Abstract
We cloned a cDNA encoding a novel (GnRH), named lamprey GnRH-II, from the sea lamprey, a basal vertebrate. The deduced amino acid sequence of the newly identified lamprey GnRH-II is QHWSHGWFPG. The architecture of the precursor is similar to that reported for other GnRH precursors consisting of a signal peptide, decapeptide, a downstream processing site, and a GnRH-associated peptide; however, the gene for lamprey GnRH-II does not have introns in comparison with the gene organization for all other vertebrate GnRHs. Lamprey GnRH-II precursor transcript was widely expressed in a variety of tissues. In situ hybridization of the brain showed expression and localization of the transcript in the hypothalamus, medulla, and olfactory regions, whereas immunohistochemistry using a specific antiserum showed only GnRH-II cell bodies and processes in the preoptic nucleus/hypothalamus areas. Lamprey GnRH-II was shown to stimulate the hypothalamic-pituitary axis using in vivo and in vitro studies. Lamprey GnRH-II was also shown to activate the inositol phosphate signaling system in COS-7 cells transiently transfected with the lamprey GnRH receptor. These studies provide evidence for a novel lamprey GnRH that has a role as a third hypothalamic GnRH. In summary, the newly discovered lamprey GnRH-II offers a new paradigm of the origin of the vertebrate GnRH family. We hypothesize that due to a genome/gene duplication event, an ancestral gene gave rise to two lineages of GnRHs: the gnathostome GnRH and lamprey GnRH-II.
THE ACQUISITION OF a hypothalamic-pituitary axis was a seminal event in vertebrate evolution leading to the neuroendocrine control of many complex functions including growth, reproduction, osmoregulation, stress, and metabolism. The synthesis and secretion of GnRH is the key neuroendocrine function in the hypothalamic regulation of the hypothalamic-pituitary-gonadal axis. In response to GnRH, gonadotropins (GTH), LH, and FSH are released from the pituitary gland, which in turn regulate gametogenesis and steroidogenesis.
GnRH was first isolated from porcine (1) and ovine (2) hypothalamic extracts. The GnRH family currently includes 25 isoforms, 14 and 11 from representative vertebrate and invertebrate species, respectively (3). To date, two to three isoforms have been identified in representative species of all classes of vertebrates (4). The number of GnRH molecular variants is surprising, particularly in view of the relative constancy of their physiological role in vertebrates (5). Growing evidence reveals almost all vertebrates synthesize at least two isoforms of GnRH in the brain (4,6,13,50,51). During the past few years, as more GnRH primary sequences and their respective cDNAs have been identified, more phylogenetic and functional studies have been done leading to proposed phylogenetic relationships of the GnRHs (3,5,9,10,11). An analysis by Fernald and White (10) suggested that there are three paralogous groups of GnRH based on phylogenetic analysis of 23 GnRH transcripts, location of expression of the GnRH within the brain, and function of the GnRH. This analysis excluded the lamprey GnRHs. In a recent review of GnRH and its receptors, Kah et al. (4) also suggested three lineages and did not include the lamprey GnRH-I and -III and cDNAs or place them into one of the three groups of GnRH. We suggested from an extensive analysis of 72 cDNA GnRH sequences including the eight cloned lamprey GnRH-III cDNAs that the vertebrate GnRHs are grouped into four lineages: GnRH 1, 2, 3, and 4 (5). Our phylogenetic analysis confirmed Fernald and White’s model of three GnRH lineages and in addition showed that lamprey GnRH-I and -III form a fourth group. Recently the identification of another invertebrate GnRH in Aplysia has suggested a fifth grouping of GnRHs in invertebrates and supports the fourth group of lamprey GnRH-I and -III (3).
The availability of the lamprey genome now offers the ability to further examine the phylogenetic relationships of the vertebrate GnRHs. Lampreys are one of only two extant representatives of the jawless vertebrate class, Agnatha. It is generally believed that two large-scale genome duplications occurred during the evolution of early vertebrates, although there is controversy on whether the two large-scale genome duplications occurred before the divergence of the hagfish and lampreys or whether there was one round before the jawless vertebrates and one round after the divergence of the jawless vertebrates (14,15,16,17,18,19). Lampreys are the most basal vertebrates for which there are demonstrated functional roles for two GnRHs (lamprey GnRH-I and GnRH-III) that act via the pituitary-gonadal axis controlling reproductive processes (20). Lamprey GnRH-I and -III are each expressed by a separate gene (5,21). There are still questions about the phylogenetic relationship between the jawless vertebrate GnRHs and the variants found in gnathostomes.
Identification and analysis of this third form of lamprey GnRH may clarify some important aspects of the evolutionary origin of the vertebrate GnRH family. In jawed vertebrates, GnRH-II is the most ubiquitously expressed of the GnRH family and has the identical primary amino acid structure in all gnathostomes, from sharks to mammals (4,5,12). As more GnRHs were identified during the past 20 yr, one of the questions that arose was the origin of the vertebrate GnRHs and the lack of a lamprey or hagfish GnRH form more closely related to any of the gnathostome GnRH lineages. The primary amino acid sequence of GnRH-II has remained unchanged from cartilaginous fish through mammals, which would suggest a strong selective pressure, however, to date its function is ambiguous (4,21,22). The expression pattern of GnRH-II varies from species to species but is generally expressed in the brain and numerous peripheral tissues (4,12). The function(s) of GnRH-II in peripheral tissues and brain have not been established. In the brain, GnRH-II predominates in extrahypothalamic regions and has been suggested to act as a neuromodulator/neurotransmitter, although there are species in which GnRH-II is found in the hypothalamic regions and acts at the pituitary in stimulating gonadotropin function (27). Here we report the identification of a novel form of GnRH from the sea lamprey, Petromyzon marinus, named lamprey GnRH-II, and considered a sister group to all forms of Gnathostome GnRH. In biological studies, lamprey GnRH-II stimulated the hypothalamic-pituitary axis. Lamprey GnRH-II was also shown to activate the inositol phosphate signaling system in transiently transfected COS7 cells with the lamprey GnRH receptor. Using in situ hybridization and immunohistochemistry (IHC), expression of lamprey GnRH-II transcript and location of GnRH-II peptide was shown in the preoptic/hypothalamic areas and unlike the other lamprey GnRHs, lamprey GnRH-II transcript was also expressed in extrahypothalamic regions in the midbrain and olfactory region. These studies provide evidence for an ancestral GnRH that has a role as a third hypothalamic GnRH in a basal vertebrate.
Materials and Methods
Screening the lamprey genome
Trace files were produced by the Genome Sequencing Center at Washington University School of Medicine (St. Louis, MO) for the sea lamprey genome sequencing project and were obtained from the National Center for Biotechnology (ftp.ncbi. nlmgov/pub/TraceDB/petromyzon_marinus), and blasted locally, using National Center for Biotechnology Information blast software (Bethesda, MD), against a custom database consisting only of GnRH preprohormone sequences. The parameter used for the searches was blastall-p, blastx-a, -e100, -v100, and -b100. The resulting sequences with high similarity were then manually reexamined for their resemblance to the GnRH preprohormone. The following are the trace identification numbers first identified with similarity to known forms of GnRH: 1170767299, 1180014463, 1194330245, 1193338384, and 1438788497.
Lamprey
For all studies, a total of 98 adult sea run lamprey were collected at the Cocheco River fish ladder in Dover, NH. The lamprey were then transported to the Anadramous Fish and Invertebrate Research Laboratory in Durham, NH, where they were maintained in a artificial spawning channel with flow-through reservoir water at ambient temperatures 10–18 C and following University of New Hampshire Institutional Animal Care and Use guidelines.
Isolation of total RNA and genomic DNA
Total RNA was isolated from liver, kidney, intestine, muscle, heart, thyroid, brain, testis, ovary, pituitary, and eye using ISOGEN (Nippon Gene Co. Ltd., Toyama, Japan), following the protocol as outlined by Amemiya et al. (22). Total RNA was then deoxyribonuclease (DNase) treated using RQ1 ribonuclease-free DNase (Promega, Madison, WI) following manufacturer protocol. Genomic DNA was isolated as described by Silver et al. (5).
RT and PCR
PCR was performed using Petromyzon marinus first-strand cDNA and genomic DNA as the templates with primers designed to the elucidated lamprey GnRH-II sequence using Primer 3 (23). The following primers were prepared by Integrated DNA Technologies, Inc. (Coralville, IA): PmF1, 5′-AATGAACGAATCGATTGCTGTCTGTC-3′, PmF2, 5′-ATGAATGAACGAATCGAT TGCTGTCTG-3′, PmR1, 5′-AAAACC CATTTGGAGAGCGTGAAC-3′, PmR2, 5′-CTCCAGCACTTGGGAGAGAACGTC-3′, Pm β-actin F, 5′-GACCTCACCGA CTACCTGATGA-3′, Pm β-actin R, 5′-TGATGTCGCGCACGATCT-3′. Using the AccessQuick RT-PCR system (Promega), 50 ng of DNase-free RNA were used in each reaction with a 0.1 μm final primer concentration in a 25.5-μl final reaction volume. Thermal cycling was performed using an Eppendorf Master Gradient thermal cycler using the following parameters: 48 C for 45 min, 94 C for 2 min followed by 35 cycles of 94 C for 1 min, primer specific annealing temperature for 30 sec and 72 C for 50 sec, and finishing with a 10 min 72 C incubation and 4 C hold. PCR of genomic DNA was done as follows: reactions were performed using Promega components consisting of 0.5 U of Taq DNA polymerase, 1 mm MgCl, 10 mm Tris-HCl (pH 9) at 25 C, 50 mm KCl, 0.1% Triton X-100, 0.2 mm deoxynucleotide triphosphate mix, and 0.1 μm forward and reverse primers. Cycling was done using an Eppendorf Mastercycler gradient, using the following reaction conditions: 35 cycles of 94 C for 50 sec, 58 C for 30 sec, and 72 C for 30 sec, concluding with a hold of 72 C for 10 min and for digoxigenin (DIG) probe synthesis: activation step of 94 C for 7 min; 94 C for 1 min, 57 C for 1 min, 72 C for 1 min, and a 72 C extension for 10 min followed with 4 C hold. All PCRs were electrophoresed in 2% agarose and visualized using a UV light box and images captured with a digital camera. DNA sequencing was performed at the Hubbard Genome Research Center at the University of New Hampshire.
Phylogenetic analysis
The alignment used to generate the neighbor joining tree consisted of 43 prepro-GnRH amino acid sequences retrieved from GenBank and the deduced prepro-lamprey GnRH-II sequence (GenBank accession no. DQ457017). The alignments were produced using MegAlign in the DNAStar software package from Lasergene Clustal W method. The tree was rooted with the prepro-Aplysia GnRH sequence. The alignments were then analyzed using phylogenetic analysis using parsimony version 4.0 β10 (24), and the trees were constructed using the neighbor joining method with 1000 bootstrap replicates.
In situ hybridization
Probe synthesis for in situ was done as previously described (25,26) with the following modifications. DIG-labeled sense and antisense RNA probes of complementary DNAs of lamprey GnRH-II were prepared to encompass the regions encoding the preprohormone using the DIG-labeled riboprobe synthesis kit (Roche, Indianapolis, IN). The primers, PmF1, PmF2, PmR1, and PmR2, were used with cDNA template derived from the brain using first-strand synthesis kit (Amersham Biosciences, Piscataway, NJ).
Hybridization was performed following the protocol of Root et al. (25) and Rubin et al. (26). A dot blot was done to determine cross-reactivity between antisense probes as previously described (25). The data showed that each probe only bound to their respective template (data not shown). Sections were analyzed using the confocal facilities at the University of New Hampshire with a LSM 510 Meta laser-scanning confocal microscope (Zeiss, Thornton, NY) with a 633-nm helium-neon laser as outlined by Trinh et al. (27) using the following parameters: filter 638–799 nm, averaging 8, unmixed, pixel time 3.37 μsec, 20× objective.
Peptide synthesis and antiserum generation
Lamprey GnRH-I and -III were purchased from American Peptide Co., Inc. (Sunnyvale, CA). The newly identified GnRH (lamprey GnRH-II) peptide was synthesized by American Peptide and purified to 95% purity. The synthesized peptide was shipped to Colcalico Biologicals (Reamstown, PA) for polyclonal antiserum production. At Colcalico Biologicals, the lamprey GnRH-II was conjugated to BSA via glutaraldehyde following the procedures described by Coligan (28). Four female New Zealand white rabbits housed at their facility were injected with the peptide (two rabbits) boosted and bled (29). All bleeds were sent to us for characterization of the polyclonal antisera.
Immunocytochemistry
Tissue preparations and immunohistochemical staining procedures were described elsewhere (30,31). Anti-lamprey GnRH-II (optimum working dilution: 1:400, no. UNH107) was applied as a primary antiserum. To confirm the specificity of the immunostaining, the following control staining procedures were performed: 1) replacement of primary antiserum with normal rabbit serum, 2) absorption of antilamprey GnRH-II with synthetic lamprey GnRH-I, -II, -III, or mixture of -I and -III (20–50 μg per 200 μl antiserum at working dilution), and 3) absorption of antilamprey GnRH-I (lot King 1467) and antilamprey GnRH-III (lot 3952), both of which had been previously validated for localization of GnRH-I and -III, respectively (29), with synthetic lamprey GnRH-II (25 μg per 100 μl antiserum at working dilution). Replacement of the primary antiserum with normal rabbit serum gave no positive reaction. Positive reaction to antilamprey GnRH-II in the hypothalamus including the preoptic nucleus was abolished only by the preabsorption with synthetic lamprey GnRH-II. After the preabsorption with synthetic lamprey GnRH-I, -III, or mixture of -I and -III, GnRH-II-positive reaction was reduced considerably but not eliminated. Moreover, both lamprey GnRH-I and -III-positive reactions were not eliminated by the preabsorption with synthetic lamprey GnRH-II, respectively. Thus, taking both data of items 2 and 3 into considerations, GnRH-II-like, but not -I-like or -III-like, material is present in the preoptic nucleus, even though antilamprey GnRH-II exhibited slight cross-reactivity to GnRH-I and -III. All results were described by use of anti-GnRH-II preabsorbed with a mixture of synthetic lamprey GnRH-I and -III.
In vitro and in vivo biological studies
The in vitro experiment followed the protocol previously outlined by Gazourian et al. (32). Briefly, ovarian tissue was removed from sea lamprey and placed in a petri dish containing Hanks’ balanced salt solution (HBSS; Sigma, St. Louis, MO) at pH 7.0 and held on ice. The ovarian tissue was cut into small sections (∼30 mg). Pituitaries were removed from decapitated lampreys and immediately placed into the same buffer. Six samples were used for each treatment. After preincubation, the ovarian sections were incubated in triplicate for 18 h at 18 C with either 300 μl lamprey GnRH-I, -II, or -III at the following concentrations: control (HBSS/HEPES) and 10, 100, or 1000 ng/ml. The cocultures of ovarian sections and pituitary (one per well) were incubated (in triplicate) in 300 μl of 1000 ng/ml lamprey GnRH-II or -III. At the end of the incubation period, the gonad tissue was blotted and then weighed, and culture media were collected frozen at −80 C until extracted and assayed for estradiol by RIA. The validation of the estradiol RIA was described for adult sea lamprey plasma by Sower et al. in 1983 (33).
The in vivo experiment followed the protocol previously described by Sower et al. (34). In brief, female sea lamprey (10 per treatment) were injected ip twice with either lamprey GnRH-II or GnRH-III at doses of 0, 50, or 100 μg/kg body weight at 24-h intervals, and then blood was sampled at 24 h. The plasma was separated from the blood and stored at −80 C until extracted and assayed for estradiol by RIA.
Inositol phosphate (IP) assay
The IP stimulation and extraction protocol followed the procedure of Silver et al. (35). Lamprey GnRH-I, lamprey GnRH-II, or lamprey GnRH-III were used for dose-response analysis; concentrations of ligand used were from 10−6 to 10−11 m. Treatments were performed in triplicate in three independent experiments. Nontransfected cells and cells transfected with blank vector were used as negative controls. The results were analyzed using Prism (GraphPad, San Diego, CA).
Estradiol RIA
The RIA for estradiol followed the procedures described previously (33). The RIA for estradiol-17β used antiestradiol-17β (S-244) obtained from G. Niswender (Colorado State University, Fort Collins, CO). The estradiol antiserum was used at a dilution of 1:85,000. The range of binding was between 24.1 and 29.3%. The intraassay and interassay coefficients of variation were 3.8 and 8.5%, respectively.
Statistics
The resulting hormone concentrations were analyzed with StatView 5.0 (SAS, Carey, NC) using one-way ANOVA followed by Fisher’s protected least significant difference test. The level of significance for differing groups was P < 0.05.
Results
Cloning and sequence analysis of prepro-lamprey GnRH-II
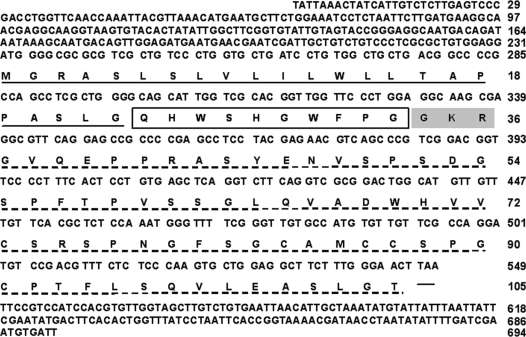
A 694-base sequence of the cDNA of lamprey GnRH-II was cloned from the sea lamprey. The cloned precursor includes 105 amino acids with a signal peptide of 23 amino acids; a GnRH decapeptide, dibasic processing site, and 69 amino acids of the GnRH-associated peptide (Fig. 1) (GenBank accession no. DQ457017). The deduced amino acid sequence of the newly identified lamprey GnRH-II in lamprey is QHWSHGWFPG (Fig. 2).
Figure 1.
Putative lamprey GnRH-II transcript map. The putative prepro-lamprey GnRH-II nucleotide and deduced amino acid sequences. Regions corresponding to known GnRHs are shown; signal peptide is underlined, the lamprey GnRH-II decapeptide is boxed, the dibasic processing site is boxed in gray and the GnRH-associated peptide is dash-underlined. The stop codon is indicated by a hyphen.
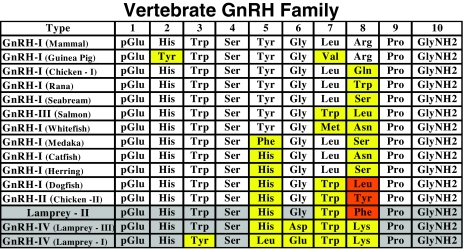
Figure 2.
Primary structure of GnRHs in vertebrates. There are currently 15 reported isoforms of GnRH in vertebrates. Yellow highlight, Amino acids changed, compared with mammalian GnRH; gray highlight, GnRHs occurring in the sea lamprey; orange highlight, single amino acid difference between dogfish, chicken-II, and lamprey GnRH.
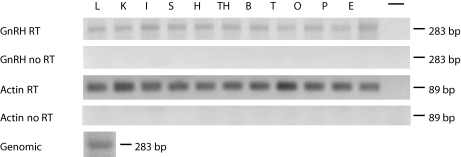
Analysis of the lamprey genome shows the gene for lamprey GnRH-II does not have introns. Primers used were designed to encompass the nucleotide sequence surrounding two introns normally present in the vertebrate GnRH gene. Results from PCR with genomic DNA experiment confirm the lack of introns (Fig. 3).
Figure 3.
Expression and analysis of lamprey GnRH-II. Expression of lamprey GnRH in several tissues is shown using RT-PCR (first panel); negative control, RNA that was not reverse transcribed (second panel). Lamprey actin was used to ensure quality of RNA (third panel) and actin without reverse transcription (RT; fourth panel). Results from PCR with genomic DNA template products (fifth panel) are the same size as those produced with RT-PCR, indicating the lack of introns. L, Liver; K, kidney; I, intestine; S, skeletal muscle; H, heart muscle; TH, thyroid; B, brain; T, testis; O, ovary; P, pituitary; E, eye; −, negative control.
Tissue expression
The lamprey GnRH-II transcript was shown to be wide spread in various tissues including liver, kidney, intestine, skeletal muscle, heart muscle, thyroid, brain, testis, ovary, pituitary, and eye (Fig. 3). Negative controls of reactions that did not have template or were not reverse transcribed did not show contamination with genomic DNA.
Phylogenetic analysis
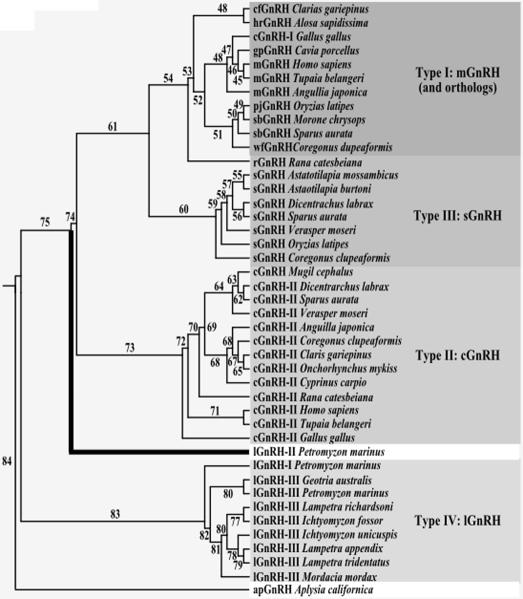
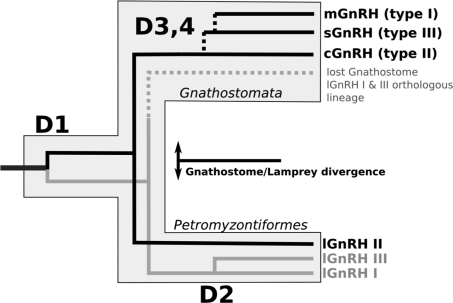
Phylogenetic analysis segregates the prepro-GnRHs into four groups: type I (mammalian and orthologs), type II (chicken-II), type III (salmon), and type IV (lamprey I and III) (Fig. 4). Further analysis suggests that lamprey GnRH II evolved from a common ancestor of all Gnathostome forms of GnRH (Fig. 5). The gene duplication events that generated the different fish and tetrapod paralogous groups (D3, D4) took place within the Gnathostome lineage, after its divergence from the ancestral agnathans. The two other forms of lamprey GnRH (I and III) can be identified now as paralogous homologs of Gnathostome GnRH and lamprey GnRH II group, resulting from a duplication within the lamprey lineage (D2) (Fig. 5).
Figure 4.
Phylogenetic analysis of GnRH precursors. The neighbor-joining method with 1000 bootstrap replicates was used to produce this phylogenetic tree using the amino acid sequences of the prepro-GnRHs (signal peptide, decapeptide, dibasic cleavage site, and GAP) and is rooted with Aplysia-GnRH. The branch of lamprey GnRH-II is highlighted with a thick black line. Numbers indicate the percentage of bootstrap replicates in which the labeled branch was reproduced. m, Mammalian; gp, guinea pig; pj, pejerry; sb, seabream; wf, whitefish; cf., catfish; hr, herring; r, rana; s, salmon; c, chicken; lamprey; ap, Aplysia.
Figure 5.
Evolution of vertebrate GnRH family of neuropeptides. This is a schematic diagram in which we hypothesize that lamprey GnRH-II evolved from a common ancestor of all Gnathostome forms of GnRH (black line). The gene duplication events that generated the different fish and tetrapod paralogous groups (D3, D4) took place within the Gnathostome lineage, after its divergence from the ancestral agnathans. The two other forms of lamprey GnRH (I and III, gray line) can be identified now as paralogous homologs of Gnathostome GnRH and lamprey GnRH II group, resulted from a duplication within the lamprey lineage (D2). This implies that there probably was a genome/gene duplication event, which gave rise to all forms of vertebrate GnRH affecting the common ancestor all of lamprey and Gnathostome isoforms (D1). The Gnathostome branch of the lamprey I and III orthologous group was probably lost during evolution (dotted gray line).
In situ hybridization
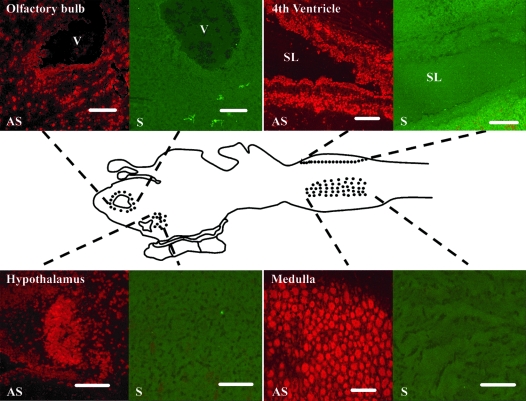
In situ hybridization showed expression of the GnRH-II transcript in the optic lobe, hypothalamus, fourth ventricle, and medulla (Fig. 6).
Figure 6.
In situ hybridization of the adult lamprey brain. In situ hybridization of lamprey brain and sagittal tissue sections show distinct cell populations expressing lamprey GnRH-II and their relative locations within the lamprey brain. AS, Antisense probe; S, sense probe; V, ventriculi lateralis; SL, sulcus limitans. Scale bar, 100 μm.
Immunohistochemistry
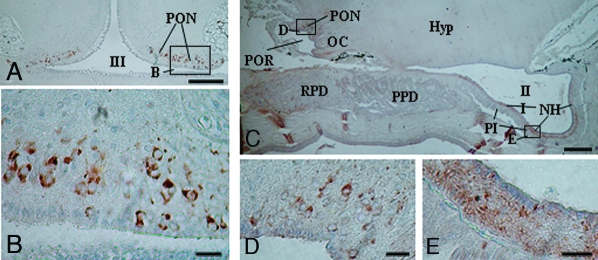
Cells stained with anti-lamprey GnRH-II were densely distributed in the arc-shaped hypothalamic area extending from the preoptic nucleus to the postoptic commissure nucleus (Fig. 7, A–D). In transverse sections through the preoptic nucleus, most immunoreactive (ir) GnRH-II cells were found in a neuron-rich zone just below the ependyma of the third ventricle (Fig. 7, A and B). Most ir-GnRH-II nerve fibers originating from cells in the arc-shaped area projected to the neurohypophysis (Fig. 7E) and formed the preopticohypophysial GnRH tract.
Figure 7.
IHC lamprey GnRH-II-like ir in the hypothalamus (hyp) of adult sea lampreys. Transverse (A) and sagittal (C) sections were stained with antilamprey GnRH-II, which was previously absorbed with a mixture of synthetic lamprey GnRH-I and -III. The areas outlined by the rectangle (A and C) are enlarged and shown in B, D, and E, respectively. Note lamprey GnRH-II-positive cell bodies in the preoptic nucleus (PON) and fibers in the neurohypophysis (NH). OC, Optic chiasm; PI, pars intermedia; POR, preoptic recess; PPD, proximal pars distalis; RPD, rostral pars distalis; III, third ventricle. Scale bars (A and C), 200 μm (A, ×50; C, ×40); B, D, and E, 20 μm (B, ×390; D, ×360; E, ×450).
In vitro and in vivo biological studies
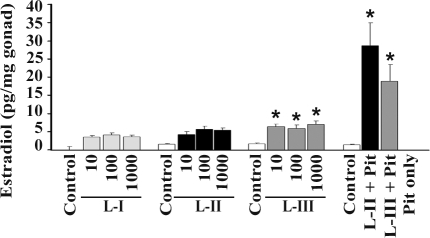
In incubations with the ovary only, lamprey GnRH-I, -II, or -III increased media estradiol concentrations when compared with control; however, only estradiol from lamprey GnRH-III-treated ovary had significantly higher estradiol (P < 0.05) (Fig. 8). The cocultures of pituitary and ovary with either lamprey GnRH-II or -III (24 ± 6.4, pg/mg ovary or 25.6 ± 4.6 pg/mg ovary, respectively, mean ± se) had significantly higher media estradiol concentrations, compared with controls (1.6 ± 0.1 pg/mg ovary) (P < 0.05). Estradiol from media in the lamprey-II GnRH-treated ovary-pituitary coculture was significantly higher (P < 0.05), compared with the lamprey GnRH-III treatment. Estradiol was not detected in medium from the pituitary only culture (Fig. 8).
Figure 8.
In vitro ovarian and ovarian-pituitary cultures. Estradiol (picograms per milligram of tissue) after ovary treatments with HBSS (pH 7.0) with 25 mm HEPES (control), lamprey GnRH-I, -II, -III (10, 100, or 1000 ng/ml), coculture of pituitary and ovary treated with lamprey GnRH-I or -III (1000 ng/ml) and pituitary in HBSS (pH 7.0) with 25 mm HEPES. Lamprey GnRH-III-treated ovary had significantly higher estradiol (P < 0.05). The cocultures of pituitary and ovary with either lamprey GnRH-II or -III had significantly higher media estradiol concentrations, compared with controls (P < 0.05). *, Significance.
In the in vivo experiments, lamprey GnRH-II (50 or 100 μg/kg) or lamprey GnRH-III (50 or 100 μg/kg) increased plasma estradiol concentrations significantly (P < 0.05), compared with control. Also, lamprey GnRH-II (50 or 100 μg/kg) significantly increased plasma estradiol concentrations, compared with lamprey GnRH-III (P < 0.05) (Fig. 9).
Figure 9.
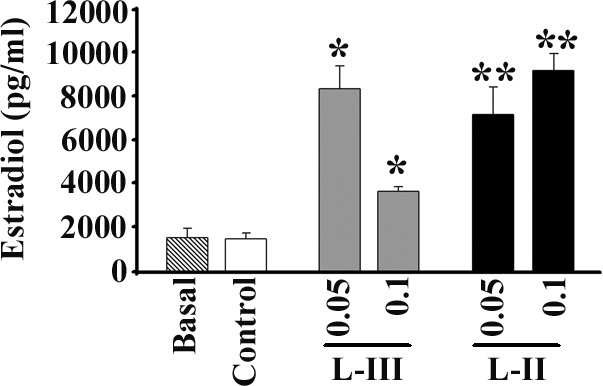
In vivo biological assay. Plasma estradiol (picograms per milliliter) following no injection (basal), injection with saline (control), lamprey GnRH-III (50 or 100 μg/kg), or lamprey GnRH-II (50 or 100 μg/kg). Lamprey GnRH-II (50 or 100 μg/kg) and lamprey GnRH-III (50 μg/kg) both increased plasma estradiol concentrations significantly (P < 0.05), compared with control. Lamprey GnRH-II (50 μg/kg) significantly increased plasma estradiol concentrations, compared with lamprey GnRH-III (P < 0.05). *, Significance when compared with control; **, significance when compared with control and l-GnRH-III.
IP assay
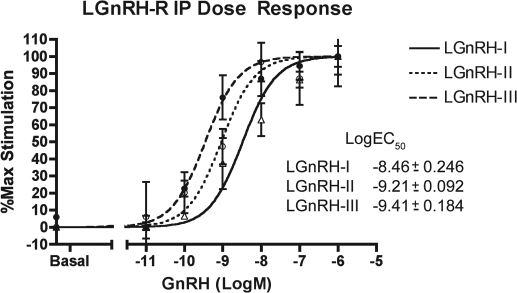
All of the peptides (lamprey GnRH-I, lamprey GnRH-II and lamprey GnRH-III) stimulated a significant response of IP accumulation in a dose-dependent manner using COS-7 cells, which were transiently transfected with the lamprey GnRH receptor. The log EC50 (represented as mean ± sem; n = 3) were lamprey GnRH-I (−8.46 ± 0.246), lamprey GnRH-II (9.21 ± 0.0921), and lamprey GnRH-III (9.41 ± 0.184) (Fig. 10).
Figure 10.
IP assay lamprey GnRH dose-response curve. The lamprey GnRH receptor was shown to stimulate the IP signaling system in a dose-dependent manner, in transiently transfected COS7 cells. Lamprey GnRH-III stimulated IP accumulation at a significantly lower log EC50 when compared with lamprey GnRH-II (P < 0.02) and lamprey GnRH-I (P < 0.001). Lamprey GnRH-II stimulated IP accumulation at a significantly lover log EC50 when compared with lamprey GnRH-II (P < 0.02). Log EC50 is shown as mean ± sem; n = 3 independent experiments.
Discussion
A 694-base cDNA encoding a prepro-GnRH has been cloned from the sea lamprey. The deduced amino acid sequence of the newly identified lamprey GnRH-II in lamprey is QHWSHGWFPG. As with all other vertebrate GnRHs, the lamprey GnRH-II is highly conserved with 10 amino acids and conserved C and N termini. Similar to GnRH-II expression in gnathostomes, lamprey GnRH-II was shown to be widely distributed and expressed in a number of tissues. In the brain, lamprey GnRH-II was shown to be located in preoptic area/hypothalamus by in situ hybridization and immunocytochemistry. In contrast to GnRH-II, generally acting as a nonhypothalamic hormone in gnathostomes, lamprey GnRH-II may have a role as a third hypothalamic form in lampreys.
We hypothesize that lamprey GnRH-II is a direct descendent of a common ancestor of all gnathostome GnRH lineages due to a duplication event that occurred before the rise of the gnathostomes. The deduced amino acid sequence of the lamprey GnRH-II in lamprey differs from gnathostome GnRH-II (chicken GnRH-II) by only one amino acid substitution. Lamprey GnRH-II has Phe8, compared with GnRH-II, which has Tyr8. Likewise, in sharks, two GnRHs have been determined, dogfish GnRH and GnRH-II, although the cDNA for dogfish GnRH has not been elucidated. The newly discovered lamprey GnRH-II is more ancestral to GnRH-II and dogfish GnRH. GnRH-II was structurally characterized in another Chondrichthyes, the ratfish, which diverged from the vertebrate lineage of about 400 million years ago (36,37). As shown in Fig. 5, we now propose that lamprey GnRH II evolved from a common ancestor of all gnathostome forms of GnRH. The gene duplication events that generated the different fish and tetrapod paralogous groups took place within the gnathostome lineage, after its divergence from the ancestral agnathans. The origins of the gnathostome forms of GnRH is debatable (13). As previously described, the GnRH family of peptides in gnathostomes is typically divided in three groups. However, more rigorous/extensive phylogenetic analysis suggests that in fact there are only two main paralogous GnRH lineages in Gnathostomes: the forebrain GnRH lineage and the midbrain lineage (13). This is a superposition of two classification criteria: on one hand, an evolutionary and a functional one, on the other hand. We consider that a clear distinction has to be made between the groupings of the GnRH isoforms based on their evolutionary ancestry and the clusters of the functional similar isoforms. The two other forms of lamprey GnRH (I and III) can now be identified as paralogous homologs (group IV) of gnathostome GnRH and lamprey GnRH II group, resulting from a duplication within the lamprey lineage. This implies that there probably was a genome/gene duplication event, which gave rise to all forms of vertebrate GnRH affecting the common ancestor of lamprey and Gnathostome isoforms. The Gnathostome branch of the lamprey I and III orthologous group was probably lost during evolution.
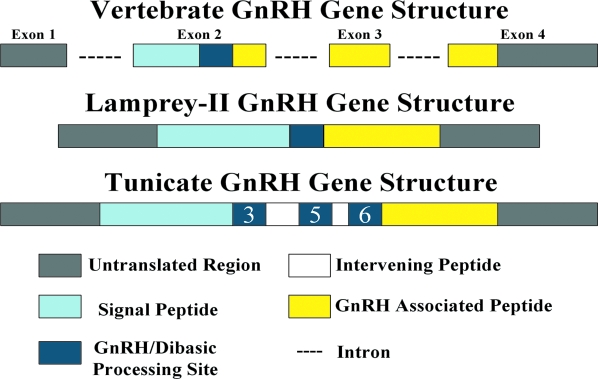
The architecture of the lamprey GnRH-II precursor is similar to that reported for other GnRH precursors consisting of a signal peptide, decapeptide, a downstream processing site, and a GnRH-associated peptide; however, the gene for lamprey GnRH-II does not have introns in comparison with the gene organization for all other vertebrate GnRHs that have three introns and four exons. Exon 1 contains the 5′ untranslated region (38). Exon 2 encodes for the signal peptide, the GnRH decapeptide, processing site, and part of the GnRH-associated peptide (GAP). The remaining GAP sequence is encoded in exon 3 and part of exon 4. The last part of exon 4 encodes the 3′ untranslated region (Fig. 11) (39). In contrast to the vertebrate GnRH gene structure, tunicate GnRH gene structure is distinctly different. In the invertebrate tunicate Ciona intestinalis, there are two genes for GnRH with each gene encoding three unique GnRH isoforms for a total of six in one animal (40). The gene for Ciona intestinalis gnrh 2 does not contain introns (40). Results from the current study showed that the gene encoding lamprey GnRH-II consists of a single exon and does not contain introns. There are various genes across vertebrates that do not contain introns (41). For some cases, it has been hypothesized that the lack of introns may be due to retro transposition (41,42). In most cases these retro genes, which are products of reverse transcription of a spliced (mature) mRNA and are characterized by lack of introns, are not functional (42). In rare cases, it has been shown that retro genes can encode for functional products (42). Thus, one possible explanation for the lack of introns for the gene encoding lamprey GnRH-II may have been the occurrence of retro transposition.
Figure 11.
GnRH gene structure of gnathostome and lamprey GnRH-I and –III. This figure depicts a schematic diagram of the genomic organization of the GnRH gene in vertebrates comprised of three introns and four exons. The lamprey GnRH-II gene consists of a single intron. For Ciona Ci-gnrh1 gene structure, Ci-gnrh1 does not have introns and consists of one exon with 5′ and 3′ flanking regions, in contrast to the three introns and four exon arrangement of the vertebrate GnRH gene.
Similar to gnathostome GnRH-II, lamprey GnRH-II also appears to be widely distributed and expressed in a variety of tissues as demonstrated by RT-PCR (8,43). In contrast to the GnRH-II being predominantly a nonhypothalamic hormone in gnathostomes, lamprey GnRH-II may have a major role as a third hypothalamic GnRH as well as having functions in other parts of the body. Supporting the notion that lamprey GnRH-II may be acting as a hypothalamic GnRH, in situ hybridization of the brain showed expression and localization of the transcript in the hypothalamus, medulla, fourth ventricle, and olfactory regions, whereas immunohistochemistry using a specific antiserum showed mostly ir-lamprey GnRH-II nerve fibers originating from cells in the arc-shaped hypothalamic/preoptic areas ending at the neurohypophysis, and proposed to form the preopticohypophysial GnRH tract. The distribution of ir-lamprey GnRH-II neurons was quite similar to distributions of lamprey GnRH-I and -III neurons, which were studied previously in the sea lamprey brain (30). In the current study, there did not appear to be ir-GnRH processed in the olfactory or midbrain regions of the adult brain. This is similar to previously reported immunohistochemical studies, both ir-lamprey GnRH-I and -III were found in the cell bodies of the rostral hypothalamus and preoptic area in larval and adult sea lamprey and not expressed in extrahypothalamic regions of the brain (30,44). Using dual-label in situ hybridization, we had shown that lamprey GnRH-I and -III mRNA are colocalized in the same cells in the preoptic nucleus/hypothalamic regions in adult lampreys (25). In the current study, it is difficult to know whether lamprey GnRH-II is colocalized in lamprey GnRH-I and/or -III cells or present in different GnRH cells because antilamprey GnRH-II used in the present study exhibited slight cross-reactivity to both GnRH-I and -III. These in situ and IHC data show that lamprey GnRH-II is expressed and processed in the hypothalamus-preoptic region.
To examine whether the lamprey GnRH-II may have a hypothalamic role, we performed biological studies and functional receptor studies. Both lamprey GnRH-I and -III have been shown by extensive functional, physiological, and immunocytochemical studies to control the pituitary-gonadal axis [reviewed in Sower (20)]. As in previous studies, plasma estradiol was measured in the lamprey as potential indicator of pituitary responsiveness to GnRH (45). Lamprey GnRH-II was shown to be biologically active as determined by significantly increased levels of plasma estradiol in the in vivo studies and significantly increased media estradiol in the coculture ovary/pituitary in vitro studies. Lamprey GnRH-III was slightly less effective, compared with lamprey GnRH-II. In these coculture ovary/pituitary studies, media estradiol was significantly potentiated, compared with media estradiol from ovary culture treated with lamprey GnRH-II. In the ovary culture only and similar to earlier studies on lamprey GnRH-I and -III (32), lamprey GnRH-II also had a slight direct effect on stimulating media estradiol, compared with controls. Administration of GnRH-II in vivo and in vitro induced significant pituitary-gonadal responses. Once a GTH RIA or ELISA is established, further studies examining the GTH response to each of the GnRHs can be done to fully understand the differential effects GnRHs on pituitary function. In addition to these biological studies, the activity of lamprey GnRH-II in a transiently transfected lamprey GnRH receptor assay was examined. Lamprey GnRH-II activated the inositol phosphate signaling pathway. Stimulation with lamprey GnRH-I, -II, or -III led to dose-dependent responses in IP in transiently transfected COS7 cells with the lamprey GnRH receptor. As has been shown in vertebrate GnRH receptors, activation of the lamprey GnRH receptor is thought to primarily stimulate the inositol 1,4,5-triphosphate second messenger system (35,46,47,48). Lamprey GnRH-III was shown to be more potent than lamprey GnRH-II; however, lamprey GnRH-II was more potent than lamprey GnRH-I. These data provide further support that the presently cloned lamprey GnRH-R is lamprey GnRH-III selective yet can be stimulated by lamprey GnRH-I or -II. These functional studies provide further evidence for a hypothalamic role of lamprey GnRH-II and its ability to act via a putative pituitary lamprey GnRH receptor.
In an evolutionary sense, the functional changes in the GnRHs appear to associate with the three types of regulation of the adenohypophysis developed in the vertebrates: the agnathan diffusional median eminence type; the teleostean direct innervation type; and the mostly highly evolved median eminence vascular type seen in amphibians, reptiles, birds, and mammals (49). The anatomical structural differences that evolved during the evolution of the vertebrates associated with the change of functions of the GnRH appear to represent adaptive evolution. As brains became larger and thicker and greater distance between the hypothalamus and pituitary and during the evolution of the GnRH peptides, the GnRHs may have been coopted for different functions. For instance, one could speculate that the ancestral GnRH that gave rise to lamprey GnRH-II and gnathostome GnRH may have originally been a hypothalamic GnRH in extinct vertebrates, and after the duplication event, the GnRH-II in gnathostomes was coopted by other cells in the brain (midbrain, olfactory region) and has different functions. In the gnathostomes, GnRH-II predominates in extrahypothalamic regions and has been suggested to act as a neuromodulator/neurotransmitter, although there are species in which GnRH-II is found in the hypothalamic regions and acts at the pituitary in stimulating gonadotropin function (7). Whereas in a basal vertebrate lamprey, GnRH-II may be predominantly a hypothalamic neurohormone, suggesting that the function of the ancestral GnRH was as a hypothalamic hormone in extinct agnathans. Overall, the lamprey hypothalamic GnRH hormones show a pattern of both ancestral and derived features based on primary amino acid structure, phylogenetic analysis, expression, and functional studies. Whereas the primary structure of GnRH has been highly conserved, there are key differences in the distribution and functions of the GnRHs in lampreys, compared with gnathostomes.
In summary, the newly discovered lamprey GnRH-II offers a new paradigm of the origin of the vertebrate GnRH family. We hypothesize that due to a genome/gene duplication event, an ancestral gene gave rise to two lineages of GnRHs: the gnathostome GnRH and lamprey GnRH-II. Due to another duplication event that could have occurred within the lamprey lineage, the lamprey GnRH-I and -III became paralogous homologs of gnathostome GnRH and lamprey GnRH II.
Acknowledgments
We thank Jason Dahlstrom for his efforts in computer cluster design and software management. We also thank the following individuals for their input and experiment assistance: Dr. Mihael Freamat, Geoff Bushold, Takayoshi Kosugi, Bernadine Schultz, Eileen Balz, Caryn Macdonald, Mike Wilmot, Dr. Mark Townley, and Crescentia Anne Healy.
Footnotes
This work was supported by National Science Foundation Grants IBN-0421923 and DBI-0618719. This is scientific contribution 2341 from the New Hampshire Agriculture Experimentation Station.
Disclosure Statement: The authors have nothing to declare.
First Published Online April 24, 2008
Abbreviations: DIG, Digoxigenin; DNase, deoxyribonuclease; GAP, GnRH-associated peptide; GTH, gonadotropins; HBSS, Hanks’ balanced salt solution; IHC, immunohistochemistry; IP, inositol phosphate; ir, immunoreactive.
References
- Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV 1971 Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun 43:1334–1339 [DOI] [PubMed] [Google Scholar]
- Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellos R, Blackwell R, Vale W, Guillemin R 1972 Primary structure of the ovine hypothalamic luteinizing hormone releasing factor (LRF). Proc Natl Acad Sci USA 69:278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tello JA, Zhang W, Tsai PS 2008 Molecular cloning, expression pattern, and immunocytochemical localization of a gonadotropin-releasing hormone-like molecule in the gastropod mollusk, Aplysia californica. Gen Comp Endocrinol 156:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kah O, Lethimonier C, Somoza G, Guilgur LG, Vaillant C, Lareyre JJ 2007 GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen Comp Endocrinol 153:346–364 [DOI] [PubMed] [Google Scholar]
- Silver MR, Kawauchi H, Nozaki M, Sower SA 2004 Cloning and analysis of the lamprey GnRH-III cDNA from eight species of lamprey representing the three families of Petromyzoniformes. Gen Comp Endocrinol 139:85–94 [DOI] [PubMed] [Google Scholar]
- Dubois EA, Zandbergen MA, Peute J, Goos HJ 2002 Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Res Bull 57:413–418 [DOI] [PubMed] [Google Scholar]
- King JA, Millar RP 1995 Evolutionary aspects of gonadotropin-releasing hormone and its receptor. Cell Mol Neurobiol 15:5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD 1998 Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci USA 95:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhar IS 2002 Cell migration and evolutionary significance of GnRH subtypes. Prog Brain Res 141:3–17 [DOI] [PubMed] [Google Scholar]
- Fernald RD, White RB 1999 Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol 20:224–240 [DOI] [PubMed] [Google Scholar]
- Morgan K, Millar RP 2004 Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol 139:191–197 [DOI] [PubMed] [Google Scholar]
- Gorbman A, Sower SA 2003 Evolution of the role of GnRH in animal (Metazoan) biology. Gen Comp Endocrinol 134:207–213 [DOI] [PubMed] [Google Scholar]
- Guilgur LG, Moncaut NP, Canario AV, Somoza GM 2006 Evolution of GnRH ligands and receptors in gnathostomata. Comp Biochem Physiol A Mol Integr Physiol 144:272–283 [DOI] [PubMed] [Google Scholar]
- Ohno S, Wolf U, Atkin NB 1968 Evolution from fish to mammals by gene duplication. Hereditas 59:169–187 [DOI] [PubMed] [Google Scholar]
- Holland PW, Garcia-Fernandez J, Williams NA, Sidow A 1994 Gene duplications and the origins of vertebrate development. Dev Suppl 125–133 [PubMed] [Google Scholar]
- Irvine SQ, Carr JL, Bailey WJ, Kawasaki K, Shimizu N, Amemiya CT, Ruddle FH 2002 Genomic analysis of Hox clusters in the sea lamprey Petromyzon marinus. J Exp Zool 294:47–62 [DOI] [PubMed] [Google Scholar]
- Vandepoele K, De Vos W, Taylor JS, Meyer A, Van de Peer Y 2004 Major events in the genome evolution of vertebrates: paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc Natl Acad Sci USA 101:1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried C, Prohaska SJ, Stadler PF 2003 Independent Hox-cluster duplications in lampreys. J Exp Zoolog B Mol Dev Evol 299:18–25 [DOI] [PubMed] [Google Scholar]
- Larhammar D, Lundin LG, Hallbook F 2002 The human Hox-bearing chromosome regions did arise by block or chromosome (or even genome) duplications. Genome Res 12:1910–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower SA 2003 The physiology of reproduction in lampreys and applications for male lamprey sterilization. J Great Lakes Res 29(Suppl 1):50–65 [Google Scholar]
- Suzuki K, Gamble RL, Sower SA 2000 Multiple transcripts encoding lamprey gonadotropin-releasing hormone-I precursors. J Mol Endocrinol 24:365–376 [DOI] [PubMed] [Google Scholar]
- Amemiya Y, Sogabe Y, Nozaki M, Takahashi A, Kawauchi H 1999 Somatolactin in the white sturgeon and African lungfish and its evolutionary significance. Gen Comp Endocrinol 114:181–190 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ 2000 Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, eds. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 365–386 [DOI] [PubMed] [Google Scholar]
- Swofford DL 1998 PAUP: phylogenetic analysis using parsimony. 4.0 ed. Champaign, IL: Illinois Natural History Survey [Google Scholar]
- Root AR, Nucci NV, Sanford JD, Rubin BS, Trudeau VL, Sower SA 2005 In situ characterization of gonadotropin-releasing hormone-I, -III, and glutamic acid decarboxylase expression in the brain of the sea lamprey, Petromyzon marinus. Brain Behav Evol 65:60–70 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, Ohtomo M, King JC 1997 Luteinizing hormone-releasing hormone gene expression differs in young and middle-aged females on the day of a steroid-induced LH surge. Brain Res 770:267–276 [DOI] [PubMed] [Google Scholar]
- Trinh LA, McCutchen MD, Bonner-Fraser M, Fraser SE, Bumm LA, McCauley DW 2007 Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic strain. Biotechniques 42:756–759 [DOI] [PubMed] [Google Scholar]
- Coligan J 2000 Peptides. In: Coligan JKA, Margulies D, Shevach EM, Stober W, eds. Current protocols in immunology. New York: John Wiley, Sons, Inc.; 9.0.0–9.8.15 [Google Scholar]
- Calvin JL, Slater CH, Bolduc TG, Laudano AP, Sower SA 1993 Multiple molecular forms of gonadotropin-releasing hormone in the brain of an elasmobranch: evidence for IR-lamprey GnRH. Peptides 14:725–729 [DOI] [PubMed] [Google Scholar]
- Nozaki M, Ominato K, Gorbman A, Sower SA 2000 The distribution of lamprey GnRH-III in brains of adult sea lampreys (Petromyzon marinus). Gen Comp Endocrinol 118:57–67 [DOI] [PubMed] [Google Scholar]
- Nozaki M, Ominato K, Shimotani T, Kawauchi H, Youson JH, Sower SA 2008 Identity and distribution of immunoreactive adenohypophysial cells in the pituitary during the life cycle of sea lampreys, Petromyzon marinus. Gen Comp Endocrinol 155:403–412 [DOI] [PubMed] [Google Scholar]
- Gazourian L, Evans EL, Hanson L, Chase F, Sower SA 2000 The effects of lamprey GnRH-I, -III and analogs on steroidogenesis in the sea lamprey (Petromyzon marinus). Aquaculture 188:147–165 [Google Scholar]
- Sower SA, Dickhoff WW, Gorbman A 1983 Ovulatory and steroidal responses in the lamprey following administration of salmon gonadotropin and agonistic and antagonistic analogues of gonadotropin-releasing hormone. Can J Zool 61:2653–2659 [Google Scholar]
- Sower SA, Moriyama S, Kasahara M, Takahashi A, Nozaki M, Uchida K, Dahlstrom JM, Kawauchi H 2006 Identification of sea lamprey GTHβ-like cDNA and its evolutionary implications. Gen Comp Endocrinol 148:22–32 [DOI] [PubMed] [Google Scholar]
- Silver MR, Nucci NV, Root AR, Reed KL, Sower SA 2005 Cloning and characterization of a functional type II gonadotropin-releasing hormone receptor with a lengthy carboxy-terminal tail from an ancestral vertebrate, the sea lamprey. Endocrinology 146:3351–3361 [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Sherwood NM, Fischer WH, Jackson BC, Rivier JE, Lee T 1991 Primary structure of gonadotropin-releasing hormone from the brain of a holocephalan (ratfish: Hydrolagus colliei). Gen Comp Endocrinol 82:152–161 [DOI] [PubMed] [Google Scholar]
- Dellovade T, Schwanzel-Fukuda M, Gordan J, Pfaff D 1998 Aspects of GnRH neurobiology conserved across vertebrate forms. Gen Comp Endocrinol 112:276–282 [DOI] [PubMed] [Google Scholar]
- Bond CT, Hayflick JS, Seeburg PH, Adelman JP 1989 The rat gonadotropin-releasing hormone: SH locus: structure and hypothalamic expression. Mol Endocrinol 3:1257–1262 [DOI] [PubMed] [Google Scholar]
- Kitahashi T, Sato H, Sakuma Y, Parhar IS 2005 Cloning and functional analysis of promoters of three GnRH genes in a cichlid. Biochem Biophys Res Commun 336:536–543 [DOI] [PubMed] [Google Scholar]
- Adams BA, Tello JA, Erchegyi J, Warby C, Hong DJ, Akinsanya KO, Mackie GO, Vale W, Rivier JE, Sherwood NM 2003 Six novel gonadotropin-releasing hormones are encoded as triplets on each of two genes in the protochordate, Ciona intestinalis. Endocrinology 144:1907–1919 [DOI] [PubMed] [Google Scholar]
- Long M, Betran E, Thornton K, Wang W 2003 The origin of new genes: glimpses from the young and old. Nat Rev Genet 4:865–875 [DOI] [PubMed] [Google Scholar]
- Makalowska I, Lin CF, Hernandez K 2007 Birth and death of gene overlaps in vertebrates. BMC Evol Biol 7:193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schalburg KR, Harrower WL, Sherwood NM 1999 Regulation and expression of gonadotropin-releasing hormone in salmon embryo and gonad. Mol Cell Endocrinol 157:41–54 [DOI] [PubMed] [Google Scholar]
- Tobet SA, Nozaki M, Youson JH, Sower SA 1995 Distribution of lamprey gonadotropin hormone-releasing hormone-III (GnRH-III) in brains in larval lampreys (Petromyzon marinus). Cell Tissue Res 279:261–267 [Google Scholar]
- Sower SA 1993 Neuroendocrine control of reproduction in lampreys. Fish Physiol Biochem 8:365–374 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Enomoto M, Ikemoto T, Endo D, Okubo K, Aida K, Park MK 2004 Molecular cloning and characterization of a gonadotropin-releasing hormone receptor in the guinea pig, Cavia porcellus. Gen Comp Endocrinol 136:208–216 [DOI] [PubMed] [Google Scholar]
- Ikemoto T, Park MK 2003 Identification and characterization of the reptilian GnRH-II gene in the leopard gecko, Eublepharis macularius, and its evolutionary considerations. Gene 316:157–165 [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP 1997 Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18:180–205 [DOI] [PubMed] [Google Scholar]
- Nozaki M, Gorbman A, Sower SA 1994 Diffusion between the neurohypophysis and the adenohypophysis of lampreys, Petromyzon marinus. Gen Comp Endocrinol 96:385–391 [DOI] [PubMed] [Google Scholar]
- Sower SA, Chiang YC, Lovas S, Conlon JM 1993 Primary structure and biological activity of a third gonadotropin-releasing hormone from lamprey brain. Endocrinology 132:1125–1131 [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Sower SA, Marshak DR, Fraser BA, Brownstein MJ 1986 Primary structure of gonadotropin-releasing hormone from lamprey brain. J Biol Chem 261:4812–4819 [PubMed] [Google Scholar]