Abstract
Adipokines, cytokines mainly produced by adipocytes, are active participants in the regulation of inflammation. Administration of zymosan (ZY) was used to investigate the regulation and role of adipokines during peritonitis in mice. Injection of ZY led to a significant increase in leptin levels in both serum and peritoneal lavage fluid, whereas a differential trend in local vs. systemic levels was observed for both resistin and adiponectin. The role of leptin in ZY-induced peritonitis was investigated using leptin-deficient ob/ob mice, with and without reconstitution with exogenous leptin. Leptin deficiency was associated with delayed resolution of peritoneal inflammation induced by ZY, because ob/ob mice had a more pronounced cellular infiltrate in the peritoneum as well as higher and prolonged local and systemic levels of IL-6, TNFα, IL-10, and chemokine (C-X-C motif) ligand 2 compared with wild-type mice. Reconstitution with exogenous leptin exacerbated the inflammatory infiltrate and systemic IL-6 levels in ob/ob mice while inhibiting production of TNFα, IL-10, and chemokine (C-X-C motif) ligand 2. In contrast with the important role of leptin in regulating each aspect of ZY-induced peritonitis, adiponectin deficiency was associated only with a decreased inflammatory infiltrate, without affecting cytokine levels. These findings point to a complex role for adipokines in ZY-induced peritonitis and further emphasize the interplay between obesity and inflammation.
ADIPOSE TISSUE IS not simply an inert storage depot for lipids but is also important in the integration of endocrine, metabolic, and inflammatory signals (1,2,3). Adipokines, such as leptin, adiponectin (APN) and resistin are active modulators of inflammation (4,5,6,7,8,9). In particular, leptin is a cytokine-like hormone important in the regulation of energy-demanding physiological processes, such as hemopoiesis, angiogenesis, wound healing, and the immune and inflammatory response (1,10,11,12,13,14,15,16,17,18). During infection and inflammation, the increase in leptin production has strongly suggested that leptin is part of the cytokine cascade that modulates the immune response (19,20). Leptin protects T cells from apoptosis and modulates T-cell proliferation and activation, macrophage phagocytosis, and expression of adhesion molecules (21). However, both pro- and antiinflammatory effects have been described for leptin. In models of T-cell-mediated inflammation, such as experimental allergic encephalomyelitis, collagen-induced arthritis, and autoimmune hepatitis, leptin-deficient ob/ob mice are protected (22,23). In contrast, in models in which inflammation is independent of adaptive immunity, such as zymosan (ZY)-induced arthritis or administration of endotoxin [lipopolysaccharide (LPS)], leptin appears to exert antiinflammatory properties (12,24).
The role of APN in regulating inflammatory responses also appears to be tissue and context specific (25,26,27). The biology of APN has mostly been investigated in the context of insulin sensitivity and atherosclerosis (28,29,30). A strong epidemiological relationship between low circulating APN and diabetes, metabolic syndrome, and cardiovascular disease has been reported (1). In accordance, several antiinflammatory effects have been described for APN, including inhibition of TNFα production and activity, inhibition of nuclear factor-κB activation, and induction of antiinflammatory cytokines as well as down-regulation of adhesion molecules (17,31,32,33,34,35,36,37). Based on these observations and on the protective role of APN in cardiovascular disease, this adipokine is generally considered as an antiinflammatory molecule. However, several reports demonstrated that APN can have proinflammatory properties (38,39,40,41,42).
To better understand the regulation of adipokines and the role these proteins play in inflammation, we challenged mice with ZY, a cell wall product of the yeast Saccharomyces cerevisiae (43). ZY is a potent stimulator of innate immunity, activating macrophages mostly through Toll-like receptor 2 (TLR2), although other receptors are also involved (44,45,46,47,48,49,50). We first evaluated the regulation of adipokines during ZY-induced peritonitis. Second, to better understand the role played by leptin and APN in ZY-induced peritonitis, we investigated the effect of ZY-induced inflammation in leptin-deficient ob/ob mice as well as APN knockout (KO) mice.
Materials and Methods
Mice
Care of mice followed institutional guidelines under protocols approved by the University of Illinois at Chicago. All mice used in these experiments had a C57BL6 background. Adiponectin KO mice were generated as previously described (51). Mice heterozygous for APN were mated and 6- to 8-wk-old littermates used for each experiment. Mice were genotyped by PCR of tail DNA, and APN deficiency was confirmed by measuring serum APN using a specific ELISA (R&D Systems, Inc., Minneapolis, MN). Age-matched female leptin-deficient (C57BL/6Job/ob) mice, their lean littermates (+/?), and C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). A group of ob/ob mice received ip injections of recombinant murine leptin (R&D Systems) for 5 d (1 mg/kg) before ZY administration.
Induction and evaluation of peritonitis
Mice were injected ip with 100 mg/kg of a sterile suspension of ZY (Sigma Chemical Co., St. Louis, MO) in saline. The dose of ZY was chosen in accord with previously published data (52,53). Control mice received an ip injection of saline. All injections were performed between 0900 and 1100 h. Approximately 0.5 ml blood was collected from the retroorbital plexus under isoflurane anesthesia at different time points after ZY injection, and serum was prepared. Mice were euthanized by cervical dislocation immediately after bleeding, and peritoneal lavage fluid (PLF) was collected, as follows. Mice were instilled with 5 ml ice-cold sterile RPMI in the peritoneal cavity, followed by gentle manual massage and removal of 3 ml fluid. The PLF was centrifuged, supernatants were collected for cytokine measurement, and the cells were resuspended in 1 ml RPMI and counted on a hemocytometer.
Cytokine and adipokine measurement
Chemokine (C-X-C motif) ligand 2 (CXCL2), leptin, APN, and resistin levels were measured using ELISA kits from R&D Systems. TNFα, IL-6, and IL-10 levels were measured using ELISA kits from e-Bioscience (San Diego, CA). The distribution of APN multimeric forms was examined in serum and PLF after fractionation as previously described (20).
Statistical analysis
Data are expressed as mean ± sem. The statistical significance of differences between treatment and control groups was determined by factorial ANOVA. Statistical analyses were performed using the XLStat software (Addinsoft, Brooklyn, NY).
Results
Effect of ZY administration on adipokine levels
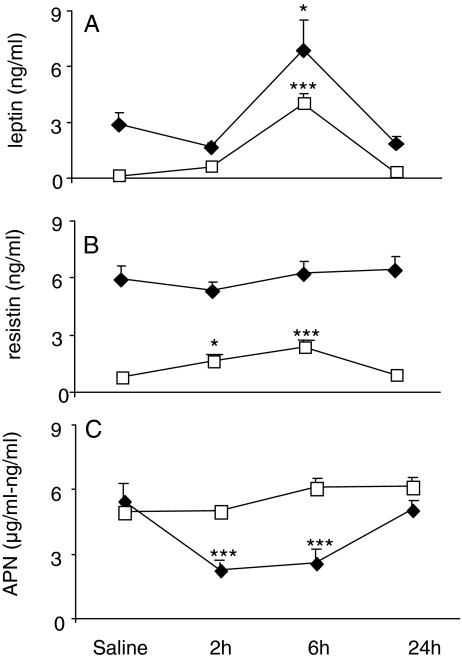
To investigate the effect of ZY-induced peritoneal inflammation on systemic and local adipokine levels, wild-type (WT) mice received an ip injection of ZY or vehicle. Basal levels of leptin, resistin, and APN were 20-, 7-, and 1000-fold higher in serum than in PLF, respectively (Fig. 1, A–C). Administration of ZY induced a significant increase in both circulating and PLF leptin at 6 h, with levels returning to baseline by 24 h (Fig. 1A). In contrast, a differential trend in local vs. systemic levels was observed for both resistin and APN. As shown in Fig. 1B, although serum resistin levels were not significantly altered at any time point after ZY administration, PLF resistin showed a 2-fold increase at 2 h followed by a 3-fold peak at 6 h, returning to baseline levels at 24 h. Finally, whereas a statistically significant reduction in serum APN levels was observed at 2 and 6 h after ZY (58 and 52% reduction, respectively), PLF APN levels did not change significantly after ZY injection (Fig. 1C). Fractionation of serum and PLF obtained from vehicle and ZY-injected mice demonstrated that the ratio of high to middle-low molecular weight (MW) APN was not significantly altered by ZY administration and did not differ between serum and PLF (data not shown).
Figure 1.
Leptin (A), resistin (B), and APN (C) levels in serum and PLF after ZY administration. WT mice were injected with ZY or vehicle. Serum (♦) and PLF (□) were collected at various times for measurement of leptin, resistin, and APN by ELISA. In C, serum APN levels are expressed as micrograms per milliliter, whereas PLF levels are expressed as nanograms per milliliter. Data are mean ± sem of four to five mice per group. *, P < 0.05; ***, P < 0.001 vs. respective vehicle.
Role of leptin in ZY-induced inflammation
To investigate whether leptin and/or obesity plays a role in modulating inflammation after administration of ZY, the response of ob/ob mice, with or without leptin replacement, was compared with that of WT animals. Leptin replacement in ob/ob mice led to serum leptin levels that were comparable to those observed in WT mice (serum leptin was 3.3 ± 1.1 vs. 2.1 ± 0.3 ng/ml in WT vs. leptin-injected ob/ob mice, respectively; data are mean ± sem; n = 3). However, this short course of leptin replacement did not induce a significant body weight loss in ob/ob mice (body weight was 47.4 ± 0.4 vs. 47.4 ± 0.7 g in ob/ob vs. leptin-replaced ob/ob mice, respectively; data are mean ± sem; n = 5). Thus, the group of leptin-replaced ob/ob mice was obese but had leptin levels comparable to those of WT mice. This group was used to differentiate between a direct effect of leptin vs. the effect of obesity on ZY-induced inflammation.
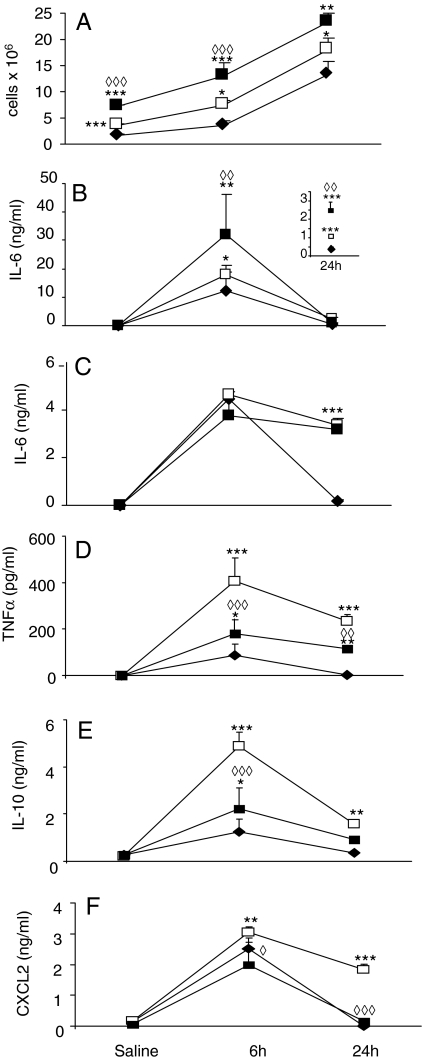
A significantly higher number of resident cells were present in the peritoneum of vehicle-injected ob/ob compared with WT mice; reconstitution with recombinant leptin led to a further increase in the number of peritoneal cells (Fig. 2A). Administration of ZY induced a significantly more pronounced infiltrate in ob/ob compared with WT mice at both 6 and 24 h, with a further increase observed in the group of ob/ob that had received exogenous leptin (Fig. 2A).
Figure 2.
Effect of ZY in ob/ob mice. WT (♦), ob/ob (□) and leptin-injected ob/ob (▪) mice were injected with ZY or vehicle. Cellular infiltrate (A) as well as serum IL-6 (B) and PLF IL-6 (C), TNFα (D), IL-10 (E), and CXCL2 (F) were evaluated at the indicated time points. The inset in B shows IL-6 levels at 24 h after ZY. Data are mean ± sem of four to five mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. WT. ◊◊, P < 0.01; ◊◊◊, P < 0.001 vs. ob/ob.
Administration of ZY induced significantly higher levels of IL-6 in both serum (Fig. 2B) and PLF (Fig. 2C) in ob/ob compared with WT mice. Although the systemic response was enhanced at both 6 and 24 h, PLF IL-6 levels were significantly higher in ob/ob compared with WT mice only at the later time point (Fig. 2, B and C). Administration of exogenous leptin to ob/ob mice further increased the systemic IL-6 response compared with non-leptin-injected ob/ob mice without significantly altering PLF IL-6 levels (Fig. 2, B and C). Thus, administration of leptin enhanced the systemic IL-6 response of ob/ob mice although not significantly altering local IL-6 levels.
In contrast with its potentiating effects on IL-6 production, leptin reconstitution of ob/ob mice reduced production of TNFα, IL-10, and CXCL2. In fact, a significantly enhanced and prolonged local response to ZY was observed for each of the three cytokines in ob/ob compared with WT mice, with leptin administration significantly dampening the heightened cytokine response of ob/ob mice (Fig. 2, D–F). A similar trend was observed when serum cytokine levels were measured (not shown).
Role of leptin in ZY-induced modulation of adipokine levels
The effect of leptin on regulation of APN and resistin levels after ZY administration was investigated using ob/ob and leptin-reconstituted ob/ob mice. Leptin administration to ob/ob mice normalized basal serum APN levels (Fig. 3A). Although administration of ZY significantly reduced circulating APN in WT mice, as already shown above in Fig. 1C, no significant change in serum APN was observed in ob/ob or leptin-replaced ob/ob mice receiving ZY. No significant changes in PLF APN levels in response to ZY were observed in any of the three groups (data not shown).
Figure 3.
APN (A) levels in serum and resistin (B) levels in PLF after ZY. WT (♦), ob/ob (□), and leptin-injected ob/ob (▪) mice were injected ip with ZY or vehicle. Circulating APN (A) as well as PLF resistin (B) were evaluated at the indicated time points. Data are mean ± sem of four to five mice per group. *, P < 0.05; ***, P < 0.001 vs. WT. ◊, P < 0.05 vs. ob/ob.
Although ZY did not significantly alter systemic resistin levels in any of the mice studied (data not shown), the kinetics of the PLF resistin response of ob/ob and leptin-replaced ob/ob mice was significantly prolonged compared with that of WT mice, which in contrast fully recovered by 24 h (Fig. 3B).
Role of APN in ZY-induced inflammation
To investigate a potential role for APN in ZY-induced inflammation, the response of WT and APN KO mice was compared. Although a significantly reduced inflammatory infiltrate was observed at 6 and 24 h after ZY in the peritoneal cavity of APN KO compared with WT mice (Fig. 4), serum and PLF cytokines, leptin, and resistin levels were not significantly different between WT and APN KO mice at any of the time points analyzed (data not shown).
Figure 4.
Reduced cellular infiltrate in APN KO mice. WT (♦) and APN-KO (⋄) mice were injected ip with ZY or vehicle. Cellular infiltrate was evaluated at the indicated time points. Data are mean ± sem of four to five mice per group. *, P < 0.05 vs. APN KO.
Discussion
The aim of the present study was to investigate the role of leptin and APN in regulating inflammation during ZY-induced peritonitis. We demonstrate that adipokines are differentially modulated after administration of ZY and that although leptin plays a major role in regulating the inflammatory response to ZY, APN deficiency has only a minor effect.
Although leptin and resistin levels were in the same order of magnitude in serum and PLF on untreated lean mice, APN′s concentration was approximately 1000-fold lower in PLF compared with serum. This major difference between serum and PLF APN levels is similar to that previously reported for other body fluids, such as bronchoalveolar lavage and cerebrospinal fluid (54,55). Mechanisms including active exclusion, increased degradation, and selective transport of low vs. high MW APN have been proposed to explain the low levels of APN present in cerebrospinal fluid (56,57). Whether these mechanisms are responsible for maintaining low levels of APN in PLF remains to be investigated, although our data obtained from fractionation studies indicate that the distribution of middle/low vs. high MW APN did not significantly differ between serum and PLF.
In agreement with previous data demonstrating that acute inflammation reduces circulating APN levels (58,59), we found a significant reduction in serum APN during ZY-induced peritonitis. Interestingly, although inflammation had a profound effect on systemic APN, no significant changes were observed in the amount of APN present in PLF after ZY injection. Lack of modulation at the local level was unique to APN, because each of the other adipokines and cytokines measured showed more pronounced changes locally than systemically in response to ZY. These results, together with data indicating a role for leptin and obesity in regulating APN levels during inflammation (see Fig. 3), point to a complex regulation of APN production and compartmentalization in the course of inflammatory responses.
The role and regulation of APN during inflammation is multifaceted and highly dependent upon the stimuli and tissues involved (60). During ZY-induced peritonitis, endogenous APN did not play a crucial role in modulating the inflammatory response. In fact, when compared with WT mice, APN KO mice had a minor reduction in cellular infiltrate, with no significant changes in terms of cytokine or adipokine levels. These data are in agreement with previous results obtained by our group using the models of inflammation induced by injection of LPS or concanavalin A and demonstrating negligible effects of APN deficiency in the regulation of cytokine production (61).
In contrast with the apparently minor role of APN in the inflammatory response to ZY, leptin significantly contributed to modulation of cellular infiltrate and cytokine production. An overall increase in the inflammatory response to ZY was observed in ob/ob compared with WT mice, including a more pronounced cellular infiltrate in the peritoneal cavity as well as higher local and systemic levels of each of the cytokines measured. Furthermore, whereas in WT mice cytokine levels returned to baseline values by 24 h after ZY, a more prolonged cytokine response, particularly at the local level, was observed in ob/ob mice, indicating failure to effectively resolve the inflammatory response, in agreement with previous data obtained using the model of ZY-induced arthritis (24). Delayed resolution of peritoneal inflammation induced by ZY was, however, not secondary to reduced IL-10 production, as previously suggested for the increased sensitivity of ob/ob mice to LPS (12), because levels of this antiinflammatory cytokine were highly elevated in ob/ob mice injected with ZY.
Whereas leptin deficiency was associated with generalized worsening of the inflammatory response, a selective effect of reconstitution with exogenous leptin was observed. Administration of leptin further increased the already elevated inflammatory infiltrate and systemic IL-6 levels in ob/ob mice. Because the schedule of leptin reconstitution used in these experiments did not induce significant weight loss, exacerbation of these parameters by leptin suggests that obesity, rather than leptin deficiency, is responsible for the differences between WT and ob/ob mice. In contrast, production of TNFα, IL-10, and CXCL2 was significantly reduced by leptin, indicating that lack of leptin is directly responsible for the increased production of these mediators in ob/ob mice.
Levels of resistin, which is mainly produced by adipocytes in mice and by macrophages in humans, are usually increased during active inflammation (62). Although ZY did not significantly alter systemic resistin levels in mice, local levels of this cytokine followed kinetics similar to that of the other cytokines measured. In particular, an overlapping pattern of local response was observed for resistin and IL-6, the kinetics of production of both being prolonged in ob/ob mice regardless of administration of exogenous leptin, suggesting similar mechanisms of regulation for these two cytokines.
In conclusion, our results further extend understanding of the complex interactions among adipokines, adipose tissue, and inflammation. We demonstrate that inflammation is an important modulator of adipokine levels in mice, while at the same time adipose tissue-derived products exert selective and potent effects on various parameters of the inflammatory response.
Footnotes
This work was supported by National Institutes of Health Grants DK061483 and DK068035 (to G.F.) and DK68037 and HL51586 (to L.C.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: APN, Adiponectin; CXCL2, chemokine (C-X-C motif) ligand 2; KO, knockout; MW, molecular weight; PLF, peritoneal lavage fluid; WT, wild type; ZY, zymosan.
References
- Fantuzzi G 2005 Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115:911–919; quiz 920 [DOI] [PubMed] [Google Scholar]
- Ronti T, Lupattelli G, Mannarino E 2006 The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 64:355–365 [DOI] [PubMed] [Google Scholar]
- Rajala MW, Scherer PE 2003 The adipocyte: at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144:3765–3773 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y 2006 Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med 3:35–42 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y 2006 The metabolic syndrome and adipocytokines. FEBS Lett 580:2917–2921 [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS 2004 Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92:347–355 [DOI] [PubMed] [Google Scholar]
- Schaffler A, Scholmerich J, Buchler C 2005 Mechanisms of disease: adipocytokines and visceral adipose tissue—emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol 2:103–111 [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B 2006 Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12 [PubMed] [Google Scholar]
- Sennello JA, Fayad R, Morris AM, Eckel RH, Asilmaz E, Montez J, Friedman JM, Dinarello CA, Fantuzzi G 2005 Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology 146:2157–2164 [DOI] [PubMed] [Google Scholar]
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS 2003 The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR 1998 Biological action of leptin as an angiogenic factor. Science 281:1683–1686 [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C 1999 Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol 276:R136–R142 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Waelput W, Guisez Y 1999 Leptin is an endogenous protective protein against the toxicity exerted by tumor necrosis factor. J Exp Med 189:207–212 [PMC free article] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM 1997 Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA 94:2557–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi R, Rifkin DB 1990 Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med 172:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cava A, Matarese G 2004 The weight of leptin in immunity. Nat Rev Immunol 4:371–379 [DOI] [PubMed] [Google Scholar]
- Sennello JA, Fayad R, Pini M, Gove ME, Fantuzzi G 2006 Transplantation of wild-type white adipose tissue normalizes metabolic, immune and inflammatory alterations in leptin-deficient ob/ob mice. Cytokine 36:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Leiter EH, La Cava A 2007 Leptin in autoimmunity: many questions, some answers. Tissue Antigens 70:87–95 [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C 2003 A role for leptin in rheumatic diseases? Ann Rheum Dis 62:913–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gomez-Reino JJ, Gualillo O 2005 Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579:295–301 [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R 2000 Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 68:437–446 [PubMed] [Google Scholar]
- Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G 2000 Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proc Natl Acad Sci USA 97:2367–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G 2002 Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol 32:552–560 [DOI] [PubMed] [Google Scholar]
- Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C 2004 Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther 6:R256–R263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G 2007 Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 132:601–614 [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y 2004 Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109:2046–2049 [DOI] [PubMed] [Google Scholar]
- Xydakis AM, Case CC, Jones PH, Hoogeveen RC, Liu MY, Smith EO, Nelson KW, Ballantyne CM 2004 Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab 89:2697–2703 [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P 2006 Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 110:267–278 [DOI] [PubMed] [Google Scholar]
- Koerner A, Kratzsch J, Kiess W 2005 Adipocytokines: leptin—the classical, resistin—the controversical, adiponectin—the promising, and more to come. Best Pract Res Clin Endocrinol Metab 19:525–546 [DOI] [PubMed] [Google Scholar]
- Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S 2005 Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 288:H2031–H2041 [DOI] [PubMed] [Google Scholar]
- Kim JY, Scherer PE 2004 Adiponectin, an adipocyte-derived hepatic insulin sensitizer regulation during development. Pediatr Endocrinol Rev 1(Suppl 3): 428–431 [PubMed] [Google Scholar]
- Lehrke M, Lazar MA 2004 Inflamed about obesity. Nat Med 10:126–127 [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Kukita T, Li YJ, Kamio N, Fukumoto S, Nonaka K, Ninomiya Y, Hanazawa S, Yamashita Y 2008 Adiponectin inhibits induction of TNF-α/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett 582:451–456 [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H 2004 Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323:630–635 [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y 1999 Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476 [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y 2000 Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102:1296–1301 [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN 2005 Adiponectin induces TNF-α and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun 335:1254–1263 [DOI] [PubMed] [Google Scholar]
- Haugen F, Drevon CA 2007 Activation of nuclear factor-κB by high molecular weight and globular adiponectin. Endocrinology 148:5478–5486 [DOI] [PubMed] [Google Scholar]
- Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, Richart C 2004 Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res 12:962–971 [DOI] [PubMed] [Google Scholar]
- Park PH, McMullen MR, Huang H, Thakur V, Nagy LE 2007 Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-α (TNF-α) expression via ERK1/2 activation and Egr-1 expression: role of TNF-α in adiponectin-stimulated interleukin-10 production. J Biol Chem 282:21695–21703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin BH, Song H 2006 Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol 120:99–105 [DOI] [PubMed] [Google Scholar]
- Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U 2006 The potential of adiponectin in driving arthritis. J Immunol 176:4468–4478 [DOI] [PubMed] [Google Scholar]
- DiCarlo FJ, Fiore JV 1958 On the composition of zymosan. Science 127:756–757 [DOI] [PubMed] [Google Scholar]
- Sanguedolce MV, Capo C, Bongrand P, Mege JL 1992 Zymosan-stimulated tumor necrosis factor-α production by human monocytes. Down-modulation by phorbol ester. J Immunol 148:2229–2236 [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A 1999 The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811–815 [DOI] [PubMed] [Google Scholar]
- Young SH, Ye J, Frazer DG, Shi X, Castranova V 2001 Molecular mechanism of tumor necrosis factor-α production in 1→3-β-glucan (zymosan)-activated macrophages. J Biol Chem 276:20781–20787 [DOI] [PubMed] [Google Scholar]
- Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y 2003 Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J Immunol 171:417–425 [DOI] [PubMed] [Google Scholar]
- Daum T, Rohrbach MS 1992 Zymosan induces selective release of arachidonic acid from rabbit alveolar macrophages via stimulation of a β-glucan receptor. FEBS Lett 309:119–122 [DOI] [PubMed] [Google Scholar]
- Noble PW, Henson PM, Lucas C, Mora-Worms M, Carre PC, Riches DW 1993 Transforming growth factor-β primes macrophages to express inflammatory gene products in response to particulate stimuli by an autocrine/paracrine mechanism. J Immunol 151:979–989 [PubMed] [Google Scholar]
- Okazaki M, Chiba N, Adachi Y, Ohno N, Yadomae T 1996 Signal transduction pathway on β-glucans-triggered hydrogen peroxide production by murine peritoneal macrophages in vitro. Biol Pharm Bull 19:18–23 [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L 2002 Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277:34658–34661 [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Sacco S, Ghezzi P, Dinarello CA 1997 Physiological and cytokine responses in IL-1β-deficient mice after zymosan-induced inflammation. Am J Physiol 273:R400–R406 [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, Perretti M 1998 Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol 63:108–116 [DOI] [PubMed] [Google Scholar]
- Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S 2007 Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab 92:1129–1136 [DOI] [PubMed] [Google Scholar]
- Holguin F, Rojas M, Hart CM 2007 The peroxisome proliferator activated receptor γ (PPARγ) ligand rosiglitazone modulates bronchoalveolar lavage levels of leptin, adiponectin, and inflammatory cytokines in lean and obese mice. Lung 185:367–372 [DOI] [PubMed] [Google Scholar]
- Ebinuma H, Miida T, Yamauchi T, Hada Y, Hara K, Kubota N, Kadowaki T 2007 Improved ELISA for selective measurement of adiponectin multimers and identification of adiponectin in human cerebrospinal fluid. Clin Chem 53:1541–1544 [DOI] [PubMed] [Google Scholar]
- Neumeier M, Weigert J, Buettner R, Wanninger J, Schaffler A, Muller AM, Killian S, Sauerbruch S, Schlachetzki F, Steinbrecher A, Aslanidis C, Scholmerich J, Buechler C 2007 Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab 293:E965–E969 [DOI] [PubMed] [Google Scholar]
- Morris AM, Sennello JA, Fayad RA, Eckel RH, Dinarello CA, Fantuzzi G 2006 T cell-mediated hepatic inflammation modulates adiponectin levels in mice: role of tumor necrosis factor α. Metabolism 55:555–559 [DOI] [PubMed] [Google Scholar]
- Tsuchihashi H, Yamamoto H, Maeda K, Ugi S, Mori T, Schimizu T, Endo Y, Hanasawa K, Tani T 2006 Circulating concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein, decrease in rats with polymicrobial sepsis. J Surg Res 134:348–353 [DOI] [PubMed] [Google Scholar]
- Fantuzzi G 2008 Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 121:326–330 [DOI] [PubMed] [Google Scholar]
- Pini M, Sennello JA, Chan L, Fantuzzi G 2006 Adiponectin deficiency does not affect the inflammatory response to endotoxin or concanavalin A in mice. Endocrinology 147:5019–5022 [DOI] [PubMed] [Google Scholar]
- Lazar MA 2007 Resistin- and obesity-associated metabolic diseases. Horm Metab Res 39:710–716 [DOI] [PubMed] [Google Scholar]