Abstract
Protein tyrosine phosphatase (PTP1B) has been implicated in the negative regulation of insulin and leptin signaling. PTP1B knockout mice are hypersensitive to insulin and leptin and resistant to obesity when fed a high-fat diet. We investigated the role of hypothalamic PTP1B in the regulation of food intake, insulin and leptin actions and signaling in rats through selective decreases in PTP1B expression in discrete hypothalamic nuclei. We generated a selective, transient reduction in PTP1B by infusion of an antisense oligonucleotide designed to blunt the expression of PTP1B in rat hypothalamic areas surrounding the third ventricle in control and obese rats. The selective decrease in hypothalamic PTP1B resulted in decreased food intake, reduced body weight, reduced adiposity after high-fat feeding, improved leptin and insulin action and signaling in hypothalamus, and may also have a role in the improvement in glucose metabolism in diabetes-induced obese rats.
INSULIN AND LEPTIN have been postulated to be signals for the long-term regulation of body weight by the brain and exert overlapping effects on hypothalamic neurons involved in energy homeostasis (1). Insulin and leptin receptors are expressed by brain neurons involved in energy intake, and administration of either peptide, directly into the brain reduces food intake, whereas deficiency of either hormone results in the converse (2). Although humans with severe obesity, due to mutation of leptin or its receptor, have been described, most forms of human obesity are characterized by normal or increased circulating levels of insulin and leptin, and are devoid of any known defect in receptors for these two adiposity signals. Thus, common forms of obesity in both humans and animal models are hypothesized to involve resistance to the actions of insulin and leptin downstream of their neuronal receptors, affecting the neural pathways that mediate their effects on energy balance (3), but the means by which they become resistant to the inputs from insulin and leptin signals are not completely known.
Protein tyrosine phosphatase (PTP1B) has been implicated in the negative regulation of insulin and leptin signaling (3,4). Previous studies have demonstrated that PTP1B knockout mice are hypersensitive to insulin and leptin and resistant to obesity when fed on a high-fat diet (4). Recently Bence et al. (5) demonstrated that neuronal PTP1B−/− mice have reduced body weight and adiposity and increased activity and energy expenditure. These authors showed that PTP1B−/− mice are hypersensitive to leptin, despite paradoxically elevated leptin levels, and also show improved glucose homeostasis. Although tissue-specific knockout mice provide important mechanistic information, in this specific case, some questions remain to be delineated in control and in obese rats: 1) the importance of local populations of neurons expressing PTP1B in mediating the central nervous system (CNS) effects of leptin and insulin, 2) whether leptin levels are also elevated in other models with selective decreases in PTP1B expression in hypothalamic areas surrounding third ventricle, and 3) which peripheral tissues are involved in the improvement in glucose homeostasis. To address these questions, we generated a selective, transient reduction in PTP1B by infusion of an antisense oligonucleotide designed to blunt the expression of PTP1B in rat hypothalamic areas surrounding the third ventricle. Our results show that hypothalamic PTP1B reduction should be sufficient to promote appreciable weight reduction and improve insulin and leptin sensitivity and signaling and glucose homeostasis in diet-induced obese (DIO) rats.
Materials and Methods
Chemicals, antibodies, and oligonucleotides
Antiinsulin receptor (IR), insulin receptor substrate (IRS)-1, IRS-2, long form of the leptin receptor (ObR), Janus kinase (Jak)-2, signal transducer and activator of transcription (Stat)-3 and phosphotyrosine antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-PTP1B (AB-1 mouse polyclonal) was purchased from Calbiochem (La Jolla, CA). pp60src C-terminal phosphoregulatory peptide (TSTEPQpYQPGENL; where pY represents phosphotyrosine) and Biomol Green reagent were purchased from Biomol (Plymouth Meeting, PA). Reagents for SDS-PAGE and immunoblotting were obtained from Bio-Rad (Richmond, CA). Tris, HEPES, phenylmethysulfonyl fluoride (PMSF), aprotinin, dithiothreitol, Triton X-I00, glycerol, Tween 20, and BSA (fraction V) were from Sigma Chemical Co. (St. Louis, MO). Protein A-Sepharose 6MB was from Pharmacia (Uppsala, Sweden), 125I-Protein A, nitrocellulose membranes HPLC-purified [3-3H]glucose and 2-deoxy-d-[1–14C]glucose (2[l4C]DG1) were obtained from Amersham (Amersham Biosciences Group, Little Chalfont, UK). The Harvard apparatus (model 11) and compact infusion pumps (model 975) were obtained from Harvard (South Natick, MA). Human recombinant insulin was from Eli Lilly Co. (Indianapolis, IN). Leptin was from Calbiochem (San Diego, CA). Ketamine hydrochloride was from Fort Dodge Laboratories Inc. (Fort Dodge, IA). Sodium thiopental was from Abbot Laboratories (North Chicago, IL). Sense and antisense phosphorthioate oligonucleotides specific for PTP1B (sense, 5′-AAA GTG CTG TTG G-3′ and antisense, 5′-CCA ACA GCA CTT T-3′) were produced by Invitrogen Corp. (Carlsbad, CA). Kits for measuring insulin and leptin were obtained from Amersham Biosciences (Biotrak, Aylesbury, UK).
Animals and surgical procedures
Adult male Wistar rats from the University of Campinas Central Animal Breeding Center were used in the experiments. All experiments were approved by the Ethics Committee at the State University of Campinas. Four-week-old male Wistar rats were divided into two groups: control rats, fed with standard rodent diet (carbohydrate: 70%; protein: 20%; fat: 10%, totaling 3.8 kcal/g), and rats with DIO, fed on a high-fat diet (carbohydrate: 38.5%; protein: 15%; fat: 46.5%, totaling 5.4 kcal/g) for 2 months.
All rats were anesthetized with ketamine hydrochloride and, after loss of corneal and pedal reflexes, were positioned on a Stoelting stereotaxic apparatus. A 23-gauge guide stainless steel cannula with an indwelling 30-gauge obturator was stereotaxically implanted intracerebroventricularly (icv) into the third ventricle by use of previously reported techniques and preestablished coordinates (6). Rats were allowed 1 wk for recovery before testing for cannula patency and position. Cannulas were considered patent and correctly positioned by dypsogenic response elicited after angiotensin II injection (7). The animals in both groups were treated with two daily doses of PTP1B antisense oligonucleotide (ASO) or sense, for 4 d. Because the treatment with PTP1B ASO reduced food intake, we also included a group of pair-fed animals, as a control for the animals treated with ASO, on standard rodent chow and a high-fat diet.
Metabolic parameters
At the end of the diet period and treatment with PTP1B-ASO or PTP1B sense oligonucleotide (PTP1B-sense), the body weight and retroperitoneal and epididymal fat pad were measured. Food was withdrawn 12 h before the experiments. Blood samples of 0.5 ml were collected from the tail for the determination of the serum concentrations of insulin and leptin levels, which were measured by RIA.
Protocol for food ingestion determination
For both groups (control and DIO), at the end of the PTP1B-ASO or PTP-1B-sense treatment period, icv cannulated rats were food deprived for 6 h (from 12 to 18 h) and at 18 h were icv treated with insulin (2.0 μl, 10−6 m), leptin (2.0 μl, 10−6 m), or saline (2.0 μl). Food ingestion was determined over the next 12 h.
Tissue extraction, immunoprecipitation, and immunoblotting
Rats were anesthetized with sodium thiopental, and as soon as anesthesia was assured by the loss of pedal and corneal reflexes, the rats were icv treated with insulin (2.0 μl, 10−6 m), leptin (2.0 μl, 10−6 m), or saline (2.0 μl) and after 15 min or 60 min subjected to craniotomy (3,8). Hypothalami were obtained and homogenized in freshly prepared ice cold buffer [1% Triton X-100, 100 mm Tris (pH 7.4), 100 mm sodium pyrophosphate, 100 mm sodium fluoride, 10 mm EDTA, 10 mm sodium vanadate, 2 mm PMSF, and 0.01 mg aprotinin/milliliter]. Insoluble material was removed by centrifugation (15,000 rpm) for 40 min at 4 C. The protein concentration of the supernatants was determined by the Bradford dye binding method (9). Aliquots of the resulting supernatants containing 2.0 mg total protein were used for immunoprecipitation with specific antibodies at 4 C overnight, followed by addition of protein A-Sepharose 6MB for 2 h. The pellets were washed three times in ice-cold buffer [0.5% Triton X-100, 100 mm Tris (pH 7.4), 10 mm EDTA, and 2 mm sodium vanadate] and then resuspended in Laemmli sample buffer (10) and boiled for 5 min, after which they were subjected to SDS-PAGE in a miniature slab gel apparatus (Mini-Protean). For total extracts, 0.2 mg of protein were separated by SDS-PAGE. Electrotransfer of proteins from the gel to nitrocellulose was performed for 120 min at 120 V in a Bio-Rad Mini-Protean transfer apparatus. Nonspecific protein binding to the nitrocellulose was reduced by preincubating the filter for 2 h in blocking buffer (5% nonfat dry milk, 10 mm Tris, 150 mm NaCl, 0.02% Tween 20) (11). The nitrocellulose blot was incubated with specific antibodies overnight at 4 C and then incubated with 125I-labeled protein A. Results were visualized by autoradiography with preflashed Kodak XAR film (Rochester, NY). Band intensities were quantified by optical densitometry of developed autoradiographs (Scion Image software; Scion Corp., Frederick, MD).
Phosphorthioate-modified oligonucleotide treatment
The sequences of sense and antisense (ASO) phosphorthioate oligonucleotides, specific for PTP1B (sense, 5′-AAA GTG CTG TTG G-3′ and antisense, 5′-CCA ACA GCA CTT T-3′), were selected among three unrelated pairs of oligonucleotides on the basis of their ability to block PTP1B protein expression, as evaluated by immunoblotting total protein extracts of hypothalamus using specific anti-PTP1B antibodies. The antisense oligonucleotide sequences were submitted to BLAST analyses (www.ncbi.nlm.nih.gov) and matched only for the Rattus norvegicus PTP1B coding sequence. Control and DIO were cannulated, housed in individual cages and treated with sense oligonucleotide (PTP1B-Sense) or PTP1B antisense oligonucleotide (PTP1B-ASO). Sense and antisense PTP1B oligonucleotides were diluted in TE buffer (10 mm Tris-HCl, 1 mm EDTA) and treatment was achieved by icv infusions, twice a day (0800 h/1800 h) with a total vol of 2.0 μl/ dose (4.0 nmol/μl) for 4 d.
Immunohistochemistry
Rat hypothalami were fixed in 4% paraformaldehyde-0.2 m PBS (PBS, pH 7.4) for 24 h and embedded in paraffin, and 5.0-μm sections were obtained. The glass-mounted sections were cleared from paraffin with xylene and rehydrated by sequential washings with graded ethanol solutions (70–100%). After permeabilization with 0.1% Triton X-100 in PBS, pH 7.4, for 10 min at room temperature, the sections were incubated with the primary antibody (mouse anti-PTP1B) in 1% BSA in PBS at 4 C in a moisture chamber. After incubation with the primary antibody, sections were washed and incubated with FITC-conjugated secondary antibodies, and analysis and photo documentation were performed using a Zeiss LSM 510 Laser Scanning Confocal Microscope, according to a previously described technique (12).
Hyperinsulinemic-euglycemic clamp studies
After 12 h of fasting, animals were anesthetized ip with a mix of ketamine (100 mg) and diazepam (0.07 mg) (0.2 ml/100 g body weight), and catheters were then inserted into the left jugular vein (for tracer infusions) and carotid artery (for blood sampling), as previously described (13). A 120-min hyperinsulinemic-euglycemic clamp procedure was conducted in anesthetized catheterized rats, as shown previously (13), and (3) with a prime continuous infusion of human insulin at a rate of 3.6 mU/kg body weight·min to raise the plasma insulin concentration to approximately 800–900 pmol/liter. Unlabeled glucose (10%) was infused at variable rates to maintain plasma glucose at fasting levels. Insulin-stimulated whole-body glucose flux was estimated using a prime continuous infusion of HPLC-purified [3-3H]glucose (10 μCi bolus, 0.1 μCi/min) throughout the clamp procedure (14).
To estimate insulin-stimulated glucose-transport activity and metabolism in skeletal muscle, 2-[14C]DG1 was administered as a bolus (10 μCi) 45 min before the end of the clamp procedure. Blood samples (20 μl) were taken at 80, 90, 100, 110, and 120 min after the start of the clamp procedure to determine plasma [3H]glucose and 2-[14C]DG1 concentrations. All infusions were performed using Harvard infusion pumps. At the end of the clamp procedure, animals were killed by a mix of ketamine and diazepam iv injection. Within 2 min, two skeletal muscles (soleus and gastrocnemius) from both hind limbs were taken. Each tissue, once exposed, was dissected out within 2 sec, weighed, frozen with liquid N2, and stored at −70 C for later analysis.
In separate experiments, the basal rates of glucose turnover were measured by continuously infusing [3-3H]glucose (0.02 μCi/min) for 120 min, and blood samples (20 μl) were taken at 100, 110, and 120 min after the start of the experiment for the determination of plasma [3H]glucose concentration. The hepatic glucose output during the clamp procedure was therefore obtained from the difference between the whole-body glucose uptake and the rate of unlabeled glucose infusion. Glucose transport activity in skeletal muscle was calculated from the plasma 2[14C]DG1 profile, according to a previously described method (3,15).
Protein tyrosine phosphatase (PTP) activity assay
The in vitro PTP1B activity assay was conducted based on a protocol previously described by Taghibiglou et al. (16). Hypothalami were removed and homogenized in solubilization buffer containing 1% Triton X-100, 20 mm, Tris (pH 7.6), 5 mm EDTA, 2 mm PMSF, 0.1 mg aprotinin/ml, 1 mm EGTA, and 130 mm NaCl. Lysates were centrifuged (15,000 rpm, 40 min, 4 C) and the supernatants were collected for immunoprecipitation with anti-IR, anti-Jak-2, and anti-PTP1B antibodies, as described previously. Immunoprecipitates were washed in PTP assay buffer [100 mm HEPES (pH 7.6), 2 mm EDTA, 1 mm dithiothreitol, 150 mm NaCl, 0,5 mg/ml BSA]. The pp60c-src C-terminal phosphoregulatory peptide (TSTEPQpYQPGENL; Biomol) was added to a final concentration of 200 μm in a total reaction volume of 60 μl in a PTP assay buffer for immunoprecipitation. The activity of total extracts (125 μg) was measured in the same manner in a total reaction volume of 60 μl in a PTP assay buffer, adding the peptide to a final concentration of 200 μm. The reaction was then allowed to proceed for 1 h at 30 C. At the end of the reaction, 40-μl aliquots were placed into 96-well plates, 100 μl of Biomol Green reagent (Biomol) was added, and absorbance was measured at 660nm.
Body composition
The carcass (without the gastrointestinal tract), was weighed and stored at −20 C for analysis of body composition. The carcass water was determined as the difference between the dry and wet weights. Total fat was extracted with petroleum ether by using a Soxhlet apparatus (17).
Data presentation and statistical analysis
Data were expressed as means ± sem accompanied by the indicated number of independent experiments. For statistical analysis, the groups were compared using a two way ANOVA with the Bonferroni test for post hoc comparisons. The level of significance adopted was P < 0.05.
Results
Intracerebroventricular PTP1B antisense oligonucleotide decreases PTP1B protein in the hypothalamus
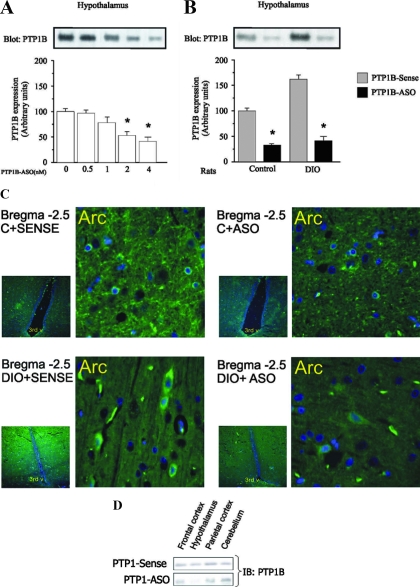
We administered PTP1B-ASO and PTP1B-sense by injection in the third ventricle of control and DIO rats and assessed the effects on PTP1B protein by immunoblotting and brain immunohistochemistry. The potency of PTP1B-ASO was tested in a dose-response experiment. Two daily doses of 0.5 nmol or 1 nmol of PTP1B-ASO did not change PTP1B expression, and the dose of 2 nmol induced a modest decrease in this enzyme, but two daily doses of 4 nmol PTP1B-ASO inhibited PTP1B expression by 70% in control rats after 4 d of treatment (Fig. 1A). Hypothalamic levels of PTP1B were increased in DIO rats, compared with control rats, and there was a marked decrease after PTP1B-ASO (Fig. 1B). We confirmed the selective inhibition of PTP1B in the hypothalamus by immunohistochemistry (Fig. 1C). Most cells staining for the enzyme were detected in the arcuate nucleus and lateral hypothalamus. In addition, some positively stained cells were seen in the paraventricular nucleus and dorsomedial hypothalamus. Substantial reductions in expression were determined in arcuate and lateral nuclei of hypothalamus, with no changes in frontal and parietal cortex, hippocampus, and cerebellum (Fig 1D). Because the numbers of positively stained neurons in other hypothalamic regions were considerably lower than in the arcuate nucleus and lateral hypothalamus, we cannot be sure of the inhibitory effect of the treatment in these regions. Nevertheless, because the enzyme PTP1B is expressed in both arcuate nucleus and lateral hypothalamus, we found it optimal to perform the injections through the third ventricle using stereotaxic coordinates that warrant delivery in the vicinity of the region of interest. At least two previous studies have shown that using the ventricular route, oligonucleotides and other drugs diffuse mostly to the areas surrounding the injection site (18,19). No oligonucleotide reaches regions such as the frontal cortex, basal forebrain, and parietal cortex. It is possible that other specific sites may have been affected by the treatment; however, the most prominent effect is likely to have occurred in the hypothalamus. PTP1B protein levels were not reduced in skeletal muscle, liver, and adipose tissue (data not shown).
Figure 1.
Immunoblotting and immunohistochemical evaluation of PTP1B distribution and expression in hypothalamus of rats treated with PTP1B-ASO. A, Immunoblotting with anti-PTP1B antibody of whole-tissue extracts from hypothalamus of rats treated with PTP1B-ASO (0, 0.5, 1, 2, 4 nm) for 4 d. B, Immunoblotting with anti-PTP1B antibody of whole-tissue extracts from hypothalamus of control and DIO rats treated with PTP1B-ASO. C, Paraformaldehyde-fixed rat hypothalamic sections (5 μm) were incubated with anti-PTP1B (visualization of immunoreactivity as described in Materials and Methods). D, Imunnoblotting of PTP1B from different areas of the CNS. Data are means ± sem of four independent experiments, i.e. four different cohorts of control rats or DIO rats. *, Control or DIO rats: P < 0.05, PTP1B-ASO vs. PTP1B sense.
Metabolic characteristics
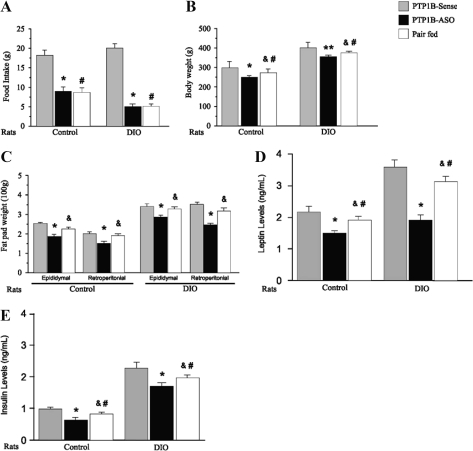
At the end of 4 d of treatment with PTP1B-ASO or PTP1B-sense, we evaluated the in vivo effect of hypothalamic PTP1B reduction on food intake, body weight, epididymal and retroperitoneal fat, and leptin and insulin levels. In control and DIO rats treated with PTP1B-ASO, there were reductions in food intake of 50 and 70%, respectively (Fig. 2A). These reductions in food intake were accompanied by a decrease in body weight in control and DIO rats after 4 d of PTP1B-ASO treatment (Fig. 2B). The reductions in body weight in pair-fed animals were less marked, compared with animals treated with PTP1B-ASO (control pair-fed: 26 ± 3 g vs. control PTP1B-ASO: 48 ± 4 g P < 0.001; DIO pair-fed: 25 ± 3 g vs. DIO PTP1B-ASO: 46 ± 4 g P < 0.001). Control and DIO rats demonstrated reduction in adiposity after treatment with PTP1B-ASO, as shown by the lower epididymal and retroperitoneal fat pad weight (Fig. 2C). The reductions in epididymal and retroperitoneal fat pad were less marked in pair-fed animals and were not significantly different, compared with their controls (Fig. 2C).
Figure 2.
Effect of icv PTP1B-ASO on metabolic parameters. A, Twenty-four hour food intake. B, Body weigh. C, Epididymal and retroperitoneal adipose tissue mass. D, serum leptin levels. E, Serum insulin levels in controls and DIO rats treated with PTP1B-ASO or PTP1B-sense or in pair-fed rats. Data are means ± sem. Each group was composed of 10–15 animals. *, Control or DIO rats: P < 0.05, PTP1B-ASO vs. PTP1B-sense; &, P < 0.05 pair-fed vs. PTP1B-ASO; #, P < 0.05 pair-fed vs. PTP1B-sense; **, P < 0.01 PTP1B-ASO vs. PTP1B sense.
To better understand these weight losses after PTP1B-ASO treatment in control and DIO rats, we carried out body composition analysis in the four groups of rats (Table 1). Carcass lipid, expressed as percentages of total body weight (carcass), was lower in controls treated with PTP1B-ASO, compared with control (PTP1B-ASO 7.5 ± 0.7% vs. control 12.3 ± 0.8%), and also in DIO rats treated with PTP1B-ASO, compared with DIO (PTP1B-ASO 16.6 ± 1.1% vs. DIO 21.3 ± 1.3% P < 0.01%; Table 1). In control or DIO rats treated with PTP1B-ASO, there was a decrease in water content, compared with their controls, but when expressed as percentage of total body weight, there was an increase. In addition, there was a decrease in free-fat mass in animals treated with PTP1B-ASO, but when expressed as percentage of body weight, no significant differences were found between the groups. In summary, the decrease in body weight in control and DIO rats treated with PTP1B-ASO was related to differences in fat, water content, and fat-free dry mass. However, when expressed as percentage of body weight, only fat content was significantly reduced in animals treated with PTP1B-ASO. In DIO pair-fed rats, the body weight composition showed intermediate values between DIO and DIO PTP1B-ASO.
Table 1.
Effect of icv PTP1B-ASO on body weight composition of control and DIO rats
| Groups | Mean carcass composition ± sem
|
|||||
|---|---|---|---|---|---|---|
| Carcass | Water | Lipid
|
Fat-free dry mass
|
|||
| Weight (g) | Percent body weight | Weight (g) | Percent body weight | |||
| Control | 266 ± 3.8 | 168.9 ± 2.2 | 32.7 ± 1.9 | 12.3 ± 0.8 | 64.4 ± 4.7 | 24.2 ± 1.7 |
| Control + ASO | 213 ± 5.9a | 147.2 ± 2.6a | 16.0 ± 1.5b | 7.5 ± 0.7a | 49.8 ± 5.6a | 23.4 ± 2.6 |
| DIO | 368 ± 9.1 | 193.6 ± 2.7 | 78.4 ± 4.6 | 21.3 ± 1.3 | 96.0 ± 5.8 | 26.1 ± 1.5 |
| DIO + ASO | 317 ± 9.1c | 187 ± 2.1c | 52.5 ± 3.2c | 16.6 ± 1.1c | 77.4 ± 5.6c | 24.4 ± 1.7 |
| DIO + PF | 349 ± 11.2 | 198 ± 2.8 | 67.0 ± 5.1 | 19.2 ± 1.4 | 83.0 ± 6.2 | 23.8 ± 1.8 |
The data are the mean ± sem, n = 7 rats/group. Carcass weight represents the total body weight minus the intestinal tract, as recorded on the last day of the experiment. Control, Rats fed with standard rodent diet; PF, pair-fed.
P < 0.001 vs. the control group.
P < 0.01 vs. the control group.
P < 0.001 vs. the DIO group.
The treatment with PTP1B-ASO for 4 d produced a biphasic response in leptin levels. Initially there was a marked increase in leptin levels in control and DIO rats in the first day of PTP1B-ASO treatment, and after the third day of PTP1B-ASO treatment, there was a decrease in leptin levels in control and DIO rats (Fig. 2D). The hypothalamic expression of PTP1B was markedly decreased since the first day of PTP1B-ASO treatment (data not shown). In addition, we observed a significant decrease in fasting insulin levels on the fourth day of PTP1B-ASO treatment in controls and DIO rats, compared with controls and DIO rats treated with PTP1B-sense, respectively (Fig. 2E). In pair-fed animals, there were significant reductions in leptin or insulin levels, compared with their controls, but these values were significantly higher, compared with PTP1B-ASO groups (Fig. 2, D and E).
Effect of reduction of PTP1B on leptin-induced satiety and leptin signal transduction
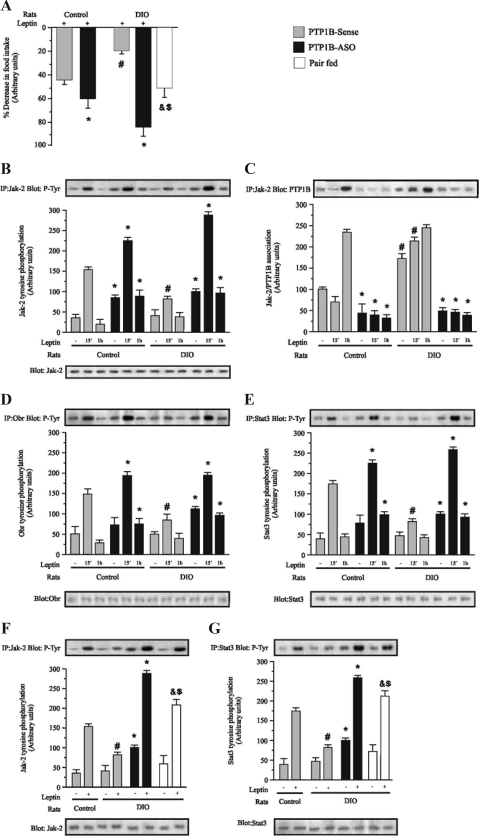
Leptin provides a robust anorexigenic signal to the hypothalamus. To evaluate the effect of hypothalamic PTP1B reduction on leptin action in the hypothalamus at the end of 4 d of treatment with PTP1B-ASO or PTP1B-sense, control and DIO rats were acutely treated with 2 μl saline or leptin (2 μl, 10−6 m) icv, and food intake was measured over the next 12 h. Leptin infusion reduced food intake by 42 and 19% in controls and DIO rats treated with PTP1B-sense, respectively, as depicted in Fig. 3A. Hypothalamic PTP1B reduction significantly increased leptin-induced suppression of food ingestion to 60 and 85%, respectively, for control and DIO rats treated with PTP1B-ASO. In pair-fed animals, leptin induced a significant decrease in food intake, compared with DIO, but this decrease was less marked than that observed in DIO PTP1B-ASO (Fig. 3A) Thus, hypothalamic PTP1B reduction led to a significant improvement in the leptin-induced anorexigenic effect, which is more evident in DIO rats. Basal Jak-2 tyrosine phosphorylation was increased in the hypothalamus of control rats treated with PTP1B-ASO. In these animals, there was also a higher increase in Jak-2 tyrosine phosphorylation after 15 min of icv leptin infusion, which was maintained at 60 min. In DIO rats leptin-induced Jak-2 phosphorylation at 15 min were reduced when compared with controls (Fig. 3B). DIO rats treated with PTP1B-ASO presented an increase in basal and leptin-induced Jak-2 tyrosine phosphorylation, and this increase was maintained 60 min after leptin infusion, similarly to that seen in of PTP1B-ASO control rats. DIO rats presented increases in the basal and leptin-stimulated association between Jak-2/PTP1B (Fig. 3C). As expected, treatment with PTP1B-ASO reduced Jak-2/PTP1B association in control and DIO rats. In control rats treated with PTP1B-ASO, there was an increase in ObR tyrosine phosphorylation after 15 and 60 min of icv leptin infusion, compared with control sense. There was a reduction in leptin-induced ObR phosphorylation in DIO rats, compared with controls. Treatment with PTP1B-ASO increased the basal and leptin-induced ObR tyrosine phosphorylation in DIO rats at 15 and 60 min after leptin infusion, compared with DIO sense (Fig. 3D). In control rats treated with PTP1B-ASO, the levels of Stat3 phosphorylation reached after 15 and 60 min of icv leptin infusion were higher, compared with control sense at the same time points. Leptin-induced Stat3 phosphorylation were reduced at 15 min in DIO rats when compared with controls. DIO rats treated with PTP1B-ASO demonstrated an increase in basal and leptin-induced Stat3 tyrosine phosphorylation at 15 and 60 min after leptin infusion, compared with DIO sense (Fig. 3E). We also performed experiments to compare the effect of PTP1B-ASO with pair-fed animals in the DIO group. Results showed that, in the pair-fed animals, the leptin-induced Jak-2 and Stat3 phosphorylation were higher than that of DIO animals but significantly lower than in the animals that received PTP1B-ASO (Fig. 3, F and G).
Figure 3.
Effect of icv PTP1B-ASO on leptin-induced satiety and leptin signaling in hypothalamus of control and DIO rats. A, leptin was icv infused, and 12-h food intake was measured in control and DIO rats treated with PTP1B-ASO or PTP1B-sense or in pair-fed rats. B, Immunoprecipitation (IP) with anti-Jak-2 and immunoblotting (IB) with anti-pY antibodies. C, IP with anti-Jak-2 and IB with anti-PTP1B. D, IP with anti-ObR and IB with anti-pY. E, IP with anti-Stat3 and IB with anti- pY. F, IP with anti-Jak-2 and IB with anti-pY. G, IP with anti-Stat3 and IB with anti-pY. Specific bands were densitometrically quantified. Data are means ± sem of four independent experiments, i.e. four different cohorts of control rats or DIO rats. The comparisons between the groups were performed by using the same time point in the respective group: *, P < 0.01 PTP1B-ASO vs. PTP1B sense; #, P < 0.05 PTP1B-sense vs. PTP1B sense; &, P < 0.05 pair-fed vs. PTP1B-ASO; $, P < 0.05 pair-fed vs. PTP1B-sense.
Effect of reduction of PTP1B on insulin-induced satiety and insulin signal transduction
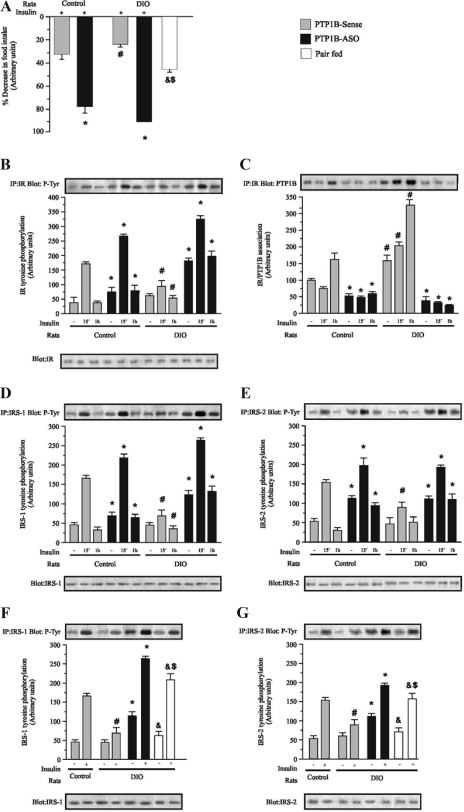
To evaluate the effect of hypothalamic PTP1B reduction upon insulin action in the hypothalamus of control and DIO rats, at the end of 4 d of treatment with PTP1B-ASO or PTP1B-sense, the animals were acutely treated with 2 μl saline or insulin (2 μl, 10−6 m) icv, and food intake was measured over the next 12 h. Insulin infusion induced reductions of 32 and 23% in food intake in controls and DIO rats treated with PTP1B-Sense, respectively, as depicted in Fig. 4A. Hypothalamic PTP1B reduction significantly increased insulin-induced suppression of food ingestion to 78 and 95%, in control and DIO rats treated with PTP1B-ASO, respectively. Thus, hypothalamic PTP1B reduction led to a significant improvement in the insulin-induced anorexigenic effect. In pair-fed animals, insulin induced a significant decrease in food intake, compared with DIO, but this decrease was less marked than that observed in DIO PTP1B-ASO. Basal IR tyrosine phosphorylation was increased in the hypothalamus of control rats treated with PTP1B-ASO. In these animals, there was also a greater increase in IR tyrosine phosphorylation after 15 min of icv insulin infusion, which was maintained at 60 min. In DIO rats insulin-induced IR phosphorylation was reduced when compared with controls (Fig. 4B). DIO rats treated with PTP1B-ASO presented an increase in basal and insulin-induced IR tyrosine phosphorylation, and this increase was maintained 60 min after insulin infusion, similar to that seen in PTP1B-ASO control rats. DIO rats presented increases in the basal and insulin-stimulated association between IR/PTP1B (Fig. 4C). As expected, treatment with PTP1B-ASO reduced IR/PTP1B association in control and DIO rats. In control rats treated with PTP1B-ASO, there was also increased basal IRS-1 tyrosine phosphorylation. In these animals, a larger increase in IRS-1 tyrosine phosphorylation was also observed after 15 and 60 min of icv insulin infusion, compared with control sense. There was a reduction in insulin-induced IRS-1 phosphorylation in DIO rats, compared with controls. Treatment with PTP1B-ASO increased the basal and insulin-induced IRS-1 tyrosine phosphorylation in DIO rats at 15 and 60 min after insulin infusion, compared with DIO sense (Fig. 4D). Basal IRS-2 tyrosine phosphorylation was increased in the hypothalamus of control rats treated with PTP1B-ASO. In these animals, the levels of IRS-2 phosphorylation reached after 15 and 60 min of icv insulin infusion were higher, compared with control sense at the same time points. Insulin-induced IRS-2 phosphorylation was reduced in DIO rats when compared with controls. DIO rats treated with PTP1B-ASO demonstrated an increase in basal and insulin-induced IRS-2 tyrosine phosphorylation at 15 and 60 min after insulin infusion, compared with DIO sense (Fig. 4E). We also performed experiments to compare the effect of PTP1B-ASO with pair-fed animals in the DIO group. Results showed that, in pair-fed animals, the insulin-induced IRS-1 and IRS-2 phosphorylations were higher, compared with DIO animals, but significantly lower than in the animals that received PTP1B-ASO (Fig. 4, F and G).
Figure 4.
Effect of icv PTP1B-ASO on insulin-induced satiety and insulin signaling in hypothalamus of control and DIO. A, insulin was icv infused, and 12-h food intake was measured in control and DIO rats treated with PTP1B-ASO or PTP1B-sense or in pair-fed animals. B, Immunoprecipitation (IP) with anti-IR and immunoblotting (IB) with anti-pY antibodies. C, IP with anti-IR and IB with anti-PTP1B. D, IP with anti-IRS-1 and IB with anti-pY. E, IP with anti-IRS-2 and IB with anti-pY. F, IP with anti-IRS-1 and IB with anti- pY. G, IP with anti-IRS-2 and IB with anti-pY. Specific bands were densitometrically quantified. Data are means ± sem of four independent experiments, i.e. four different cohorts of control rats or DIO rats. Control or DIO rats comparing the same time in the respective group: *, P < 0.01 PTP1B-ASO vs. PTP1B sense; #, P < 0.05 PTP1B-sense vs. PTP1B-sense; &, P < 0.05 pair-fed vs. PTP1B-ASO; $, P < 0.05 pair-fed vs. PTP1B-sense.
Effect of the reduction of PTP1B on glucose metabolism
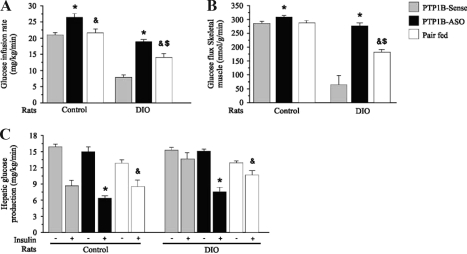
To further understand glucose use in control and DIO rats treated icv with PTP1B-ASO and PTP1B-sense, we performed hyperinsulinemic-euglycemic clamp studies. The procedure was performed with tracer infusions to examine the effects of PTP1B-ASO in the hypothalamus on the metabolism of glucose in liver and skeletal muscle tissue. The glucose infusion rate necessary to maintain euglycemia at fasting levels in the presence of a constant infusion of insulin (3.6 mU/kg body weight·min) was slightly higher in control rats treated with PTP1B-ASO, compared with controls treated with PTP1B-sense, respectively (Fig. 5A). There was a significant reduction in the glucose infusion rate in DIO rats, which was reversed when the animals where treated with PTP1B-ASO. Insulin-induced glucose uptake was quantified using 2-deoxyglucose uptake analysis in skeletal muscle tissue. The glucose uptake by skeletal muscle was significantly reduced in DIO rats treated with PTP1B-sense, and this alteration was completely reversed in DIO rats treated with PTP-1B-ASO (Fig. 5B). The glucose infusion rate and glucose uptake in muscle of pair-fed DIO rats were higher than in DIO rats but lower than in DIO rats treated with PTP1B-ASO. Figure 5C demonstrates that the insulin-induced decrease in hepatic glucose output was blunted in DIO rats treated with PTP1B-sense, but when these animals were treated with PTP1B-ASO, the insulin effect was completely reversed. The insulin-induced decrease in hepatic glucose production in pair-fed DIO rats reached intermediate values between those of the DIO and DIO animals that received PTP1B-ASO.
Figure 5.
A, Steady-state glucose infusion rates obtained from averaged rates of 90–120 min of 10% unlabeled glucose infusion during hyperinsulinemic-euglycemic clamp procedures in the control, DIO, and pair-fed rats. B, Glucose transport in skeletal muscle tissue evaluated by 2- deoxyglucose uptake during the last 45 min of the hyperinsulinemic-euglycemic clamp studies. C, Basal and insulin-stimulated rates of hepatic glucose production during the hyperinsulinemic euglycemic clamp procedures in awake rats. Data are means ± sem of four independent experiments, i.e. four different cohorts of control rats or DIO rats. DIO or control rats comparing the same time in the respective group: *, P < 0.05 PTP1B-ASO vs. PTP1B-sense; &, P < 0.05 pair fed vs. PTP1B-ASO; $, P < 0.05 pair-fed vs. PTP1B-sense.
Hypothalamus PTPase and PTP1B activities
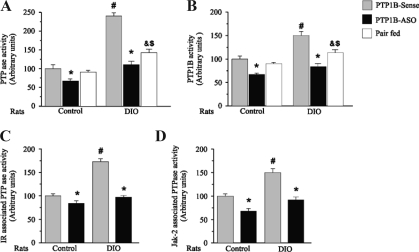
PTPase activity increases in the insulin-resistant states, correlating with increased PTP1B protein expression (18). Consistent with this finding, we observed an increase in hypothalamic PTPase activity in DIO rats, compared with control rats. After treatment with PTP1B-ASO, PTPase activity decreased in control and DIO rats (Fig. 6A). To specifically investigate PTP1B activity, we measured the activity of this enzyme in immunoprecipitates of PTP1B. Results demonstrate that PTP-1B activity was increased in the hypothalamus of DIO rats, compared with controls rats. After treatment with PTP1B-ASO, a reduction in PTP1B activity was observed in control and DIO rats (Fig. 6B). In DIO pair-fed animals, the PTPase and PTP1B activities showed intermediate values between those of the DIO and DIO animals that received PTPT1B-ASO.
Figure 6.
Effect of icv PTP1B-ASO on PTPase and PTP1B activities in control and DIO rats. Total PTPase activity and PTPase activities in immunoprecipitates were assayed by incubation with the pp60c-src C-terminal phosphoregulatory peptide (TSTEPQpYQPGENL), as described in Materials and Methods. A, Total PTPase activity in hypothalamus. B, PTPase activity in PTP1B immunoprecipitates. C, PTPase activity in IR immunoprecipitate. D, PTPase activity in Jak-2 immunoprecipitates. Data are means ± sem of four independent experiments, i.e. four different cohorts of control rats or DIO rats. Control or DIO rats comparing the same time in the respective group: *, P < 0.05 PTP1B-ASO vs. PTP1B-sense; #, P < 0.05 PTP1B-sense vs. PTP1B-sense; &, P < 0.05 pair-fed vs. PTP1B-ASO; $, P < 0.05 pair-fed vs. PTP1B-sense.
In addition to total PTPase activity, we also measured PTPase activity in IR and Jak-2 immunoprecipitates and found an increase in IR- and Jak-2-associated PTPase activity in DIO rats, compared with control rats, respectively (Fig. 6, C and D). After treatment with PTP1B–ASO, there was a decrease in these associated activities in control and DIO rats.
Discussion
Resistance to leptin and insulin is likely to play a central role in obesity; however the mechanism by which insulin and leptin signaling becomes impaired is poorly understood. Genetic and biochemical evidence for an important role of PTP1B as a negative regulator of insulin and leptin-induced metabolic actions has emerged in recent years (20). Several studies have examined PTP1B expression in rodents and humans with insulin resistance, diabetes, and obesity. Many reports have shown increased expression and/or activity of PTP1B in these states (21,22,23,24). Mice lacking PTP1B are protected from DIO and are hypersensitive to leptin,; however, the site and mechanism of these effects remain controversial. Studies using PTP1B-ASO, which lowers PTP1B levels in liver and fat, suggest that this phosphatase regulates glucose homeostasis and adiposity primarily through actions on these tissues. Bence et al. (5), however, recently demonstrated that neuronal PTP−/− mice have reduced body weight and adiposity and increased activity and energy expenditure. These authors showed that PTP−/− mice are hypersensitive to leptin, despite paradoxically elevated leptin levels, and also show improved glucose homeostasis. In contrast, adipose PTP1B deficiency increases body weight and PTP1B deletion in liver or muscle does not affect weight. This important study demonstrates that PTP1B regulates body mass and adiposity through the brain.
To initially investigate the importance of local populations of neurons expressing PTP1B in mediating the CNS effects of leptin and insulin in control and DIO rats, we have herein generated a selective, transient reduction in PTP1B by infusion of an antisense oligonucleotide designed to blunt the expression of PTP1B in hypothalamic areas surrounding the third ventricle of rats.
DIO rats have a marked increase in PTP1B protein expression in the hypothalamus. The treatment of these DIO rats with PTP1B-ASO icv induced a marked and selective decrease in PTP1B expression in discrete hypothalamic nuclei, mainly in arcuate and lateral hypothalamic nuclei. The selective decrease in hypothalamic PTP1B protein in rats receiving PTP1B-ASO resulted in a rapid decrease in food intake, reduced body weight, and reduced adiposity in control and DIO animals. These data suggest an improvement in leptin sensitivity, which was confirmed by the finding that, in addition to a basal reduction in food intake in rats treated icv with PTP1B-ASO, an enhanced suppression of 12 h food intake was also observed in response to leptin.
Leptin signals in the hypothalamus by binding to a type I cytokine receptor (the leptin receptor, ObR), and activating the associated tyrosine kinase Jak-2 (23). Activated Jak-2, in turn, phosphorylates itself and residues Tyr985 and Tyr1138 within the ObR cytoplasmic tail. Tyr1138 recruits and activates the transcription factor Stat3 (25). This cascade of events leads to translocation of STAT3 into the nucleus and transcriptional regulation of neuropeptides and various leptin target genes (26,27,28).
Leptin terminates its own action through phosphorylation of Tyr985 of leptin receptor and induction of suppressor of cytokine signaling 3 (27). The mechanisms controlling and terminating the leptin signal transduction also include dephosphorylation and inactivation of signaling proteins, mediated by PTPs (28,29). The transient character of the tyrosine phosphorylation of Jak-2 and Stat3 reinforces the involvement of PTPs as negative regulators of this signaling pathway. PTP1B, a well-known inhibitor of insulin action, also terminates leptin signaling through inactivation of JAK2 (25). Our data show an increase in tyrosine phosphatase activity, an increase in PTPase activity associated with Jak-2, and a decrease in JaK-2 tyrosine phosphorylation levels in DIO rats. Because JAK2 represents the initial kinase mediating all aspects of downstream leptin signaling, a reduction in JAK2 phosphorylation would be predicted to attenuate leptin-dependent regulation of multiple pathways including STAT3 phosphorylation. The leptin resistance in DIO rats was also characterized by a less evident increase in ObR and Stat3 tyrosine phosphorylation evoked by leptin. These alterations were completely reversed in DIO rats treated with PTP1B-ASO. These results suggest that a reduction in hypothalamic PTP1B is sufficient to improve leptin sensitivity and signaling in DIO rats.
In the present study, we demonstrate that the selective decrease in hypothalamic PTP1B protein in rats receiving PTP1B-ASO resulted in decreased food intake, reduced body weight, reduced adiposity after high-fat feeding, and improved leptin action and signaling. At the molecular level, there was a decrease in insulin-induced IR tyrosine phosphorylation and an increase in PTP1B activity associated with IR in the hypothalamus of DIO. Downstream, there was also a reduction in insulin-induced IRS-1 and IRS-2 tyrosine. Our results, in accordance with previous data on neuronal Ptpn1−/− mice (5), show that the primary effect of a reduction on PTP1B protein expression in hypothalamus is an increase in leptin levels. This increase was evident on the first day of PTP1B ASO treatment. The mechanisms that might regulate this increase in leptin levels are not known. Recent evidence suggests parasympathetic innervation of some fat depots (30), which may regulate adipocyte leptin production. Additionally and/or alternatively, as previous suggested (5), neuronal PTP1B could regulate secretion of an unknown humoral factor that controls adipokine secretion. Interestingly, on the second and third day of PTP1B ASO treatment, there is a gradual decrease in leptin levels, with a marked decrease on the fourth day. This decrease might reflect the reduction in food ingestion and/or the body weight of the animals, which are known mechanisms that induce a decrease in leptin levels.
In addition to leptin, insulin is also able to reduce food intake (8), and PTP1B is a negative regulator of insulin signaling (31). As in peripheral tissues, neuronal insulin action involves the pathway IR/IRS/phosphatidylinositol 3-kinase (PI3K) signal transduction pathway. Thus, hypothalamic IR/IRS/PI3K increases after either icv or systemic insulin administration (32) or the inhibitory effect of icv insulin on both food intake (6,32) and hepatic glucose production (33) can be blocked by icv pretreatment with PI3K inhibitor. Thus, we also investigated the effect of icv insulin infusion on food intake and insulin signaling in these rats. DIO and control rats treated icv with PTP1B-ASO and demonstrating a reduced basal food intake still showed a clear suppression in 12 h food intake in response to icv insulin infusion. At the molecular level, there was a decrease in insulin-induced IR tyrosine phosphorylation and an increase in PTP1B activity associated with IR in the hypothalamus of DIO. Downstream there was also a reduction in insulin-induced IRS-1 and IRS-2 tyrosine phosphorylation levels. These alterations may have an important role in insulin resistance in the hypothalamus of obese rats. Although in obese animals there is an increase in IRS-1 serine phosphorylation in hypothalamus (6), which can contribute to insulin resistance, our data showed that the treatment of DIO rats with PTP1B-ASO reversed insulin resistance in the hypothalamus of obese rats and also improved insulin signaling in control rats. Because IRS-1/2 are substrates of the insulin receptor, it is possible that the down-regulation of PTP1B improved insulin receptor tyrosine kinase activity, culminating with an increase in insulin-induced IRS-1/2 tyrosine phosphorylation levels. Several have demonstrated that, although insulin resistance is mediated by different mechanisms, the disruption of one of these may restore insulin signaling almost completely (32,34,35).
To assess the impact of hypothalamic PTP1B down-regulation on the peripheral action of insulin, we performed hyperinsulinemic-euglycemic clamp studies in conscious rats. As a result of physiological increases in plasma insulin concentrations, the rate of glucose infusion required to maintain the plasma glucose at basal levels was similar in PTP1B antisense-treated rats and in controls on standard rodent chow. However, the reduction in the rate of glucose infusion observed in DIO rats was completely reversed when these DIO animals were treated icv with PTP1B-ASO. The action of insulin on glucose metabolism includes stimulation of glucose uptake and inhibition of glucose production. The insulin action on peripheral glucose uptake was reduced, and there was also a less evident decrease in hepatic glucose production in DIO rats. These alterations were completely reversed when the DIO animals were treated icv with PTP1B-ASO. Thus, muscle and hepatic insulin action were normalized in DIO rats after selective attenuation of hypothalamic PTP1B expression. We cannot exclude the possibility that the improvement in glucose metabolism is related to the dramatic reduction of the body weight in DIO PTP1B-ASO, but it is also possible that the improvement in insulin and leptin actions in hypothalamus might have a role in the control of liver glucose metabolism, as demonstrated during recent years (36,37,38,39,40).
Although the effects of PTP1B reduction on leptin and insulin sensitivity and signaling may have been influenced by an important reduction in food intake in these animals, most of the effects were only partially observed in pair-fed animals, suggesting that this reduction in food intake may have a role, but certainly does not account entirely for the effects observed in body and fat weight, on insulin and leptin signaling and sensitivity and on the improvement in glucose metabolism in liver and muscle. These results are in accordance with previous data that showed that, in PTP1B knockout mice or even neuronal PTP1B knockout mice, there is also an increase in energy expenditure that might have an effect on the body weight and body composition of these animals (5,41,42).
In conclusion, we have demonstrated an important role of hypothalamic PTP1B in the modulation of energy balance, insulin and leptin action, and glucose metabolism. Thus, the reduction in hypothalamic PTP1B should be sufficient to promote an appreciable weight reduction and access to the brain may also be necessary to optimally improve insulin and leptin sensitivity and glucose homeostasis.
Acknowledgments
We thank Mr. Luis Janieri, Mr. Márcio Alves da Cruz, and Mr. Jósimo Pinheiro for their technical assistance.
Footnotes
Disclosure Statement: All the authors have nothing to declare.
First Published Online May 8, 2008
Abbreviations: ASO, Antisense oligonucleotide; 2[l4C]DG1, 2-deoxy-d-[1–14C]glucose; CNS, central nervous system; DIO, diet-induced obesity; icv, intracerebroventricular; IR, insulin receptor; IRS, insulin receptor substrate; Jak, Janus kinase; ObR, long form of the leptin receptor; PMSF, phenylmethysulfonyl fluoride; PTP, protein tyrosine phosphatase; Stat, signal transducer and activator of transcription; PTP1B, protein tyrosine phosphatase 1B; PTP1B-sense, PTP1B sense oligonucleotide.
References
- Schwartz WM, Porte D 2005 Diabetes, obesity and the brain. Science 307:375–379 [DOI] [PubMed] [Google Scholar]
- Johnson TO, Ermolieff J, Jirousek MR 2002 Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov 1:696–709 [DOI] [PubMed] [Google Scholar]
- Prada PO, Zecchin HG, Gasparetti AL, Torsoni MA, Ueno M, Hirata AE, Corezola do Amaral ME, Hoer NF, Boschero AC, Saad MJ 2005 Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 146:1576–1587 [DOI] [PubMed] [Google Scholar]
- Goldstein BJ 2002 Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J Clin Endocrinol Metab 87:2474–2480 [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB 2006 Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 8:917–924 [DOI] [PubMed] [Google Scholar]
- Carvalheira JB, Siloto RM, Ignacchitti I, Brenelli SL, Carvalho CR, Leite A, Velloso LA, Gontijo JA, Saad MJ 2001 Insulin modulates leptin-induced STAT3 activation in rat hypothalamus. FEBS Lett 500:119–124 [DOI] [PubMed] [Google Scholar]
- Johnson AK, Epstein AN 1975 The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res 86:399–418 [DOI] [PubMed] [Google Scholar]
- Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ 2005 Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res 13:48–57 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Saad MJ, Maeda L, Brenelli SL, Carvalho CR, Paiva RS, Velloso LA 1997 Defects in insulin signal transduction in liver and muscle of pregnant rats. Diabetologia 40:179–186 [DOI] [PubMed] [Google Scholar]
- Torsoni MA, Carvalheira JB, Pereira-Da-Silva M, de Carvalho-Filho MA, Saad MJ, Velloso LA 2003 Molecular and functional resistance to insulin in hypothalamus of rats exposed to cold. Am J Physiol Endocrinol Metab 285:216–223 [DOI] [PubMed] [Google Scholar]
- Prada P, Okamoto MM, Furukawa LN, Machado UF, Heimann JC, Dolnikoff MS 2000 High- or low-salt diet from weaning to adulthood: effect on insulin sensitivity in Wistar rats. Hypertension 35:424–429 [DOI] [PubMed] [Google Scholar]
- Yang C, Coker KJ, Kim JK, Mora S, Thurmond DC, Davis AC, Yang B, Williamson RA, Shulman GI, Pessin JE 2001 Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest 107:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leturque AP, Burnol AF, Penicaud L, Girard J 1985 A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 228:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K 2002 Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem 277:793–803 [DOI] [PubMed] [Google Scholar]
- Ventrucci G, Silva RGL, Mello RAM, Marcondes GCCM 2004 Effects of a leucine-rich diet on body composition during nutritional recovery in rats. Nutrition 20:213–217 [DOI] [PubMed] [Google Scholar]
- Chauhan BN 2002 Trafficking of intracerebroventricularly injected antisense oligonucleotides in the mouse brain. Antisense Nuclei Ac Drug Dev 12:353–357 [DOI] [PubMed] [Google Scholar]
- Engelhard HH 1994 Antisense oligodeoxynucleotide technology: potential use for the treatment of magnant brain tumors. Cell Mol Neurobiol 14:475–486 [Google Scholar]
- Lund KI, Hansen AJ, Andersen SH, Moller HPN, Billestrup N 2005 Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signaling. J Mol Endocrinol 34:339–351 [DOI] [PubMed] [Google Scholar]
- Ahmad Fand Goldstein BJ 1995 Increased abundance of specific skeletal muscle protein tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metabolism 44:1175–1184 [DOI] [PubMed] [Google Scholar]
- Dadke SS, Li HC, Kusari AB, Begum N, Kusari J 2000 Elevated expression ans activity of protein-tyrosine phosphatase 1B in skeletal muscle of insulin-resistant type II diabetic Goto-Kakizaki rats. Biochem Biophys Res Commun 274:583–589 [DOI] [PubMed] [Google Scholar]
- Venable CL, Frevert EU, Kim YB, Fischer BM, Kamatkar S, Neel BG, Kahn BB 2000 Overexpression of protein-tyrosine phosphatase-1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/protein kinase B activation. J Biol Chem 275:18318–18326 [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Prada PO, de Souza CT, Faria MC, Picardi PK, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira J 2006 Reversal of diet-induced insulin resistance with a single bout of exercise: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG 2002 PTP1B regulates leptin signal transduction in vivo. Dev Cell 2:385–387 [DOI] [PubMed] [Google Scholar]
- Lavens D, Piessevaux J, Tavernier J 2006 Negative regulation of leptin receptor signalling. Eur Cytokine Netw 17:211–219 (Review) [PubMed] [Google Scholar]
- Ahima RS, Qi Y, Singha NS, Jackson MB, Scherer PE 2006 Brain adipocytokine action and metabolic regulation. Diabetes 55:145–154 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers Jr MG 2005 Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8:566–570 [DOI] [PubMed] [Google Scholar]
- Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, Kroeger PE, White DW, Jirousek MR, Trevillyan JM 2002 Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol 195:109–118 [DOI] [PubMed] [Google Scholar]
- Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM 2002 Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—functional implications. J Clin Invest 110:1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser JM, Tremblay LM, Kennedy PB 1999 Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548 [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Seeley RJ, Schwartz MW 2003 Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52:227–231 [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L 2002 Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5:566–572 [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS 2002 A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ 2005 S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 54:959–967 [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Obici S, Accilli D, Rossetti L 2005 Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest 115:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L 2005 A brain-liver circuit regulates glucose homeostasis. Cell Metab 1:53–61 [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juanez R, Obici S, Schwartz GJ, Bryan J, Aguilar- Bryan L, Rossetti L 2005 Hypothalamic KATP channels control hepatic glucose production. Nature 434:1026–1031 [DOI] [PubMed] [Google Scholar]
- Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M 2006 Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 4:231–232 [DOI] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic DP, Chaubey PV, Lee-Loy A, McGlade JC, Kennedy PB, Tremblay LM 2002 Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Cell Dev 2:497–503 [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB 2000 Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20:5479–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]