Abstract
PTH is the only currently available anabolic therapy for osteoporosis. In clinical practice, the skeletal response to PTH varies and because therapy is limited to 2 yr, approaches to maximize the therapeutic response are desirable. Rac2 is a small GTPase that is expressed only in hematopoietic tissue. Rac2−/− mice have a slight increase in bone mass and osteoclasts isolated from these animals have reduced basal resorptive activity and reduced chemotaxis. To evaluate the anabolic response to PTH in Rac2−/− mice, we treated 18 Rac2−/− and 17 control, age-matched wild-type animals once daily for 28 d with 80 ng/g body weight of h(1–34)PTH. Treatment resulted in significantly greater increments in spinal, femur, and total bone density in the Rac2−/− as compared with wild-type animals. Microcomputed tomography analysis demonstrated greater increases in trabecular thickness and cortical thickness in the knockout mice. Interestingly, histomorphometric analysis showed an equivalent increase in osteoblast and osteoclast number in response to PTH treatment in both groups of animals. However, as judged by changes in serum markers, the resorptive response to PTH was impaired. Thus, telopeptide of type 1 collagen was 15.9 ± 6.9 ng/ml after PTH treatment in the knockout animals and 26.8 ± 11.1 ng/ml in the PTH-treated wild-type group. In contrast, serum aminoterminal propeptide of type 1 collagen and osteocalcin were equivalent in both groups. We conclude that, in the genetic absence of Rac2, the anabolic response to PTH is increased. This appears to be due to attenuated resorptive activity of osteoclasts.
THE ONLY SKELETAL anabolic agent currently approved for the treatment of low bone mass in the United States is human PTH(1–34) (teriparatide). Daily injection of PTH increases bone mass in postmenopausal women, men, and patients with glucocorticoid-induced osteoporosis (1,2,3). It has been shown to reduce the risk of fracture in postmenopausal women and steroid-treated patients with low bone mass (3,4).
The mechanism by which PTH stimulates new bone formation is unclear. It is thought that PTH directly stimulates new bone formation by increasing osteoblast differentiation from precursor cells and/or activating quiescent osteoblasts (5). It has also been proposed that PTH inhibits osteoblast apoptosis, thereby effectively prolonging the anabolic action of these cells (6). Recently evidence has emerged to suggest that a preosteoclast progenitor cell is also a target for PTH’s anabolic effect and that in the complete absence of the osteoclast lineage, the ability of the hormone to stimulate new bone formation is abrogated (7,8).
Histomorphometric analyses have established that daily sc PTH administration dramatically increases osteoblast number as well as the bone formation and mineral apposition rates (9,10). On the other hand, daily sc PTH administration induces a transient increase in receptor activator of nuclear factor-κB ligand production by osteoblasts and an increase in osteoclastic resorptive activity, whether assessed by osteoclast number or resorption area (11,12).
The relationship between the anabolic and catabolic actions of daily sc PTH administration has not been studied in detail at the histologic level. In a short-term study of postmenopausal women with osteoporosis who were given daily sc PTH(1–34) injections, bone formation markers increased within the first month of treatment, whereas the increase in bone resorption markers was delayed, peaking only after 6 months of therapy (13,14). It has been reported that the most robust response to the anabolic effect of PTH occurs in the first 6–12 months of therapy and that the subsequent response is attenuated (15,16). Long-term clinical trails to assess the duration and rate of response to anabolic PTH treatment have not been reported, but it is reasonable to assume that the concomitant increase in bone resorption induced by daily sc PTH treatment might limit the ability of this treatment to increase bone mass. However, attempts to inhibit bone resorption during anabolic PTH therapy have generally resulted in a reduction in the rate of bone accrual, not an improvement. Indeed in clinical studies, concomitant treatment of osteoporotic patients with PTH and a bisphosphonate resulted in significant early blunting of the anabolic response to PTH (17,18). In addition, in ovariectomized rats treated with PTH, bone formation parameters were reduced when cotreatment was provided with either estrogen or a bisphosphonate (19). Interestingly, a role for the osteoclast in bone anabolism is emerging. In addition to the above-mentioned data indicating that an early osteoclast precursor may be a target for PTH (7,8), it is now clear that osteoclasts make a variety of potentially important anabolic factors including TGF-β, IGFs, and bone morphogenetic proteins (20,21,22,23). Thus, the diminution in the anabolic response to PTH in the setting of potent antiresorptive therapy may be due to suppression of the bone-resorbing activity of osteoclasts due to inhibition of some other anabolic function of osteoclasts or a combination of both.
Rac2 is a member of the Rho family of small GTPases and its expression is largely restricted to hematopoietic cells. Osteoclasts and preosteoclasts isolated from Rac2 knockout mice show a reduced basal rate of bone resorption and defective migration in response to the chemoattractant, colony-stimulating factor 1 (24,25). We and others have reported that these animals have a slight increase in trabecular bone mass (24,25). In our hands, histologic analyses indicate that osteoclast number tends to be elevated in Rac2 knockout animals (25), suggesting that the resorptive and chemotactic defects of Rac2−/− osteoclasts are responsible for the skeletal phenotype. We therefore wondered whether these subtle defects in Rac2−/− osteoclasts would result in a more robust skeletal response to daily sc PTH administration in these animals.
Materials and Methods
Experimental animals
Rac2 knockout mice were kindly provided by Dr. David Williams (Hematology Division, Children’s Hospital, Cincinnati, OH). C57BL/6 mice were purchased from the Charles River Laboratories, Inc. (Wilmington, MA). Mice were fed standard chow ad libitum and were maintained and used according to National Institutes of Health guidelines. The two groups of animals were maintained in the same room of the same animal care facility and received identical animal husbandry. This study was approved by the Yale Animal Care and Use Committee.
Treatment protocol
Eighteen 3-month-old Rac2 knockout and 17 3-month-old C57BL/6 mice were randomly assigned to receive either a daily sc dose of vehicle (10 mm acetic acid containing 2% heat inactivated mouse serum) or 80 ng/g body weight of human PTH(1–34) reconstituted in vehicle for 4 wk. Animals were weighed every 7 d, and the dose of PTH was adjusted for any change in weight. On d 1, 15, and 29, total body and regional bone mineral density (BMD) were determined by PIXImus (see below). On d 29, the day the animals were killed, animals were deeply anesthetized and serum samples collected from each mouse for determination of osteocalcin, the aminoterminal propeptide of type 1 collagen (P1NP) and the carboxyterminal cross-linking telopeptide of type 1 collagen (CTX). Animals were then killed by cervical dislocation and the left femur isolated from each animal, fixed in 70% ETOH, and subsequently scanned by microcomputed tomography (microCT). The left tibia was harvested, fixed in Millonig’s fixative, embedded in methylmethacrylate, and processed for histomorphometric analyses.
Bone densitometry by PIXImus
Serial BMD determinations of spine, femur, and total body were obtained using a PIXImus densitometer (GE-Lunar Corp., Madison, WI) running software version 1.45. The coefficient of variation for total BMD as measured in our laboratory is 1.0 ± 0.2%. Scan acquisition time is 5 min/animal and is performed on anesthetized animals in the prone position. The spine window is a rectangle spanning a length of the spine from L1 to the beginning of the sacrum. The femur window encompasses the entire right femur of each mouse. The total body window is defined as the whole-body image minus the calvarium, mandible, and teeth. BMD values are expressed in grams per square centimeter. Anesthesia for densitometric measurements was accomplished using a ketamine/xylazine mixture administered ip at a dose of 50 and 5 mg/kg, respectively. After scanning, mice were warmed under a heating lamp until they regained consciousness.
MicroCT
Femur morphometry was quantified using cone beam microfocus x-ray computed tomography (μCT40; Scanco Medical AG, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 μA, collecting 1000 projections per rotation at 300 msec integration time. Three-dimensional, 16-bit gray-scale images were reconstructed using standard convolution back-projection algorithms with Shepp and Logan filtering and rendered within a 12.3-mm field of view at a discrete density of 578,704 voxels/mm3 (isometric 12 μm voxels). Segmentation of bone from marrow and soft tissue was performed in conjunction with a constrained Gaussian filter to reduce noise, applying hydroxyapatite-equivalent density thresholds of 470 and 710 mg/cm3 for the trabecular and cortical compartments of the femur, respectively. Volumetric regions for trabecular analysis were selected within the endosteal borders of the distal femoral metaphysis to include the secondary spongiosa located 1 mm from the growth plate and extending 1 mm proximally. Cortical morphometry was quantified and averaged volumetrically through 50 serial cross-sections (600 μm) extending distally from the diaphyseal midpoint between proximal and distal growth plates.
Bone histomorphometry
Undecalcified tibiae were embedded in methylmethacrylate using the rapid embedding method, cut into 4-μm longitudinal sections, mounted on chrome-alum gelatin-coated slides, and stained with 2% toluidine blue (pH 3.7) in citric acid buffer for light microscopy as we have previously reported (26). Sections were examined in a blinded fashion using the Osteomeasure software program (Osteometrics, Atlanta, GA) to quantify histomorphometric parameters. These parameters are as described by Parfitt et al. (27). All measurements were taken at a final magnification of ×250 beginning at a constant distance from the growth plate of the tibiae. The trabecular bone of the proximal tibia was analyzed for each mouse.
Assays for markers of bone turnover
Serum osteocalcin was measured by a species-specific RIA as previously described (28,29). Serum P1NP was measured using a species-specific ELISA (Immuno Diagnostics Systems Ltd., Fountain Hills, AZ). Serum CTX was measured using the RatLaps ELISA kit (Nordic Bioscience Diagnostics A/S, Herlev, Denmark).
Statistics
Two-way ANOVA was used with post hoc Bonferroni-corrected tests to assess the effect of Rac2 deletion, PTH treatment and the interaction of the two. Paired or unpaired t test was used with Welch’s correction where indicated in the text or figure legends. Data presented are the mean ± sd and the error bars in figures reflect sem, unless otherwise noted.
Results
Baseline characteristics of animals and tolerance of treatment
Tables 1 and 2 describe the baseline characteristics of the animals in the four treatment groups. Baseline body weight and regional and total body bone density were similar in all four groups. Percent body fat was also similar in all groups. In general, animals tolerated the study protocol well. No animals died during the treatment protocol. The mean weight gain in the four groups of animals were as follows: Rac2−/−-PTH treatment, 1.23 ± 0.85 g; Rac2−/−-vehicle treatment, 0.57 ± 1.02 g; wild-type-PTH treatment, 2.14 ± 1.78 g; wild-type-vehicle treatment, 0.71 ± 1.87 g. The extent of weight gain was similar in the two vehicle-treated groups and in the two PTH-treated groups.
Table 1.
Body weight and percent body fat at baseline and body weight after d 29 of treatment
| Group | n | Initial body weight (g) | Final body weight (g) | Increase in body weight (g) | Baseline percent body fat |
|---|---|---|---|---|---|
| Knockout | |||||
| PTH | 11 | 25.0 ± 4.2 | 26.2 ± 4.2a | 1.23 ± 0.85 | 17.1 ± 2.4 |
| Vehicle | 8 | 22.7 ± 3.1 | 23.3 ± 2.6 | 0.57 ± 1.02 | 15.3 ± 2.2 |
| Wild type | |||||
| PTH | 10 | 25.1 ± 4.4 | 27.2 ± 3.1a | 2.14 ± 1.78 | 16.1 ± 1.5 |
| Vehicle | 8 | 23.7 ± 3.9 | 24.4 ± 2.9 | 0.71 ± 1.87 | 17.4 ± 3.7 |
P < 0.001, compared with initial body weight.
Table 2.
BMD (by dual-energy x-ray absorptiometry) at baseline in the four groups
| Group | n | Spine | Femur | Total body |
|---|---|---|---|---|
| Knockout | ||||
| PTH | 11 | 0.0561 ± 0.0038 | 0.0702 ± 0.0062 | 0.0484 ± 0.0027 |
| Vehicle | 8 | 0.0609 ± 0.0035 | 0.0699 ± 0.0063 | 0.0486 ± 0.0021 |
| Wild type | ||||
| PTH | 10 | 0.0581 ± 0.0043 | 0.0740 ± 0.0083 | 0.0497 ± 0.0024 |
| Vehicle | 8 | 0.0572 ± 0.0039 | 0.0694 ± 0.0044 | 0.0485 ± 0.0022 |
Changes in bone density with treatment
As assessed by PIXImus, BMD increased significantly with PTH treatment in both knockout and wild-type mice in each of the measured parameters: spine, femur, and total body. Vehicle-treated animals showed only minor fluctuations in BMD over the 4-wk treatment period with no consistent changes noted in the mean spine BMD values. There were slight but equivalent increases in femur and total body BMD in both the Rac2−/−-vehicle treatment and wild-type-vehicle treatment groups.
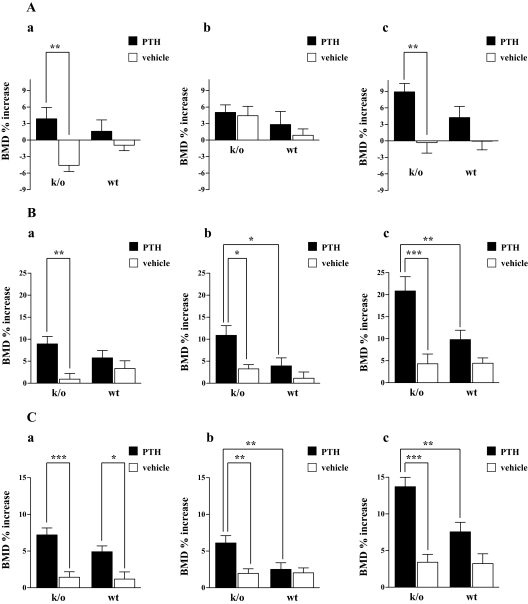
Figure 1, A (c), B (c), and C (c) summarize the mean percent change from baseline in spine, femur, and total body BMD in the four treatment groups at the end of the treatment period. As summarized in Fig. 1, B (c) and C (c), the increment in femur and spinal BMD was greater with PTH treatment in the knockout animals, compared with the PTH-treated wild-type animals. As shown in Fig. 1A (c), there was no significant change from baseline in spinal BMD in either group with vehicle treatment (P = NS for both). Furthermore, the mean change from baseline was no different between the two groups (knockout: −0.32 ± 5.0% vs. wild type: −0.047 ± 4.1%; P = NS). In contrast, compared with the baseline value, spinal bone density increased with PTH treatment in both wild-type and knockout animals (8.9 ± 5.0% for the knockout group, P < 0.001 and 4.3 ± 6.4% for the wild-type group, P = 0.06). Notably the mean increment in spinal BMD in the knockout animals was more than twice that seen in the wild-type group (8.9 vs. 4.3%), although this difference did not reach statistical significance by two-way ANOVA (P = 0.20).
Figure 1.
Percent increase from baseline in spine (A), femur (B), and total body (C) in PTH-treated (solid bars) and vehicle-treated (open bars) Rac2−/− and wild-type mice at the end of 29 d of treatment. The changes in bone density during the first 15 d treatment are shown in A (a), B (a), and C (a). The increases in bone density between d 15 and d 29 are shown in panel b of these three respective parts of the figure. Panel c in each of the three parts of the figure represents the cumulative change over the entire 29 d of treatment. **, P < 0.01; ***, P < 0.001. k/o, Knockout; wt, wild type.
The difference in response to treatment was even more pronounced in the femur [Fig. 1B (c)]. At this site, vehicle treatment led to slight but similar increases in femur BMD in the two groups. Compared with baseline, vehicle treatment led to a 4.3 ± 5.9% increase in femur BMD in the wild-type animals and a similar 4.4 ± 3.2% increase in the knockout animals. There was no statistically significant difference in these two mean values (P = NS). PTH treatment led to a significant increase in femur BMD in both treatment groups (20.9 ± 10.8% in the knockout group, P < 0.001 and 9.8 ± 6.7% for the wild-type animals, P = 0.001). The mean increment in femur BMD in the knockout animals was 2.1-fold greater than that seen in the wild-type group (20.9 vs. 9.8%), and this difference was highly significant by two-way ANOVA (P = 0.002).
The difference in response to treatment was also significant in the total body [Fig. 1C (c)]. At this site, vehicle treatment led to slight but similar increases in BMD in the two groups. Compared with baseline, vehicle treatment led to a 3.2 ± 3.5% increase in total body BMD in the wild-type animals and a similar 3.4 ± 2.8% increase in the knockout animals. There was no statistically significant difference in these two mean values (P = NS). As was the case in the spine and femur, PTH treatment led to a significant increase in total body BMD in both treatment groups (13.7 ± 4.2% in the knockout group, P < 0.001 and 7.6 ± 4.2% for the wild-type animals, P < 0.001). As was the case for the femur, the mean increment in total body BMD in the knockout animals was significantly greater than that seen in the wild-type group (13.7 ± 4.2 vs. 7.6 ± 4.2%; P = 0.0007 by two-way ANOVA). The augmented response to PTH treatment was not apparent in the first 14 d of treatment. As shown in Fig. 1, A (a), B (a), and C (a), there was a trend toward a greater percent increase in bone density at all three measured skeletal sites when PTH-treated knockout animals were compared with PTH-treated wild-type mice. These differences were not statistically significant. However, during the second 14 d of treatment (b in each of the portions of Fig. 1) the percent increase in bone density was statistically significantly greater in both the femur and total body in PTH-treated knockout animals, compared with PTH-treated wild-type animals.
MicroCT analysis
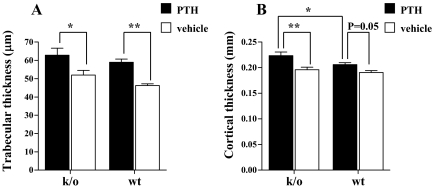
We sought to further analyze the response to PTH treatment in the two groups by microCT. Figure 2, A and B, shows the results of microCT analyses of trabecular BMD in the left femoral metaphysis and the left femoral cortex in wild-type and knockout animals after 28 d of treatment. By two-way ANOVA, there was a statistically significant effect of PTH treatment on trabecular thickness (P < 0.001). Thus, the mean trabecular thickness was 58.9 ± 4.7 vs. 46.3 ± 2.4 μm in PTH-treated vs. vehicle-treated wild-type animals (P < 0.01) and 62.8 ± 10.3 vs. 52.0 ± 6.8 μm, respectively, in the knockout mice (P < 0.05). PTH treatment led to a greater mean value for trabecular thickness in the knockout animals (62.8 vs. 58.9 μm, knockout vs. wild type), although this did not quite reach statistical significance by two-way ANOVA (P = 0.07). Consistent with our earlier histomorphometric findings (25), there was a trend toward an increased trabecular bone volume in the vehicle-treated knockout animals vs. the vehicle-treated wild-type animals (Fig. 2A).
Figure 2.
Trabecular thickness (A) and cortical thickness (B) after 28 d of treatment with PTH (solid bars) or vehicle (open bars) in Rac2−/− and wild-type animals. *, P < 0.05; **, P < 0.01. k/o, Knockout; wt, wild type.
By two-way ANOVA, there was also statistically significant effect of PTH treatment on cortical thickness (P < 0.001). The mean cortical thickness in PTH-treated wild-type animals was significantly higher than in the vehicle-treated animals of the same genotype (0.21 ± 0.01 vs. 0.19 ± 0.01 mm; P = 0.05). PTH also significantly increased cortical thickness in the knockout animals 0.22 ± 0.02 vs. 0.20 ± 0.01 mm; P < 0.01). The mean cortical thickness in the PTH-treated knockout animals was significantly greater than the value in the wild-type animals, 0.22 ± 0.02 vs. 0.21 ± 0.01 mm by two-way ANOVA (P < 0.05). Consistent with a more robust response to PTH treatment in the knockout animals was the finding that the mean percent increase in cortical thickness in the PTH-treated knockout group (compared with vehicle treatment) was greater than that in the wild-type animals (13.8 vs. 8.0%, respectively) (Fig. 2B).
Histomorphometric analyses
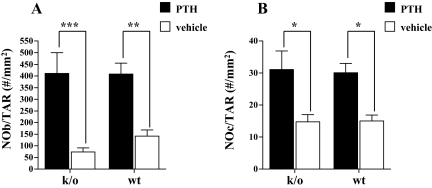
As might be anticipated and consistent with the known effect of PTH on parameters of bone formation and bone resorption, histomorphometric analysis of the trabecular bone envelope in the left tibiae demonstrated significantly increased numbers of osteoblasts and osteoclasts in both knockout and wild-type animals after PTH treatment when compared with mean values in vehicle-treated animals of the same genotype. Thus, there was a statistically significant effect of PTH treatment on both the number of osteoblasts per tissue area and the number of osteoclasts per tissue area by two-way ANOVA (P < 0.01). The number of osteoblasts per tissue area increased 558% with PTH treatment (over vehicle controls) in the knockout animals, compared with a 289% increase in the wild-type mice; absolute values at the end of treatment were similar in both groups of PTH-treated animals (411 ± 200 vs. 408 ± 104; knockout PTH vs. wild-type PTH; P = 0.5; Fig. 3A). The number of osteoclasts per tissue area increased to a similar extent in both knockout and wild-type animals with PTH treatment (211 vs. 200%, respectively); absolute values at the end of treatment were again similar in both groups of PTH-treated animals: 31.1 ± 12.8 vs. 30.1 ± 6.4; knockout PTH vs. wt PTH; P = 0.44; Fig. 3B). Thus, the cellular response to PTH appeared to be equivalent in both groups of animals. However, an equivalent increase in cell number does not necessarily mean an equivalent increase in cellular activity. We therefore used bone turnover markers as a means of assessing the impact of PTH treatment on osteoblast and osteoclast activity.
Figure 3.
The number of osteoblasts per total area (NOb/TAR; A) and the number of osteoclasts per total area (NOc/TAR; B) after 28 d of treatment with PTH (solid bars) or vehicle (open bars) in Rac2−/− and wild-type animals. *, P < 0.05; **, P < 0.01; ***, P < 0.001. k/o, Knockout; wt, wild type.
Bone turnover markers
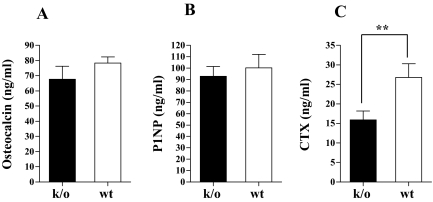
In untreated animals at baseline, there were no significant differences in mean serum levels of CTX or osteocalcin when values in Rac2−/− mice were compared with those in wild-type animals. Figure 4, A–C, summarizes the data for serum assays of bone turnover in PTH-treated knockout and wild-type animals. Serum osteocalcin levels were similar in PTH-treated knockout and wild-type animals with mean values of 67.7 ± 28.3 and 78.4 ± 11.5 ng/ml, respectively (P = 0.17; Fig. 4A). Consistent with these data, there was no significant difference in the mean serum levels of the formation maker, P1NP, in the PTH-treated knockout and wild-type animals, (92.7 ± 28.7 vs. 100.3 ± 37.3 ng/ml; PTH-treated knockout vs. PTH-treated wild-type; P = 0.30; Fig. 4B). In contrast, serum levels of CTX, a marker of bone resorption, were significantly lower in PTH-treated knockout mice than in the PTH-treated wild-type animals (15.9 ± 6.9 vs. 26.8 ± 11.1 ng/ml; P < 0.05; Fig. 4C).
Figure 4.
Mean serum osteocalcin (A), mean serum P1NP (B), and mean serum CTX (C) in Rac2−/− (solid bar) and wild-type animals (open bars) after 28 d of PTH treatment. **, P < 0.01. k/o, Knockout; wt, wild type.
Discussion
Our findings indicate that in the genetic absence of Rac2, the anabolic response to PTH is significantly increased. By PIXImus, there were significantly greater increases in femur and total BMD in response to PTH treatment in knockout vs. wild-type animals. Indeed, in the femur, the increase in BMD in the knockout animals was more than 2-fold that seen in the wild-type animals. A similar augmentation of the anabolic response to PTH was also seen in the total body BMD. The changes in the spine did not reach statistical significance, although similar trends were observed there. More detailed analysis of the different skeletal envelopes in the femur indicated that the more pronounced anabolic response to PTH in the absence of Rac2 was seen in both trabecular and cortical envelopes. In both these skeletal compartments, the anabolic response was greater in the Rac2 knockout animals, although this change was significant only in the cortex, in which the increase in cortical thickness was greater in the knockout animals.
Our histomorphometric data suggest that the cellular response to PTH is unimpaired in the absence of Rac2, but the turnover markers suggest that the resorptive activity of osteoclasts is diminished. This led us to hypothesize that the mechanism for the increased anabolic response to PTH in the Rac2−/− animals is due to a slight diminution in the resorptive response to PTH consequent to the reduced resorptive activity of Rac2−/− osteoclasts. This is consistent with our prior work in which we demonstrated diminished chemotactic activity and resorptive activity of Rac2−/− osteoclasts (25). Consistent with our findings, Wang et al. (24) also observed reduced resorptive activity in osteoclast-like cells prepared from Rac2−/− animals. However, Wang et al. reported diminished numbers of mature osteoclasts in bone from Rac2−/− animals, whereas we report that osteoclast numbers are the same or higher in Rac2−/− mice (Fig. 3B and Refs. 24 and 25). We believe this difference may relate to differences in the way osteoclast number was quantified in the two studies. We used the standard histomorophometric technique in which multinucleated osteoclasts were identified and counted. In contrast, Wang et al. relied on tartrate-resistant acid phosphatase (TRAP)-stained bone sections to identify osteoclasts. They also noted that the appearance of TRAP is delayed during osteoclastogenesis in Rac2 knockout mice. This suggests they may have underestimated osteoclast number because, based on their data, not all osteoclasts would necessarily stain for TRAP.
There are three Rac isoforms: 1, 2, and 3. Rac2 is largely restricted in its expression of hematopoietic cells (30,31,32,33). Thus, our finding that the principal cellular change in bone in Rac2−/− animals is in the osteoclast, is consistent with the known cellular distribution of this isoform. It is interesting, however, that the histomorphometric data suggested a somewhat more robust increase in the number of osteoblasts per total area with PTH treatment in the knockout, compared with the wild-type animals. Whether this trend indicates that there may also be an effect at the level of the osteoblast is not clear. Arguing against this possibility is the fact that serum levels of P1NP and osteocalcin were equivalent after PTH treatment in Rac2−/− and knockout animals. Although a reduction in resorptive activity could explain the augmented anabolic response, the fact that osteoclasts also elaborate potentially anabolic factors like IGF-I, bone morphogenetic proteins, and TGF-β leaves open the possibility that altered Rac2 expression in these cells could in some way lead to an enhanced release of bone tropic factors from osteoclasts.
Teriparatide is currently the only anabolic therapy available for the treatment of severely reduced bone loss. Its efficacy in the treatment of skeletal fragility disorders, such as postmenopausal osteoporosis and steroid-induced osteoporosis, has now been established. Despite this, there is variability in the response to this drug in clinical practice, and ways to optimize or even augment its therapeutic efficacy would be particularly useful. Given the restricted tissue expression of Rac2 and the very limited skeletal phenotype of Rac2−/− mice, targeting Rac2 for drug discovery seems plausible.
Footnotes
This work was supported by Grant DE01245 from National Institute of Dental and Craniofacial Research (to K.I.) and the Yale Core Center for Musculoskeletal Disorders Grant P30AR46032. T.K. is the recipient of a Brown-Coxe fellowship at Yale.
Disclosure Statement: The authors have nothing to declare.
First Published Online May 8, 2008
Abbreviations: BMD, Bone mineral density; CTX, cross-linking telopeptide of type 1 collagen; microCT, microcomputed tomography; P1NP, aminoterminal propeptide of type 1 collagen; TRAP, tartrate-resistant acid phosphatase.
References
- Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP 2000 Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, Dalsky GP, Marcus R 2007 Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441 [DOI] [PubMed] [Google Scholar]
- Dobnig H, Turner RT 1995 Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136:3632–3638 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC 1999 Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, Taichman RS, McCauley LK 2005 Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology 146:4584–4596 [DOI] [PubMed] [Google Scholar]
- Demiralp B, Chen HL, Koh AJ, Keller ET, McCauley LK 2002 Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology 143:4038–4047 [DOI] [PubMed] [Google Scholar]
- Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R 2002 Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res 17:808–816 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW 2006 A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res 21:366–373 [DOI] [PubMed] [Google Scholar]
- Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE 2001 Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142:4047–4054 [DOI] [PubMed] [Google Scholar]
- Locklin RM, Khosla S, Turner RT, Riggs BL 2003 Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 89:180–190 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Ostbye T, Adachi JD, Steer BM 1993 An evaluation of several biochemical markers for bone formation and resorption in a protocol utilizing cyclical parathyroid hormone and calcitonin therapy for osteoporosis. J Clin Invest 91:1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlot M, Meunier PJ, Boivin G, Haddock L, Tamayo J, Correa-Rotter R, Jasqui S, Donley DW, Dalsky GP, Martin JS, Eriksen EF 2005 Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 20:1244–1253 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK 2005 Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Bilezikian JP 2003 The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med 19:415–432 [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ 2003 The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM 2003 The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Yen CF, Qi H, Dann LM 1993 Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831 [DOI] [PubMed] [Google Scholar]
- Baylink DJ, Finkelman RD, Mohan S 1993 Growth factors to stimulate bone formation. J Bone Miner Res 8(Suppl 2):S565–S572 [DOI] [PubMed] [Google Scholar]
- Hayden JM, Mohan S, Baylink DJ 1995 The insulin-like growth factor system and the coupling of formation to resorption. Bone 17:93S–98S [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K 2007 Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res 22:487–494 [DOI] [PubMed] [Google Scholar]
- Garimella R, Tague SE, Zhang J, Belibi F, Nahar N, Sun BH, Insogna K, Wang J, Anderson HC 2008 Expression and synthesis of bone morphogenetic proteins by osteoclasts: a possible path to anabolic bone remodeling. J Histochem Cytochem 56:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M 2008 Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res 23:260–270 [DOI] [PubMed] [Google Scholar]
- Itokawa T, Zhu M, Troiano N, Insogna K 2005 Rac-2 knockout mice have increased bone mass and osteoclasts with reduced resorptive activity. J Bone Miner Res 20:S83 [Google Scholar]
- Kacena MA TN, Wilson KM, Coady CE, Horowitz M 2004 Evaluation of two different methylmethacrylate processing, infiltration and embedding techniques on the histological, histochemical and immunohistochemical analysis of murine bone specimens. J Histotechnol 27:119–130 [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Clough ME, Carpenter TO 1992 Development and validation of a radioimmunoassay for mouse osteocalcin: paradoxical response in the Hyp mouse. Endocrinology 130:1909–1915 [DOI] [PubMed] [Google Scholar]
- Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K 2005 The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146:1983–1990 [DOI] [PubMed] [Google Scholar]
- Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R 1989 Rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem 264:16378–16382 [PubMed] [Google Scholar]
- Moll J, Sansig G, Fattori E, van der Putten H 1991 The murine rac1 gene: cDNA cloning, tissue distribution and regulated expression of rac1 mRNA by disassembly of actin microfilaments. Oncogene 6:863–866 [PubMed] [Google Scholar]
- Shirsat NV, Pignolo RJ, Kreider BL, Rovera G 1990 A member of the ras gene superfamily is expressed specifically in T, B and myeloid hemopoietic cells. Oncogene 5:769–772 [PubMed] [Google Scholar]
- Haataja L, Groffen J, Heisterkamp N 1997 Characterization of RAC3, a novel member of the Rho family. J Biol Chem 272:20384–20388 [DOI] [PubMed] [Google Scholar]