Abstract
Atrial natriuretic peptide (ANP) regulates arterial blood pressure and volume. Its guanylyl cyclase-A (GC-A) receptor is expressed in vascular endothelium and mediates increases in cGMP, but the functional relevance is controversial. Notably, mice with endothelial-restricted GC-A deletion [EC GC-A knockout (KO) mice] exhibit significant chronic hypervolemic hypertension. The present study aimed to characterize the endothelial effects of ANP and their relevance for the acute regulation of intravascular fluid volume. We studied the effect of ANP on microvascular permeability to fluorescein isothiocyanate-labeled albumin (BSA) using intravital microscopy on mouse dorsal skinfold chambers. Local superfusion of ANP (100 nm) increased microvascular fluorescein isothiocyanate-BSA extravasation in control but not EC GC-A KO mice. Intravenous infusion of synthetic ANP (500 ng/kg·min) caused immediate increases in hematocrit in control mice, indicating intravascular volume contraction. In EC GC-A KO mice, the hematocrit responses were not only abolished but even reversed. Furthermore, acute vascular volume expansion, which caused release of endogenous cardiac ANP, did not affect resting central venous pressure of control mice but rapidly and significantly increased central venous pressure of EC GC-A KO mice. In cultured lung endothelial cells, ANP provoked cGMP-dependent protein kinase I-mediated phosphorylation of vasodilator-stimulated phosphoprotein. We conclude that ANP, via GC-A, enhances microvascular endothelial macromolecule permeability in vivo. This effect might be mediated by cGMP-dependent protein kinase I-dependent phosphorylation of vasodilator-stimulated phosphoprotein. Modulation of transcapillary protein and fluid transport may represent one of the most important hypovolemic actions of ANP.
THE HEART IS involved in the regulation of arterial blood pressure and blood volume by the secretion of two natriuretic peptides (NPs), atrial (ANP) and B-type natriuretic peptides (1,2). These NPs activate a common guanylyl cyclase-A (GC-A) receptor expressed in a wide variety of tissues, thereby increasing intracellular cGMP concentrations (3). NPs are secreted from atrial granules into the circulation in response to acute or chronic atrial stretch to act as antihypertensive and antihypervolemic factors via GC-A in distant organs (2,4). A second specific receptor subtype for NPs is the C receptor (NPR-C), a clearance receptor that is devoid of guanylyl cyclase activity and that mediates the cellular internalization and degradation of NPs (5). It may also participate in mediating some of their cellular actions by means of coupling to G proteins and negative modulation of adenylyl cyclase activity (6).
The important physiological role of the ANP/GC-A system in blood pressure/volume homeostasis has been emphasized by the phenotype of various genetic mouse models. Targeted deletion of the peptide (ANP−/−) or its receptor (GC-A−/−) leads to severe, chronic arterial hypertension and hypervolemia (7,8,9). Remarkably, hypervolemic hypertension in GC-A−/− mice is apparent even under conditions of normal dietary salt intake (8,9). The hypovolemic actions of ANP are complex and involve the kidneys (diuresis, natriuresis, inhibition of renin), adrenals (inhibition of aldosterone), and central nervous system (reduction of salt and water appetite). Additionally, both GC-A and NPR-C are densely expressed on vascular endothelia (10), but the biological function is controversial. To elucidate to what extent the GC-A-mediated endothelial effects of ANP participate in the regulation of systemic blood pressure and volume, we recently generated mice with conditional, endothelium-restricted deletion of the GC-A gene (EC GC-A KO) (11,12). Notably, despite full preservation of the direct vasodilating effects of ANP, EC GC-A KO mice exhibited significant chronic arterial hypertension and hypervolemia (11). Indirect measurements with iodinated albumin suggested that ANP stimulates endothelial macromolecule permeability and thereby regulates transvascular fluid balance, these actions being abolished in EC GC-A KO mice (11,13 and comprehensively reviewed in Ref. 14). The present study aimed to further characterize these endothelial effects, their relevance for the acute maintenance of blood volume homeostasis by ANP and the participating intracellular signaling pathways acting downstream to GC-A/cGMP in microvascular endothelium.
Materials and Methods
ANP receptor-deficient mice
Mice with conventional, global (GC-A−/−), or conditional endothelial-restricted deletion of the GC-A receptor for ANP (EC GC-A KO mice with homologous loxP/Tie2-Cre-mediated recombination of the GC-A gene) were generated and genotyped as described (8,11). Wild-type (GC-A+/+) and floxed GC-A littermates (with normal GC-A expression levels) served as respective controls.
The experiments were conducted in accordance with the German legislation on protection of animals and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, revised 1985). They were approved by the local governmental animal care committee.
Preparation of the dorsal skinfold chamber for microvascular permeability studies
For observation of the sc microcirculation, skinfold chambers were implanted as described (15). Briefly, the animals were anesthetized by ip injection of ketamine [50 mg/kg body weight (BW)] and xylazine (5 mg/kg BW). Subsequently two symmetrical titanium frames were implanted on the extended dorsal skinfold of the mice so that they sandwiched the double layer of skin. One layer of skin was then completely removed in a circular area of about 15 mm in diameter, and the remaining layers (consisting of striated skin muscle, sc tissue, and skin) were covered with a removable coverslip incorporated into one of the titanium frames. After 3 d recovery, evaluation of the permeability response was carried out in mice sedated with diazepam (10 mg/kg) and immobilized in a plexiglas tube on a microscope stage (Olympus, Hamburg, Germany). The glass window of the chamber was removed for local superfusion of modified Krebs bicarbonated solution [composition (in millimoles): 120 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 12.5 NaHCO3, 11 glucose (pH 7.4) gassed with 95% N2 and 5% CO2, temperature 34 C] at a rate of 1.2 ml/min.
Fluorescein isothiocyanate (FITC)-labeled albumin (BSA; Sigma, Deisenhofen, Germany) was dissolved in PBS at a concentration of 10 mg/ml. The free fluorescent dye in the solution was removed by passing through a size exclusion column. After tail vein injection of 0.1 ml FITC-albumin, intravital fluorescence microscopy was performed using a modified microscope with a 100 W mercury lamp and a fluorescence filter for FITC for epiillumination. The microscopic images were recorded by a charge-coupled device camera and transferred to a computer system for off-line evaluation.
The responses to local superfusion with ANP (100 nm; Bachem, Weil am Rhein, Germany) or to histamine (80 μm; Sigma) were compared with vehicle superfusion (Krebs solution). Topical application of agents or vehicle was always started 2 min after iv administration of FITC-albumin and continued for 30 min.
Microcirculatory analysis
Quantitative off-line analysis of the microscopic images was performed by means of the computer-assisted image analysis system Cell-D (Olympus). The observer was blinded to the genotype and treatment group. Macromolecular leakage was assessed using a ×20 water immersion objective in six different randomly selected microvascular regions by determining densitometrically gray levels in the tissue directly adjacent to the vessel wall of postcapillary venules [interstitial fluorescence (I)]. The same preselected areas were observed every 2 min during the first 10 min and every 5 min during the resting 20 min. The relative changes from their baseline values were reported. Interstitial fluorescence at start of the experiment (I0, immediately after tail vein injection of FITC-albumin) was set 1. Extravasation (I) at the indicated time points (It) was then calculated as I = It/I0.
Acute effects of exogenous ANP on hematocrit
These and the following invasive studies were conducted in anesthetized mice with an indwelling jugular catheter (16). Body temperature was maintained at 36–37 C by a heating pad. Either synthetic mouse ANP (500 ng/kg BW·min) or the selective NPR-C ligand cANP(4–23) (des[Gln18, Ser19, Gly20, Leu21, Gly22] (ANP 4–23)-NH2; 100 nm; Bachem) (5,17) was infused at a rate of 2.5 μl/kg BW per hour for 60 min via a microinfusion pump (Harvard Apparatus, distributed by FMI GmbH, Seeheim, Germany). Hematocrit was measured before and at 30 and 60 min of infusion by collection of a drop of blood into a hematocrit capillary via a tail vein and by spinning it in a microfuge.
Acute effects of volume expansion on central venous pressure
To study the contribution of endothelial GC-A to the acute moderation of intravascular fluid volume by endogenous ANP, we tested the effects of acute vascular volume expansion. Mice were anesthetized with isoflurane (2.5%). Both jugular veins were cannulated with polyethylene tubing (inside diameter 0.8 mm). One section of tubing was cannulated for infusion studies, and the other one was connected to a Statham pressure transducer (placed at heart level). The proper placement of the central venous catheter was judged by the shape of the pressure curve and its respiratory fluctuations. Basal central venous pressure (CVP) was determined during infusion with lactated Ringer’s solution containing 4.5% BSA at a rate of 4.3 μl/g BW per hour. After 15 min, the rate was increased to 114 μl/g BW per hour for 15 min to give a bolus of about 3% of BW. The infusion was continued at 4.3 μl/g BW per hour for another 30 min (18). This protocol has been shown to increase cardiac ANP release and plasma ANP (18).
Assay of plasma ANP levels
In an additional series of experiments, mice were either subjected to the above volume load protocol or infused at the low rate. Fifteen minutes later, the mice were killed and blood was collected by cardiac puncture for determination of circulating ANP levels by RIA as described (19).
Cell culture
Microvascular endothelial cells were isolated from lungs of animals 3–4 months old (20). For each experiment, primary cultures of both genotypes were started simultaneously. Animals were killed by cervical dislocation, and lungs were collected in ice-cold DMEM. Peripheral lung tissue was minced and digested for 1 h at 37 C in 0.1% collagenase-A (Biochrom, Berlin, Germany). The digest was passed through a blunt 14-gauge needle and filtered through a 130-μm steel mesh. Cells were pelleted at 300 × g and resuspended in medium containing 20% fetal bovine serum, 40% DMEM, 40% Ham’s F-12, 50 μg/ml endothelial mitogen (Biomedical Technologies, Stoughton, MA), 2 mm l-glutamine, 100 μg/ml heparin, and 100 U/100 μg/ml penicillin-streptomycin and plated in 0.1% gelatin-coated T75 flasks. Cells were washed after 24 h and cultured for 2–4 d. Magnetic beads were coated with antimouse CD102 (Becton Dickinson, Heidelberg, Germany) antibody (5 μg/4 × 106 beads, Dynabeads M-450; Invitrogen, Karlsruhe, Germany). Per flask, 4 × 106 beads were added and incubated for 1 h at 4 C. Cells were trypsinized and selected in a magnetic field for 10 min. Cultures were grown to confluence and selected twice before being plated for experiments. By following this procedure, cells used in the experiments were, on average, 10 d in culture.
Determination of endothelial cGMP contents
Cells were incubated with ANP (1–100 nm) for 10 min. Then the incubation medium was aspirated and intracellular cGMP was extracted with ice-cold 70% (vol/vol) ethanol. After centrifugation (3000 × g, 5 min, 4 C), the supernatants were dried in a speed vacuum concentrator, resuspended in sodium acetate buffer [50 mm (pH 6.0)], and acetylated, and then cGMP contents were quantified by RIA.
Western blotting
Endothelial proteins were resolved by 10% SDS-PAGE. Electrophoresis and immunoblotting were performed as previously described (21). Antibodies were against GC-A (generated in our laboratory), cGMP-dependent protein kinase I [PKG I; (21)], the cytoskeletal protein vasodilator-stimulated phosphoprotein (VASP), and VASP phosphorylated at the PKG I-preferred site, Ser235 (Cell Signaling, Frankfurt, Germany) (21).
Data analysis
Results are shown as the mean ± sem. Differences between treatments (ANP vs. vehicle) or genotypes were evaluated with an unpaired Student’s t test. The serial changes in hematocrit and central venous pressure were analyzed by a repeated-measures ANOVA followed by Bonferroni and Student-Newman-Keuls post hoc test for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Microvascular permeability responses to ANP are abolished in EC GC-A KO mice
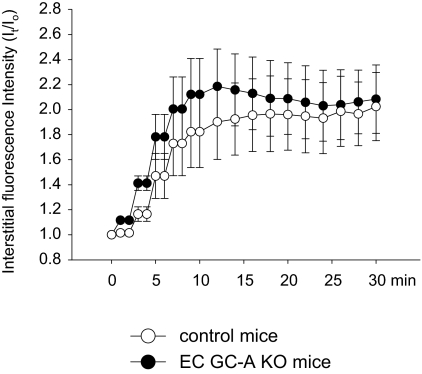
First, we applied intravital microscopy to study the permeability of postcapillary venules to FITC-albumin. As shown in Fig. 1, in all genotypes on average no significant changes in interstitial fluorescence intensity were observed during the 30-min superfusion of the skinfold preparation with Krebs solution (vehicle; Fig. 1, A–D). This indicated that the basal leakiness of the microvasculature to albumin was very low. In wild-type, GC-A+/+ mice, topical application of ANP (continuous superfusion with 100 nm ANP, 30 min) led to a mild, progressive increase in interstitial fluorescence intensity, indicating significantly increased microvascular permeability to albumin (Fig. 1A). In mice with global GC-A deletion (GC-A−/−), the stimulatory effect of topically applied ANP on FITC-albumin extravasation was abolished (Fig. 1B). To dissect the role of the endothelium in the permeability effects of ANP, the same experimental protocol was applied to EC GC-A KO and corresponding control littermates. Local superfusion of ANP (100 nm, 30 min) significantly increased interstitial FITC-albumin deposition in control mice (by ∼1.4-fold within 30 min) (Fig. 1C) and did not affect interstitial fluorescence in EC GC-A KO mice (Fig. 1D).
Figure 1.
Effects of ANP on microvascular permeability to FITC-albumin in mice with dorsal skinfold chambers. Relative changes in interstitial fluorescence intensity were evaluated during 30 min of continuous local ANP (100 nm) or vehicle superfusion. A, ANP increases albumin extravasation in wild-type (GC-A+/+) mice. B, The permeability responses to ANP are abolished in mice with global GC-A deletion (GC-A−/−). C, ANP increases albumin extravasation in control mice (floxed GC-A, with normal GC-A expression levels). D, The permeability responses to ANP are abolished in mice with conditional, endothelial GC-A deletion (EC GC-A KO) (n = 7–9 mice per genotype and treatment). *, P < 0.05 vs. vehicle.
Last, we examined the permeability responses of EC GC-A KO and respective control mice to the inflammatory stimulus histamine. As demonstrated in Fig. 2, topical application of histamine (80 μm) provoked about a 2-fold increases in interstitial FITC-albumin deposition in both control and EC GC-A KO mice. Notably, the increases in interstitial fluorescence evoked by histamine were faster and much more pronounced, compared with the increases observed in wild-type mice in response to ANP.
Figure 2.
Effects of histamine on microvascular permeability to FITC-albumin in EC GC-A KO and corresponding control mice. Relative changes in interstitial fluorescence intensity were evaluated during 30 min of continuous local histamine (80 μm) superfusion (n = 7 mice per genotype).
Acute effects of exogenous ANP on hematocrit are reversed in EC GC-A KO mice
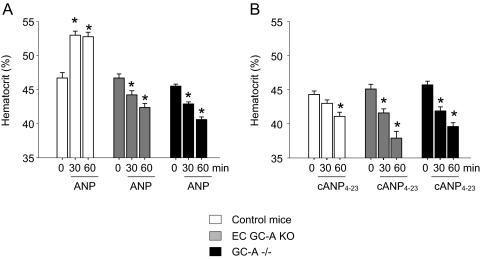
To study the contribution of endothelial GC-A to the acute systemic, hypovolemic effects of ANP, we tested the effect of exogenous iv ANP administration on hematocrit as well as the effects of an acute intravascular volume load on central venous pressure (see next section). Baseline hematocrit values did not differ between genotypes (Fig. 3). Infusion of ANP at a dose of 500 ng/kg BW·min to control mice caused a significant, immediate increase in hematocrit, indicating an acute contraction of plasma volume (Fig. 3A). Similar effects of ANP have been reported in many other experimental studies (22). In contrast, ANP infusion failed to increase and surprisingly even diminished hematocrit in mice with either global (GC-A−/−) or endothelial-restricted ablation of GC-A (EC GC-A KO mice) (Fig. 3A). To elucidate whether this dual action of ANP involves the NPR-C (or clearance receptor), the specific NPR-C agonist cANP(4–23) (5,17) was tested. As demonstrated in Fig. 3B, infusion of cANP(4–23) (250 ng/kg BW·min) significantly diminished hematocrit in control and even more in EC GC-A KO and GC-A−/− mice.
Figure 3.
A, Effect of iv ANP (500 ng/kg BW· min, as an infusion) on hematocrit values of control mice (with unaltered GC-A expression levels) and mice with either endothelial (EC GC-A KO) or global GC-A deletion (GC-A−/−). Note that at 30 and 60 min of ANP infusion, control mice show significant increases in hematocrit. Notably, this effect was not only abolished but also even reversed in EC GC-A KO and GC-A−/− mice. B, The selective NPR-C ligand cANP(4–23) (250 ng/kg BW·min, as an infusion) significantly decreased hematocrit in mice from all three genotypes (n = 6–8 per genotype). *, P < 0.05, compared with basal values at time 0.
Acute vascular volume expansion increases central venous blood pressure in EC GC-A KO mice
Under baseline conditions (when starting the experiments with the low rate infusion), jugular venous pressure (CVP) was not different in GC-A−/−, EC GC-A KO, and respective control mice (see Fig. 4). CVP did not change during intravascular volume expansion in wild-type (GC-A+/+) mice (Fig. 4A, high infusion rate). However, it increased immediately and significantly (by 2.2 ± 0.3 mm Hg) in mice with global GC-A deletion (GC-A−/−). CVP in GC-A−/− mice remained elevated during the whole subsequent experimental observation time (Fig. 4A). This demonstrated that the ANP/GC-A system is indispensable to prevent increases in CVP in response to acute intravascular volume expansion. However, these observations did not permit us to differentiate among cardiovascular, renal, or neurohumoral effects of ANP, which all could contribute to prevent increases in CVP in wild-type mice (all being abolished in GC-A−/− mice). We therefore applied the same experimental protocol to EC GC-A KO and corresponding control mice. Again, whereas the latter tolerated an acute intravascular volume overload without changes in CVP, in EC GC-A KO mice, volume expansion led to significant increases in CVP (by 2.4 ± 0.3 mm Hg) (Fig. 4B). In contrast to GC-A−/− mice, the increases in CVP in EC GC-A KO mice partly reversed about 20 min after the high rate infusion (Fig. 4B).
Figure 4.
Effect of acute vascular volume expansion on jugular venous blood pressure. A, Volume expansion in wild-type (GC-A+/+) mice had no effect on jugular venous pressure. In contrast, in mice with global GC-A deletion (GC-A−/−), volume expansion caused a rapid and significant increase in venous pressure, which did not fully reverse during the experimental observation time. B, Effect of acute vascular volume expansion on jugular venous blood pressure of control and EC GC-A KO mice. Whereas the former tolerated intravascular volume expansion without changes in jugular venous pressure, EC GC-A KO mice responded with significant increases in venous pressure, which partly reversed during the following observation time (n = 7–9 per genotype). *, P < 0.05 vs. baseline; #, P < 0.05 vs. respective GC-A+/+ or control mice.
EC GC-A KO mice exhibit increased circulating plasma ANP levels
To confirm that acute intravascular volume expansion caused release of the cardiac granules containing ANP, plasma concentrations of immunoreactive ANP were estimated at baseline conditions and after volume expansion. ANP levels were higher in EC GC-A KO, compared with control littermates (0.51 ± 0.048 vs. 0.34 ± 0.049 ng/ml, respectively; P < 0.05). Volume overload significantly increased circulating ANP in both genotypes: to 1.7 ± 0.34*§ in EC GC-A KO and to 1.1 ± 0.1§ ng/ml in control mice (n = 6 per genotype and condition; *, P < 0.05 vs. control mice; §, P < 0.05 vs. baseline conditions).
The ANP/GC-A/PKG I system stimulates the phosphorylation of VASP in cultured microvascular endothelial cells
The following experiments with primary cultured murine microvascular lung endothelial cells (MLECs) aimed to investigate the possible role of PKG I in the permeability actions of ANP. ANP (1–100 nm, 10 min incubation) increased intracellular cGMP in wild-type (GC-A+/+) but not GC-A-deficient MLECs (Fig. 5A). Notably, the expression levels of PKG I were significantly increased in the latter (Fig. 5B). ANP stimulated the phosphorylation of VASP at Ser235, the PKG I preferred site (23), only in GC-A+/+ and not GC-A−/− MLEC (Fig. 5C).
Figure 5.
A. Effect of ANP on intracellular cGMP content of MLECs. ANP increased cGMP in wild-type (GC-A+/+) but not GC-A−/− MLECs. B and C, Western blot analyses. GC-A protein was present in primary cultures of GC-A+/+ and absent in GC-A−/− cells. PKG I was present in MLECs from both genotypes, expression levels being significantly increased in GC-A-deficient cells. ANP stimulates the phosphorylation of VASP at the PKG I preferred site, Ser235, in GC-A+/+ but not GC-A−/− MLECs.
Discussion
Deficiency in endothelial ANP/GC-A signaling, as present in EC GC-A KO mice, results in chronic hypervolemic hypertension, which is probably due to a diminished microvascular endothelial permeability for macromolecules such as albumin and thereby altered transvascular fluid balance (11,12). Here we used this model to investigate the permeability effects of ANP in vivo and the role of the cardiac-endothelial axis in the acute maintenance of intravascular volume homeostasis. Intravital microscopy studies on dorsal skinfold chambers demonstrated that topical ANP increases extravasation of fluorescently (FITC)-labeled albumin. In mice with either global or endothelial-restricted deletion of GC-A this effect was totally absent, demonstrating that the ANP-induced acute increases in microvascular macromolecule permeability are mediated by endothelial GC-A. As demonstrated, these effects were much milder, compared with the effects of the classic inflammatory stimulus histamine.
ANP regulates arterial blood volume not only chronically but also in an acute fashion, i.e. in situations of acute volume expansion or sudden increases in blood pressure/volume, which cause a release of atrial granules from the heart (1,2,18). In fact, increased atrial wall tension produced by volume expansion is the dominant stimulus for ANP release, and the ANP/GC-A signaling pathway has often been thought of as an acute sensor of changes in blood volume (1,2). To study the role of endothelial GC-A in the acute maintenance of blood plasma volume homeostasis, we first mimicked the sudden cardiac release of ANP by iv application of synthetic ANP. As shown, iv ANP caused immediate increases in hematocrit in control mice, indicating contraction of intravascular volume (22). Remarkably this effect was completely abolished and even reversed in EC GC-A KO mice, demonstrating that the presence of endothelial GC-A is an absolute prerequisite for this acute hypovolemic action of ANP.
In a second series of experiments, mice were subjected to acute vascular volume expansion to provoke a rapid endogenous release of cardiac ANP (18). Control mice were able to compensate this volume overload and did not exhibit changes in CVP. Mice with either global (GC-A−/−, data not shown) or EC GC-A KO reacted to volume expansion with even greater increases in circulating ANP levels. Despite this, very prompt and significant increases in CVP were observed. We conclude that the communication between the heart (ANP) and endothelium (GC-A) is indispensable for the very acute maintenance of intravascular pressure/volume homeostasis. Together with our intravital microscopy studies, these observations suggest that cardiac ANP can acutely increase plasma protein escape across capillary walls. This increases interstitial oncotic pressure and ultimately can shift fluid from the intravascular to the interstitial compartment in an acute, immediate fashion to maintain a constant intravascular volume. These compensatory (endothelial) responses are abolished in GC-A−/− and EC GC-A KO mice, resulting in increased CVP as indirect index of intravascular volume expansion. Notably, in EC GC-A KO mice the increases in CVP were partly reversible, whereas GC-A−/− mice exhibited significantly increased CVP during the whole 30-min observation period. We postulate that the preserved renal and neurohumoral effects of ANP led to a delayed normalization of the volume expansion in the former mice, effects, which are all abolished in the global GC-A−/− mice (18).
Which cellular pathways mediate the effects of ANP on endothelial permeability? The exact mechanism of how cGMP regulates vascular permeability is poorly understood but might involve modulation of cAMP levels in endothelial cells. Endothelial cells express cGMP-inhibited phosphodiesterase (PDE)-3A and cGMP-stimulated PDE2A. A very recent study showed a biphasic effect of ANP/cGMP on cAMP levels of cultured human umbilical vein endothelial cells, low concentrations of ANP/cGMP potentiating (via PDE3A), and higher concentrations reversing (via PDE2A) the inhibitory effect of cAMP on thrombin-induced permeability (24). He et al. (25,26) had already shown that increased cGMP stimulates PDE2 and lowers cAMP, resulting in [Ca2+]i-independent increases in permeability in rat and frog mesenteric vessels. Another possible action of cGMP is through its ability to stimulate PKG I and thereby the phosphorylation of VASP, a protein associated with focal adhesion sites and adherens junctions (23). Indeed, our experiments with primary cultured microvascular lung endothelial cells showed that ANP stimulates the phosphorylation of VASP at the PKG I preferred site, Ser235. Although expression of PKG I was significantly increased in GC-A-deficient endothelia, the effects of ANP on PKG I-mediated VASP phosphorylation were abolished. This demonstrated that ANP, via cGMP and PKG I, leads to VASP phosphorylation in microvascular endothelia. However, the exact role of PKG I and VASP in regulating vascular permeability in vivo and how these and/or cAMP-dependent pathways are affected by ANP/GC-A/cGMP signaling in endothelial cells of different vascular beds is not known and will be a subject of our future studies.
Intriguingly, the hematocrit responses to ANP in mice with either global or endothelial ablation of GC-A were not only abolished but paradoxically even reversed. This suggests an acute expansion (not contraction) of plasma volume in response to ANP in the absence of GC-A. The inhibitory effect of ANP on hematocrit was mimicked by the selective NPR-C agonist cANP(4–23), indicating the involvement of NPR-C. Historically, the physiological effects of ANP have been attributed almost without exception to GC-A-dependent elevations in cGMP. Because of its short cytoplasmic domain and lack of guanylyl cyclase activity, NPR-C is classically regarded as a clearance receptor that binds all natriuretic peptides (ANP, B-type natriuretic peptide, and C-type natriuretic peptide) with similar affinity and removes them from the circulation (5). However, recent findings indicate that NPR-C activation can couple to G-proteins and initiate signal transduction pathways such as inhibition of adenylyl cyclase activity and opening of G protein-activated inwardly rectifying K+ channels (GIRK) (5,6,27). In vitro studies showed that NPR-C activation can increase endothelial barrier properties (17,28). In vivo, NPR-C stimulation was shown to inhibit endothelial-neutrophil interactions, which, of course, also play a critical role in endothelial barrier functions (29) as well as VEGF-induced permeability (28). Moreover, NPR-C gene-deficient mice exhibit a mild hypotension and decreased intravascular volume, as indicated by chronically increased hematocrit (30). We therefore hypothesize that ANP might exert dual effects on endothelial barrier functions. Via GC-A, it raises permeability of unstimulated endothelial cells in specific vascular beds, such as the (sub)cutaneous microcirculation, which has an important physiological role in volume homeostasis. Via NPR-C (preferentially when GC-A signaling is impaired, e.g. under pathophysiological conditions), NPs could diminish endothelial permeability and expand intravascular fluid volume.
In conclusion, the present study demonstrates that endothelial GC-A is critically involved in the acute hypovolemic actions of ANP. Unlike other natriuretics, which act via the kidneys to reduce interstitial fluid volume with little changes in plasma volume, these extrarenal, endothelial actions enable ANP to reduce plasma volume preferentially (14). Our observations in EC GC-A KO mice emphasize the (patho)physiological importance of this very unique property of ANP, demonstrating that a defect in endothelial GC-A signaling prevents appropriate and immediate resetting of intravascular volume after an acute overload.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Sonderforschungsbereich 688 (to M.K.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: ANP, Atrial natriuretic peptide; BW, body weight; CVP, central venous pressure; EC GC-A KO, endothelium-restricted deletion of the GC-A gene; FITC, fluorescein isothiocyanate; GC-A, guanylyl cyclase-A; I, interstitial fluorescence; MLEC, microvascular lung endothelial cell; NP, natriuretic peptide; NPR-C, NP C receptor; PDE, phosphodiesterase; PKG I, cGMP-dependent protein kinase I; VASP, vasodilator-stimulated phosphoprotein.
References
- de Bold AJ, Ma KK, Zhang Y, de Bold ML, Bensimon M, Khoshbaten A 2001 The physiological and pathophysiological modulation of the endocrine function of the heart. Can J Physiol Pharmacol 79:705–714 [PubMed] [Google Scholar]
- Kuhn M 2003 Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93:700–709 [DOI] [PubMed] [Google Scholar]
- Drewett JG, Garbers DL 1994 The family of guanylyl cyclase receptors and their ligands. Endocr Rev 15:135–162 [DOI] [PubMed] [Google Scholar]
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML 1990 Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70:665–699 [DOI] [PubMed] [Google Scholar]
- Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA 1987 Physiological role of silent receptors of atrial natriuretic factor. Science 238:675–678 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR, Makhlouf GM 2000 G(i-1)/G(i-2)-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am J Physiol 278:G974–G980 [DOI] [PubMed] [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O 1995 Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267:679–681 [DOI] [PubMed] [Google Scholar]
- Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A 1995 Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378:65–68 [DOI] [PubMed] [Google Scholar]
- Skryabin BV, Holtwick R, Fabritz L, Kruse MN, Veltrup I, Stypmann J, Kirchhof P, Sabrane K, Bubikat A, Voss M, Kuhn M 2004 Hypervolemic hypertension in mice with systemic inactivation of the (floxed) guanylyl cyclase-A gene by αMHC-Cre-mediated recombination. Genesis 39:288–298 [DOI] [PubMed] [Google Scholar]
- Leitman DC, Andresen JW, Kuno T, Kamisaki Y, Chang JK, Murad F 1986 Identification of multiple binding sites for atrial natriuretic factor by affinity cross-linking in cultured endothelial cells. J Biol Chem 261:11650–11655 [PubMed] [Google Scholar]
- Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M 2005 Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest 115:1666–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry FR 2005 Atrial natriuretic peptide: an essential physiological regulator of transvascular fluid, protein transport, and plasma volume. J Clin Invest 115:1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RS, Trippodo NC, MacPhee AA, Martinez AJ, Barbee RW 1990 High-dose atrial natriuretic factor enhances albumin escape from the systemic but not the pulmonary circulation. Circ Res 67:461–468 [DOI] [PubMed] [Google Scholar]
- Renkin EM, Tucker VL 1996 Atrial natriuretic peptide as a regulator of transvascular fluid balance. News Physiol Sci 11:138–143 [Google Scholar]
- Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K 1993 Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol 143:1055–1062 [PMC free article] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M 2002 Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99:7142–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A, Noll T, Bach C, Piper HM, Willenbrock R, Höhnel K, Haller H, Luft FC 1998 Atrial natriuretic peptide clearance receptor participates in modulating endothelial permeability. Am J Physiol 275(5 Pt 2):H1818–H1825 [DOI] [PubMed] [Google Scholar]
- Kishimoto I, Dubois SK, Garbers DL 1996 The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: acute handling of sodium and water in response to volume expansion. Proc Natl Acad Sci USA 93:6215–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M 2003 Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 111:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone Jr MA, Ertl G, Huang PL 2004 Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol 286:C1195–C1202 [DOI] [PubMed] [Google Scholar]
- Gambaryan S, Butt E, Marcus K, Glazova M, Palmetshofer A, Guillon G, Smolenski A 2003 cGMP dependent protein kinase type II regulates basal level of aldosterone secretion from zona glomerulosa cells without activating StAR gene expression. J Biol Chem 278:29640–29648 [DOI] [PubMed] [Google Scholar]
- Almeida FA, Suzuki M, Maack T 1986 Atrial natriuretic factor increases hematocrit and decreases plasma volume in nephrectomized rats. Life Sci 39:1193–1199 [DOI] [PubMed] [Google Scholar]
- Smolenski A, Poller W, Walter U, Lohmann SM 2000 Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase I. J Biol Chem 275:25723–25732 [DOI] [PubMed] [Google Scholar]
- Surapisitchat J, Jeon KI, Yan C, Beavo JA 2007 Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res 101:811–818 [DOI] [PubMed] [Google Scholar]
- He P, Zeng M, Curry FE 1998 cGMP modulates basal and activated microvessel permeability independently of [Ca2+]i. Am J Physiol 274:H1865–H1874 [DOI] [PubMed] [Google Scholar]
- He P, Zeng M, Curry FE 2000 Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol 278:H1124–H1133 [DOI] [PubMed] [Google Scholar]
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ 2003 Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 100:1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2002 Deciphering vascular endothelial cell growth factor/vascular permeability factor signalling to vascular permeability. Inhibition by atrial natriuretic peptide. J Biol Chem 277:44385–44398 [DOI] [PubMed] [Google Scholar]
- Scotland RS, Cohen M, Foster P, Lovell M, Mathur A, Ahluwalia A, Hobbs AJ 2005 C-type natriuretic peptide inhibits leucocyte recruitment and platelet-leucocyte interactions via suppression of P-selectin expression. Proc Natl Acad Sci USA 102:14452–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O 1999 The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 96:7403–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]