Abstract
Estrogen, acting via estrogen receptor (ER)α, regulates serum gonadotropin levels and pituitary gonadotropin subunit expression. However, the cellular pathways mediating this regulation are unknown. ERα signals through classical estrogen response element (ERE)-dependent genomic as well as nonclassical ERE-independent genomic and nongenomic pathways. Using targeted mutagenesis in mice to disrupt ERα DNA binding activity, we previously demonstrated that ERE-independent signaling is sufficient to suppress serum LH levels. In this study, we examined the relative roles of ERE-dependent and -independent estrogen signaling in estrogen regulation of LH, FSH, prolactin, and activin/inhibin subunit gene expression, pituitary LH and FSH protein content, and serum FSH levels. ERE-independent signaling was not sufficient for estrogen to induce pituitary prolactin mRNA or suppress pituitary LHβ mRNA, LH content, or serum FSH in estrogen-treated ovariectomized mice. However, ERE-independent signaling was sufficient to reduce pituitary glycoprotein hormone α-subunit, FSHβ, and activin-βB mRNA expression. Together with previous serum LH results, these findings suggest ERE-independent ERα signaling suppresses serum LH via reduced secretion, not synthesis. Additionally, ERE-dependent and ERE-independent ERα pathways may distinctly regulate steps involved in the synthesis and secretion of FSH.
LH AND FSH ARE SYNTHESIZED and secreted by the pituitary gonadotrope in response to pulsatile GnRH released into the hypophyseal portal vasculature from the terminals of hypothalamic GnRH neurons at the median eminence (1,2). FSH stimulates ovarian follicle development, and LH stimulates steroidogenesis and ovulation. In turn, follicle-derived steroids (estrogen and progesterone) and peptide hormones (inhibins) provide negative feedback at the level of the pituitary and/or hypothalamus to limit further gonadotropin stimulation (3,4,5,6). In this way, negative feedback maintains circulating gonadotropins at low levels throughout most of the estrous cycle until the afternoon of proestrus when, in response to increased estrogen secreted from preovulatory follicles, feedback switches to positive. This results in a surge release of GnRH, and subsequently LH and FSH, and the initiation of ovulation (7). In the rat, a secondary FSH surge occurs after ovulation, which likely depends on the reduction of circulating inhibin (8) and functions to recruit the next cohort of follicles.
The gonadal steroids are critically important to the neuroendocrine regulation of gonadotropins. In the female, their removal by ovariectomy (OVX) results in increased expression of gonadotropin subunits and serum concentration of gonadotropins. Estrogen treatment is sufficient to decrease the post-OVX increases in the common glycoprotein hormone α-subunit (αGSU) and specific β-subunit (LHβ and FSHβ) expression (9,10,11,12,13). There are at least two forms of estrogen receptor ER), ERα (ESR1) and ERβ (ESR2). The use of murine ER targeted deletion technology has identified a predominant role for ERα (14) in providing estrogen negative neuroendocrine feedback regulation of LH (4) and FSH (14,15,16). This result has been confirmed with selective ER agonists (17). However, other than a critical role for ERα, the mechanisms underlying estrogen negative feedback remain largely unknown.
ERα, like other nuclear hormone receptors, binds ligand, translocates into the nucleus, and induces the transcription of target genes through binding directly at estrogen-responsive elements (EREs) in regulatory regions of DNA (18). It has become increasingly recognized that ERα also signals through ERE-independent pathways. These include protein-protein interactions to modulate the activity of other transcription factors at their cognate sites in DNA [e.g. activator protein 1 (AP-1) and nuclear factor-κB] (19) and nongenomic membrane-initiated signaling pathways (20). Previously, we generated a mutant ERα with two amino acid substitutions in the first zinc finger of the DNA binding domain. This AA mutant receptor cannot bind to the ERE consensus sequence and lacks ERE-dependent but has retained ERE-independent activity (21). Using this AA mutant we created a nonclassical ER knock-in (NERKI) mouse model (22). Through breeding with the ERα knockout (ERαKO), ERE-independent signaling was selectively restored to the ERα null background (NERKI/ERαKO). This genetic model, when paired with a castration and estrogen-replacement paradigm, enables the assignment of the in vivo effects of estrogen to either the ERE-dependent or -independent ERα signaling pathways.
We recently found that the ERE-independent pathway is sufficient to convey substantial (70%) estrogen negative feedback regulation of serum LH (23). In the current study, the relative roles of ERα pathways in conferring estrogen negative feedback suppression of serum FSH have been investigated. Additionally, the ability of ER pathways to convey estrogen effects on pituitary prolactin, gonadotropin, and activin/inhibin subunit gene expression as well as LH and FSH pituitary contents have been examined.
Materials and Methods
Animal treatment and tissue collection
Animals were maintained and experiments conducted in accord with the accepted standards of humane animal care (24). Animal use procedures were approved by the Northwestern University Animal Care and Use Committee. NERKI mice were created on a 129SvJ background (22), whereas ERαKO mice obtained from Dr. Pierre Chambon were on a C57BL/6 background (25). ERα−/AA used in these experiments are the result of the AA mutant allele crossed seven to 11 generations onto the ERαKO C57BL/6 background. Mice were maintained on a 14-h light, 10-h dark cycle with standard chow (Harlan Teklad, 7912) and water available ad libitum. Mice used in this study were adult females from 8–13 wk of age.
To enable comparisons with previous serum LH results (23), the same estrogen replacement paradigm was used here to characterize negative feedback regulation of serum FSH and gonadotropin subunit expression. This paradigm produced physiological circulating estradiol levels similar to those observed at proestrus (26), 48.6 ± 6.5 pg/ml (n = 13). Briefly, mice were transferred to a low phytoestrogen diet (Harlan Teklad, 2019S) 1 d before surgery. Between 0800 and 1000 h on the day of surgery (d 0), females were anesthetized by ip injection of 200 mg/kg 2,2,2-tribromoethanol (Sigma Chemical Co., St. Louis, MO; T48402) in vehicle, 0.9% sodium chloride (Sigma, S8776), and 2% tert-amyl alcohol (Sigma, 240486), and ovaries were surgically removed. Mice were implanted with prepared SILASTIC brand silicon capsules (Dow Corning, Midland, MI) (27) containing either silicone vehicle for OVX or silicone with 2.5 μg 17β-estradiol (E2) for OVX + E2 groups. On d 6 after OVX between 0900 and 1000 h, animals were injected sc with either 0.1 ml sesame oil (Sigma, S3547) for OVX groups or 0.1 ml sesame oil containing 1 μg estradiol benzoate (Sigma, E8515) for OVX + E2 groups. On d 7 after OVX between 0800 and 1000 h, mice were deeply anesthetized by acute exposure to halothane (Halocarbon Laboratories, River Edge, NJ) vapors and immediately killed by exsanguination. Blood was collected from the abdominal aorta using a 25-gauge needle. Dissected anterior pituitary tissue was immediately frozen in liquid nitrogen and then stored at −80 C until further processing.
Because ERα−/− and ERα−/AA do not cycle and are in constant diestrus (23), ovary-intact female ERα+/+ mice were cycled to diestrus before being killed to better control for fluctuations caused by the estrous cycle. Briefly, female mice were individually housed for at least 1 wk. Subsequently, vaginal smears were performed daily at 1000 h for 10–20 d to confirm cycling, and a predominance of leukocytes in the vaginal smear was used to identify females in diestrus for killing (28). Ovary-intact female mice were killed, and blood was collected between 0800 and 1000 h, as described above.
Serum and pituitary extract preparation and hormone assays
Blood was allowed to coagulate for 90 min at room temperature and then centrifuged at 2000 × g for 15 min. Serum was transferred to a fresh tube and stored at −20 C until assayed. Sera were randomized and assayed for FSH by RIA at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Intraassay coefficient of variation was less than 18.1%. Groups contain data from five to eight animals, except for ERα−/− OVX + E2, which only has data from three animals.
Pituitary extracts were prepared using a previously described method (16) with slight modifications. Briefly, 500 μl ice-cold Dulbecco’s PBS (Life Technologies, Inc., Rockville, MD; 14190) containing protease inhibitor cocktail (Roche, Indianapolis, IN; 11697498001) was added to frozen pituitary tissue. Samples were immediately lysed using two rounds of sonification, 2 sec each 0.2 on/0.2 off at 32% amplitude. Lysates were then frozen in a dry-ice/ethanol bath and then thaw-fractured four times. Cellular debris was removed by centrifugation at 2000 rpm for 5 min at 4 C, and supernatant was transferred to a clean tube. For LH, extract was diluted 1:10 in PBS and kept frozen until assayed. Samples were diluted an additional 1:10 to be within assay reportable range. For FSH, pituitary extracts were diluted 1:25 for intact, 1:200 for OVX, and 1:100 for ERα+/+, 1:200 for ERα−/−, and 1:150 for ERα−/AA OVX + E2. Samples were randomized and assayed for LH by mouse LH sandwich immunoradiometric assay and FSH by RIA at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Intraassay coefficients of variance were less than 5.7 and 14.2%, respectively. Total pituitary content was back-calculated from the dilution factor and percentage of total volume assayed. Groups contain data from four to 12 animals.
RNA isolation and semiquantitative RT-PCR
Tissue was homogenized using a Polytron and 500 μl Trizol (Invitrogen Carlsbad, CA; 15596-018) reagent. RNA was extracted according to the manufacturer’s protocol using chloroform (Sigma, C2432) and the addition of linear acrylamide (Ambion, Austin, TX; 9520) to the aqueous phase to facilitate precipitation. DNA contamination was removed from RNA using RQ1 ribonuclease-free deoxyribonuclease I (Promega, Madison, WI; M6101) according to the manufacturer’s instructions, followed by extraction using acid phenol:chloroform (pH 4.5) (Ambion, 9720) followed by ethanol precipitation. Pellets were resuspended in 10 μl nuclease-free water. RT was performed using 4 μl after adding Powerscript reverse transcriptase (Clontech, Palo Alto, CA; S2314) according to the manufacturer’s instructions with 500 ng oligo (deoxythymidine)12–18 primer (Invitrogen, Y01212).
Semiquantitative PCR was performed using iQ Supermix (Bio-Rad, Hercules, CA; 170-8862) for LHβ and FSHβ using 100 nmol/μl of each primer and 100 μm probe (supplemental table, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) and a two-step program: 3 min at 95 C, followed by 35 cycles of 30 sec at 90 C and then 30 sec at 60 C on the iCycler My iQ single color real-time detection system (Bio-Rad). For all other genes, semiquantitative PCR was performed using iQ SYBR Green Supermix (Bio-Rad, 170-8882) with 600 pm each primer (supplemental table), 2 μl cDNA at either 1:100 (prolactin) or 1:5 (all others) using a three-step program: 3 min at 95 C, followed by 35 cycles of 30 sec at 95 C, 45 sec at annealing temperature (supplemental table), and 1 min at 72 C. The absence of contaminating genomic DNA was confirmed using no RT enzyme cDNA reaction control samples amplified with the housekeeping gene ribosomal protein L19 (RPL19) primer and primer/probe sets. Primer specificity was confirmed by PCR product size determined by agarose gel electrophoresis and direct sequencing of excised bands (QIAGEN, Valencia, CA; 28706) performed at the Genomics Core Facility at Northwestern University.
Threshold cycle (Ct) data were normalized to median RPL19 Ct before calculating ΔCt. When dilution of cDNA was required, PCR was performed for RPL19 with the same diluted cDNA to control for variability introduced during dilution. Relative fold change was calculated using the difference, or ΔCt, for each individual from the mean Ct of the designated control group (either ERα+/+ intact at diestrus for LHβ and FSHβ or ERα−/− intact for prolactin) with one ΔCt cycle defined as a 2-fold change.
Statistics
The Shapiro-Wilks and Bartlett’s tests were used to determine normality and variance heteroskedasticity of the data sets. When the data did not meet the assumptions of normal distribution and equal variance, they were transformed using the Box-Cox family of transformations (29,30). Data were analyzed for genotype-treatment interaction effects using a two-way ANOVA (α = 0.05). Multiple comparisons between genotype and treatment groups were conducted using the post hoc Fisher’s least significant difference (LSD) test with P < 0.05 as the minimum criterion to declare statistical significance. Data transformed using the powers of −0.2 to 0.2, including the log, are presented as geometric means derived using the Taylor series expansion ± se. Data transformed by power values outside of this range are presented using the arithmetic mean ± se.
Results
Genotype-treatment interaction effects
All data, with the exception of pituitary LH content, showed genotype-treatment interaction effects by two-way ANOVA (α = 0.05): for prolactin, F(4,42) = 22.91, P < 0.0001; for LHβ, F(4,42) = 6.73, P = 0.0003; for αGSU, F(4,41) = 20.97, P < 0.0001; for LH content, F(4,54) = 0.9, P = 0.472; for FSHβ, F(4,42) = 18.37, P < 0.0001; for FSH content, F(4,54) = 9.49, P < 0.0001; for serum FSH, F(4,40) = 6.81, P < 0.0001; for inhibin-α, F(4,39) = 3.15, P = 0.025; for activinβB, F(4,39) = 3.46, P = 0.016; and for activinβA, F(4,39) = 10.02, P < 0.0001. Results from posttest Fisher’s LSD multiple comparisons are detailed below.
Effects on pituitary prolactin gene expression
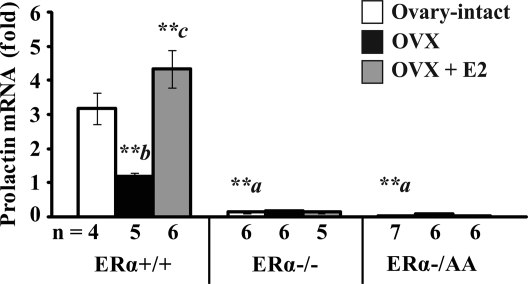
Initially, the effect of estrogen on prolactin, a gene stimulated by estrogen via ERα binding to an ERE, was examined to confirm that the AA mutant receptor conveys only ERE-independent ERα activity in vivo. Prolactin expression was 63% reduced after OVX when compared with ovary-intact ERα+/+ mice (P < 0.01; Fig. 1, left). Estrogen treatment of OVX ERα+/+ mice increased prolactin expression 4.3-fold to near ovary-intact levels (P < 0.01). In contrast, prolactin expression was 96% reduced in ovary-intact ERα−/− and 99% reduced in ovary-intact ERα−/AA compared with ovary-intact ERα+/+ mice at diestrus (P < 0.01). Furthermore, mRNA for prolactin was not decreased by OVX or induced by estrogen (Fig. 1, center and right). Thus, the ERα ERE-dependent induction of prolactin gene expression is absent in mice with isolated ERE-independent ERα signaling, similar to the null.
Figure 1.
ERE-dependent signaling is required for estrogen to induce pituitary prolactin mRNA expression. Real-time quantitative PCR was used to determine prolactin mRNA expression levels in pituitary tissues from female mice carrying wild-type (+/+), null (−/−), or a mutant ERα with ERE signaling disrupted on the null background (−/AA) that are ovary-intact (white), OVX (black), or OVX + E2 (gray). Data were indexed to ERα+/+ OVX. Significant genotype-treatment interaction effects [F(4,42) = 22.91; P < 0.0001] were identified in the prolactin expression data by two-way ANOVA (α = 0.05). Significant differences were examined by the post hoc Fisher’s LSD multiple comparison test. Differences between genotypes are denoted by a (vs. ovary-intact ERα+/+) and within genotype response to treatment by b (OVX vs. intact) and c (E2 vs. OVX). **, P < 0.01.
Effects on pituitary LH subunit expression and pituitary LH content
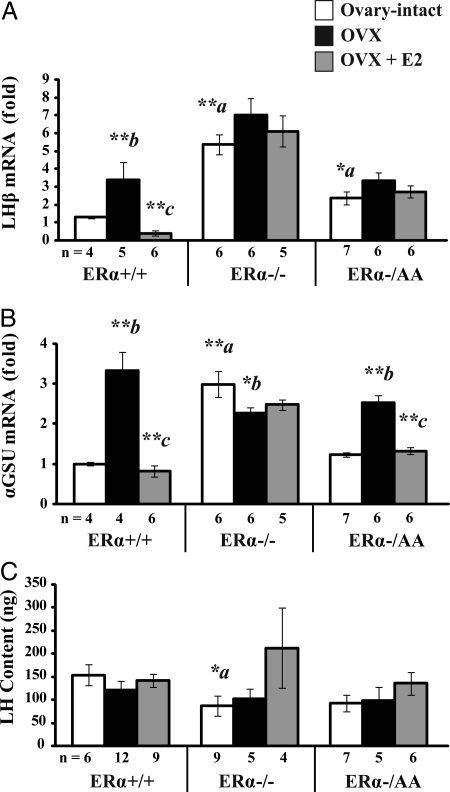
The effect of ERE-independent ERα estrogen signaling on LH subunit expression was examined to assess whether estrogen negative feedback on LH might occur as a result of decreased pituitary LH synthesis. In ERα+/+ mice, LHβ expression increased 3.4-fold after OVX, and estrogen treatment completely suppressed the post-OVX increase (P < 0.01; Fig. 2A, left). LHβ expression was 5.4-fold higher in ovary-intact ERα−/− compared with ERα+/+ at diestrus (P < 0.01), and levels did not significantly change after OVX or in response to estrogen treatment (P > 0.1; Fig. 2A, center). In ovary-intact ERα−/AA mice, LHβ expression was 2.4-fold elevated compared with ERα+/+ (P < 0.05) but 56% lower than in ERα−/− (P < 0.01). Similar to ERα−/−, ERα−/AA LHβ expression showed no significant change in response to either OVX or estrogen treatment (P > 0.1; Fig. 2A, right). Thus, estrogen suppression of pituitary LHβ expression requires ERE-dependent pathway signaling.
Figure 2.
ERE-dependent signaling is required for estrogen to suppress pituitary LHβ but not αGSU mRNA expression. Real-time quantitative PCR was used to determine LHβ (A) and αGSU (B) gene expression, and LH sandwich immunoradiometric assay was used to determine LH content in pituitary tissues from female mice carrying wild-type (+/+), null (−/−), or a mutant ERα with ERE signaling disrupted on the null background (−/AA) that are ovary-intact (white), OVX (black), or OVX + E2 (gray). Expression data were indexed to intact ERα+/+ cycled to diestrus. Significant genotype-treatment interaction effects were detected in LHβ [F(4,42) = 6.73; P = 0.0003] and αGSU expression (F(4,41) = 20.97; P < 0.0001) but not LH content [F(4,54) = 0.9; P = 0.472] data by two-way ANOVA (α = 0.05). Significant differences were examined by the post hoc Fisher’s LSD multiple comparison test. Differences between genotypes are denoted by a (vs. ovary-intact ERα+/+) and within genotype response to treatment by b (OVX vs. intact) and c (E2 vs. OVX). *, P < 0.05; **, P < 0.01.
Expression levels of the common αGSU were also measured. In ERα+/+ mice, αGSU expression increased 3.3-fold after OVX, and this rise was completely suppressed by estrogen treatment (P < 0.01; Fig. 2B, left). αGSU levels were 3.0-fold elevated in ovary-intact ERα−/− mice compared with ERα+/+ at diestrus, and expression did not significantly increase but rather decreased in response to OVX (P < 0.05). Also, levels did not decrease in response to estrogen treatment (P > 0.1; Fig. 2B, center). αGSU levels were similar in ovary-intact ERα−/AA mice compared with ERα+/+ mice at diestrus (P > 0.1). αGSU expression in ERα−/AA increased 2.1-fold in response to OVX, and the post-OVX rise was 93% decreased after estrogen treatment (P < 0.01; Fig. 2B, right). This result indicates the ERE-independent ERα signaling conveys estrogen suppression of pituitary αGSU.
Total pituitary LH content was measured in each of the genotypes and treatment conditions. Ovary-intact ERα−/− showed a 43% reduction in pituitary LH content when compared with diestrous ERα+/+ (P < 0.05). Ovary-intact ERα−/AA was not different from intact ERα+/+ at diestrus. Furthermore, ERα+/+, ERα−/−, or ERα−/AA pituitary LH content was not altered in response to OVX or estrogen replacement (each P > 0.1; Fig. 2C). Therefore, despite having distinct suppressive effects on LH subunit expression, estrogen signaling via ERα pathways did not result in reduced pituitary LH protein content.
Effects on pituitary FSHβ expression, FSH content, and serum FSH levels
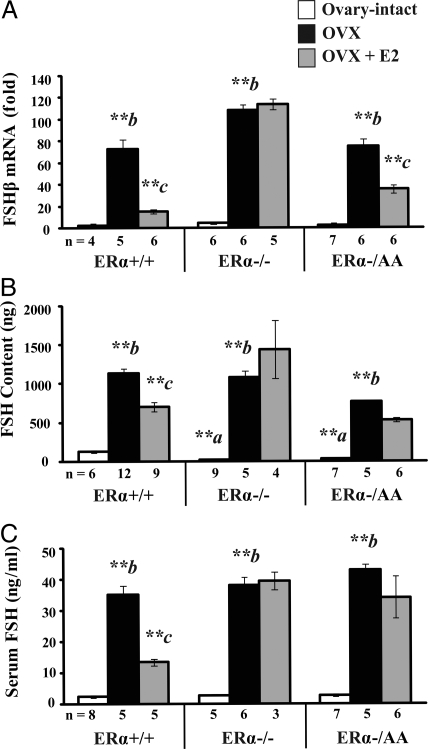
Basal pituitary FSHβ expression was similar between ovary-intact ERα+/+ cycled to diestrus and ovary-intact ERα−/− and ERα−/AA (P > 0.1). After OVX, FSHβ mRNA increased more than 70-fold in all genotypes in comparison with ovary-intact ERα+/+ mice (P < 0.01). FSHβ was reduced 82% by estrogen treatment in ERα+/+ (P < 0.01; Fig. 3A, left) and was unchanged by estrogen treatment in ERα−/− (P > 0.1; Fig. 3A, center). Similar to ERα+/+, FSHβ mRNA was reduced, although just 55% by estrogen treatment in OVX ERα−/AA (P < 0.01; Fig. 3A, right). Thus, the ERE-independent ERα signaling conveyed estrogen suppression of FSHβ. Also, FSHβ is further reduced by the addition of other ovarian factors in ERα+/+ and ERα−/AA (P < 0.01; Fig. 3A, compare OVX + E2 to intact).
Figure 3.
ERE-independent signaling mediates estrogen suppression of pituitary FSHβ expression but pituitary FSH protein or serum FSH. Real-time quantitative PCR was used to determine FSHβ gene expression in pituitary tissues (A), and RIA was used to determine pituitary FSH content (B) and serum FSH levels (C) in female mice carrying wild-type (+/+), null (−/−), or a mutant ERα with ERE signaling disrupted on the null background (−/AA) that are ovary-intact (white), OVX (black), or OVX + E2 (gray). Expression data were indexed to intact ERα+/+ cycled to diestrus. Significant genotype-treatment interaction effects were detected in FSHβ expression [F(4,42) = 18.37; P < 0.0001], FSH content [F(4,54) = 9.49; P < 0.0001], and serum FSH [F(4,40) = 6.81; P < 0.0001] by two-way ANOVA (α = 0.05). Significant differences were examined by the post hoc Fisher’s LSD multiple comparison test. Differences between genotypes are denoted by a (vs. ovary-intact ERα+/+) and within genotype response to treatment by b (OVX vs. intact) and c (E2 vs. OVX). *, P < 0.05; **, P < 0.01.
Pituitary FSH content was not significantly different in ovary-intact ERα−/− and ERα−/AA compared with intact ERα+/+ at diestrus (P > 0.1; Fig. 3B, center and right). FSH content was elevated in response to OVX in all genotypes (P < 0.01). Estrogen treatment reduced the post-OVX rise in pituitary FSH content by 43% in ERα+/+ (P < 0.01) but not ERα−/− or ERα−/AA (P > 0.08). FSH content in OVX ERα−/AA was significantly less than ERα+/+ and ERα−/− (P < 0.05). Furthermore, FSH content in OVX, estrogen-treated ERα−/AA mice was not elevated compared with OVX, estrogen-treated ERα+/+ (P > 0.05). Thus, it is unclear whether ERE-dependent signaling was required for estrogen suppression of FSH content or the post-OVX increase was lessened in presence of isolated ERE-independent ERα signaling. FSH content was further reduced by the addition of other ovarian factors in ERα+/+ mice (P < 0.01; Fig. 3B, left, compare OVX + E2 to intact).
Serum FSH levels were not different in ovary-intact ERα−/− or ERα−/AA compared with intact ERα+/+ at diestrus (P > 0.1; Fig. 3C). Serum FSH increased in response to OVX in all genotypes (P < 0.01). This post-OVX rise in serum FSH was reduced 66% by estrogen replacement in ERα+/+ (P < 0.01; Fig. 3C, left). However, there was no response to estrogen treatment in ERα−/− or ERα−/AA, and both were elevated compared with ERα+/+ (P > 0.1; Fig. 3B, center and right). Thus, ERE-dependent ERα signaling was required for estrogen suppression of serum FSH. Also, as with subunit expression and pituitary content, serum FSH in ERα+/+ is further decreased by the addition of other ovarian factors (P < 0.05; Fig. 3C, left, compare OVX + E2 to intact).
Effects on pituitary inhibin and activin subunit gene expression
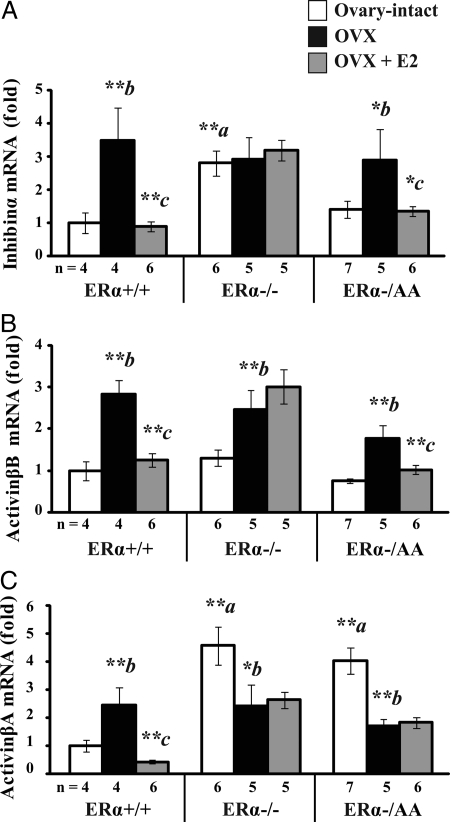
Activin is composed of two β-subunits (βA/βA, βB/βB, or βA/βB) in contrast to inhibin, which consists of one α- and one β-subunit (α/βA or α/βB) (31). In the ERα+/+, inhibin-α subunit expression was 3.5-fold higher after OVX when compared with ovary-intact controls at diestrus (P < 0.01). This post-OVX increase was completely suppressed by estrogen treatment (P < 0.01; Fig. 4A, left). Ovary-intact ERα−/− had 2.8-fold greater inhibin-α expression compared with gonad-intact ERα+/+ at diestrus (P < 0.01), and levels did not change in response to OVX or estrogen replacement (P > 0.1; Fig. 4A, center). Ovary-intact ERα−/AA had similar inhibin-α expression levels compared with ovary-intact ERα+/+ at diestrus (P > 0.1), and levels increased 1.9-fold in response to OVX (P < 0.05). This post-OVX rise was completely suppressed by estrogen treatment (P < 0.01; Fig. 4A, right), indicating that pituitary inhibin subunit expression is suppressed through an ERE-independent ERα pathway.
Figure 4.
ERE-independent signaling mediates estrogen suppression of pituitary activinβB and inhibin-α but not activinβA subunit expression. Real-time quantitative PCR was used to determine inhibin-α (A), activinβB (B), and activinβA (C) mRNA levels in pituitary tissues from female mice carrying wild-type (+/+), null (−/−), or a mutant ERα with ERE signaling disrupted on the null background (−/AA) that are ovary-intact (white), OVX (black), or OVX + E2 (gray). Data were indexed to ERα+/+ OVX. Significant genotype-treatment interaction effects were detected in inhibin-α [F(4,39) = 3.15; P = 0.025], activinβB [F(4,39) = 3.46; P = 0.016], and activinβA [F(4,39) = 10.02; P < 0.0001] by two-way ANOVA (α = 0.05). Significant differences were examined by the post hoc Fisher’s LSD multiple comparison test. Differences between genotypes are denoted by a (vs. ovary-intact ERα+/+) and within genotype response to treatment by b (OVX vs. intact) and c (E2 vs. OVX). *, P < 0.05; **, P < 0.01.
ERα+/+ pituitary activinβB expression increased 2.8-fold in response to OVX (P < 0.01). This post-OVX increase was reduced 86% by estrogen treatment (P < 0.01; Fig. 4B, left). Ovary-intact ERα−/− had similar activinβB levels as ERα+/+ cycled to diestrus (P > 0.1) that were increased 2.5-fold by OVX (P < 0.01) but did not decline in response to estrogen treatment (P > 0.1; Fig. 4B, center). Ovary-intact ERα−/AA had similar activinβB expression levels as ovary-intact ERα+/+ cycled to diestrus (P > 0.1). Like ERα+/+, activinβB expression in ERα−/AA increased 1.8-fold after OVX (P < 0.01) and was 74% reduced by estrogen treatment (P < 0.01; Fig. 4B, right). Thus, the signaling through the ERE-independent ERα pathway was sufficient to convey estrogen suppression of pituitary activinβB gene expression.
Pituitary activinβA expression was increased 2.5-fold after OVX in ERα+/+ compared with ovary-intact mice cycled to diestrus (P < 0.01). This post-OVX increase was completely reversed by estrogen treatment (P < 0.01; Fig. 4C, left). Gonad-intact ERα−/− had 4.6-fold greater activinβA expression than ovary-intact ERα+/+ cycled to diestrus (P < 0.01). Levels were reduced 47% after OVX (P < 0.05) but did not change after estrogen treatment (P > 0.1; Fig. 4C, center). Ovary-intact ERα−/AA had 4.0-fold higher activinβA expression compared with ovary-intact ERα+/+ cycled to diestrus (P < 0.01). Levels were reduced 58% after OVX (P < 0.01) and, like ERα−/−, were not reduced further by estrogen treatment (P > 0.1; Fig. 4C, right). Thus, the ERE-dependent ERα pathway is required for estrogen suppression of pituitary activinβA gene expression.
Follistatin can antagonize pituitary activin signaling to reduce serum FSH and FSHβ (32,33). However, similar to previous results in ERαKO and wild-type mice (14), follistatin expression in the anterior pituitary samples was below the limit of detection (data not shown).
Discussion
In summary, in the absence of other gonadal factors, estrogen signaling via the ERE-independent pathway conveyed estrogen suppression of pituitary αGSU, FSHβ, activinβB, and inhibin-α gene expression. In contrast, the ERE-dependent pathway was required for estrogen induction of pituitary prolactin mRNA and suppression of serum FSH, pituitary FSH protein content, and LHβ and activinβA mRNA. These results suggest ERE-independent estrogen negative feedback suppression of serum LH (23) is caused by reduced secretion, not synthesis. Additionally, estrogen signaling via the ERE-dependent and -independent ERα pathways distinctly suppresses serum FSH and FSHβ subunit expression, possibly through the modulation of pituitary activin.
Rat pituitary prolactin mRNA expression is increased by estrogen treatment (34,35). This induction requires the DNA binding region of the ER and an ERE located 1 kb upstream of the prolactin gene coding sequence (36,37). A role for ERα and not ERβ was suggested by a reduction of pituitary prolactin mRNA levels in ERαKO but not ERβKO mice. Furthermore, the induction of prolactin mRNA by estrogen treatment observed in OVX wild-type female pituitary tissues was completely absent in ERαKO mice (15). Here, decreased pituitary prolactin expression after targeted deletion of ERα was confirmed. Furthermore, prolactin expression was not increased as a result of estrogen treatment in the ERα null mice and mice with isolated ERE-independent ERα signaling. This lack of an induction of prolactin expression by estrogen treatment exemplifies the selectivity by which the AA mutant ERα conveys the ERE-independent ERα signaling in vivo. Of note, decreased lactotrope cell number and growth was previously shown in ERαKO mice (38) and thus may account for the further reduction of prolactin expression in the ERα null and isolated ERE-independent ERα compared with OVX wild-type mice.
Estrogen negative feedback was shown to be due in part to decreased transcription of gonadotropin subunit mRNA in OVX, estrogen-replaced rats (9,10). This feedback occurs through ERα because mice with ERα targeted deletion exhibit increased αGSU (14,38) and LHβ (14,16,38) expression levels. Here, elevated αGSU and LHβ mRNA expression was confirmed in ovary-intact ERαKO mice. Additionally, estrogen suppression of LHβ expression required signaling through the ERE-mediated pathway, whereas the ERE-independent signaling was sufficient to mediate estrogen suppression of αGSU. These results are consistent with in vitro studies that showed ER binding to a region containing an imperfect ERE in the rat LHβ promoter (39) and estrogen suppression of an αGSU promoter construct despite the lack of an ERE or a high-affinity binding site (40). Estrogen did not suppress αGSU mRNA levels in rat pituitary cells in vitro (41,42) or in animals treated with a GnRH antagonist (43). Thus, estrogen’s suppressive effects on αGSU and LHβ expression were proposed to be indirect through a suppression of hypothalamic GnRH. This interpretation is supported by a recent report of normal basal serum LH levels in the pituitary-specific ERα knockout mouse, ERαflox/flox αGSUcre (44).
In the current study, estrogen suppression of LHβ and αGSU gene expression was not paralleled by decreased pituitary LH content in the wild type. Also, despite elevations in subunit expression, the ERαKO females did not exhibit increased pituitary LH content. In fact, ovary-intact ERαKO mice had reduced pituitary LH content. However, this appears to be due to an ovary-derived factor other than estrogen because it is lost after OVX and does not return with estrogen treatment. Despite reduced LH content, ovary-intact ERαKO mice continue to have elevated serum LH. Thus, pituitary LH content appears to be present in excess and not directly influenced by estrogen effects on subunit expression. This interpretation is in accord with previous studies that reported LH to be more highly regulated at the level of secretion than subunit gene expression (45,46) by ERα (17). Therefore, the previous observation of an ERE-independent estrogen suppression of serum LH (23) was likely caused by reduced LH secretion, not synthesis.
In contrast to LH, negative feedback regulation of FSH is primarily exerted by ovarian inhibin and pituitary follistatin by suppression of intrapituitary activin (33,47). However, in the absence of other ovarian factors, estrogen can partially suppress serum FSH and FSHβ expression. This is illustrated by ovary-intact ERαKO mice, which have similar serum FSH (16) and pituitary FSHβ (14,38) expression levels when compared with wild-type mice. After OVX, these levels increase similar to wild type. However, estrogen treatment fails to reduce the post-OVX increase serum FSH in ERαKO mice, suggesting a requirement for ERα (15). The current report confirmed ovary-intact ERαKO mice to have normal pituitary FSHβ expression, FSH content, and serum FSH that increase after OVX and are not reduced by estrogen treatment. Similarly, ovary-intact females with isolated ERE-independent ERα signaling had normal serum FSH, pituitary FSHβ expression, and FSH content levels that became elevated after OVX. Unlike ERαKO, estrogen treatment of OVX mice with isolated ERE-independent ERα signaling suppressed pituitary FSHβ expression. However, estrogen failed to suppress serum FSH in these mice. This result confirms the primary suppression of FSH by an ovarian factor other than estrogen, likely inhibin, and that estrogen suppression occurs via ERα. Furthermore, it indicates ERE-independent ERα signaling is capable of providing estrogen negative feedback suppression of pituitary FSHβ, whereas the estrogen suppression of serum FSH requires ERE-dependent ERα signaling.
The mechanism by which estrogen suppresses FSHβ gene expression is complex and may vary by species. Estrogen was shown to suppress FSHβ expression in primary pituitary cultures in the sheep, pig, and human. This suppression of FSHβ occurred in the presence of cycloheximide blockade of new protein synthesis (48), suggesting estrogen suppression occurred by a direct effect on gene expression. A region that confers estrogen suppression in the ovine FSHβ promoter was identified and shown not to have a detectable ERE or to bind ER (49). Furthermore, studies showed that GnRH and activin induction of FSHβ expression involve AP-1 sites in the promoter (50,51,52). Here, estrogen suppression of FSHβ is shown to occur via an ERE-independent ERα pathway. This result indicates that investigations of estrogen suppression of FSHβ expression should not be confined to regulatory regions with identifiable EREs. In previous studies, both wild-type and AA mutant ERα were shown to convey an estrogen-dependent suppression of an AP-1 reporter (21). Thus, perhaps ERE-independent ERα signaling acts through a tethered mechanism to suppress AP-1-mediated FSHβ induction by GnRH and/or activin.
The FSHβ promoter sequence responsible for estrogen suppression in the ewe is not conserved in the rat, suggesting a different mechanism in rodents. In particular, there is strong evidence that estrogen suppression of FSH may act indirectly through either altered hypothalamic GnRH release or suppression of intrapituitary activin. Activin increased the number of rat FSHβ primary transcripts ex vivo (53) and activation of a rat FSHβ promoter construct in vitro (54). Moreover, antibody neutralization of activinβB decreased FSH secretion in rat primary pituitary cultures (55). Also, activin subunit expression was suppressed by estrogen treatment in primary pituitary cultures from the ewe (56) coincident with decreased FSHβ gene expression and FSH secretion (57). Estrogen was further shown to suppress activin subunit mRNA in ovarian tissue and reporter constructs in vitro (58). ActivinβB expression was shown to be elevated in pituitary tissues from ERαKO mice (14), further suggesting estrogen suppression occurs via ERα. Here, activinβA and activinβB expression were shown to be elevated in ERαKO mice. Furthermore, ERE-independent ERα signaling pathway was shown to be sufficient to convey the estrogen-dependent suppression of activinβB, whereas activinβA required ERE-dependent signaling. Activin subunits can heterodimerize with inhibin-α to form inhibin. Estrogen treatment, however, did not induce but rather suppressed pituitary inhibin-α expression. Thus, the ERE-independent estrogen suppression of FSHβ may have occurred in response to reduced pituitary activinβB gene expression.
Estrogen suppression of FSHβ but not serum FSH was observed in female mice with isolated ERE-independent ERα signaling. This differed from wild-type mice, which exhibited estrogen suppression at both levels. However, pituitary FSH protein content in OVX, estrogen-treated mice with isolated ERE-independent ERα signaling is similar to wild-type. Thus, ERE-dependent suppression of serum FSH likely occurs through reduced secretion or circulating FSH half-life, rather than further suppression of synthesis (59,60). The dichotomous effect of ERE-independent estrogen signaling on FSHβ and serum FSH was unexpected. Previous studies have suggested that FSH secretion occurs through a constitutive pathway (61) and changes in FSHβ mRNA are directly reflected in serum levels (45). A lack of dissociation between estrogen suppression of FSH synthesis and secretion in wild-type mice may indicate that the ERE-dependent pathway is generally present and able to decrease the secretion of FSH. Of note, similar reduction of FSHβ but not serum FSH was observed in male mice with targeted deletion of both inhibin-α and activin receptor II after castration (62). Thus, this effect may have been produced by estrogen effects on pituitary activin and inhibin subunit expression. Or, the lack of suppression of serum FSH may have been caused by gene dosage effects because only one and not two mutant alleles were introduced into mice. This alternate explanation also applies to the lack of ERE-independent estrogen suppression observed for pituitary LHβ and activinβA gene expression.
The ERE-independent pathway appeared permissive, whereas the ERE-dependent pathway was suppressive to serum FSH. It is known that there is an increased ratio of FSH to LH release at the time of the secondary FSH surge (63). Thus, perhaps a switch from ERE-dependent to independent ERα signaling occurs at this time. However, the relevance of estrogen in the suppression of FSH in the ovary-intact animal is unclear. It is most likely that a decrease in circulating inhibin after ovulation plays the predominant role in the establishing a permissive environment for FSH release.
We have previously shown that the ERE-independent ERα pathway suppresses serum LH (23). In contrast, here we report the ERE-dependent ERα pathway is required for estrogen suppression of serum FSH. These results are consistent with a recent report that showed nongenomic estrogen signaling is capable of suppressing serum LH but not FSH in OVX ewes (64). Results from a gonadotroph/thyrotroph cell-specific ERαKO mouse suggest estrogen negative feedback on LH occurs at a suprapituitary, likely hypothalamic level (44). In contrast, this report provides further evidence that estrogen negative feedback regulation of FSH may occur, in part, through a modulation of pituitary activin. However, it remains unclear whether estrogen negative feedback of FSH might also include effects at the level of the hypothalamus. To this point, variation in GnRH pulse frequency has been described during the menstrual cycle and was shown to distinctly regulate LH and FSH (1,65,66). Thus, estrogen signaling through ERE-independent and ERE-dependent ERα signaling pathways, possibly through hypothalamic or mixed hypothalamic and pituitary effects, may act to differentially regulate serum LH and FSH.
In conclusion, data presented here suggest the previously reported ERE-independent estrogen negative feedback on LH (23) was likely due to reduced secretion. Additionally, the ERE-independent ERα signaling was sufficient to convey suppression of FSHβ expression, but the ERE-dependent ERα signaling was required to suppress serum FSH (Fig. 5). Finally, estrogen signaling through ERE-dependent and -independent ERα pathways may act to either coordinately or distinctly regulate serum LH and FSH. Therefore, the modulation of ERα signaling, in addition to changes in ovarian-derived inhibin, progesterone, pituitary activin, hypothalamic GnRH, or a putative FSH-releasing factor, may enable the distinct regulation of LH and FSH during the female mouse estrous cycle.
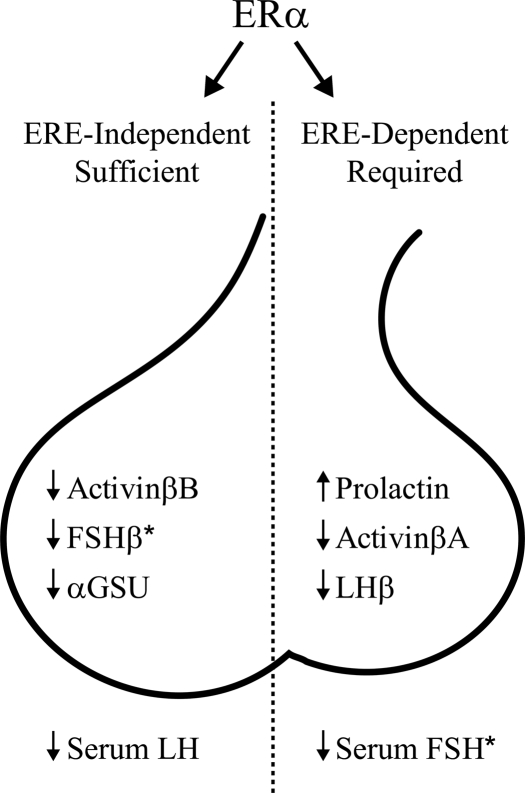
Figure 5.
ERE-independent and ERE-dependent ERα signaling pathways distinctly convey estrogen negative feedback on gonadotropin subunit expression and secretion. The ERE-independent pathway conveys estrogen suppression of FSHβ expression, whereas estrogen suppression of serum FSH requires ERE-dependent signaling. These effects may be mediated through distinct ERα pathway effects on activin/inhibin subunit expression. In contrast, estrogen pathways distinctly suppressed LHβ expression but did not decrease pituitary LH content. Thus, our previous observation of an ERE-independent ERα-mediated estrogen suppression of serum LH (23) likely occurred as the result of reduced secretion and not synthesis, possibly via altered hypothalamic GnRH. *, Serum FSH and FSHβ expression levels in ovary-intact vs. OVX + E2 wild-type mice suggest that ovarian factors besides estrogen, likely inhibin, are additionally required for full suppression.
Supplementary Material
Acknowledgments
ERαKO mice were a generous gift from Dr. Pierre Chambon at the Institut de Génétique et de Biologie Moléculaire et Cellulaire, Institut Clinique de la Souris, CNRS/INSERM/ULP, Collège de France, Illkirch Cedex, France. We acknowledge Lisa Fisher, Jennifer Wedgewood, and Thomas Kotlar for their support and thank Dr. Francisco J. López for his technical assistance.
Footnotes
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development (NICHD) Grant PO1 HD21921, Training Grant T32 GM008061, and NICHD (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934, and University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: AP-1, Activator protein 1; Ct, threshold cycle; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; ERαKO, ERα knockout; αGSU, glycoprotein hormone α-subunit; LSD, least significant difference; NERKI, nonclassical ER knock-in; OVX, ovariectomy; RPL19, ribosomal protein L19.
References
- Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E 1981 Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- Knobil E 1981 Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol Reprod 24:44–49 [DOI] [PubMed] [Google Scholar]
- Levine JE 1997 New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Gregg DW, Schwall RH, Nett TM 1991 Regulation of gonadotropin secretion and number of gonadotropin-releasing hormone receptors by inhibin, activin-A, and estradiol. Biol Reprod 44:725–732 [DOI] [PubMed] [Google Scholar]
- Horvath JE, Helyes Z, Flerko B 1996 Gonadectomy modifies the gender specific pattern of desensitization of pituitary cells by gonadotropin-releasing hormone in the superfusion system. Acta Biol Hung 47:195–205 [PubMed] [Google Scholar]
- Henry HL, Norman AW 2003 Encyclopedia of hormones. Amsterdam and Boston: Academic Press [Google Scholar]
- Schwartz NB, Channing CP 1977 Evidence for ovarian “inhibin”: suppression of the secondary rise in serum follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc Natl Acad Sci USA 74: 5721–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1988 Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology 122:1842–1846 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Badger TM, Chin WW 1987 Sex steroid hormone regulation of follicle-stimulating hormone subunit messenger ribonucleic acid (mRNA) levels in the rat. J Clin Invest 80:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Suhr A, Marshall JC 1990 Gonadal regulation of gonadotropin subunit gene expression: evidence for regulation of follicle-stimulating hormone-β messenger ribonucleic acid by nonsteroidal hormones in female rats. Endocrinology 127:798–806 [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Knight CD, Shupnik MA, Haisenleder DJ, Aloi J, Kirk SE, Yasin M, Marshall JC 1993 Ovariectomy and inhibin immunoneutralization acutely increase follicle-stimulating hormone-β messenger ribonucleic acid concentrations: evidence for a nontranscriptional mechanism. Endocrinology 132:1297–1304 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF 1999 Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor α. Endocrine 11:137–143 [DOI] [PubMed] [Google Scholar]
- Lindzey J, Jayes FL, Yates MM, Couse JF, Korach KS 2006 The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-α. J Endocrinol 191:309–317 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, de Las Mulas JM, Bellido C, Navarro VM, Aguilar R, Garrido-Gracia JC, Malagon MM, Tena-Sempere M, Blanco A 2006 Gonadotropin-secreting cells in ovariectomized rats treated with different oestrogen receptor ligands: a modulatory role for ERβ in the gonadotrope? J Endocrinol 188:167–177 [DOI] [PubMed] [Google Scholar]
- O'Malley BW, Tsai MJ 1992 Molecular pathways of steroid receptor action. Biol Reprod 46:163–167 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL 2001 Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL 2002 An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.), Committee on Care and Use of Laboratory Animals Guide for the care and use of laboratory animals. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD 1975 The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE 1999 Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140:3653–3658 [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP 2002 Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614 [DOI] [PubMed] [Google Scholar]
- Box GE, Cox DR 1964 An analysis of transformations. J Royal Stat Soc Ser B 26:211–252 [Google Scholar]
- Box GHWJ 1974 Correcting inhomogeneity of variance with power transformation weighting. Technometrics 16:385–389 [Google Scholar]
- Bernard DJ, Chapman SC, Woodruff TK 2001 Mechanisms of inhibin signal transduction. Recent Prog Horm Res 56:417–450 [DOI] [PubMed] [Google Scholar]
- Phillips DJ, de Kretser DM 1998 Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol 19:287–322 [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Hedger MP, Loveland KL, Phillips DJ 2002 Inhibins, activins and follistatin in reproduction. Hum Reprod Update 8:529–541 [DOI] [PubMed] [Google Scholar]
- Stone RT, Maurer RA, Gorski J 1977 Effect of estradiol-17β on preprolactin messenger ribonucleic acid activity in the rat pituitary gland. Biochemistry 16:4915–4921 [DOI] [PubMed] [Google Scholar]
- Ryan R, Shupnik MA, Gorski J 1979 Effect of estrogen on preprolactin messenger ribonucleic acid sequences. Biochemistry 18:2044–2048 [DOI] [PubMed] [Google Scholar]
- Maurer RA, Notides AC 1987 Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol Cell Biol 7:4247–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman ML, Adler S, Nelson C, Greene GL, Evans RM, Rosenfeld MG 1988 A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol Endocrinol 2:14–21 [DOI] [PubMed] [Google Scholar]
- Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG 1997 Role of estrogen receptor-α in the anterior pituitary gland. Mol Endocrinol 11:674–681 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Weinmann CM, Notides AC, Chin WW 1989 An upstream region of the rat luteinizing hormone β gene binds estrogen receptor and confers estrogen responsiveness. J Biol Chem 264:80–86 [PubMed] [Google Scholar]
- Keri RA, Andersen B, Kennedy GC, Hamernik DL, Clay CM, Brace AD, Nett TM, Notides AC, Nilson JH 1991 Estradiol inhibits transcription of the human glycoprotein hormone α-subunit gene despite the absence of a high affinity binding site for estrogen receptor. Mol Endocrinol 5:725–733 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gharib SD, Chin WW 1989 Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 3: 474–480 [DOI] [PubMed] [Google Scholar]
- Shupnik MA 1996 Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod 54:279–286 [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Fallest PC 1994 Pulsatile GnRH regulation of gonadotropin subunit gene transcription. Neurosci Biobehav Rev 18:597–599 [DOI] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C 2008 Pituitary gonadotroph estrogen receptor α (ERα) is necessary for fertility in females. Endocrinology 149:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JE, Clements JA, Funder JW, Clarke IJ 1989 Regulation of follicle-stimulating hormone β and common α-subunit messenger ribonucleic acid by gonadotropin-releasing hormone and estrogen in the sheep pituitary. Neuroendocrinology 50:321–326 [DOI] [PubMed] [Google Scholar]
- Mercer JE, Phillips DJ, Clarke IJ 1993 Short-term regulation of gonadotropin subunit mRNA levels by estrogen: studies in the hypothalamo-pituitary intact and hypothalamo-pituitary disconnected ewe. J Neuroendocrinol 5:591–596 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW 2006 Pituitary actions of ligands of the TGF-β family: activins and inhibins. Reproduction 132: 207–215 [DOI] [PubMed] [Google Scholar]
- Phillips CL, Lin LW, Wu JC, Guzman K, Milsted A, Miller WL 1988 17β-Estradiol and progesterone inhibit transcription of the genes encoding the subunits of ovine follicle-stimulating hormone. Mol Endocrinol 2:641–649 [DOI] [PubMed] [Google Scholar]
- Miller CD, Miller WL 1996 Transcriptional repression of the ovine follicle-stimulating hormone-β gene by 17β-estradiol. Endocrinology 137:3437–3446 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL 1997 Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL 1998 Transcriptional activation of the ovine follicle-stimulating hormone β-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology 139:4455–4465 [DOI] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL 2001 Transcriptional regulation of the ovine follicle-stimulating hormone-β gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology 142:2267–2274 [DOI] [PubMed] [Google Scholar]
- Weiss J, Guendner MJ, Halvorson LM, Jameson JL 1995 Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136:1885–1891 [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB 2005 Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- Corrigan AZ, Bilezikjian LM, Carroll RS, Bald LN, Schmelzer CH, Fendly BM, Mason AJ, Chin WW, Schwall RH, Vale W 1991 Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology 128:1682–1684 [DOI] [PubMed] [Google Scholar]
- Nett TM, Turzillo AM, Baratta M, Rispoli LA 2002 Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol 23:33–42 [DOI] [PubMed] [Google Scholar]
- Baratta M, West LA, Turzillo AM, Nett TM 2001 Activin modulates differential effects of estradiol on synthesis and secretion of follicle-stimulating hormone in ovine pituitary cells. Biol Reprod 64:714–719 [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE 2007 Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology 148:1968–1976 [DOI] [PubMed] [Google Scholar]
- Robertson DM, Foulds LM, Fry RC, Cummins JT, Clarke I 1991 Circulating half-lives of follicle-stimulating hormone and luteinizing hormone in pituitary extracts and isoform fractions of ovariectomized and intact ewes. Endocrinology 129:1805–1813 [DOI] [PubMed] [Google Scholar]
- Stanton PG, Burgon PG, Hearn MT, Robertson DM 1996 Structural and functional characterisation of hFSH and hLH isoforms. Mol Cell Endocrinol 125:133–141 [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR 2003 The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reproduction 61(Suppl):463–476 [PubMed] [Google Scholar]
- Kumar TR, Agno J, Janovick JA, Conn PM, Matzuk MM 2003 Regulation of FSHβ and GnRH receptor gene expression in activin receptor II knockout male mice. Molecular and cellular endocrinology 212:19–27 [DOI] [PubMed] [Google Scholar]
- Fallest PC, Schwartz NB 1990 Pituitary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) responses to gonadotropin-releasing hormone during the rat estrous cycle: an increased ratio of FSH to LH is secreted during the secondary FSH surge. Biol Reprod 43:977–985 [DOI] [PubMed] [Google Scholar]
- Arreguin-Arevalo JA, Nett TM 2006 A nongenomic action of estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone in ovariectomized ewes. Biol Reprod 74:202–208 [DOI] [PubMed] [Google Scholar]
- Knobil E 1980 The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88 [DOI] [PubMed] [Google Scholar]
- Savoy-Moore RT, Swartz KH 1987 Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv Exp Med Biol 219:641–645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.