Abstract
To study the effects of gestational exposure to estrogen on early gonadal differentiation, pregnant mice were treated by sc injection of diethylstilbestrol (DES) or vehicle from embryonic day (E) 8.5 to E14.5, and gonads at E11.5, E12.5, and E14.5 were examined. Quantitative real-time RT-PCR and in situ hybridization revealed that mRNA levels of steroidogenic factor 1 (SF-1), a key regulator of gonadal differentiation, and several male gonad-specific genes, including Müllerian-inhibiting substance (MIS), steroidogenic acute regulatory protein, cholesterol side-chain cleavage cytochrome P450, and Cerebellin 1 precursor protein, were significantly decreased in the DES-treated testis, compared with the control testis at E12.5 and/or E14.5. Immunohistochemistry demonstrated that the staining intensities for SF-1 and MIS in Sertoli cells were apparently reduced in the DES-treated testis, compared with those of the controls, at E12.5 and E14.5. Because MIS, steroidogenic acute regulatory protein, cholesterol side-chain cleavage cytochrome P450, and Cerebellin 1 precursor protein are activated under the regulation of SF-1, the down-regulation of these factors may be due to reduced SF-1 expression. Immunohistochemistry for laminin-1 demonstrated that ovigerous cords in the DES-treated ovary were smaller than those in controls at E14.5. Moreover, the number of 5-bromo-2′deoxyuridine-5-monophosphate-labeled cells in the DES-treated testis was significantly reduced at E12.5 and E14.5, compared with controls, and that in the DES-treated ovary remained higher than that in the control ovary at E14.5. The results suggest that exogenous estrogens can alter sex-specific genetic pathways governing early differentiation and cell proliferation of both male and female gonads.
GONADAL DIFFERENTIATION is critical for the development of sexual phenotype. The earliest event is testicular cord formation that becomes visible in male gonads at embryonic day (E) 12.5 in mice, accompanied by differentiation of two male-specific somatic cells, namely Sertoli and Leydig cells. Sertoli cells are the first cell type to differentiate in the fetal testis and produce an essential male factor called Müllerian-inhibiting substance (MIS; also known as anti-Müllerian hormone), which induces degeneration of the Müllerian duct. After Sertoli cell differentiation, Leydig cells differentiate from mesenchymal cells in the interstitial region of the testis between the testicular cords and synthesize testosterone, which is essential for masculinization of the internal and external genitalia. In females, no apparent morphological changes occur during this time, but cord-like structures, namely ovigerous cords, become distinguishable around E14.5. Another obvious difference between male and female gonads is the drastic increase in the size of male gonads relative to female gonads. These sex-specific morphological events proceed under genetic control during a limited short-time window (1). In mammals, male-specific events are triggered by the expression of the sex-determining gene on the Y chromosome (Sry) (2). After Sry expression, numerous genes have been identified to show a sexually dimorphic expression pattern in embryonic gonads starting from around E11.5 (3,4,5). However, the functions of most genes and molecular mechanisms regulating gonadal differentiation remain largely unclarified.
Gestational exposure to estrogenic compounds including diethylstilbestrol (DES) causes malformations of male reproductive organs, such as formation of hypoplastic testis and epididymal cysts, cryptorchidism, and the persistence of Müllerian duct remnants in humans and rodents (6,7). Because the development of the male reproductive tract is dependent on three hormones produced by the embryonic testis, namely MIS, testosterone, and insulin-like factor 3, impaired hormone production is thought to be responsible for such malformations. The majority of these studies focused on such hormone-dependent events occurring in the mid- to late gestational periods, whereas early events of testicular cord formation are believed to be regulated by genes largely insensitive to estrogens (7). However, an in vitro study has shown the inhibitory effects of estradiol and DES on the number and activity of somatic and germ cells in an organ culture from rat testis at E14.5, the time when testicular differentiation is initiated (8). A study of estrogen receptor (ER)-α knockout (KO) mice has suggested the role of endogenous estrogen in regulating fetal Leydig cell development via ERα (9). The orphan nuclear receptor steroidogenic factor 1 (SF-1), a key regulator of development of the reproductive system (10), is expressed in gonads of both sexes from the earliest stage of development (11). Two studies showed that the expression of this gene in the embryonic testis after DES treatment was altered, although results are inconsistent (12,13). These studies suggest that the earliest events of testicular development are sensitive to estrogens.
Development of the female reproductive tract has also been shown to be estrogen responsive, and organs and several genes thought to be involved in the mechanisms of development have been identified (14,15,16). Ovarian differentiation is represented by follicle formation occurring around the time of birth. Our previous studies showed that exposure of newborn female rats to estrogens can inhibit follicular formation and alter the expression of several genes including SF-1, MIS, cholesterol side-chain cleavage cytochrome P450 (P450scc), and the steroidogenic acute regulatory protein (StAR) during early postnatal development (17,18,19). It has recently been revealed that a female-specific molecular program is in progress in embryonic female gonads as early as E11.5 (5,20). To date, however, there are few studies on the effects of estrogens on embryonic ovary.
The aim of the present study was to determine the molecular and cellular targets of estrogens in the initial events of gonadal differentiation. For this study, pregnant mice were treated by sc injection of DES [5 μg/kg body weight (bw) per day] or vehicle (control), from E8.5 to E14.5, and analyses were carried out for both male and female gonads of the control and DES-treated mice during developmental stages at E11.5, E12.5, and E14.5. Quantitative real-time RT-PCR (qPCR) was used to quantify the expression levels of genes. The genes analyzed included two key regulators of gonadal differentiation, namely SF-1 (10) and the dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on the X chromosome gene 1 (Dax-1) (21,22); two male-specific markers, namely Sry high-mobility group box-related gene 9 (Sox9) and desert hedgehog (Dhh) (3,4,5); two female-specific markers, namely wingless-related mouse mammary tumor virus integration site 4 (Wnt4) (23) and follistatin (Fst) (24); and two ERs, namely types ERα and ERβ. For male gonads, several genes thought to be regulated by SF-1, including MIS (25), P450scc (26), StAR (27), and the Cerebellin 1 precursor protein (Cbln 1) (28). The localizations of several of these genes were further analyzed using in situ hybridization (ISH) and/or immunohistochemistry (IHC). Moreover, we studied the effects of DES on gonadal structures including testicular and ovigerous cords in male and female gonads, respectively, by immunofluorescence for laminin-1. Finally, we analyzed the effects of DES on cell proliferation by an assay using the 5-bromo-2′deoxyuridine-5-monophosphate (BrdU)-labeling method for both sex gonads.
Materials and Methods
Animals and treatments
All animals were handled in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Yokohama City University School of Medicine. Pregnant C57BL/6J mice were purchased from CLEA Japan, Inc. (Tokyo, Japan) and maintained under standard conditions. The day after mating was designated as E0.5. DES (Sigma Chemical Co., St. Louis, MO) dissolved in olive oil, or olive oil alone (control), was administrated by sc injection to pregnant mice at E8.5-E14.5 of gestation at a daily dose of 5 μg/kg of maternal bw. This dose was previously reported to cause reproductive tract abnormalities later in life (29). Control and DES-treated pregnant mice were killed at E11.5 (n = 9, control; n = 9, DES), E12.5 (n = 9, 9), and E14.5 (n = 10, 11), and embryos were dissected.
Embryos at E11.5 and E12.5 were more accurately staged by counting the tail somites (ts) at the time of dissection (30). By this method, embryos with 18 ts (±2 ts) were considered as E11.5 and those with 30 ts (±3 ts) as E12.5. E14.5 embryos were staged by examining forelimb and hindlimb morphologies (31). Paired gonad/mesonephros complexes dissected from embryos at E11.5, and gonads, which were separated from mesonephroi from embryos at E12.5 and E14.5, were frozen immediately in liquid nitrogen and used for RNA extraction. Dissection instruments were cleaned with RNaseZap wipes (Ambion Inc., Austin, TX) between embryos. One or two embryos of each sex per litter were placed in 4% paraformaldehyde for histology, ISH, and IHC. Using a tail removed from each embryo, sexing was performed by PCR to check the presence or absence of the Y chromosome-specific gene Sry (32).
Total RNA preparation and qPCR
Two gonad/mesonephros complexes or gonads from the same animal were pooled. Total RNA was isolated and residual genomic DNA was digested using the MELT total RNA isolation system (Ambion). The quantity and quality of purified RNA were evaluated by spectroscopy. cDNA was produced using the ExScript reverse transcription reagent kit (Takara Bio Inc., Shiga, Japan) from 1 μg total RNA using T16 primer and 18S rRNA-specific oligos.
qPCR was performed using an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA) using standard TaqMan technology employing fluorescence monitoring. Predesigned gene-specific primers and probes for SF-1 (Mm00446826_m1), Dax-1 (Mm00431729_m1), Sox9 (Mm00448840_m1), Dhh (Mm00432820_g1), Wnt4 (Mm00437341_m1), Fst (Mm00514982_m1), ERα (Mm00433149_m1), ERβ (Mm00599819_m1), MIS (Mm00431795_g1), StAR (Mm00441558_m1), P450scc (Mm00490735_m1), Cbln 1 (Mm00517177_m1), β-actin (Mm00607939_s1), and 18S rRNA control reagents (APL-4308329) were obtained from Applied Biosystems, using Premix Ex Taq (Perfect Real Time; Takara) at a final reaction volume of 25 μl/well in 96-well plates. Negative controls were run for every primer/probe combination. Each PCR experiment was carried out in triplicates on two separate pooled RNA samples from gonads of three to five embryos and average cycle thresholds (Cts) were obtained. To determine the relative amount of PCR products, the Ct of 18S rRNA was subtracted from those of genes of interest to obtain ΔCt. ΔCt of the DES-treated samples was compared with that of the oil control samples, and the difference was assigned as ΔΔCt. The fold change between the two samples was then calculated as 2−ΔΔCt.
ISH
Sense and antisense riboprobes for SF-1 (33), MIS (25), P450scc (34), StAR (27), and Sox9 (35) were generated with T7, T3, and SP6 RNA polymerases and the SureSite T7 RNA probe kit (Novagen Inc., Madison, WI). ISH was performed using the SureSite hybridization reagents kit (Novagen). Emulsion-coated slides were exposed for 3 wk at 4 C. ISH was performed at least twice per tissue sample with each probe. Control experiments used a sense probe and signals remained below the background.
IHC
Embryos fixed in 4% paraformaldehyde at 4 C overnight were embedded in paraffin. Sagittal sections (6 μm) were cut and mounted onto silane-coated slides. Tissue sections were deparaffinized in xylenes and descending ethanols. Antigen retrieval was performed by microwave boiling slides for 5 min in 0.01 m citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked by immersion in 3% (vol/vol) hydrogen peroxide in PBS for 15 min, and the slides were washed three times in PBS. The sections were incubated in a blocking solution, 5% Block Ace (Dainippon Pharmaceutical Co., Ltd., Tokyo, Japan), at room temperature for 30 min and subsequently incubated with a diluted primary antibody overnight at 4 C. Rabbit anti-SF-1 antibody (17; 1:5000 dilution), rabbit anti-P450scc antibody (AB1244, Chemicon International, Inc., Temecula, CA; 1:5000 dilution), goat anti-MIS antibody (sc-6886; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:5000 dilution), and rabbit anti-Sox9 antibody (sc-20095; Santa Cruz; 1:500 dilution) were used as primary antibodies. After washing with PBS +0.1% Triton X-100, sections were incubated with either N-histofine simple stain mouse MAX PO (R) (Nichirei Biosciences, Tokyo, Japan), N-histofine simple stain mouse MAX PO (G) (Nichirei), or N-histofine mouse stain (Nichirei), followed by staining using the VectaDAB substrate kit (Vector Laboratories, Burlingame, CA). On each occasion, sections from at least three animals in each treatment group at three time points were processed in parallel for comparison of immunostaining on at least two separate occasions to ensure reproducibility of results. For negative controls, tissue sections were incubated without the addition of primary antibody.
Immunofluorescence for laminin-1
After dewaxing, antigen retrieval, blocking of endogenous peroxidase activity, and incubation in a blocking solution, the sections were incubated overnight with rabbit anti-laminin-1 antibody (provided by Hajime Sawada, Department of Histology and Cell Biology, Yokohama City University Graduate School of Medicine, Yokohama, Japan) at 4 C. After incubation with the primary antibody, the sections were washed in PBS and incubated with Alexa Fluor 488 goat antirabbit IgG secondary antibody (Molecular Probes, Inc., Eugene, OR) for 60 min at room temperature.
BrdU incorporation
To label proliferating cells, BrdU (B-500, Sigma; 10 mg/kg bw) was administrated by ip injection 2 h before pregnant mice were scheduled to be euthanized. BrdU-labeled DNA was visualized by IHC with a 1:500 dilution of a monoclonal antibody against BrdU (B2531; Sigma). Germ cells were identified by their large round nuclei (supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Labeled nuclei of somatic cells and germ cells were counted in sections in an area spanning a width of 150 μm and extending 35 μm from the top of the coelomic epithelium into the gonad. Three to five gonadal areas from three to five embryos in each treatment at each time points were counted. Counting was carried out three times using three independent sets of gonadal area. To minimize counting error, counting was performed independently by three persons who had no knowledge about the treatment, and the results were similar.
Statistical analysis
Data are presented as mean ± sem. Statistical analysis was performed using Microsoft Excel97 SR-2 (Microsoft Corp., Redmond, WA). At each developmental stage, the significance of differences in gene expression between the control and DES-treated groups was evaluated using one-way ANOVA (P < 0.05).
Results
Quantification of gene expression
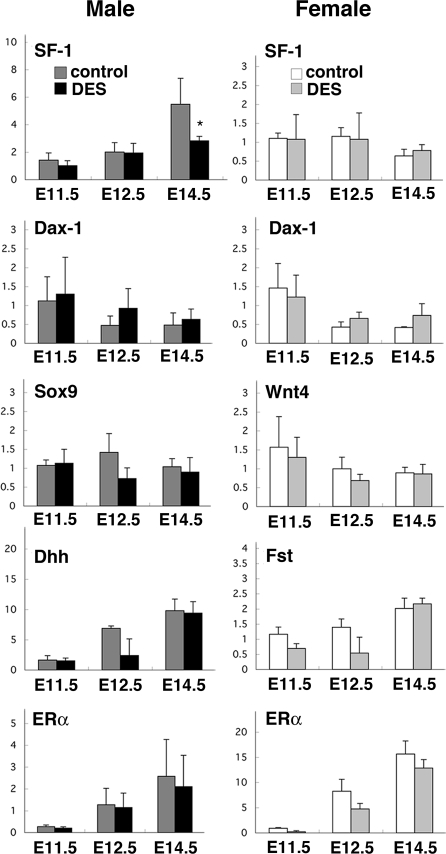
Using qPCR analysis, we quantified the change in the expression of several genes considered responsible for early gonadal development, in response to in utero DES treatment. As shown in Fig. 1, the mRNA levels of SF-1, which are up-regulated in the male gonad, compared with the female gonad after E12.5 (11), were significantly reduced in the male gonad of the DES-treated group at E14.5, compared with the control group (F7,22 = 13.35, P < 0.05), whereas those in the female gonad were similar in the two treatment groups at any developmental stage (P = 0.43). Dax-1 is involved in molecular pathways for gonadal differentiation (21,22), and its expression profile during gonadal development is very similar to that of SF-1 (36,37). The levels of Dax-1 mRNA did not change significantly after DES treatment in either male or female gonads at any developmental age examined (male: P = 0.85; female: P = 0.38; Fig. 1). The expression levels of two early male markers, namely Sox9 and Dhh, both of which are highly expressed in the male gonad but are absent in the female gonad (3,4,5), remained similar in the male gonads of the control and DES-treated groups examined (Sox9: P = 0.10, Dhh: P = 0.57; Fig. 1). Two female markers, namely Wnt4 and Fst, both of which are activated specifically in embryonic female gonads (23,24), were expressed at similar levels in oil- and DES-treated female gonads (Wnt4: P = 0.63; Fst: P = 0.08; Fig. 1).
Figure 1.
mRNA levels of several regulators of gonadal differentiation. qPCR was performed to analyze gene expression in male (left panels) and female (right panels) gonads at E11.5, E12.5, and E14.5 from mice exposed in utero to vehicle or DES. Graphs show mRNA levels normalized to the levels of 18S rRNA, and each bar shows mean ± sem of three to five embryos. *, P < 0.05 vs. control.
We also measured the mRNA levels of ERα and ERβ, two major genes involved in estrogen signaling. Gonads of both sexes possessed mRNAs of ERα (Fig. 1) but not of ERβ (data not shown) at ages from E11.5 to E14.5. The expression levels of ERα in DES-treated animals tended to be lower than in control animals but were not significantly different in the gonad of either sex at any age examined (male: P = 0.96; female: P = 0.61).
We further examined several other genes whose expression is male gonad-specific during early testicular differentiation. The expression levels of MIS, a functional marker of Sertoli cells, were significantly lower in DES-treated testis than control testis at E12.5 and E14.5 (F2,24 = 5.55, P < 0.05; Fig. 2). The expression levels of StAR and P450scc, two markers of steroidogenesis by interstitial Leydig cells, and Cbln 1, which is expressed by interstitial cells of male but not female gonads (5,38), were significantly reduced in the male gonad of DES-treated mice at E12.5 or E14.5 (StAR: F2,20 = 15.07, P < 0.05; P450scc: F2,19 = 22.99, P < 0.05; Cbln 1: F2,14 = 4.19, P < 0.05; Fig. 2).
Figure 2.
mRNA levels of early markers of testicular differentiation. qRT-PCR was performed to analyze expressions of MIS, P450scc, StAR, and Cbln 1 as early markers of testicular differentiation in developing testis at E11.5, E12.5, and E14.5 from mice exposed in utero to vehicle or DES. Graphs show mRNA levels normalized to the levels of 18S rRNA, and each bar shows mean ± sem of three to five embryos. *, P < 0.05 vs. control.
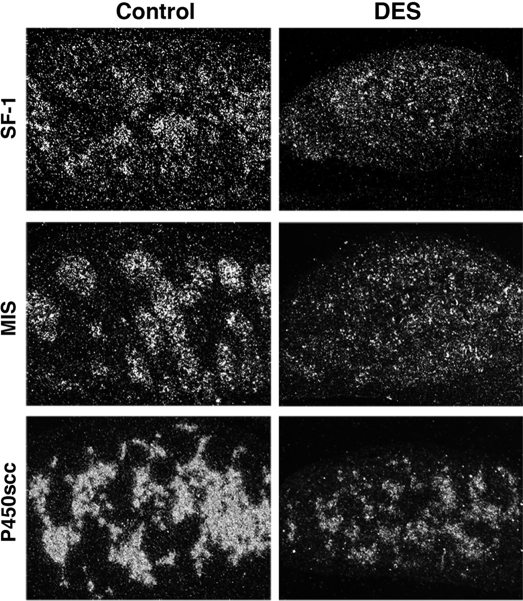
Consistent with the qPCR results, ISH analysis showed an apparent reduction of the mRNAs of SF-1, MIS, and P450scc in the DES-treated testis, compared with the oil-treated control testis at E14.5 (Fig. 3).
Figure 3.
Localization of mRNAs of SF-1, MIS, and P450scc in DES-treated testis. In situ hybridization using 35S-labeled cRNA probe was performed to analyze localization of mRNAs of SF-1, MIS, and P450scc on sections of fetal mouse testis at E14.5. Left and right panels show testis from mice treated in utero with vehicle and DES, respectively (original magnification, ×33).
Localization of proteins
To further extend our qPCR and ISH data, we examined the cellular localization of several factors in the developing testis of control and DES-treated mice, using IHC. SF-1-immunoreactivity (ir) was detected in cellular nuclei of the testis of both treatment groups, and the expression profile in the control testis was consistent with that shown in a previous study (37). At E11.5, SF-1-ir was detected in somatic cells, and the localization, staining intensity, and number of positive cells were similar in the two treatment groups (Fig. 4, A and B). In E12.5 testis of control mice, SF-1-ir was present in Sertoli cells within the cords and in the majority of interstitial cells, some of which were very heavily stained (Fig. 4C). At this stage, the results of IHC for SF-1 were somewhat variable between testes in the DES-treated group. The staining intensity for SF-1 in most of the DES-treated testes was similar to controls (data not shown). However, the SF-1 staining intensity was apparently reduced in some cells in a few DES-treated testes (Fig. 4D). At E14.5, SF-1-ir was detected in both Sertoli and Leydig cells in the control testis with stronger ir in Leydig cells than in Sertoli cells (Fig. 4, E and G), whereas in the DES-treated testis, SF-1-ir was observed in Leydig cells but was nearly undetectable in Sertoli cells (Fig. 4, F and H). MIS was localized to the cytoplasm of Sertoli cells in both the control and DES-treated testes at both E12.5 and E14.5, but the staining was much weaker in the DES-treated testis than the controls (Fig. 5). In contrast, intense nuclear immunostaining of Sox9 in Sertoli cells was detected in the testis of both treatment groups (Fig. 5). Although the qPCR results revealed a significant reduction in the expression level of P450scc mRNA in the DES-treated testis, compared with controls, the staining for P450scc was strong in the testes of both treatment groups. IHC for P450scc-stained Leydig cells in the testis of both oil- and DES-treated embryos, and the staining intensities and numbers of P450scc-positive cells were not apparently different between the two treatment groups at E14.5 (Fig. 5). Probably, the differences in the expression level of the P450scc protein between the two treatment groups could not be clearly distinguished by this nonquantitative IHC method because the P450scc protein levels were much higher than the threshold level detectable by this method.
Figure 4.
Localization of SF-1 protein in DES-treated testis. Immunohistochemistry for SF-1 was performed on sections of fetal mouse testis at E11.5 (A and B), E12. 5 (C and D), and E14.5 (E–H) from mice exposed in utero to vehicle (left panels) or DES (right panels). i, Interstitial region; tc, testicular cords (original magnifications, ×33 in E, F, and ×132 in A–D, G, and H).
Figure 5.
Localization of MIS, Sox9, and P450scc proteins in DES-treated testis. IHC for MIS, Sox9, and P450scc proteins was performed on sections of fetal mouse testis at E12.5 (MIS) and E14.5 (MIS, Sox9, and P450scc) from mice exposed in utero to vehicle (left panels) or DES (right panels) (original magnification, ×132).
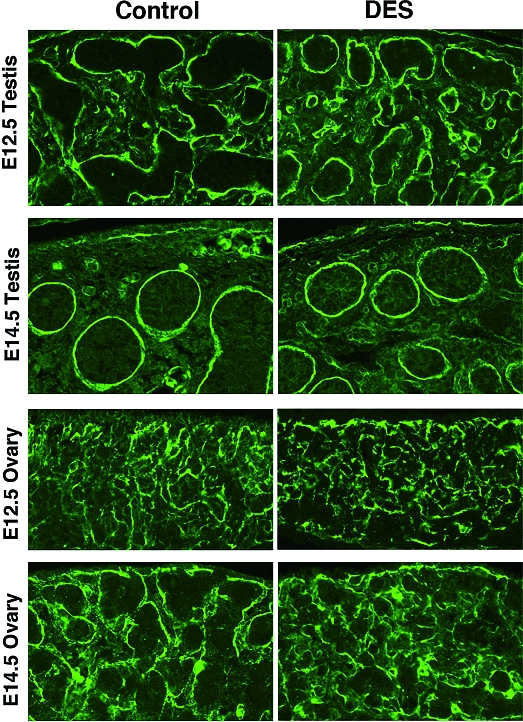
Gonadal structures
The size and shape of the gonads of either sex at E11.5 and E12.5 were similar between the control and DES-treated groups. At E14.5, DES-treated testis was smaller than control testis (Fig. 4, E and F). The shape of control gonads of both sexes changed from sausage-like to oval at this time, whereas some of the DES-treated testis and ovary still maintained a sausage-like shape. To assess the structural organization of gonads, we examined the expression of laminin-1 (Fig. 6). Both oil- and DES-treated testes at E12.5 showed numerous cords clearly outlined by laminin-1 staining. At E14.5, testicular cords became larger and the characteristic male-specific coelomic vessel was clearly visible in the mesenchyme just beneath the coelomic epithelium. No apparent differences in size or shape of the testicular cord were detected between the two treatment groups at E12.5 or E14.5. In females, both oil- and DES-treated ovaries showed a similar arrangement of laminin-1 staining at E12.5. At E14.5, cord-like structures, namely ovigerous cords, were readily distinguished in the control ovary, whereas those in the DES-treated ovary were markedly smaller and less organized than those in the control ovary.
Figure 6.
Localization of laminin-1 in DES-treated gonad. Male and female gonads after in utero exposure to vehicle (left panels) or DES (right panels) were analyzed by immunofluorescence for laminin-1. Laminin-1 is localized to delineate testicular cords in the testis and ovigerous cords in the ovary (original magnification, ×66).
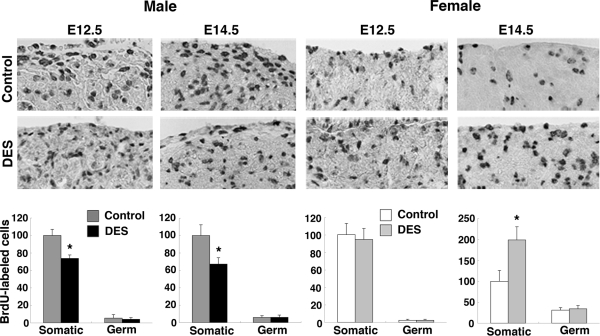
Cell proliferation
To examine the effect of DES treatment on cell proliferation, we performed BrdU-labeling experiments as described in Materials and Methods. The results of IHC for BrdU were quantitatively analyzed. Counts of BrdU-labeled nuclei within the set area were determined for somatic and germ cells (Fig. 7). In the male gonad, the number of BrdU-labeled somatic cells in the testis was similar in the two treatment groups at E11.5 (data not shown) but was significantly lower in the DES-treated group than the control group at E12.5 and E14.5 (F1,4 = 31.88, testis (P < 0.05). In females, the number of BrdU-labeled somatic cells remained the same in control and DES-treated ovaries at E11.5 and E12.5 but was significantly higher in the DES-treated ovary than in the control ovary at E14.5 (F2,7 = 5.20, P < 0.05). Only a few germ cells were labeled with BrdU, and the number of labeled cells in the gonad of either sex at any developmental stage was not significantly different between groups.
Figure 7.
Analyses of cell proliferation in DES-treated gonad. Embryos were labeled transplacentally with BrdU, and BrdU incorporation into gonads was assessed as described in Materials and Methods. Upper panels show representative photomicrographs of sections of testis (left panels) and ovary (right panels) after in utero exposure to vehicle or DES (original magnification, ×66). Lower panels show quantitative analyses of the results of IHC for BrdU. In each graph, the relative number of BrdU-labeled cells is scaled such that the BrdU-labeled somatic cell number of the control group is equal to 100. Three to five gonadal areas from three to five embryos in each treatment were counted, and each bar shows mean ± sem. *, P < 0.05 vs. control.
Discussion
The expression levels of SF-1, a key regulator of gonadal differentiation, and several male gonad-specific genes including MIS, P450scc, StAR, and Cbln 1, were reduced in the DES-treated testis at E12.5–E14.5, when morphological changes became first recognizable in the testis. At E12.5, although qPCR showed no significant changes in the mRNA levels of SF-1 between control and DES-treated testes, some of the DES-treated testes exhibited a clear reduction in the immunostaining intensity for SF-1 in some cells. Many cells, particularly fetal Leydig cells, are actively differentiating in the testis at this stage, and each cell is at a different stage of differentiation. SF-1 is expressed not only in Sertoli and Leydig cells but also in indifferent interstitial cells, and the expression level of SF-1 should be different between cell types and also between individual cells. Therefore, the variable IHC results may reflect these differences, suggesting that the action of DES on testicular SF-1 expression is different between cell types and/or in individual cells, depending on the differentiating stage. We further demonstrate that ovigerous cord formation in the embryonic ovary is inhibited and that cell proliferation in both male and female gonads is affected. These results suggest that early events of gonadal differentiation for both sexes are sensitive to estrogens. The dose of DES used in the present study is much higher than the level of endogenous estrogen during the early embryonic period (6). The effects of DES and estrogen depend on various conditions such as dose and timing (8,39), and the binding affinity of DES to ER is 3–4 times higher than that of estrogen (40). Therefore, the effects of in utero DES on gonadal differentiation may be different from those of endogenous estrogen exposure.
The effects of prenatal treatment with estrogens on the expression of SF-1 in the embryonic testis have been reported. Our results are consistent with those of a previous study, in which in utero exposure to DES decreased the expression level of SF-1 in the rat fetal testis at E17, the age corresponding to E15 in mice (12). However, several studies detected no significant change in SF-1 expression in the mouse testis after gestational treatment with DES or 17β-estradiol (41,42,43). The embryonic ages analyzed are different between these studies and the present study (E16.5 and later vs. E12.5-14.5). In fact, we did not detect a significant change in SF-1 expression in the DES-treated testis at E16.5 (data not shown). Therefore, it is possible that the testicular SF-1 expression was transiently reduced by in utero treatment of DES at E14.5 but recovered to the normal level by E16.5. Another previous study showed results that were in contrast to ours. This previous study showed that the expression levels of both SF-1 and MIS increased in the mouse testis at E13.5 after in utero treatment of DES at E9–16 (13). Although the timing and period of DES treatment and the age of embryos analyzed were similar between the previous (13) and present studies, there are several differences, including doses (100 vs. 5 μg/kg), mouse strains (FVB vs. C57BL/6J), and methods of analysis (ribonuclease protection assay vs. qPCR). In our preliminary experiments, all C57BL/6J mouse embryos were found dead after exposure to DES at 100 μg/kg (data not shown). Furthermore, total RNA was isolated from the whole fetus in the previous study, whereas it was isolated from the dissected gonad in the present study. Because SF-1 is expressed in not only the gonad but also the adrenal and pituitary and brain of fetal mice (11,36,37), the expression levels of SF-1 in the previous study included the expression levels in all tissues. Thus, the discrepancies between the two studies may be due to these different experimental conditions.
The present study for the first time demonstrates that genes expressed in not only Leydig cells (StAR, P450scc, and Cbln 1) but also Sertoli cells (MIS) are significantly reduced in the DES-treated testis at E12.5 and/or E14.5. These results suggest that DES can affect the differentiation of both cell types at the earliest time of testicular differentiation. Considering that Sertoli cells are the first cell type to differentiate and that differentiation of fetal Leydig cells is controlled by Sertoli cells (44), it is also possible that DES affects only Sertoli cell differentiation, and Leydig cell differentiation is secondarily affected by the impaired Sertoli cells. Because these genes are thought to act under the regulation of SF-1 (25,26,27,28), the decrease in SF-1 expression could explain the decrease in the expression of these genes in the testis of DES-treated mice. Similar results were obtained by Majdic et al. (12,45), demonstrating that in utero exposure to DES decreased the expressions of several steroidogenic markers including Cyp17 and StAR in the Leydig cells of the rat fetal testis at E17.
Despite the reduction in the expression of the genes, testicular cord formation seemed to proceed normally during the period between E12.5 and E14.5, based on the immunofluorescence analysis using laminin-1. In the present study, the expression level of SF-1 in the DES-treated testis was reduced to approximately half the level in the control testis, a level similar to that in the gonad of SF-1 heterozygous mutant mice. In the male gonads of heterozygous SF-1-deficient mice, the expressions of MIS and P450scc were reduced transiently between E11.5 and E13.5 but recovered by E14.5; testicular cord formation was normal, indicating that half of the action of SF-1 can cause transient reduction in the expression level of target genes, although such level is still sufficient for testicular cord formation (46). Previous morphological studies also failed to detect apparent abnormalities in the DES-treated testis before E14 but found abnormalities such as the appearance of hyperplastic areas of Leydig cells, delay in testicular descent, and incomplete regression or persistence of the Müllerian duct in the DES-treated male embryo at E16.5 and later in development (13,42,43,45,47). Therefore, morphological changes in the DES-treated testis may have occurred at later developmental stages not examined in the present study. Here we show for the first time that ovigerous cords were smaller in the DES-treated ovary than the control ovary at E14.5, suggesting that the embryonic ovary is also a target of estrogens. In contrast to testicular differentiation, however, very little is known about the molecular regulation of embryonic ovarian development (20). Because keratins (K) 8, K18 and K19, are expressed and suggested to be involved in ovigerous cord formation (5,48), estrogens may affect their gene expression.
The difference in size is also one of the earliest morphological changes observed between male and female gonads, and this is thought to be due to increased cell proliferation in the male pathway (49). A study using an organ culture system showed that DES reduces the number of three main cell types, namely gonocytes, Sertoli cells, and Leydig cells, in the rat testis explanted at E14.5, and the reduction is thought to be due to reduced cell proliferation and enhanced apoptosis (8). We observed that DES-treated testis was smaller than control testis at E14.5. This may reflect the reduced proliferation of somatic cells in the DES-treated testis, compared with the control testis at E12.5 and E14.5. In females, the number of proliferating somatic cells in the DES-treated ovary was significantly higher than that in the control ovary. These results suggest that DES affects the proliferation of somatic cells during the early stages of gonadal development of both sexes in a sex-specific manner. Molecular mechanisms regulating sex-specific cell proliferation of differentiating gonads remain elusive. Because SF-1 is involved in sexually dimorphic gonadal growth (50), the reduction of SF-1 expression in the DES-treated testis may be part of the mechanisms responsible for the inhibitory effects of DES on testicular growth. A recent study using DNA microarray suggested that the smaller size of female gonads relative to male gonads may be due to decreased cellular proliferation caused by the overexpression of cyclin-dependent kinase inhibitors (Cdkn), including Cdkn 1a, Cdkn 1b, and Cdkn 1c by somatic cells overexpressed in developing female gonads at E11.5–E13.5 (5). Currently it remains unknown whether estrogens affect the expression of these genes.
The qPCR results indicate that ERα but not ERβ is expressed at a very low level in the gonad of both sexes from E11.5 through E14.5. These results are comparable with those of an ISH study in which ERα and ERβ mRNAs were detected from E12.5 and E16.5, respectively, in the gonads of both sexes (51). Similar results were also obtained from previous IHC studies showing that ERα-ir was faint in the testis and ovary at E14.5 (52) and that ERβ-ir was not detected in the gonad of either sex until E16.5 (53). In ERαKO mice, testosterone levels and the expression levels of several steroidogenic genes including StAR, P450scc, and P450c17 were increased in the testis as early as E13.5, and the levels were not altered by DES exposure in ERα KO mice, suggesting that the role of endogenous estrogen is the regulation of gonadal differentiation by suppressing genes involved in steroidogenesis via ERα (9). Furthermore, an in vitro study showed that MIS activation by ERα was dependent on the concentration of estradiol and that this activation was inhibited by SF-1 (54). Based on these studies, it seems possible that estrogens change the interaction between SF-1 and ERα to regulate genes involved in gonadal differentiation, although the expression levels of gonadal ERα mRNA were not significantly altered by DES treatment.
It is well known that in utero exposure to DES causes physiological effects in the male reproductive system, such as cryptorchidism, incomplete regression of the Müllerian ducts, decreased fertility rate, and hypoproduction of FSH, LH, and testosterone in perinatal and adult mice, even long after the cessation of DES exposure (13,41,42,44,55). Although the dose of DES used in the present study is much lower than those used in most of the previous studies, a few studies examined the male reproductive system of late embryonic and postnatal mice exposed in utero to DES at doses similar to our study. Prostate enlargement was observed in adult male offspring treated in utero with very low doses of DES (≤2 μg/kg) from E11 to E17 (55). A significant decrease in the intratesticular testosterone levels was observed in E18 mice exposed in utero to DES (10 μg/kg) from E10.5 to E17.5 (43). In this previous study, Western blot analysis showed that the intensity of the StAR signal also decreased markedly, suggesting that the decrease in the expression of this gene is related to the decreased testosterone levels in the DES-exposed testis. In another study, male offspring exposed in utero to DES at a daily dose of 10 μg/kg from E9 to E16 showed a significant reduction in testicular weights and sperm head count and concentration, accompanied by a moderate increase in the number and size of Leydig cells, compared with the control values on postnatal d 60 and 100. This suggests that the observed increase in the number and size might reflect hyperplasia/hypertrophy of Leydig cells, which is associated with reduced testosterone synthesis (56). Although we did not examine the reproductive organs on later stages of development of the DES-treated mice, these previous results strongly indicate that the in utero exposure to DES at the condition used in the present study can cause long-term effects. In reality, it is considered that humans can be exposed to a combination of various estrogenic chemicals with different activities, and physiological effects can vary between individuals depending on exposure conditions such as chemical dose, developmental time, and period of exposure. Therefore, even though the effects of exposure to a low dose of one chemical may not be evident, plural chemicals could cause much greater effects than those expected from exposure to each chemical. A recent study has shown a drastic down-regulation of genes required for testicular decent and steroidogenesis in fetal Leydig cells in control but not ERα KO mice at E18.5, by in utero exposure to 17β-estradiol or DES, suggesting that ERα mediates the down-regulation at the late gestational stage (57).
Supplementary Material
Acknowledgments
We thank Hajime Sawada for providing rabbit anti-laminin-1 antibody and Michio Ono for technical assistance.
Footnotes
Disclosure Statement: The authors have nothing to declare.
First Published Online April 24, 2008
Abbreviations: BrdU, 5-Bromo-2′deoxyuridine-5-monophosphate; bw, body weight; Cbln, Cerebellin 1; Ct, cycle threshold; Dax-1, dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on the X chromosome gene 1; DES, diethylstilbestrol; Dhh, desert hedgehog; E, embryonic day; ER, estrogen receptor; Fst, follistatin; IHC, immunohistochemistry; ir, immunoreactivity; ISH, in situ hybridization; K, keratin; KO, knockout; MIS, Müllerian-inhibiting substance; P450scc, cholesterol side-chain cleavage cytochrome P450; qPCR, quantitative real-time RT-PCR; SF-1, steroidogenic factor 1; Sox9, Sry high mobility group box-related gene 9; Sry, sex-determining gene on the Y choromosome; StAR, steroidogenic acute regulatory protein; Wnt4, wingless-related mouse mammary tumor virus integration site 4.
References
- Swain A, Lovell-Badge R 1999 Mammalian sex determination: a molecular drama. Genes Dev 13:755–767 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R 1991 Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121 [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Hart GT, Washburn LL, Recknagel AK, Eicher EM 2004 Using real time RT-PCR analysis to determine multiple gene expression patterns during XX and XY mouse fetal gonad development. Genes Expr Patterns 5:141–5149 [DOI] [PubMed] [Google Scholar]
- Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD 2005 Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod 72:492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD 2005 Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol 287:361–377 [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Habert R 2006 Estrogen effects on fetal and neonatal testicular development. Reproduction 132:527–538 [DOI] [PubMed] [Google Scholar]
- Sharpe RM 2006 Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab 20:91–110 [DOI] [PubMed] [Google Scholar]
- Lassurguere J, Livera G, Habert R, Jegou B 2003 Time- and dose-related effects of estradiol and diethylstilbestrol on the morphology and function of the fetal rat testis in culture. Toxicol Sci 73:160–169 [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R 2005 Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology 146:2454–2461 [DOI] [PubMed] [Google Scholar]
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP 2002 Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57:19–36 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL 1994 Developmental expression of mouse steroidogenic factor 1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, Saunders PT 1997 Maternal oestrogen/xenoestrogen exposure alters expression of steroidogenic factor-1 (SF-1/Ad4BP) in the fetal rat testis. Mol Cell Endocrinol 127:91–98 [DOI] [PubMed] [Google Scholar]
- Visser JA, McLuskey A, Verhoef-Post M, Kramer P, Grootegoed JA, Themmen AP 1998 Effect of prenatal exposure to diethylstilbestrol on Müllerian duct development in fetal male mice. Endocrinology 139:4244–4251 [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA 1998 Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet 20:228–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS 2000 In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing müllerian system. FASEB J 14:1101–1108 [DOI] [PubMed] [Google Scholar]
- Huang WW, Yin Y, Bi Q, Chiang TC, Garner N, Vuoristo J, McLachlan JA, Ma L 2005 Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol 19:669–682 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda M, Hayashi S 2001 Neonatal estrogen exposure inhibits steroidogenesis in the developing rat ovary. Dev Dyn 221:443–453 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S 2002 Increased expression of Müllerian-inhibiting substance correlates with inhibition of follicular growth in the developing ovary of rats treated with E2 benzoate. Endocrinology 143:304–312 [DOI] [PubMed] [Google Scholar]
- Nagai A, Ikeda Y, Aso T, Eto K, Ikeda MA 2003 Exposure of neonatal rats to diethylstilbestrol affects the expression of genes involved in ovarian differentiation. J Med Dent Sci 50:35–40 [PubMed] [Google Scholar]
- Yao HH 2005 The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 230:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R 1998 Dax1 antagonizes Sry action in mammalian sex determination. Nature 391:761–767 [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL 2003 Dax1 regulates testis cord organization during gonadal differentiation. Development 130:1029–1036 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP 1999 Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Koga M, Buscaglia ML, Simmons DM, Bicsak TA, Ling N 1989 Follistatin gene expression in the ovary and extragonadal tissues. Mol Endocrinol 3:651–659 [DOI] [PubMed] [Google Scholar]
- Shen WH, Moor CC, Ikeda Y, Parker KL, Ingraham HA 1994 Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651–661 [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL 1992 Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol 8:1249–1258 [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ 1997 Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol 11:138–147 [DOI] [PubMed] [Google Scholar]
- Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM 2005 Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci 25:4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC 1980 Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res 40:3988–3999 [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R 1995 Expression of Sry, the mouse sex determining gene. Development 121:1603–1614 [DOI] [PubMed] [Google Scholar]
- Kaufman MH 1992 The atlas of mouse development. London: Academic Press, Inc. [Google Scholar]
- McClive PJ, Sinclair AH 2001 Rapid DNA extraction and PCR-sexing of mouse embryos. Mol Reprod Dev 60:225–226 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL 1993 Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7:852–860 [DOI] [PubMed] [Google Scholar]
- Keeny DS, Ikeda Y, Waterman MR, Parker KL 1995 Cholesterol side-chain cleavage cytochrome P450 gene expression in primitive gut of the mouse embryo does not require steroidogenic factor-1. Mol Endocrinol 9:1091–1098 [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R 1996 Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 14:62–68 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, Tamai KT, Sassone-Corsi P, Lovell-Badge R, Camerino G, Parker KL 1996 Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol 10:1261–1272 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Kusaka M, Mukai T, Hisano S, Morohashi K 2001 Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn 220:363–374 [DOI] [PubMed] [Google Scholar]
- Coveney D, Ross AJ, Slone JD, Capel B 2007 A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene Expr Patterns 7:82–92 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV 1997 Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA 94:2056–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Emmen JM, McLuskey A, Adham IM, Engel W, Verhoef-Post M, Themmen AP, Grootegoed JA, Brinkmann AO 2000 Involvement of insulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryptorchidism. Endocrinology 141:846–849 [DOI] [PubMed] [Google Scholar]
- Nef S, Shipman T, Parada LF 2000 A molecular basis for estrogen-induced cryptorchidism. Dev Biol 224:354–361 [DOI] [PubMed] [Google Scholar]
- Guyot R, Odet F, Leduque P, Forest MG, Le Magueresse-Battistoni B 2004 Diethylstilbestrol inhibits the expression of the steroidogenic acute regulatory protein in mouse fetal testis. Mol Cell Endocrinol 220:67–75 [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL 2007 Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology 148:3704–3710 [DOI] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, O'Shaughnessy PJ, Saunders PT 1996 Expression of cytochrome P450 17α-hydroxylase/C17-20 lyase in the fetal rat testis is reduced by maternal exposure to exogenous estrogens. Endocrinology 137:1063–1070 [DOI] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL 2005 Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development 132:2415–2423 [DOI] [PubMed] [Google Scholar]
- Perez-Martinez C, Garcia-Iglesias MJ, Ferreras-Estrada MC, Bravo-Moral AM Espinosa-Alvarez J, Escudero-Diez A 1996 Effects of in utero exposure to zeranol or diethylstilboestrol on morphological development of the fetal testis in mice. J Comp Pathol 114:407–418 [DOI] [PubMed] [Google Scholar]
- Appert A, Fridmacher V, Locquet O, Magre S 1998 Patterns of keratins 8, 18 and 19 during gonadal differentiation in the mouse: sex- and time-dependent expression of keratin 19. Differentiation 5:273–284 [DOI] [PubMed] [Google Scholar]
- Schmahl J, Capel B 2003 Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol 258:264–276 [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B 2000 Sry induces cell proliferation in the mouse gonad. Development 127:65–73 [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B 1999 Expression of estrogen receptor α and β during mouse embryogenesis. Mech Dev 81:163–167 [DOI] [PubMed] [Google Scholar]
- Nielsen M, Bjornsdottir S, Hoyer PE, Byskov AG 2000 Ontogeny of oestrogen receptor α in gonads and sex ducts of fetal and newborn mice. J Reprod Fertil 118:195–204 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR 2000 Expression of estrogen receptor β is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62:310–317 [DOI] [PubMed] [Google Scholar]
- Chen G, Shinka T, Kinoshita K, Yan HT, Iwamoto T, Nakahori Y 2003 Roles of estrogen receptor α (ERα) in the regulation of the human Müllerian inhibitory substance (MIS) promoter. J Med Invest 50:192–198 [PubMed] [Google Scholar]
- Warita K, Sugawara T, Yue ZP, Tsukahara S, Mutoh K, Hasegawa Y, Kitagawa H, Mori C, Hoshi N 2006 Progression of the dose-related effects of estrogenic endocrine disruptors, an important factor in declining fertility, differs between the hypothalamo-pituitary axis and reproductive organs of male mice. J Vet Med Sci 68:1257–1267 [DOI] [PubMed] [Google Scholar]
- Traina ME, Rescia M, Urbani E, Mantovani A, Macrì C, Ricciardi C, Stazi AV, Fazzi P, Cordelli E, Eleuteri P, Leter G, Spanò M 2003 Long-lasting effects of lindane on mouse spermatogenesis induced by in utero exposure. Reprod Toxicol 17:25–35 [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S 2007 Estrogen receptor α is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology 148:5507–5519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.