Abstract
We characterized the enzymes that catalyze the deiodination of T4 to T3 in the male reproductive tract. Testis, epididymis (EPI), seminal vesicles, prostate, bulbourethral glands, spermatozoa, and semen were taken from sexually mature rats (300 g). Iodothyronine 5′-deiodinase (5′-D) activity was quantified by the radiolabeled-iodide-release method. 5′-D activity was 10-fold higher in EPI and semen than in the rest of the tissues. In EPI, semen, and prostate, the enzymatic activity was completely inhibited by 1 mm 6-n-propyl-2-thiouracil, whereas in the other tissues the inhibition was partial (50%). The high susceptibility to 6-n-propyl-2-thiouracil inhibition, a ping-pong kinetic pattern, and low cofactor (Michaelis Menten constant for dithiothreitol = 0.7 mm) and high substrate (Michaelis Menten constant for reverse T3 = 0.4 μm) requirements indicate that EPI 5′-D corresponds to type 1 deiodinase (D1). Real-time RT-PCR amplification of D1 mRNA in this tissue confirms this conclusion. The highest EPI D1 expression occurred at the onset of puberty and sexual maturity, and in the adult, this activity was more abundant in corpus and caput than in the caudal region. EPI D1 expression was elevated under conditions of hyperthyroidism and with addition of 17β-estradiol. Our data also showed a direct association between D1 and a functional epididymis marker, the neutral α-glucosidase enzyme, suggesting that local generation of T3 could be associated with the development and function of EPI and/or spermatozoa maturation. Further studies are necessary to analyze the possible physiological relevance of 5′-D in the male reproductive system.
FOR MANY YEARS, iodothyronines or thyroid hormones (TH) were not viewed as major regulators of reproductive function, but now it is widely accepted that they play a crucial role in sexual maturation and reproductive function (1,2). In males, iodothyronines are essential during critical periods of testicular development, when they modulate the proliferation and terminal differentiation of Sertoli and Leydig cells (3,4). The nuclear receptor for TH (TRα1) is highly expressed in spermatogonias and spermatocytes, suggesting its participation in spermatogenesis (5). However, knowledge about the impact of these hormones on reproductive organs other than gonads is limited. Prepubertal hypothyroidism is accompanied by puberty delay, reduction in testis weight, atrophy in the secretory epithelium of the epididymis (EPI) and accessory glands, and reduced number and forward motility of spermatozoids (6,7,8,9).
Some of the main genomic effects of TH are mediated by the interaction of T3 with its nuclear receptor (10). The local bioavailability and the consequent biological effects of T3 are determined by various factors, including hormone transport across membranes and its activation and inactivation by enzymatic deiodination in peripheral tissues. Based on functional, biochemical, and molecular criteria, three deiodinases involved with production and clearing of iodothyronines have been identified: type 1 (D1), type 2 (D2), and type 3 (D3). D1 functions as either an outer 5′-deiodinase (5′-D, or activation)- or inner (5-D or inactivation)-ring deiodinase, whereas D2 and D3 act exclusively on the outer and inner ring, respectively. D1 and D2 activity catalyze the conversion from T4 to T3, and D1 also participates in the deiodination of T3 to 3,3′-T2. D1 and D3 inactivate T4 and T3 by converting them to their inactive forms reverse T3 (rT3) and 3,3′-T2, respectively. D2 also deiodinates rT3 to 3,3′-T2 (11,12). D1 is abundant in liver, kidney, and thyroid (13,14), but lower activities are also expressed in pituitary (15), lactating mammary gland (16), intestine (17), perirenal fat (18), and prostate (19). Michaelis Menten constant (Km) values for substrates of D1 are in the micromolar range; its preferred substrate in vitro is rT3; it shows a ping-pong kinetic pattern and requires low thiol concentrations as cofactor [∼5 mm dithiothreitol (DTT)]. D1 is highly sensitive to inhibition by 6-n-propyl-2-thiouracil (PTU) and is positively regulated by T3 at the pretranslational level (20). D2 catalyzes exclusively 5′-D activity, shows a sequential kinetic pattern with T4 as preferred substrate, requires higher cofactor concentrations (∼20 mm DTT), and it is resistant to inhibition by PTU (11,12). D2 is located mainly in brain (21), brown adipose tissue (22), human skeletal muscle (23), and vascular smooth muscle (24). It is increased in hypothyroidism and decreased in hyperthyroidism. Both pre- and posttranslational mechanisms are involved in its regulation by thyroid state (25).
The physiological relevance of locally generated T3 in reproductive physiology is not well understood. Studies in female rabbits, mares, and sows reveal the presence of 5′-D activity in the follicular fluid from the ovary, suggesting that local T3 production plays an important role in granulose cell functions (26). During pregnancy, D1 and D2 activities are strongly induced in the mammary gland and uterus, respectively (16,27). In mammary gland, the activity persists during lactation and disappears at weaning (16). In males, the testis expresses predominantly D2 enzyme and increases in mRNA or activity correlate with the generation and differentiation of germ cells (28,29,30). In extragonadal tissues, recent studies from our laboratory have shown that the prostate of pubescent rat expresses D1 activity (19). Differential expression of D1 and D2 activity in the reproductive tract is consistent with the notion that these enzymes are expressed and regulated by the physiological state in an organ-specific manner. Therefore, this study was undertaken to analyze the 5′-D activity in the male reproductive tract. Expression of 5′-D was detected in all tissues analyzed, showing high activity in EPI. This activity exhibits a deiodinase type 1 enzymatic pattern and is stimulated by hyperthyroidism and 17β-estradiol supplement. Also, changes in D1 are positively correlated with the functional epididymis marker, the neutral α-glucosidase enzyme, suggesting that local generation of T3 could be associated with the development and/or function of EPI.
Materials and Methods
Reagents
125I-rT3 (1174 μCi/μg), 125I-T3 (1200 μCi/μg), 125I-T4 (1400 μCi/μg), and 3H-17β-estradiol were purchased from PerkinElmer Life Sciences (Boston, MA). DTT was obtained from Calbiochem (La Jolla, CA). Thyronines, PTU, methimazole (MMI), p-chloroamphetamine hydrochloride, and oligonucleotides were obtained from Sigma (St. Louis, MO). Testosterone was from Organon (Mexico City, Mexico), and 17β-estradiol from Schering Plough Corp. (Mexico City, Mexico). All other reagents were of the highest purity commercially available.
Animals
Adult male rats [Wistar, 300–350 g body weight (bw)] were housed in stainless steel cages under controlled temperature (22 ± 1 C) and lighting conditions (12-h light, 12-h dark cycle; lights on 0700–1900 h). They had free access to rat chow (Purina, Richmond, CA) and tap water. Surgical procedures were conducted under ketamine (30 mg/kg bw) and xylazine (6 mg/kg bw) anesthesia. Rats were killed by decapitation. Euthanasia of animals was reviewed and approved by an ad hoc ethics committee from the Universidad Nacional Autónoma de México. The testes (Te), EPI, seminal vesicles (SV), bulbourethral glands (BG), and prostates (P) were excised, defatted, weighed, immediately frozen in dry ice, and stored at −70 C. In some assays, the EPI were dissected into the caput, corpus, and caudal regions, and liver was used as positive control for D1 activity. Blood was collected, and the serum was separated by centrifugation (1500 × g, 15 min) and stored at −70 C until the assay.
Experiments
I. Identification of 5′-D activity in the male reproductive tract.
Enzymatic activity was analyzed in T, EPI, SV, BG, and P. PTU-sensitive activity was calculated as the difference between total and PTU-resistant activity (1 mm).
II. Kinetic characterization of 5′-D activity in homogenates of EPI.
Characterization included determination of the optimal assay conditions: protein concentration (50–500 μg), incubation time (1–4 h), incubation temperature (4–45 C), and cofactor concentration (0.1–5 mm). We measured the affinity for rT3 or T4 as substrate (1–4000 nm) and the inhibition by different concentrations of PTU (0.1–1.0 mm). The substrate and cofactor dependence were analyzed using the Lineweaver-Burk transformation (31).
III. Effect of thyroid status on EPI D1 expression.
To determine the effects of altered thyroid status on D1 expression (activity and mRNA), the tissues were collected from control, T4-treated, and MMI-treated rats. Hyperthyroidism was induced by adding T4 (6 μg/ml) and hypothyroidism by adding MMI (0.05%) to the drinking water for 3 wk. The thyroid state was confirmed by measuring the bw gain and the circulating T3 levels. In this study, liver was used as positive control for the D1 enzyme.
IV. D1 expression during development, maturation, and aging of EPI.
We analyzed D1 activity in neonatal (1 wk), prepubescent (3 and 5 wk), pubescent (6 and 7 wk), adult (3 and 8 months), and old rats (2 yr). The enzymatic assays were performed with and without PTU (1.0 mm).
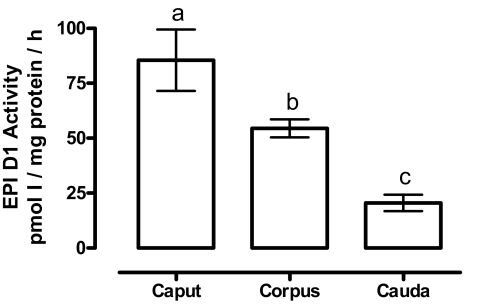
V. Segment-specific distribution of D1 expression in EPI.
In adult rats (3 months), the enzymatic activity was determined in the caput, corpus, and cauda.
VI. Modulation of EPI D1 expression by sex hormones.
These experiments were performed to determine whether androgens and estrogens participate in regulating D1 expression (activity and mRNA). We evaluated the effects of androgen ablation and hormonal replacement in castrated rats (Cx) with no other treatment or treated with testosterone (T, 3 mg) and/or 17β-estradiol (E2; 20 μg). Sesame oil was used as vehicle (V). Rats were bilaterally castrated via the scrotal route. Sham-operated rats were subjected to the same surgical procedure except that the testes were not removed. The groups were the following: sham + V, sham + T, sham + E2, sham + T + E2, Cx + V, Cx + T, Cx + E2, Cx + T + E2. Hormonal replacement began 1 wk after surgery. Both hormones were administered im in a slow-delivery oil solution twice: once on d 0 and once on d 7. Rats were killed at d 15. The steroidogenic state was confirmed by measuring the circulating levels of T and E2.
VII. Neutral α-glucosidase (NAG) activity during different physiological conditions.
NAG activity is widely used as a marker of EPI function (32); therefore, this experiment was performed to analyze whether D1 enzyme is associated with epididymal function. NAG activity was determined at different ages (5 and 12 wk and 2 yr old) and hormonal manipulations (thyroid status, castration, and E2 replacement).
VIII. Identification and comparison of 5′-D activity in spermatozoa and semen.
Spermatozoa were obtained by pricking the caput or caudal region of the EPI with a needle and allowing the sperm to swim into buffered saline (pH 7.6). Spermatozoid preparations were agitated and centrifuged at 750 × g for 5 min, and the supernatant was discarded. The presence of spermatozoids was confirmed by light microscopy. The pellet was resuspended and sonicated on ice in 0.01 m HEPES (pH 7.6, containing 0.32 m sucrose and 1.0 mm EDTA). We also tested for the presence of 5′-D activity in semen. Semen was obtained from ejaculations induced by administration of p-chloroamphetamine hydrochloride to anesthetized rats. This drug was dissolved in physiological saline and given ip at a dose of 2.5 mg/kg (33). Semen was homogenized on ice in 0.01 m HEPES (pH 7.6, containing 0.32 m sucrose and 1.0 mm EDTA) and centrifuged (1500 × g, 20 min at 4 C) to precipitate the spermatozoids. All assays were performed with the supernatant in the absence or presence of 1 mm PTU.
Analytical procedures
5′-D activity.
Tissues were homogenized in 10 vol (wt/vol) of cold 0.01 m HEPES buffer (pH 7.5) containing 0.32 m sucrose, 10 mm DTT, and 1 mm EDTA. Crude homogenates were centrifuged at 1500 × g for 20 min at 4 C. In the supernatant, enzymatic activity was determined by a modification of the release of radiolabeled iodide method (14,19) 125rT3 was purified by passage through a column (Sep-Pak, C-18 cartridges; Millipore Waters, Milford, MA). The standard assay contained 50 μl of homogenate (150 and 1.0 μg of protein for EPI and liver, respectively) and 50 μl of radiolabeled mix (2 nm 125I-rT3 and 5 mm DTT) in a final volume of 100 μl. For EPI and liver assays, nonradiolabeled rT3 was added at 0.4 and 0.5 μm, respectively. After incubation (1–1.5 h at 37 C), released acid-soluble 125I was isolated by chromatography on Dowex 50W-X2 columns (Bio-Rad, Richmond, CA) and counted with a γ-counter. Parallel control tubes without homogenate were included in each assay. Proteins were measured by the Bradford method (Bio-Rad). Results are expressed as picomoles or nanomoles I released per milligram protein per hour.
NAG activity.
The activity of this enzyme was determined by a commercial colorimetric kit (Roche, Mannnheim Germany). Briefly, tissue was homogenized in 5 vol (wt/vol) of cold phosphate buffer [0.05 m (pH 6.8)]. Crude homogenates were centrifuged at 1500 × g for 20 min at 4 C. The supernatant (100 μg of protein) was incubated for 2 h at 37 C with 4-nitrophenyl-α-glucopyranoside as substrate plus an inhibitor of the acidic form of the enzyme (catanospermine). The enzyme product (p-nitrophenol) was evaluated at 405 nm. Activity is reported as micromoles per milligram protein per hour.
Hormone levels. Serum T3 and E2 were quantified by homologous RIA (19,34). T was determined with a commercial enzyme immunoassay (Assay Designs, Inc., Ann Arbor, MI).
RNA purification and mRNA expression.
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA). Single-strand cDNA synthesis was performed on 2.0 μg of total RNA using oligo deoxythymidine as primer and Super Script for the reverse transcription. Quantitative real-time PCR was performed on an Applied Biosystems 7500 instrument using SBYR green PCR master mix (Applied Biosystems, Foster City, CA). Standard curves using the cDNA equivalents of 1, 10, and 100 were analyzed in each assay plate and used to calibrate the relative expression of D1 in each sample; calculations were made using the Applied Biosystems software based on threshold values. The cDNA equivalent of 10 ng of RNA was used for the PCR of each sample. The cycle conditions were 5 min at 94 C, 30 sec at 94 C, 30 sec at 55 C, and 45 sec at 72 C for 40 cycles followed by the melting curve protocol to verify the specificity of amplicon generation. All values were normalized using the housekeeping gene cyclophylin A as an internal control. Comparable efficiency was observed, and r2 was greater than 0.99. The primers used are shown in Table 1.
Table 1.
Sequences of primers used in the RT-PCR amplification
| Target | Length (bp) | Sequence | GenBank |
|---|---|---|---|
| D1 | 561 | 66–82, CTT GGA GGT GGC TAC GG | X57999 |
| 627–610, CTG GCT GCT CTG GTT CTG | |||
| Cyc | 520 | 7–27, AGA CGC CGC TGT CTC TTT TCG | M19533 |
| 527–507, CCA CAC AGT CGG AGA TGG TGA TC |
Cyc, Cyclophylin; D1, deiodinase type 1.
Statistical analysis
All results are expressed as the mean ± sem. Data were analyzed by the Student’s t test or one- or two-way ANOVA and multiple comparisons. An asterisk or different letters were used to identify significant differences between means (P ≤ 0.05).
Results
EPI expresses the highest 5′-D activity in the male reproductive system
Figure 1 shows the results of a preliminary survey of 5′-D activity in male reproductive tissues. The results show that this activity is present in all tissues examined. Activity in EPI was 10-fold higher than in any other tissue tested. PTU greatly decreased (>90%) the 5′-D activity in the EPI and P. In contrast, in Te, SV, and BG, the activity was only partially inhibited (50%) by PTU. The elevated 5′-D activity found in EPI prompted us to characterize its biochemical and functional properties.
Figure 1.
Regional distribution of 5′-D activity in the male reproductive tract. The assay was performed with 2.0 nm 125I-rT3, 150 μg protein, and 20 mm DTT in the absence or presence of 1.0 mm PTU. Incubation was 3 h at 37 C. Data were analyzed by a two-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 5).
EPI expresses D1 activity
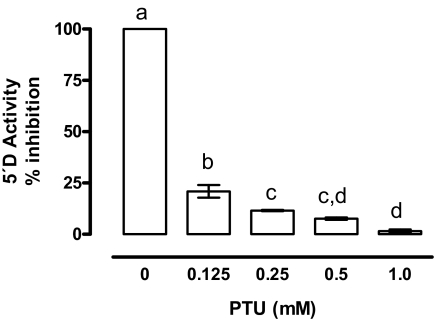
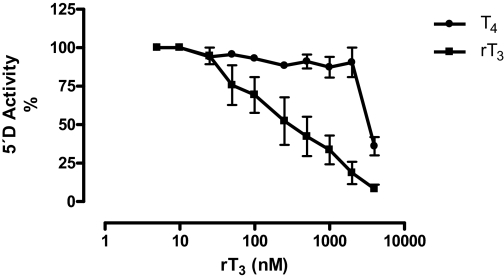
We used 2 nm 125I-rT3 as substrate to define initial assay conditions. The enzymatic activity was linear between 25 and 200 μg of protein and 0.30 and 3.0 h of incubation. Maximal rates of 5′-D were obtained between 30 and 37 C and with 2.5 mm DTT (data not shown). 5′-D activity was greatly reduced (80%) in the presence of 0.25 mm PTU (Fig. 2). Substrate affinity assays showed that rT3 was an 8-fold better substrate than T4, based on the concentrations required to cause 50% suppression of deiodination using [125I]rT3 as substrate (Fig. 3). In contrast, deiodination of 125-T4 was minimal (<1.0%), and it did not change with increasing concentrations of unlabeled T4 or T3 (data not shown). Therefore, subsequent assays were performed using only rT3 as substrate. EPI 5′-D activity was directly proportional to the rT3 and DTT concentrations (Fig. 4A). The kinetic pattern is apparently of the ping-pong type (Fig. 4B). From the double reciprocal plot, kinetic constants for rT3 were calculated to be: Km = 0.4 μm and maximal velocity = 250 pmol I− released per milligram protein per hour (Fig. 4C). Taken together, these results indicate that the characteristics of the 5′-D activity correspond to those of the type D1 enzyme.
Figure 2.
Inhibitory effect of PTU on EPI 5′-D activity. Data were normalized with respect to the 100% value corresponding to incubations performed in the absence of PTU, using 2 nm 125-rT3 and 5 mm DTT. Incubation was 1.5 h at 37 C. Data were analyzed by a one-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.01, n = 3 independent experiments, each in duplicate).
Figure 3.
Substrate affinity assay for EPI 5′-D activity. A 100% value corresponds to incubations performed in the presence of 125I-rT3 (70,000 cpm) without unlabeled iodothyronines. A wide range of rT3 and T4 concentrations were tested (5–4000 nm) using 5 mm DTT and 100 μg protein. Incubation was 1.5 h at 37 C (n = 3 independent experiments, each in duplicate).
Figure 4.
Kinetic parameters of EPI 5′-D activity. A, The rT3 concentrations were 125, 250, 500, and 1500 nm in the presence of 0.312, 0.625, 1.25, 2.5, and 5 mm DTT. The assay was performed with 50 μg of protein. Incubation was 1.5 h at 37 C. B, Lineweaver-Burk plot. C, The replots of intercepts from these data yielded the Km constants (n = 3 independent experiments, each in duplicate).
EPI D1 expression responds to hyper- but not hypothyroidism
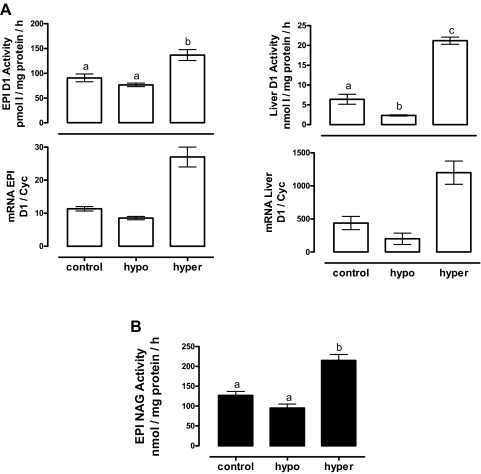
The most important regulatory factor for deiodinases is the thyroid hormone status; therefore, we analyzed the effect of hyper- and hypothyroidism on EPI D1 expression. As expected, the circulating T3 levels were significantly elevated in the T4-treated group (2-fold) and reduced in MMI-treated rats (6-fold) vs. the control group, confirming the thyroid states. Hyperthyroidism had no effect on EPI or bw, but hypothyroidism reduced (7-fold) the gain in body weight (Table 2). The results in Fig. 5A show that hyperthyroidism increased D1 activity in both liver (3-fold) and EPI (2-fold). In contrast, hypothyroidism decreased the activity in liver (60%) and EPI (10%), but the latter reduction was not significant. The changes of mRNA D1 expression were parallel to those of the activity (Fig. 5A). NAG activity showed a significant increase in hyperthyroidism without modifications in hypothyroidism (Fig. 5B).
Table 2.
Circulating T3 levels, EPI weight, and bw in control, T4-, and MMI-treated rats
| Groups | T3 (ng/dl) | EPI weight (mg/100 g bw) | bw increase (%) |
|---|---|---|---|
| Control | 64 ± 7.5a | 1.15 ± 0.07a | 40 ± 2.4a |
| T4 treated | 117 ± 6.7c | 1.12 ± 0.03a | 35 ± 3.3a |
| MMI treated | 10 ± 2.2b | 1.41 ± 0.05b | 5.2 ± 1.5b |
The weights were registered at the end of the experiment. Data were analyzed with a one-way ANOVA, and differences between groups were evaluated by the Tukey test. Different letters indicate significant differences between groups (P < 0.001, n = 6).
Figure 5.
Effect of thyroid status on D1 expression (A) and NAG activity (B). Liver was used as positive control for D1 activity. Optimal assay conditions for EPI and liver D1 activity were used (see Materials and Methods). NAG was measured by a colorimetric test. The data were analyzed with a one-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 6). Two independent RNA samples were used for mRNA analysis. Hypo, Hypothyroidism; hyper, hyperthyroidism.
The increased expression of EPI D1 correlates with the onset of puberty
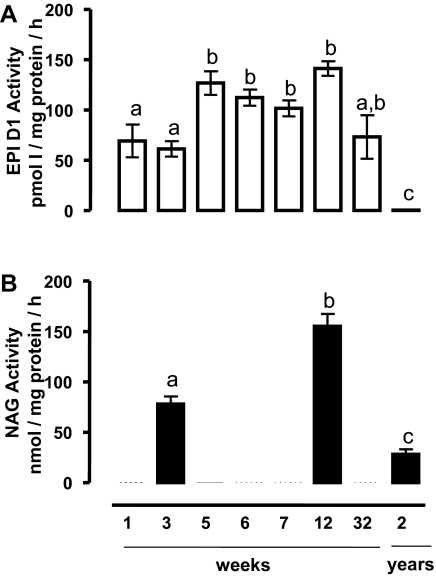
Figure 6A shows that D1 activity is present at all physiological stages of development except in old rats, in which the activity was virtually undetectable. The activity was detected in the neonatal period, increased substantially during the first 5 wk, and reached the highest values during the prepubertal stage. There were no significant differences between prepubertal (5 wk), pubertal (6 and 7 wk), and young adult rats (12 wk). In contrast, D1 activity decreased significantly in old rats (2 yr). Figure 6B summarizes the NAG activity in pubescent, adult, and old animals and shows a clear cut association between D1 and NAG expression. At all physiological stages, PTU caused clear inhibition of the D1 activity (data not shown).
Figure 6.
Temporal profile of D1 and NAG activity at different stages of development and functionality of EPI. A, Optimal conditions for D1 activity were used (see Materials and Methods). B, NAG was measured by a colorimetric test. The data were analyzed with a one-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 6).
D1 is preferentially expressed in the caput EPI of adult rats
Our results show that distribution of D1 activity is heterogeneous. D1 activity was 2-fold higher in caput than corpus and 5-fold higher in caput than in the cauda region (Fig. 7).
Figure 7.
Segment-specific distribution of EPI D1 activity. Optimal assay conditions for D1 activity were used (see Materials and Methods). The data were analyzed with a one-way ANOVA. Different superscript letters indicate significant differences between groups (P ≤ 0.001, n = 5).
EPI D1 expression is E2 dependent
We analyzed the combined effects of castration and hormonal replacement on EPI D1 activity. The results in Table 3 show that castration is accompanied by a reduction in EPI weight as well as in circulating levels of T and E2. T replacement increased its own circulating levels and partially reversed the EPI weight loss caused by castration. In contrast, E2 replacement had no effect on EPI weight but, as expected, it increased the circulating E2 levels. Figure 8 shows that castration was accompanied by a significant decrease in EPI D1 expression (both activity and mRNA transcripts); E2 but not T replacement raised the expression of EPI D1 to normal values, as also observed when the two steroids were administered simultaneously. Figure 9A shows that castration significantly reduced D1 activity in caput and corpus but had no effect on cauda. E2 replacement increased D1 activity in all segments to levels above the basal values. It has been documented that NAG expression is also regionalized, showing the highest levels in caput (35). To correlate the functional status of EPI with D1 regulation, we measured the NAG activity in the EPI caput from castrated and E2-replaced animals. Figure 9 shows a positive association between the two enzymes.
Table 3.
Effect of castration and hormonal replacement
| Groups | Treatments | EPI weight (g/100 g bw) | T (ng/ml) | E2 (pg/ml) |
|---|---|---|---|---|
| Sham | Vehicle | 0.27 ± 0.01a,b | 1.8 ± 0.30a | 142 ± 12a |
| T | 0.30 ± 0.015a | 5.0 ± 0.67b | Nontested | |
| E2 | 0.23 ± 0.008b | Nontested | 490 ± 58b | |
| Cx | Vehicle | 0.041 ± 0.007c | 0.20 ± 0.04c | 60 ± 12a |
| T | 0.15 ± 0.024d | 7.9 ± 1.2b | Nontested | |
| E2 | 0.062 ± 0.010c | Nontested | 1009 ± 153c | |
| T + E2 | 0.17 ± 0.018d | 8.1 ± 1.0b | 793 ± 62b,c |
Data were analyzed with a two-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 5).
Figure 8.
Effects of castration and sex hormones on EPI D1 activity. A, Optimal assay conditions for D1 activity were used (see Materials and Methods). The data were analyzed with a one-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 5). B, Two independent RNA samples were used for mRNA analysis.
Figure 9.
Effect of E2 replacement on EPI D1 and NAG activity. A, Optimal assay conditions for EPI D1 activity were used (see Materials and Methods). B, NAG was measured in caput by a colorimetric test. The data were analyzed with a one-way ANOVA, and differences between means were evaluated by the Tukey test. Different letters indicate significant differences between groups (P ≤ 0.05, n = 5).
D1 activity is present in epididymal tissue and semen but not in spermatozoa
Our results show that D1 activity is concentrated predominantly in epididymal tissue but not spermatozoa. A very low 5′-D activity that is not sensitive to PTU was detected in spermatozoa. In contrast, our data show that semen contains an elevated 5′-D activity, which was almost completely inhibited by 1.0 mm PTU (Fig. 10).
Figure 10.
5′-Deiodinase activity in EPI, spermatozoa (Spz), and semen. Spz were obtained from the caput and caudal regions of the EPI. Assays were performed with 150 μg protein, 2.0 nm 125I-rT3, and 20 mm DTT in the presence or absence of 1 mm PTU. Incubation was 3 h at 37 C. The data were analyzed by the Student’s t test. *, P ≤ 0.001 for PTU vs. control (n = 3).
Discussion
In this study, we showed for the first time a differential expression of 5′-D activity in several tissues of the male reproductive tract. Te, BG, and SV expressed both PTU-sensitive and PTU-insensitive enzymes, whereas P and EPI selectively expressed only PTU-sensitive deiodinase activity. One of the most surprisingly findings was the high EPI 5′-deiodinase activity. The complete inhibition by PTU, low DTT requirement, preference for rT3 as substrate, ping-pong kinetic pattern, and identification of mRNA for D1 strongly support the notion that the deiodinase present in rat EPI is type 1 (11,12,20,36). In addition, the increase in EPI 5′-D in response to T4 treatment is consistent with the well-documented fact that T3 increases D1 activity by a transcriptional mechanism (20,37). In this study, the elevated levels of circulating T3, as well as the typical response of liver D1 activity to T4 treatment, confirm the hyperthyroid state of the animals. Our data show that EPI was less sensitive than liver to the effects of hypothyroidism. The relative insensitivity of EPI D1 to hypothyroidism could be due to the presence of a larger pool of TH in this tissue; however, this point remains to be demonstrated.
Another possibility is that T3 is not the main regulator of EPI D1 activity. Indeed, we found that E2 replacement in castrated rats had a stimulatory effect on EPI D1, restoring the activity to normal levels in caput, corpus, and cauda; however, T replacement did not normalize it. These data are in agreement with studies from our and other laboratories, which have shown that D1 activity is up-regulated by E2 in prostate and pituitary (19,38), consistent with the well-known organ-specific regulation of this enzyme. Previous data from our laboratory show that in males, E2 treatment had no effect on liver D1 activity (19). However, a previous report demonstrated that E2 does regulate liver D1 activity in ovariectomized rats, indicating the existence of a sexual dimorphism (38,39,40). The mechanism of E2 action on the D1 enzyme remains unknown at the present time. Although high concentrations of E2 and its receptors (estrogen receptors) have been reported in EPI (41), the D1 gene sequence does not contain E2 response elements, suggesting that E2 has an indirect effect, i.e. on membranes and/or by modulating the action of other hormones. For example, it is well established that E2 administration increases the circulating prolactin levels (42). Previous studies from our own laboratory have shown stimulatory or inhibitory prolactin effects on prostate and mammary gland D1 activity, respectively (19,43). The complex, organ-specific regulation of D1 becomes more evident with the null effect of T replacement on EPI D1, which contrasts with its inhibitory effect on prostate D1 (19) and its stimulatory action on liver D1 from castrated rats (37,44).
According to the current concept, D1 could be an enzyme that cannot efficiently generate circulating T3 under euthyroid conditions (45), although it cannot be ruled out that T3, generated locally by D1, could have a local effect in D1-expressing tissues. The positive correlation between NAG and D1 expression support the notion that D1 is a local source of T3 in EPI. Moreover, the significant increase of NAG shown in the present work during hyperthyroidism, as well as its reduction during food restriction (32), suggests that thyroid hormones could participate in the expression of this enzyme. NAG activity has been used as a functional marker of EPI. This enzyme hydrolyzes glucosidic linkages, participating in modification of epididymal fluid and/or plasma membrane remodeling associated with sperm maturation. Similar to D1, NAG activity is highest in caput, increases around puberty, and decreases after castration and during aging (32,35,46). The high local T3 production (D1 expression) found in pubescent and adult rats could be associated with the onset of differentiation and function of EPI (47). It is possible that the clear-cut regionalization of EPI D1 (caput to cauda) is related to specific metabolic processes that occur in each segment. In the caput, reabsorption of fluids and secretion of components take place, processes that are involved with maturation of spermatozoids. During their transit from the caput to the cauda, spermatozoids undergo significant biochemical modification. This posttesticular remodeling leads to the acquisition of their forward motility and their ability to recognize and fertilize oocytes (48,49). The elevated D1 activity found in EPI is in accord with the fact that both the number and forward motility of sperm recovered from cauda were significantly lower in hypothyroid rats (50,51). Moreover, it has been reported that thyroid hormones are essential to maintain the integrity of the epithelium in adult rats (52). In contrast, the low D1 activity found in the cauda could be associated with maintaining metabolic quiescence of the male gametes and preventing premature sperm activation, given that the cauda acts as a sperm reservoir and exhibits a temperature 3 C lower than the caput (53).
EPI D1 activity was detected as early as the first postnatal week. Although the secretory epithelium had not yet differentiated, it is possible that the local T3 production could be related to the formation of the blood-epididymis-barrier, which occurs at this time (54). It has been demonstrated that neonatal hypothyroidism reduces the levels of the gap junction protein connexin 43, an essential component of the EPI barrier (55). On the other hand, the low EPI D1 activity found in old rats is consistent with the diminution of NAG activity with age. Liver and kidney D1 activity as well as other biochemical markers are also reduced in aging rats (56,57).
We showed that D1 was present in epididymal tissue, whereas Te, SV, and BG express two deiodinases: PTU sensitive and PTU insensitive. Our results also showed the existence of a high D1 activity in semen, but not spermatozoa, whose PTU-insensitive activity (possible D2), is very low. This observation agrees with a previous report showing: 1) the existence of high 5′-D activity in boar seminal plasma (28), 2) the presence of D2 activity in testis from different species (28,29,30), and 3) highly expressed D2 activity in elongated spermatids from adult rats (30). Together, these data lead us to propose that during spermatogenesis, spermatids generate their own T3 by means of the D2 enzyme, but during the EPI maturation period, cells of the caput generate high concentrations of T3 via D1 that remains active in semen. It is well known that some proteins from these tissues are secreted in an apocrine manner by the epithelium and are sequestered in exosomes, called epididymosomes (58).
Recent reports have shown that fertility of D1 or D2 KO mice is unaffected (59,60). However, these finding are not surprising because, in these models as in other hormonal knockout animals, compensatory mechanisms could be involved. Indeed, in D1 knockout, circulating levels of T4, and metabolism and excretion of both T3 and T4 are greatly increased. It is possible that these compensatory mechanisms are sufficient to maintain adequate levels of thyronines in epididymis and other tissues. It would be interesting to know whether the fertility of the double D1 and D2 knockout model is also unaffected.
In conclusion, we have demonstrated the expression of 5′-D activity in the male rat reproductive tract. Although further experiments are necessary to understand the role of deiodinases in EPI and accessory glands, we suggest that locally produced T3 may exert an effect on the maturation, metabolism, and viability of spermatozoa by regulating the secretions of these tissues.
Acknowledgments
We thank Felipe Ortíz and Martín García for animal care; Alberto Lara and Omar González for computer assistance; Pilar Galarza and Rafael Silva for their bibliographic assistance, Leonor Casanova for academic support, and Dorothy Pless for proofreading this manuscript.
Footnotes
This work was supported in part by CONACYT 44976-M, 52077), PAPIIT UNAM; (IN201207). N.A. was supported by fellowships from CONACYT (181306) and DGEP-UNAM (504005923).
Disclosure Statement: All authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: BG, Bulbourethral gland; bw, body weight; Cx, castrated; 5′-D, 5′-deiodinase; D1, deiodinase type 1; D2, deiodinase type 2; D3, deiodinase type 3; DTT, dithiothreitol; E2, 17β-estradiol; EPI, epididymis; Km, Michaelis Menten constant; MMI, methimazole; NAG, neutral α-glucosidase; P, prostate; PTU, 6-n-propyl-2-thiouracil; rT3, reverse T3; SV, seminal vesicle; T, testosterone; Te, testis; TH, thyroid hormone; V, vehicle; Vmax, maximal velocity.
References
- Jannini EA, Ulisse S, D'Armiento M 1995 Thyroid hormone and male gonadal function. Endocr Rev 16:443–459 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Holsberger DR, Witorsch RJ, Sylvester PW, Meredith JM, Treinen KA, Chapin RE 2004 Thyroid hormone, glucocorticoids, and prolactin at the nexus of physiology, reproduction, and toxicology. Toxicol Appl Pharmacol 194:309–335 [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Mendis-Handagama SM, Mason JI 2000 Effects of tri-iodothyronine on testicular interstitial cells and androgen secretory capacity of the prepubertal rat. Biol Reprod 63:493–502 [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Cooke PS 2005 Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res 322:133–140 [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Morrison JR, O'Bryan MK, Song Q, Wreford NG 2000 Developmental expression of thyroid hormone receptors in the rat testis. Biol Reprod 62:664–669 [DOI] [PubMed] [Google Scholar]
- Elzanaty S, Richthoff J, Malm J, Giwercman A 2002 The impact of epididymal and accessory gland function on sperm motility. Hum Reprod 17:2904–2911 [DOI] [PubMed] [Google Scholar]
- Maran RRM, Aruldhas MM 2002 Adverse effects of neonatal hypothyroidism on Wistar rat spermatogenesis. Endocr Res 28:141–151 [DOI] [PubMed] [Google Scholar]
- Del Rio AG, Blanco AM 1999 Changes in the epididymal ultrastructure in hypothyroid rats. Arch Androl 43:197–201 [DOI] [PubMed] [Google Scholar]
- Kala N, Ravisankar B, Govindarajulu P, Aruldhas MM 2002 Impact of foetal-onset hypothyroidism on the epididymis of mature rats. Int J Androl 25:139–148 [DOI] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR 2003 Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol 213:1–11 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Kester MHA, Peeters RP, Visser TJ 2005 Biochemical mechanisms of thyroid hormone deiodination. Thyroid 15:787–798 [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Utiger RD 1978 Iodothyronine metabolism in rat liver homogenates. J Clin Invest 61:459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JL, Rosenberg IN 1980 Iodothyronine 5′deiodinase from rat kidney: substrate specificity and the 5′-deiodination of reverse triiodothyronine. Endocrinology 107:1376–1383 [DOI] [PubMed] [Google Scholar]
- Baur A, Bauer K, Jarry H, Kohrle J 1997 3,5-Diiodo-l-thyronine stimulates type 1 5′deiodinase activity in rat anterior pituitaries in vivo and in reaggregate cultures and GH3 cells in vitro. Endocrinology 138:3242–3248 [DOI] [PubMed] [Google Scholar]
- Aceves C, Valverde-RC 1989 Type I 5′monodeiodinase activity in the lactating mammary gland. Endocrinology 124:2818–2820 [DOI] [PubMed] [Google Scholar]
- Bates JM, St Germain DL, Galton VA 1999 Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology 140:844–851 [DOI] [PubMed] [Google Scholar]
- Nicol F, Lefranc H, Arthur JR, Trayhurn P 1994 Characterization and postnatal development of 5′-deiodinase activity in goat perirenal fat. Am J Physiol 267:R144–R149 [DOI] [PubMed] [Google Scholar]
- Anguiano B, López A, Delgado G, Aceves C 2006 Deiodinase type 1 is expressed in prostate of pubescent rats and is modulated by prolactin and sex hormones. J Endocrinol 190:363–371 [DOI] [PubMed] [Google Scholar]
- Berry MJ, Kates AL, Larsen PR 1990 Thyroid hormone regulates type 1 deiodinase messenger RNA in rat liver. Mol Endocrinol 4:743–748 [DOI] [PubMed] [Google Scholar]
- Kaplan MM, McCann UD, Yaskoski KA, Larsen PR, Leonard JL 1981 Anatomical distribution of phenolic and tyrosyl ring iodothyronine deiodinases in the nervous system of normal and hypothyroid rats. Endocrinology 109:397–402 [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR 1983 Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712–713 [DOI] [PubMed] [Google Scholar]
- Salvatore D, Bartha T, Harney JW, Larsen PR 1996 Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3331 [DOI] [PubMed] [Google Scholar]
- Mizuma H, Murakami M, Mori M 2001 Thyroid hormone activation in human vascular smooth muscle cells. Expression of type II iodothyronine deiodinase. Circ Res 88:313–318 [DOI] [PubMed] [Google Scholar]
- Gereben B, Salvatore D 2005 Pretranslational regulation of type 2 deiodinase. Thyroid 15:855–864 [DOI] [PubMed] [Google Scholar]
- Slebodzinski AB 2005 Ovarian iodide uptake and triiodothyronine generation in follicular fluid. The enigma of the thyroid ovarian interaction. Domest Anim Endocrinol 29:97–103 [DOI] [PubMed] [Google Scholar]
- Galton VA, Martinez E, Hernandez A, St. Germain EA, Bates JM, St. Germain DL 2001 The type 2 iodothyronine deiodinase is expressed in the rat uterus and induced during pregnancy. Endocrinology 142:2123–2128 [DOI] [PubMed] [Google Scholar]
- Brzezinska-Slebodzinska E, Slebodzinski-AB, Kowalska K 2000 Evidence for the presence of 5′-deiodinase in mammalian seminal plasma and for the increase in enzyme activity in the prepubertal testis. Int J Androl 23:218–224 [DOI] [PubMed] [Google Scholar]
- Sambroni E, Gutieres S, Cauty C, Guiguen Y, Breton B, Lareyre JJ 2001 Type II iodothyronine deiodinase is preferentially expressed in rainbow trout (Oncorhynchus mykiss) liver and gonads. Mol Reprod Dev 60:338–350 [DOI] [PubMed] [Google Scholar]
- Wajner SM, Wagner M dos S, Melo RCN, Parreira GG, Chiarini-Garcia H, Bianco AC, Fekete C, Sanchez E, Lechan RM, Maia AL 2007 Type 2 iodothyronine deiodinase is highly expressed in germ cells of adult rat testis. J Endocrinol 194:47–54 [DOI] [PubMed] [Google Scholar]
- Copeland RA 1996 Enzymes. New York: Wiley-VCH USA; 93–119 [Google Scholar]
- Martini AC, Molina RI, Vincenti LM, Santillan ME, Stutz G, Ruiz RD, Fiol de Cuneo M 2007 Neutral α-glucosidase activity in mouse: a marker of epididymal function? Reprod Fertil Dev 19:563–568 [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Watanabe C, Ando R, Furuta S, Sakurada S, Yoshimura H, Iwanaga T, Kimura Y 2000 Characterization of p-chloroamphetamine-induced penile erection and ejaculation in anesthetized rats. Life Sci 67:3031–3039 [DOI] [PubMed] [Google Scholar]
- Herrera MR, Luna M, Romero RC 1993 Obtención de anticuerpos contra progesterona y estradiol, estandarización del radioinmunoanálisis y validación en suero de rumiantes. Vet Mex 24:223–229 [Google Scholar]
- Kalla NR, Kaur S, Ujwal N, Metha U, Joos H, Frick J 1997 α-Glucosidase activity in the rat epididymis under different physiological conditions. Int J Androl 20:92–95 [DOI] [PubMed] [Google Scholar]
- Sun BC, Harney JW, Berry MJ, Larsen PR 1997 The role of active cysteine in catalysis by type 1 iodothyronine deiodinase. Endocrinology 138:5452–5458 [DOI] [PubMed] [Google Scholar]
- Zhang CY, Kim S, Harney JW, Larsen PR 1998 Further characterization of thyroid hormone response elements in the human type 1 iodothyronine deiodinase gene. Endocrinology 139:1156–1163 [DOI] [PubMed] [Google Scholar]
- Lisboa PC, Curty FH, Moreira RM, Oliveira KJ, Pazos-Moura CC 2001 Sex steroids modulate rat anterior pituitary and liver iodothyronine deiodinase activities. Horm Metab Res 33:532–535 [DOI] [PubMed] [Google Scholar]
- Riese C, Michaelis M, Mentrup B, Gotz F, Kohrle J, Schweizer U, Schomburg L 2006 Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology 147:5883–5892 [DOI] [PubMed] [Google Scholar]
- Marassi MP, Fortunato RS, Matos da Silva AC, Pereira VS, Carvalho DP, Rosenthal D, Correa da Costa VM 2007 Sexual dimorphism in thyroid function and type 1 iodothyronine deiodinase activity in pre-pubertal and adult rats. J Endocrinology 192:121–130 [DOI] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R, Oliveira C, Cho N, Nakaia M, Carnes K 2001 Estrogens and epididymal function. Reprod Fertil Dev 13:273–283 [DOI] [PubMed] [Google Scholar]
- De las Heras F, Negro-Vilar A 1979 Effect of aromatizable androgens and estradiol on prolactin secretion in prepuberal male rats. Arch Androl 2:135–139 [DOI] [PubMed] [Google Scholar]
- Aceves C, Pineda O, Ramírez I, Navarro L, Valverde-RC 1999 Mammary type 1 deiodinase is dependent on the suckling stimulus: differential role of norepinephrine and prolactin. Endocrinology 140:2948–2953 [DOI] [PubMed] [Google Scholar]
- Miyashita K, Murakami M, Iriuchijima T, Takeuchi T, Mori M 1995 Regulation of rat liver type 1 iodothyronine deiodinase mRNA levels by testosterone. Mol Cell Endocrinol 115:161–167 [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR 2005 Type 2 iodothyronine deiodinase is the mayor source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzanaty S 2007 Association between age and epididymal and accessory sex gland function and their relation to sperm motility. Arch Androl 53:149–156 [DOI] [PubMed] [Google Scholar]
- Marty MS, Chapin RE, Parks LG, Thorsrud BA 2003 Development and maturation of the male reproductive system. Birth Defects Res B Dev Reprod Toxicol 68:125–136 [DOI] [PubMed] [Google Scholar]
- Robaire B, Viger RS 1995 Regulation of epididymal epithelial cell functions. Biol Reprod 52:226–236 [DOI] [PubMed] [Google Scholar]
- Gatti J-L, Castella S, Dacheux F, Ecroyd H, Métayer S, Thimon V, Dacheux J-L 2004 Post-testicular sperm environment and fertility. Anim Reprod Sci 82–83:321–339 [DOI] [PubMed] [Google Scholar]
- Del Rio AG, Quiros MC 1983 Thyroid gland and epididymal function in rats. II. Sperm motile efficiency. Arch Androl 11:25–28 [DOI] [PubMed] [Google Scholar]
- Del Rio AG, Blanco AM, Niepomniscze H, Carizza C, Parera F 1998 Thyroid gland and epididymal sperm motility in rats. Arch Androl 41:23–26 [DOI] [PubMed] [Google Scholar]
- Del Rio AG, Palaoro LA, Canessa OE, Blanco AM 2003 Epididymal cytology changes in hypothyroid rats. Arch Androl 49:247–255 [PubMed] [Google Scholar]
- Cooper TG 1999 Epididymis. In: Knobil E, Neill JD, ed. Encyclopedia of reproduction. San Diego: Academic Press; 1–17 [Google Scholar]
- Agarwal A, Hoffer AP 1989 Ultrastructural studies on the development of the blood-epididymis barrier in immature rats. J Androl 10:425–431 [DOI] [PubMed] [Google Scholar]
- St. Pierre N, Dufresne J, Rooney AA, Cyr DG 2003 Neonatal hypothyroidism alters the localization of gap junctional protein connexin 43 in the testis and messenger RNA levels in the epididymis of the rat. Biol Reprod 68:1232–1240 [DOI] [PubMed] [Google Scholar]
- Correa da Costa VM, Rosenthal D 1996 Effect of aging on thyroidal and pituitary T4-5′deiodinase activity in female rats. Life Sci 59:1515–1520 [DOI] [PubMed] [Google Scholar]
- Viger RS, Robaire B 1995 Gene expression in the aging brown Norway rat epididymis. J Androl 16:108–117 [PubMed] [Google Scholar]
- Sullivan R, Saez F, Girouard J, Frenette G 2005 Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis 35:1–10 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St. Germain DL, Galton VA 2001 Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 12:2137–2148 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St. Germain E, Parlow AF, St. Germain DL, Galton VA 2006 Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147:580–589 [DOI] [PubMed] [Google Scholar]