processing (e.g. PVN, dorsomedial hypothalamus, LH, and medial hypothalamus) mediates central GLP-1R agonist effects irrespective to the site of agonist delivery. This perspective, expressed in many central GLP-1R agonist application studies is supported by patterns of GLP-1 immunoreactivity (16,60), central GLP-1-induced neuronal activation (7,61) and the organization of hypothalamic projections from the proglucagon expressing neurons in the NTS (47). Of the studies examining effects of CNS GLP-1R ligand application, the study of greatest relevance to the discussion of our hindbrain ventricular data is that of Kinzig et al. (17), which show that fourth ventricular application of GLP-1 (7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36) suppresses food intake at doses below that required for intake suppression with lateral ventricle delivery. Moreover, Kinzig et al. (17) demonstrated that the mediation of central GLP-1R-driven intake inhibition and visceral illness production are dissociable. That is, activation of caudal brainstem GLP-1Rs reduces food intake without inducing illness, whereas activation of forebrain GLP-1R via third and lateral ventricular injection results in a conditioned taste aversion, presumably through activation of CeA GLP-1R (17). Having generated suppression of food intake after forebrain and caudal brainstem application of GLP-1, Kinzig et al. (17) concluded that anorectic actions of CNS GLP-1 are mediated by processing in both caudal brainstem and hypothalamic nuclei. Analogous to the conclusions of Kinzig et al. (17), Yamamoto et al. (44) suggest similar caudal brainstem-hypothalamic communication requirements, specifically that circulating GLP-1 activates GLP-1-responsive neurons in the AP and NTS, which in turn activate neurons expressing GLP-1R in the hypothalamus and brainstem, leading to coordinated endocrine and autonomic effects on HR and blood pressure. We showed that central, hindbrain ventricular Ex-4 administration, in proximity to the endogenous central source of proglucagon in the NTS (14) and to caudal brainstem GLP-1R (16), engages endemic efferent circuits mediating the suppressive effects on intake and Tc by Ex-4 and does not require ascending forebrain input and processing.
The current findings are inconsistent with the conclusion that the intake inhibition induced by central GLP-1 delivery requires activation of and processing by hypothalamic circuits (18,61,62). These reports indicate that GLP-1 engages the hypothalamus and activates PVN neurons to modulate sympathetic outflow controlling for energy expenditure (44) via monosynaptic hypothalamo-spinal pathways (63). Whereas hypothalamic activation is observed in neurologically intact rats after peripheral or brain application of GLP-1R ligands (3,7,21,43), the findings in CD rats indicate that the contribution of GLP-1R-triggered sympathetic output pathways from forebrain structures in intact rats is complementary to the output pathways endemic to, and effects mediated by, the caudal brainstem. The finding that CD rats had an enhanced hypothermic response to fourth icv Ex-4 administration, compared with control rats, suggests that forebrain processing may in fact dampen or fine-tune the sympathetic/vagal outputs to hindbrain GLP-1R ligands.
The chronic decerebrate strategy involves the elimination of all neural connections between the forebrain and caudal brainstem. Undoubtedly, such a global disconnection results in differences between CD and intact rats. Yet the strength of the approach lies in its ability to document similarities (not differences) between the responses of intact brain rats and chronic decerebrates. Therefore, it is noteworthy that food intake, gastric emptying, Tc, and HR of CD and pair-fed intact rats are comparable under vehicle injection, baseline conditions. This indicates that integrations performed in the caudal brainstem contribute to the function of a broad range of physiological control systems. As stated above, however, the elimination of neural connections with the forebrain resulted in a notable difference between CD and intact rats. Activity counts after vehicle injection for CD rats were significantly greater than for control rats. This difference in vehicle values rather than differences for the ip or the fourth icv Ex-4 conditions between CD and control rats likely contribute to the group differences observed. A possible explanation for the elevated baseline activity counts in CDs, compared with controls, comes from the fact that energy expenditure measurements were made in the light phase, during which control rats’ baseline activity is very low because the majority of intact rats’ physical activity occurs during the dark phase. Unlike controls, CD rats are neurologically blind and do not show the differential pattern of rest/activity behavior associated with light/dark phases observed in intact rats. Instead, CD rats show a relatively consistent pattern of activity across a 24-h period, which here is shown to be elevated, compared with control rats’ resting behavior in the light phase, but is likely reduced, compared with control rats’ dark-phase activity. Thus, the absence of an activity effect of Ex-4 in controls may reflect a floor effect on activity in the light phase. Further experiments are certainly warranted to examine the effects on spontaneous activity after ip and fourth icv Ex-4 in the dark phase of neurologically intact controls.
The observed tachycardia to ip Ex-4 treatment may be mediated by peripheral vs. central sites of ligand action. In addition to caudal brainstem GLP-1R-mediated output controlling for HR, it is possible that when applied to the periphery, Ex-4 directly activated GLP-1R on the heart (64). However, a variety of data supports the notion that central sympathetic outflows (7) and vagal efferent (8,51) pathways mediate peripheral GLP-1R triggered cardiac effects. Future examination of HR variability in the time domain (65,66), a function of interbeat interval and systolic blood pressure, may provide a basis for determining the differential contributions of the sympathetic nervous system and vagal efferent pathways to Ex-4-generated cardiac effects because HR variability is considered a proxy of vagal efferent activity. Additionally, tachycardia is often associated with nonanesthesia hypothermia (67,68,69), and it has often been asserted that tachycardia is a thermoregulatory compensatory response to hypothermia along with vasoconstriction, increased glomerular filtration rate, and overall increase in metabolism in attempts to restore Tc to normal. We cannot completely rule out the possibility that the observed tachycardia in control and CD rats after Ex-4 treatment was a secondary thermoregulatory response to the hypothermic effects of GLP-1R activation. Nonetheless, the latency of hypothermia and tachycardia were not statistically different, suggesting that the GLP-1R-mediated tachycardia response is not a compensatory response to hypothermia. Future investigations using various surgical and ex vivo preparations are certainly warranted to further investigate the mechanisms of GLP-1’s cardiac effects.
In conclusion, the overall pattern of results demonstrate that caudal brainstem processing is sufficient to mediate peripheral GLP-1R-triggered inhibition of intake, core temperature, and gastric emptying rates as well as tachycardia and that hypothalamic/forebrain processing is not required for response production. The same pattern was observed with hindbrain-delivered fourth icv agonist injection.
Acknowledgments
We thank Lisa Maeng, Grace Lee, and Jolanta Jozefara for their technical assistance. We also thank Monell Chemical Senses Center for use of their spectrophotometer.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK21397 (to H.J.G.) and DK077484 (to M.R.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 17, 2008
Abbreviations: aCSF, Artificial cerebrospinal fluid; AP, area postrema; ARC, arcuate nucleus; BPM, beats per minute; CD, collicular decerebrate; CeA, central nucleus of the amygdala; CNS, central nervous system; Ex-4, exendin-4; GLP-1, glucagon like peptide 1; GLP-1R, GLP-1 receptor; HR, heart rate; icv, intracerebroventricular; LH, lateral hypothalamus; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; PVN, paraventricular nucleus; Tc, core temperature.
References
- Creutzfeldt W 2001 The entero-insular axis in type 2 diabetes—incretins as therapeutic agents. Exp Clin Endocrinol Diabetes 109(Suppl 2):S288–S303 [DOI] [PubMed] [Google Scholar]
- Mojsov S, Kopczynski MG, Habener JF 1990 Both amidated and nonamidated forms of glucagon-like peptide I are synthesized in the rat intestine and the pancreas. J Biol Chem 265:8001–8008 [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR 2005 The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB 1997 Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol 273:G920–G927 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD 2005 Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- Osaka T, Endo M, Yamakawa M, Inoue S 2005 Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides 26:1623–1631 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK 2002 Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan JM, Eng J, Rodriguez R, Blazquez E 1999 Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol 277:E784–E791 [DOI] [PubMed] [Google Scholar]
- Barragan JM, Rodriguez RE, Blazquez E 1994 Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol 266:E459–E466 [DOI] [PubMed] [Google Scholar]
- Barragan JM, Rodriguez RE, Eng J, Blazquez E 1996 Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept 67:63–68 [DOI] [PubMed] [Google Scholar]
- Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S 1989 Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 38:902–905 [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C 1999 Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 276:R1541–R1544 [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C 1999 Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C 1997 Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77:257–270 [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP 1995 Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7:2294–2300 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P 1999 Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ 2002 The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V 2003 Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284:R1427–R1435 [DOI] [PubMed] [Google Scholar]
- Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R 2005 Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA 2006 Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 30:1332–1340 [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL 2005 Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146:3748–3756 [DOI] [PubMed] [Google Scholar]
- Norgren R 1978 Projections from the nucleus of the solitary tract in the rat. Neuroscience 3:207–218 [DOI] [PubMed] [Google Scholar]
- Norgren R, Leonard CM 1973 Ascending central gustatory pathways. J Comp Neurol 150:217–237 [DOI] [PubMed] [Google Scholar]
- Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD, Dhillo WS, Stanley SA, Ghatei MA, Bloom SR 2006 Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun 350:298–306 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V 2003 Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27:313–318 [DOI] [PubMed] [Google Scholar]
- Ma X, Bruning J, Ashcroft FM 2007 Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci 27:7125–7129 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ 2006 Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147:1365–1376 [DOI] [PubMed] [Google Scholar]
- Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B 1993 Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting β-cells. J Biol Chem 268:19650–19655 [PubMed] [Google Scholar]
- Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A 2006 Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia 49:1247–1253 [DOI] [PubMed] [Google Scholar]
- Briones M, Bajaj M 2006 Exenatide: a GLP-1 receptor agonist as novel therapy for type 2 diabetes mellitus. Expert Opin Pharmacother 7:1055–1064 [DOI] [PubMed] [Google Scholar]
- Doggrell SA 2006 Recent evidence of sustained benefit with exenatide in type 2 diabetes. Expert Opin Pharmacother 7:2003–2006 [DOI] [PubMed] [Google Scholar]
- Yoo BK, Triller DM, Yoo DJ 2006 Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother 40:1777–1784 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R 1978 Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201:267–269 [DOI] [PubMed] [Google Scholar]
- Daniels D, Markison S, Grill HJ, Kaplan JM 2004 Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci 24:11457–11462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ 1988 Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci 102:934–941 [DOI] [PubMed] [Google Scholar]
- Aziz A, Anderson GH 2003 Exendin-4, a GLP-1 receptor agonist, interacts with proteins and their products of digestion to suppress food intake in rats. J Nutr 133:2326–2330 [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Siemers W, Grill HJ 1994 Ingestion, gastric fill, and gastric emptying before and after withdrawal of gastric contents. Am J Physiol 267:1257–1265 [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Wohn A, White WO, Schwartz GJ, Moran TH 1999 Inhibition of gastric emptying by bombesin-like peptides is dependent upon cholecystokinin-A receptor activation. Regul Pept 84:101–106 [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Netterville LA, McHugh PR, Moran TH 1991 Gastric loads potentiate inhibition of food intake produced by a cholecystokinin analogue. Am J Physiol 261:R1141–R1146 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Moore RL, Shah SM, Covasa M 2004 5-HT3 receptors participate in CCK-induced suppression of food intake by delaying gastric emptying. Am J Physiol Regul Integr Comp Physiol 287:R817–R823 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ 2004 Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127:546–558 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK 2003 Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ 1997 Decreased intake of a liquid diet in nonfood-deprived rats following intra-PVN injections of GLP-1 (7–36) amide. Pharmacol Biochem Behav 58:673–677 [DOI] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ 1998 PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 274:R23–R29 [DOI] [PubMed] [Google Scholar]
- Vrang N, Hansen M, Larsen PJ, Tang-Christensen M 2007 Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149:118–126 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Card JP, Schwaber JS, Miselis RR 1989 Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci 9:1985–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC 2004 Gastrointestinal mechanisms of satiation for food. Physiol Behav 81:249–273 [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW 2006 Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55:3387–3393 [DOI] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA 2007 Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 292:G1474–G1482 [DOI] [PubMed] [Google Scholar]
- Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA 2007 Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res 1162:76–84 [DOI] [PubMed] [Google Scholar]
- Viltart O, Sartor DM, Verberne AJ 2006 Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull 71:51–59 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Covasa M 2006 Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 1088:120–130 [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM 1999 Differential control of sympathetic drive to the rat tail artery and kidney by medullary premotor cell groups. Brain Res 834:196–199 [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM 2001 Cold-activated raphe-spinal neurons in rats. J Physiol 535:841–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF 1993 Raphe pallidus excites a unique class of sympathetic preganglionic neurons. Am J Physiol 265:R82–R89 [DOI] [PubMed] [Google Scholar]
- Morrison SF 1999 RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol 276:R962–R973 [DOI] [PubMed] [Google Scholar]
- Nautiyal KM, Dailey M, Brito N, Brito MNd A, Harris RB, Bartness TJ, Grill HJ Energetic responses to cold temperatures in rats lacking forebrain-caudal brainstem connections. Am J Physiol Regul Integr Comp Physiol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK 1988 Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol 271:519–532 [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR 1996 A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR 1999 Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140:244–250 [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM 1976 Direct hypothalamo-autonomic connections. Brain Res 117:305–312 [DOI] [PubMed] [Google Scholar]
- Bullock BP, Heller RS, Habener JF 1996 Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137:2968–2978 [DOI] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME 2001 Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280:R1007–R1015 [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM 2003 Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30:769–778 [DOI] [PubMed] [Google Scholar]
- Danzl D 2002 Hypothermia. Semin Respir Crit Care Med 23:57–68 [DOI] [PubMed] [Google Scholar]
- Fisher LA, Brown MR 1984 Bombesin-induced stimulation of cardiac parasympathetic innervation. Regul Pept 8:335–343 [DOI] [PubMed] [Google Scholar]
- Dubose DA, Morehouse DH, Rufolo DM, Leon LR 2006 Hypothermia (HYP) induces tachycardia, not bradycardia in free-ranging (FR) rats. FASEB J 20:A1450 (Meeting Abstract) [Google Scholar]