Abstract
The G-protein coupled receptor GPR54 and its ligand, KiSS-1-derived peptide kisspeptin-54, appear to play an important role in the mechanism of puberty. This study measures the release of kisspeptin-54 in the stalk-median eminence (S-ME) during puberty and examines its potential role in the pubertal increase in LHRH-1 release in female rhesus monkeys. First, developmental changes in release of kisspeptin-54 and LHRH-1 were assessed in push-pull perfusate samples obtained from the S-ME of prepubertal, early pubertal, and midpubertal female rhesus monkeys. Whereas LHRH-1 levels in 10-min intervals had been measured previously for other experiments, kisspeptin-54 levels in 40-min pooled samples were newly measured by RIA. The results indicate that a significant increase in kisspeptin-54 release occurred in association with the pubertal increase in LHRH-1 release and that a nocturnal increase in kisspeptin-54 release was already observed in prepubertal monkeys and continued through the pubertal period. Second, we measured kisspeptin-54 release in the S-ME of midpubertal monkeys at 10-min intervals using a microdialysis method. Kisspeptin-54 release in the S-ME was clearly pulsatile with an interpulse interval of about 60 min, and approximately 75% of kisspeptin-54 pulses were correlated with LHRH-1 pulses. Finally, the effect of kisspeptin-10 on LHRH-1 release was examined with the microdialysis method. Kisspeptin-10 infusion through a microdialysis probe significantly stimulated LHRH-1 release in a dose-dependent manner. Collectively, the results are consistent with the hypothesis that kisspeptin plays a role in puberty.
BECAUSE A DELAY in or absence of puberty in patients with mutations in the gene encoding G protein coupled receptor 54 (GPR54) has been reported (1,2,3), GPR54 and its cognate ligand, kisspeptin-54 (also called metastin1–54), have been proposed to play a significant role in the mechanism of the onset of puberty (4,5,6,7,8). GPR54 is a member of the G protein-coupled seven-transmembrane receptor family and the metastasis suppressor gene KiSS-1-derived peptide, kisspeptin-54, is its ligand (9,10,11). The C-terminal region of kisspeptin-54 is responsible for receptor binding, and short forms of the peptide, kisspeptin-10, -13, -14, and -15, are all biologically active (9,10,11). Moreover, LHRH-1 neurons express GPR54 (12,13), and kisspeptins stimulate LH and FSH release (13,14,15,16). Hypothalamic expression of KiSS mRNA increases with puberty (13,14), and GPR54 mRNA expression increases with puberty (14). Importantly, mice lacking the GPR54 gene failed to undergo puberty and had immature gonads (1,17). Recently, it has been reported that mice lacking the KiSS-1 gene also exhibited abnormal timing of puberty, although their phenotypes were not as severe as those in GPR54 knockout mice (18,19). These reports are consistent with the hypothesis that the GPR54 and KiSS-1 genes are responsible for the onset of puberty. Nonetheless, to support this hypothesis, several critical questions remain to be answered. Is kisspeptin-54 release in the stalk-median eminence (S-ME) pulsatile? Does kisspeptin-54 release in the S-ME change at puberty? Do kisspeptins stimulate LHRH-1 release in vivo in primates? To answer to these questions, we conducted the present study. We found that kisspeptin-54 was released in a pulsatile manner in the S-ME, kisspeptin-10 stimulated LHRH-1 release, and kisspeptin-54 release increased along with the pubertal increase in LHRH-1 release.
Materials and Methods
Animals
All animals used in this study were born and raised in the Wisconsin National Primate Research Center. They were housed in pairs (cages 172 × 86 × 86 cm) in rooms with 12 h light (0600–1800 h)and 12 h dark (1800–0600 h), except for experiments 2 and 3, and controlled temperature (22 C). During the entire course of experiments 2 and 3, the animals were kept in a room where the lighting condition was shifted to lights on from 0300–1500 h and lights off from 1500–0300 h. The animals were fed a standard diet of Harlan 20% Protein Primate Diet once every morning, supplemented with fresh fruit several times per week. Water was available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the National Institutes of Health and U.S. Department of Agriculture.
Experiment 1: developmental changes in release of kisspeptin-54 and LHRH-1 in the S-ME
In the first experiment, we asked whether kisspeptin-54 is detectable in samples collected from the S-ME of female rhesus monkeys and, if so, whether developmental changes in kisspeptin-54 release occur along with the pubertal increase in LHRH-1 release. Two sets of push-pull perfusates, which were collected from female rhesus monkeys at the three stages of puberty using the push-pull perfusion method were used. LHRH-1 levels in the perfusates had been measured previously for other studies. The first set of samples were collected at 0900–1100 h from 10 prepubertal, eight early pubertal, and nine midpubertal females at 17.2 ± 0.4, 23.0 ± 1.0, and 38.3 ± 2.0 months of age, respectively, at 10-min intervals using the push-pull perfusion method. The body weights of these animals were 2.3 ± 0.1, 3.1 ± 0.1, and 5.0 ± 0.4 kg, respectively. The second set of samples were collected at 0600–1200 h and 1800–2400 h from six prepubertal, six early pubertal, and five midpubertal females at 16.6 ± 1.3, 24.5 ± 1.1, and 38.7 ± 2.2 months of age, respectively, at 10-min intervals using the push-pull perfusion method. Their body weights were 2.4 ± 0.2, 3.4 ± 0.3, and 4.7 ± 0.3 kg, respectively. The pubertal stages were defined as described previously (20). Because of a small sample volume left after LHRH-1 assay, four consecutive (a 40-min period) samples were pooled for kisspeptin-54 assay. For comparison, mean LHRH-1 levels during the corresponding 40-min period measured previously were calculated from four successive 10-min values. Because surgical procedures and the push-pull perfusion method have been extensively reported (21,22), we will not describe them in this article.
Experiment 2: release pattern of kisspeptin-54 in the S-ME
Because we found that kisspeptin-54 was released in the S-ME from experiment 1, in the second experiment, we examined whether kisspeptin-54 is released in a pulsatile manner using the microdialysis method. Four female rhesus monkeys at 29.1 ± 1.2 months of age (body weight 3.6 ± 0.1 kg) received cranial pedestal implantation as described previously (22). A custom-made microdialysis probe with a polyarylethersulfone membrane (CMA 12; CMA, Stockholm, Sweden) was inserted into the S-ME, and dialysates for kisspeptin-54 assay were collected at 10-min intervals for up to 12 h. Kisspeptin-54 levels in dialysates were assessed by a specific RIA (16). Each animal was examined one to three times. The mean age of sampling was 34.8 ± 1.2 months. This experiment was conducted in a room with lights on from 0300–1500 h and lights off from 1500–0300 h.
Experiment 3: the effect of kisspeptin-10 infusion into the S-ME on LHRH-1 release
In the third experiment, we tested whether human kisspeptin-10 [Kiss-1 (112–121)-amide; Phoenix, Belmont, CA] infusion into the S-ME induced LHRH-1 release using the microdialysis method. The same four midpubertal females were used for this experiment. Human kisspeptin-10 at 0.1, 1, and 10 nm dissolved in central nervous system perfusion fluid by CMA (see below) or vehicle alone was infused through the microdialysis probe for 10 min in a random order, whereas dialysates were continuously collected. Each animal was examined repeatedly with two to three doses up to three times. LHRH-1 levels in dialysates were assessed by RIA. This experiment was also conducted in a room with lights on from 0300–1500 h and lights off from 1500–0300 h.
Microdialysis experiments
Implantation of cranial pedestal.
Before experiments, monkeys were well adapted to the primate chair, the experimental environment, and the investigator, as described previously (20,21). They were implanted with a cranial pedestal under isoflurane anesthesia, similar to those described previously for push-pull perfusion method (20,21). Animals were allowed to recover for at least 1 month before experimentation.
Insertion of a guide cannula and a microdialysis probe.
On the day of experiment, the monkey was placed in the stereotaxic apparatus under ketamine (15 mg/kg body weight) and medetomidine (0.03–0.05 mg/kg body weight) anesthesia. The custom-made guide cannula (CMA 12) consisted of a stainless steel shaft (76.0 mm in length, 0.91 mm outer diameter) and a removable stainless steel stylet (96.0 mm in length, 0.6 mm outer diameter), which extruded 20 mm from the guide cannula tip. It was inserted into the skull 5 mm above the S-ME with a hydraulic microdrive unit (MO95-B; Narshige, Tokyo, Japan). The microdrive unit allowed for accurate three-dimensional adjustment of the tip location. The x, y, and z coordinates for the S-ME were calculated using ventriculographs and the final radiographs taken during cranial pedestal implantation surgery. Cannula placement was confirmed with radiographic visualization, as described previously (22,23). After placement of the guide cannula, the monkey was removed from the stereotaxic apparatus and placed into a primate chair. Once the monkey was properly placed in the chair, the inner stylet was removed from the guide cannula, and the custom-made microdialysis probe (a stainless steel shaft 96.0 mm in length, 0.6 mm outer diameter), fitted with a membrane (5 mm in length, 0.5 mm outer diameter), was inserted into the S-ME through the guide cannula as described previously (23). To reverse the effects of medetomidine, atipamazole (0.15–0.25 mg/kg) was injected (iv) into the animal. The animal was fully awake within 1 h after probe insertion.
In vivo microdialysis perfusion.
A perfusion fluid consisting of 147 mm NaCl, 2.7 mm KCl, 1.2 mm CaCl2, and 0.85 mm MgCl2, purchased from CMA/Microdialysis with bacitracin (4 U/ml) added, was infused through the inflow tubing at 2 μl/min with the CMA/102 microdialysis pump (Stockholm, Sweden) outfitted with a 1- or 2.5-ml Hamilton gas tight syringe (Reno, NV). Perfusates were continuously collected at 10-min intervals for up to 12 h through the outflow tubing into 12- × 75-mm borosilicate tubes containing 280 μl RIA buffer (solution containing 0.1% gelatin in 0.01 m PO4; 0.15 m NaCl; 0.1% NaN3, pH 7.4) on ice with a fraction collector (model FC203B; Gilson, Middleton, WI). The perfusate samples were immediately frozen on dry ice and stored at −80 C. During the entire experiment, monkeys were placed in proximity to a companion monkey, given constant access to food and water, and were provided frequently with fruit, cereal, raisins, and other snacks.
RIAs for kisspeptin-54 and LHRH-1
Kisspeptin-54 in 150 μl from pooled push-pull perfusates (experiment 1) or 20 μl from microdialysates (experiment 2) were measured by RIA using antiserum GQ2, as described previously (16). Briefly, GQ2 was raised in a sheep immunized with synthetic human kisspeptin-54 conjugated to BSA by glutaraldehyde. This antisera cross-reacts 100% with human kisspeptin-54, kisspeptin-14, and kisspeptin-10. Although this antibody does not discriminate kisspeptin-54 from smaller kisspeptin molecules, we used kisspeptin-54 throughout the article, because currently kisspeptin-54 is the only known peptide form for the GPR54 ligand in human circulation (24). GQ2 cross-reacts less than 0.01% with any other related human RFamide peptide, including human RFamide-related peptide (RFRP)1, human RFRP2, human RFRP3, prolactin-releasing peptide, human neuropeptide FF, and human neuropeptide AF as well as LHRH-1 (16). It is important to emphasize that GQ2 was not cross-reactive with human RFRP1 and RFRP3, which are also known to be gonadotropin-inhibiting hormones in the avian (25). Synthetic human kisspeptin-54 [Kiss-1 (68–121)-amide; Bachem, Torrance, CA] was used for both radiolabeled antigen and the reference standard. A custom-ordered [125I]kisspeptin-54 was produced by Bachem. The antigen-antibody complex was precipitated with a donkey antisheep-γ-globulin. The sensitivity was 0.05 pg/tube at a 95% confidence limit, and intra- and interassay coefficients of variation were 10.1 and 14.3%, respectively. We also confirmed that neither artificial cerebrospinal fluid used for push-pull perfusion nor perfusion fluid used for microdialysis interferes with the kisspeptin-54 assay. LHRH-1 RIA was conducted with antisera, either R42 or R1245, kindly provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO), as described previously (21). Assay sensitivity was 0.02 pg/tube, and intra- and interassay coefficients of variation were 8.1 and 11.3%, respectively. For co-measurement of kisspeptin-54 and LHRH-1 (experiment 2), 20-μl microdialysates were divided into two aliquots (10 μl each).
Statistical analysis
Developmental changes in kisspeptin-54 release (experiment 1) were evaluated with two-way ANOVA unrepeated measure followed by Tukey’s multiple comparison test.
Peaks of kisspeptin-54 in dialysates (experiment 2) were identified using the PULSAR algorithm (26). Intraassay coefficents of variation for kisspeptin-54 assay were described by the equation y = 0.0491x2− 0.1726x + 0.1813. The cutoff criteria for pulse determination, G1, G2, G3, G4, and G5 were 3.8, 2.6, 1.9, 1.5, and 1.2, respectively. Parameters of pulsatile kisspeptin-54 release were calculated for each experiment as follows: 1) mean kisspeptin-54 release was derived from the mean of all kisspeptin-54 values; 2) pulse amplitude of kisspeptin-54 release was defined as the difference between peak and trough; 3) interpulse interval was defined as the intervals between peaks of kisspeptin-54 pulses; and 4) total release was calculated by adding the kisspeptin-54 values of a given period. Subsequently, mean ± sem for each parameter was calculated. The difference in the mean, pulse amplitude, and total release between the lights-on period and lights-off period were examined by paired t test. Correlation between kisspeptin and LHRH-1 pulses was determined by a method described previously (27,28). Because we expect that kisspeptin stimulates LHRH-1 release in the hypothalamus, with this method, kisspeptin pulses were considered to be correlated with LHRH-1 pulses if a kisspeptin peak occurred at the same time as or 10 min preceding a LHRH-1 peak. As a control, a set of kisspeptin data from a monkey was cross-compared with a set of mismatched LHRH-1 data from another monkey. Subsequently, the frequency of coincidence from the experiment in which kisspeptin and LHRH-1 were measured in the same sample was compared with the frequency of random coincidence from a mismatched data set. Statistical significance was evaluated with Student’s t test.
The effect of kisspeptin-10 on LHRH-1 release (experiment 3) was examined by comparing the two points before and after kisspeptin (or vehicle) challenge using two-way ANOVA (variables: treatment and dose) followed by Tukey’s multiple comparison test. Because each trial had a different combination of kisspeptin doses, we treated each experiment as one entry. If the same dose was tested twice in an experiment, the mean of the two was used as an entry. No animal contributed more than twice in each dose. All statistical analyses were conducted with raw values. Differences were considered significant at P < 0.05.
Results
Experiment 1: developmental changes in release of kisspeptin-54 and LHRH-1 in the S-ME
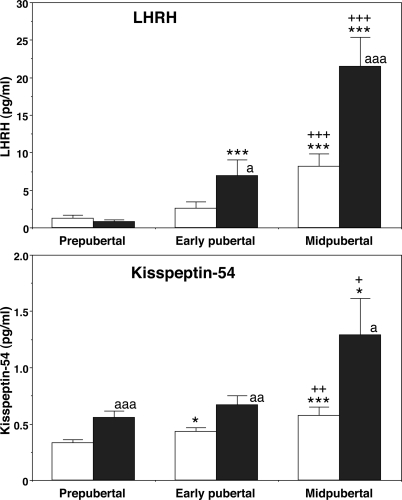
The first set of samples collected from prepubertal, early pubertal, and midpubertal monkeys contained 0.36 ± 0.09, 0.70 ± 0.19, and 1.00 ± 0.15 pg/ml kisspeptin-54, respectively. Previously measured LHRH-1 levels in the corresponding samples were 1.51 ± 0.51, 3.66 ± 0.99, and 5.57 ± 1.88 pg/ml, respectively. Statistical analysis indicated that kisspeptin-54 concentrations in the midpubertal monkeys were significantly higher (P < 0.01) than those in prepubertal monkeys, and LHRH-1 concentrations in the midpubertal and early pubertal monkeys were significantly higher (P < 0.05) than those in prepubertal monkeys.
To confirm the initial findings described above and to further examine whether a nocturnal increase in kisspeptin-54 release occurs in association with the nocturnal increase in LHRH-1 release during puberty, we measured kisspeptin-54 in push-pull perfusates obtained in the morning (0600–1200 h) and evening (1800–2400 h). Kisspeptin-54 levels in 40-min pooled samples showed that both morning and evening levels significantly (P < 0.01 for morning, P < 0.05 for evening) increased during the course of puberty (Fig. 1). Post hoc analysis further indicated that morning levels in early pubertal monkeys were significantly higher than those in prepubertal monkeys, and both morning and evening levels of midpubertal monkeys were also significantly higher than those in prepubertal and early pubertal monkeys (Fig. 1). In addition, the nocturnal increase in kisspeptin-54 release was already observed in prepubertal monkeys and continued through the pubertal period.
Figure 1.
Developmental changes in the release of LHRH-1 (top) and kisspeptin-54 (bottom) in push-pull perfusates. Both peptides were measured in the same samples. Kisspeptin-54 levels gradually increased along with the pubertal increase in LHRH-1 release. The nocturnal increase in kisspeptin-54 release was already observed in prepubertal monkeys and continued through the pubertal period. In contrast, the nocturnal increase in LHRH-1 release occurred in early and midpubertal monkeys but not in prepubertal monkeys. The number of animals at the prepubertal, early pubertal, and midpubertal stages was six, six, and five, respectively. White bars, morning values; black bars, evening values. *, P < 0.05 vs. prepubertal; ***, P < 0.001 vs. prepubertal; +, P < 0.05 vs. early pubertal; ++, P < 0.01 vs. early pubertal; +++, P < 0.001 vs. early pubertal; a, P < 0.05 vs. morning; aa, P < 0.01 vs. morning; aaa, P < 0.001 vs. morning.
LHRH-1 levels during the corresponding 40-min period indicated that both morning and evening levels significantly (P < 0.001 for both morning and evening) increased during the course of puberty. Post hoc analysis indicated that both morning and evening levels in midpubertal monkeys were significantly higher than those in prepubertal and early pubertal monkeys, and evening levels in early pubertal monkeys were significantly higher than those in prepubertal monkeys. The nocturnal increase in LHRH-1 release was observed in early and midpubertal monkeys but not in prepubertal monkeys.
Experiment 2: release pattern of kisspeptin-54 in the S-ME
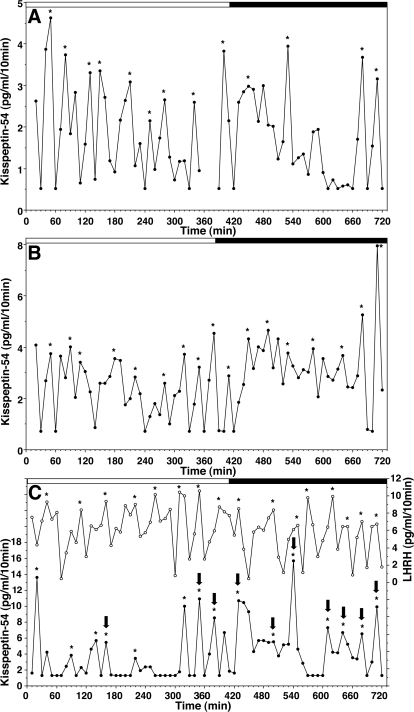
Changes in kisspeptin-54 levels in the S-ME measured by the microdialysis method suggested that kisspeptin-54 release was clearly pulsatile (Fig. 2). Pulse interval, mean release, and pulse amplitude were 59.3 ± 14.8 min, 2.1 ± 0.3 pg/ml, and 3.8 ± 1.0 pg/ml, respectively (n = 7). Moreover, interpulse interval (59.3 ± 14.8 min) of kisspeptin-54 release in midpubertal females was similar to that (55.8 ± 10.9 min) of LHRH-1 release obtained from adult ovariectomized females with the microdialysis method (Table 1; Ref. 23). Importantly, kisspeptin-54 levels, increased shortly after lights-off (Fig. 2, A and B). Statistical analysis indicated that total release of kisspeptin-54 (68.0 ± 16.6 pg/ml) during the 5-h period after lights-off was significantly (P < 0.05) higher than that (30.1 ± 6.9 pg/ml) during the 5-h period before lights-off. Both mean release (2.9 ± 0.5 pg/ml) and pulse amplitude (5.0 ± 1.4 pg/ml) during the dark phase had a tendency to be higher than those during the light phase (1.8 ± 0.3 and 2.7 ± 0.5 pg/ml, respectively), although the values were not statistically significant (P > 0.05).
Figure 2.
Kisspeptin-54 release in the S-ME assessed by the microdialysis method was pulsatile. In A and B, kisspeptin-54 alone was measured in microdialysate samples, whereas in C, both kisspeptin-54 (•) and LHRH-1 (○) were measured in the same microdialysate samples. Kisspeptin-54 and LHRH-1 pulses, indicated by asterisks, were identified using the PULSAR algorithm. Kisspeptin-54 pulses correlated with LHRH-1 pulses, defined in Materials and Methods, are indicated by arrows on the top of kisspeptin pulses. Lighting conditions are indicated on the top of each graph (white bar for the lights-on period and black bar for the lights-off period). Note that in all three cases, total release of kisspeptin-54 during a 5-h period of the dark phase was significantly higher than that during the light phase. The missing data between 370 and 390 min in A was due to a technical problem during the sample collection.
Table 1.
Comparison of kisspeptin-54 release and LHRH-1 release measured by microdialysis in female rhesus monkeys
| Kisspeptin-54 | LHRH-1a | |
|---|---|---|
| Mean (pg/ml) | 2.1 ± 0.3 | 9.9 ± 3.9 |
| Baseline (pg/ml) | 1.1 ± 0.1 | 3.8 ± 2.1 |
| Amplitude (pg/ml) | 3.8 ± 1.0 | 10.6 ± 4.3 |
| Interpulse interval (min) | 59.3 ± 14.8 | 55.8 ± 10.9 |
| n | 7 | 4 |
Results are shown as mean ± sem.
From ovariectomized adult females (23).
To assess the coordinated release of LHRH-1 and kisspeptin-54, we measured kisspeptin-54 and LHRH-1 in the same samples. As shown in Fig. 2C, data suggested that kisspeptin-54 and LHRH-1 pulses had a tendency to occur with a similar timing. Statistical analysis indicated that kisspeptin-54 peaks occurred at the same time as or 10 min before LHRH-1 peaks 76.5 ± 2.4% of the time (n = 3), and this coincidence was significantly higher (P < 0.02) than random coincidence (45.4 ± 6.6%, n = 3).
Experiment 3: the effect of kisspeptin-10 infusion into the S-ME on LHRH-1 release
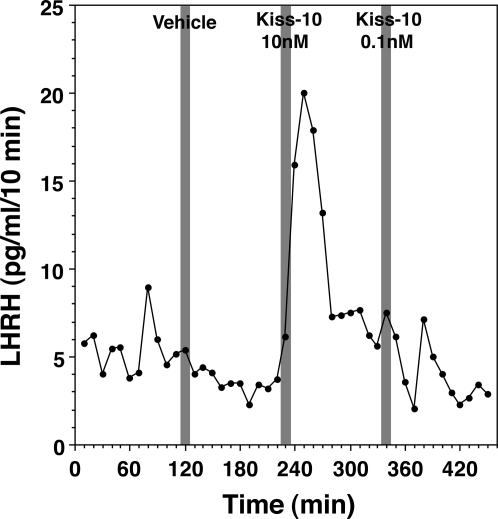
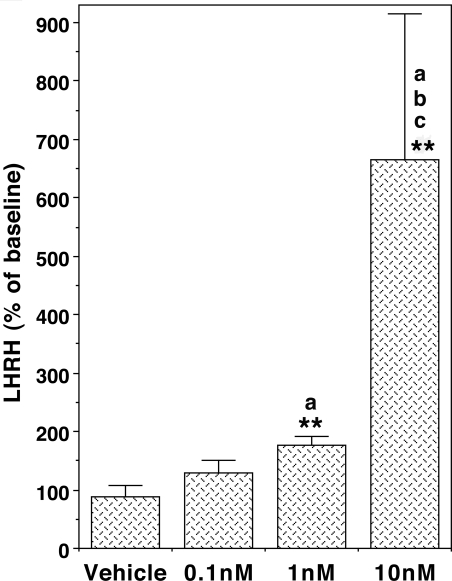
Next we examined whether human kisspeptin-10 stimulated LHRH-1 release using the microdialysis method. Kisspeptin-10 at 1 and 10 nm infusion through a microdialysis probe consistently stimulated LHRH-1 release, whereas kisspeptin-10 at 0.1 nm stimulated LHRH-1 release in most of the cases (four of five cases, Fig. 3). Statistical analysis suggested that there was a significant interaction between treatment and dose (P < 0.01), and the kisspeptin-10 induced LHRH-1 release was a dose dependent (P < 0.01, Fig. 4). Post hoc analysis indicated that kisspeptin-10 at doses of 1 and 10 nm resulted in a significant increase in LHRH-1 release (P < 0.01 and P < 0.05, respectively), whereas the LHRH-1 response to kisspeptin-10 at 0.1 nm was not significant. Based on our previous assessment that the dialysis membrane passes about 10% of the peptide (23), it is estimated that the threshold concentration of kisspeptin-10 to induce LHRH-1 release in the S-ME was 0.1 nm.
Figure 3.
An example of the effects of human kisspeptin-10 on LHRH-1 release in a pubertal female monkey. Kisspeptin-10 at 10 or 0.1 nm concentration or vehicle was perfused through a dialysis membrane (as indicated by a shaded bar), and LHRH-1 levels in dialysates obtained from the S-ME were measured by RIA. Note that the local concentration of kisspeptin-10 in the tissue was estimated to be 1 or 0.01 nm, respectively, based on a 10% recovery rate through the membrane. Kisspeptin-10 stimulated LHRH-1 release.
Figure 4.
Kisspeptin-10 infusion to the S-ME induced LHRH-1 release in a dose-responsive manner. The kisspeptin-10-induced LHRH-1 responses are expressed as a percentage of before kisspeptin-10 challenge. The overall dose-response curve was significant at P < 0.01. **, P < 0.01 vs. baseline before kisspeptin-10 in each dose; a, P < 0.05 vs. vehicle; b, P < 0.05 vs. 0.1 nm; c, P < 0.05 vs. 1 nm. n = 5 in all groups.
Discussion
The results of the present study suggest that 1) a significant increase in kisspeptin-54 release occurred in association with the pubertal increase in LHRH-1 release; 2) a nocturnal increase in kisspeptin-54 release was already observed in prepubertal monkeys and continued through the pubertal period, whereas a nocturnal increase in LHRH-1 release occurred in early pubertal and midpubertal but not in prepubertal monkeys; 3) kisspeptin-54 release in the S-ME is pulsatile with an interpulse interval of about 60 min and about 75% of kisspeptin-54 pulses occurred with LHRH-1 pulses; and 4) kisspeptin-10 stimulates LHRH-1 release in a dose-dependent manner.
An increased release in LHRH-1 from the hypothalamus is essential for the onset of puberty. Pulsatile administration of LHRH-1 induces menarche and first ovulation in sexually immature female monkeys (29), and an increase in LHRH-1 release in the S-ME occurs at the onset of puberty in female monkeys (20). However, the mechanism triggering the pubertal increase in LHRH-1 release is still unclear (30). It has been hypothesized that activation of the GPR54 signaling mechanism plays an important role in the onset of puberty. 1) Hypothalamic expression of both GPR54 and KiSS-1 mRNA are significantly elevated around the time of puberty in male and female rats (14) and in ovarian intact pubertal female monkeys (13). 2) The number of LHRH-1 neurons responding to kisspeptin-10 increases with developmental age in mice, and LH response to intracerebroventricular injection of kisspeptin in juvenile mice is higher than in adult mice (31). 3) The number of kisspeptin-positive neurons in the anteroventral periventricular nucleus of mice increases at the age of puberty (32). 4) Central administration of kisspeptin results in precocious puberty in female rats (33) and precocious LH elevation in prepubertal male monkeys (34). Therefore, if this hypothesis is correct, kisspeptin release would increase along with the pubertal increase in LHRH-1 release. In the present study, we measured kisspeptin-54 levels in two sets of our archived samples, which were previously collected from the S-ME of prepubertal, early pubertal, and midpubertal monkeys using a push-pull perfusion method, from which we had already measured LHRH-1 several years ago. In the first set of samples obtained during the morning (0900–1100 h), kisspeptin-54 levels increased with the developmental increase in LHRH-1 release. In the second set of samples, we were able to assess kisspeptin-54 levels in samples collected both during the morning (0600-1200 h) and evening (1800–2400 h). To our surprise, kisspeptin-54 levels not only gradually increased during the progress of puberty in parallel with the pubertal increase in LHRH-1 release, but kisspeptin-54 levels during the evening were also higher than in the morning in animals at all developmental stages, i.e. prepubertal and early and midpubertal stages. Although additional studies are required, these observations are indicative for the role of kisspeptin in puberty.
Because we needed to pool archived samples for kisspeptin-54 assay, we were not able to assess the pattern of kisspeptin-54 release in the S-ME. Thus, we conducted experiment 2 with the microdialysis method. Kisspeptin-54 release in the S-ME in midpubertal monkeys was pulsatile with an interpulse interval of about 60 min, which is very similar to that of LHRH-1 release in ovariectomized adult female monkeys. Interestingly, kisspeptin-54 release increased shortly after the lights went off. At this point, it is unclear whether this phenomenon is unique to only midpubertal monkeys or all other developmental stages, i.e. neonatal, prepubertal (juvenile), early pubertal, and fully mature stages. It is further unclear whether the kisspeptin-54 response to the lights-off signal is a simple reflex of kisspeptin neurons to optic input or a part of entrained circadian rhythms of kisspeptin neurons. Nonetheless, these observations from experiment 2 along with the nocturnal increase in kisspeptin-54 release from experiment 1 suggest that the prominent nocturnal increase in LHRH-1 release in pubertal monkeys (20,35) might be attributable to the LHRH-1 neuronal system being directly coupled to the light-sensitive kisspeptin neuronal system.
A significant number of studies show that central or systemic administration of kisspeptin stimulates LH release in a dose-dependent manner in vivo in various species including in humans [1,12,13,16,36; see also review by Seminara (6)] and LHRH-1 release in vitro (37). In experiment 3, we observed that kisspeptin-10 stimulated LHRH-1 release in a dose-dependent manner in vivo. To our knowledge, this is the first report of direct evidence showing that kisspeptin-10 administered locally into the S-ME stimulates LHRH-1 release in animals in vivo. Based on the similar pulse frequency of kisspeptin-54 release and LHRH-1 release in experiment 2 and the ability of kisspeptin-10 to stimulate LHRH-1 release in a dose-dependent manner, we can hypothesize that pulsatile kisspeptin-54 release is associated with pulsatile LHRH-1 release. Indeed, our data shown in Fig. 2C suggest that approximately 75% of kisspeptin-54 peaks occurred at the same time as or 10 min preceding thr LHRH-1 peaks, although the correlation between kisspeptin-54 and LHRH-1 pulses was not as tight as we have seen between neuropeptide Y and LHRH-1 pulses (27).
The pubertal increase in LHRH-1 release is independent from the pubertal increase in the ovarian steroid estrogen, because the pubertal increase in LHRH-1 release is observed in ovariectomized females (35) and estrogen suppresses LHRH-1 release after, not before, the onset of puberty (38). However, the question of whether the increase in kisspeptin-54 release with female puberty observed in this study is due to the pubertal increase in circulating estrogen or developmental change in the hypothalamus remains to be investigated. For example, whereas the influence of gonadal steroids on kisspeptin gene expression has extensively been reported (4,5,6,7,8,39), the data by Shahab et al. (13) that the pubertal increase in KiSS-1 mRNA, but not GPR54 mRNA, expression was observed in gonadectomized male monkeys, indicating that the gonadal steroid-independent maturational change in the GPR54 signaling system occurs in male monkeys. However, in that study (13), the authors did not examine whether the developmental increase in KiSS-1 mRNA levels was independent from the pubertal increase in gonadal steroid, estrogen, in female monkeys. Because there are some sex differences in the mechanism of the onset of puberty in primates (30,40,41), it will be important to investigate the possible role of estrogen in the pubertal increase in kisspeptin-54 release in the future.
In our previous and present studies, we did not observe a nocturnal increase in LHRH-1 release in prepubertal monkeys (30), although a nocturnal increase in LH (and presumably LHRH-1) has been described in prepubertal children (41). Nonetheless, our observation that the nocturnal increase in kisspeptin-54 precedes the nocturnal increase in LHRH-1 release is consistent with the hypothesis that kisspeptin plays a role in the mechanism of the onset of puberty, and our findings lead to several important questions. How much of a role does kisspeptin/GPR54 signaling play in the timing of puberty? Does an increase in kisspeptin release trigger the pubertal increase in LHRH increase? Does a decrease in prepubertal γ-aminobutyric acid (GABA) inhibition precede the pubertal increase in kisspeptin release, or does the pubertal increase in kisspeptin release trigger GABA disinhibition before the onset of puberty? Does the pubertal increase in glutamate release coincide with kisspeptin increase? We need answers to these questions before establishing the critical role of kisspeptin in puberty, because previously we found that a decrease in GABAergic inhibition followed by an increase in glutamatergic stimulation is critical for the onset of puberty (30).
Acknowledgments
We express our sincere appreciation to our veterinarians, especially to Drs. Saverio Capuano and Kevin Brunner, and CPI staff, especially Karla Nephew for support of this project.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01HD15433 and R01HD11355 and was made possible to perform by NIH support (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: GABA, γ-Aminobutyric acid; GPR54, G-protein coupled receptor 54; RFRP, RFamide-related peptide; S-ME, stalk-median eminence.
References
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA 2005 Novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 90:1849–1855 [DOI] [PubMed] [Google Scholar]
- Colledge WH 2004 GPR54 and puberty. Trends Endocrinol Metab 15:448–453 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Kuohung W 2005 KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty. Endocrine 26:277–284 [DOI] [PubMed] [Google Scholar]
- Seminara SB 2005 Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol 26:131–138 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA 2007 Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511 [DOI] [PubMed] [Google Scholar]
- Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H 2007 Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 8:21–29 [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M 2001 Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC 2001 AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M 2001 The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM 2005 Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2005 Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146:156–163 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2005 Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 146:1689–1697 [DOI] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR 2005 Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL 2003 The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH 2007 Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Wei Le W, Hoffman GE, Seminara SB 2007 Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- Watanabe G, Terasawa E 1989 In vivo release of luteinizing hormone releasing hormone (LHRH) increases with puberty in the female rhesus monkey. Endocrinology 125:92–99 [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E 1988 Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- Terasawa E 1994 In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perifusion. In: Conn PM, Levine JE, eds. Methods in neurosciences San Diego: Academic Press; 184–202 [Google Scholar]
- Frost SI, Keen KL, Levine JE, Terasawa E 2008 Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neuorsci Methods 168:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M 2003 Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab 88:914–919 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ 2000 A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 275:661–667 [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- Woller MJ, McDonald JK, Reboussin DM, Terasawa E 1992 Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology 130:2333–2342 [DOI] [PubMed] [Google Scholar]
- Tannenbaum PL, Schultz-Darken NJ, Woller MJ, Abbott DH 2007 Gonadotrophin-releasing hormone (GnRH) release in marmosets. II. Pulsatile release of GnRH and pituitary gonadotrophin in adult females. J Neuroendocrinol 19:354–363 [DOI] [PubMed] [Google Scholar]
- Wildt L, Marshall G, Knobil E 1980 Experimental induction of puberty in the infantile rhesus monkey. Science 207:1373–1375 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- Han S, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone (GnRH) neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2004 Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Dipietro MJ 2006 Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- Chongthammakun S, Claypool LE, Terasawa E 1993 Ovariectomy increases in vivo LHRH release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol 5:41–50 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Chongthammakun S, Terasawa E 1993 Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF 2006 Puberty in nonhuman primates and humans. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. 3rd ed. San Diego: Elsevier; 2177–2230 [Google Scholar]
- Grumbach MM, Styne DM 1998 Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Williams RH, Foster DW, Kroenenberg H, Larsen PR, Zorab R, eds. Williams textbook of endocrinology. 9th ed. Philadelphia: WB Saunders; 1509–1625 [Google Scholar]