Abstract
Hepcidin plays an essential role in maintaining normal iron homeostasis outside the brain. This recently discovered iron regulation hormone is predominantly expressed in the liver, and regulated by iron and hypoxia. As an antimicrobial peptide, this hormone is also elevated during infections and inflammation. In this study we investigated the expression of hepcidin mRNA and protein in different brain regions, including the cortex, hippocampus, striatum, and substantia nigra, and the effects of lipopolysaccharide (LPS) on the expression of hepcidin using quantitative real-time RT-PCR and immunofluorescence analysis. Our data provided further evidence for the existence of hepcidin in all the regions we examined. We also demonstrated for the first time that LPS administration by iv injection can regulate the expression of hepcidin mRNA and protein not only in peripheral organs such as the liver, but also in the brain. LPS induced a significant increase in the expression of hepcidin mRNA and protein in the cortex and substantia nigra, but not in the hippocampus and striatum, indicating a regionally specific regulation of LPS on hepcidin in the brain. The relevant mechanisms and the functions of hepcidin in the brain remain to be elucidated.
IRON SERVES AS an essential trace element for many aspects of the physiological activities of mammalian cells, including those in the brain (1). Both iron deficiency and iron excess can lead to cellular dysfunctions, therefore, maintaining normal iron homeostasis is crucial (2,3). Accumulated data have shown that hepcidin, a recently discovered iron regulation hormone, plays an essential role in maintaining normal iron homeostasis outside the central nervous system (CNS) (4,5). This hormone controls plasma iron concentration and tissue iron distribution by inhibiting intestinal iron absorption, iron recycling by macrophages, and iron mobilization from hepatic stores (4,5,6).
This peptide is mainly synthesized by the liver, distributed in extracellular fluid and excreted in urine (7,8,9). Mice that fail to express hepcidin have elevated body iron stores, presumably due to hyperabsorption associated with decreased iron in tissue macrophages (10,11,12). The synthesis of hepcidin is homeostatically increased by iron loading (9,13) and decreased by anemia and hypoxia (5,6). As an antimicrobial peptide, hepcidin is also elevated during infections and inflammation (6), causing a decrease in serum iron levels and contributing to the development of anemia of inflammation, probably as a host defense mechanism to limit the availability of iron to invading microorganisms (14). In agreement with the potential role for hepcidin in the host defense, hepcidin mRNA was increased in the liver of the lipopolysaccharide (LPS)-treated mice and in the LPS-treated hepatocytes (9). In vitro stimulation of fresh human hepatocytes with a panel of cytokines showed a strong induction of hepcidin mRNA by IL-6 (15), indicating that IL-6 might be a mediator of hepcidin induction by inflammation.
Although hepcidin is predominantly expressed in the liver, it is also detectable in the brain (9). The widespread distribution of hepcidin in the CNS (16,17) implies that hepcidin might also have a key role in iron homeostasis and might function as an antimicrobial peptide in the brain. Currently, the function and functional mechanisms of hepcidin, the mechanisms of its regulation, and the linkage between hepcidin and other iron transport proteins in the CNS are unknown. Thus, in this study we investigated the effects of LPS treatment on the expression of hepcidin in different brain regions, including the cortex, hippocampus, striatum, and substantia nigra, to find out whether the expression of hepcidin in the brain corresponds to the peripheral administration of LPS. Our data provided further evidence for the existence of hepcidin in all the regions we examined and demonstrated for the first time that the administration of LPS by iv injection can regulate the expression of hepcidin mRNA not only in peripheral organs such as the liver, but also in the brain.
Materials and Methods
Materials
Unless otherwise stated, all chemicals, including LPS, were obtained from Sigma Chemical Co. (St. Louis, MO). Agarose and Tris were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA). Advantage RT-for-PCR kit was from Promega Corp. (Madison, WI). The RNeasy Mini kit and QIAEX II gel extraction kit were from QIAGEN, Inc. (Valencia, CA). The commercial kit to determine serum iron and total iron binding capacity (TIBC) was purchased from Stanbio Laboratory (Boerne, TX) and the SmartCycler from Cepheid Co. (Sunnyvale, CA). Male Sprague Dawley rats weighing 300–350 g (∼8 wk old) were supplied by the Centralized Animal Facilities of The Hong Kong Polytechnic University and treated according to regulatory issues to laboratory animals for biomedical research. The Health Department of Hong Kong Government and Animal Ethics Committee of The Hong Kong Polytechnic University approved the use of animals for this study. All animals were housed in pairs in stainless steel cages at 21 ± 2 C, and were provided free access to food (catalog no. 5001, the Laboratory Rodent Diet; PMI Nutrition International Inc., Brentwood, MO) and distilled water at all times. Rooms were in a cycle of 12-h light (0700–1900 h) and 12-h darkness (from 1900–0700 h).
Experimental design and sampling of blood and tissues
To conduct time-course analysis of the expression of hepcidin mRNA in the liver and different brain regions in rats injected with LPS, male Sprague Dawley rats were iv injected with sterile saline (the control) or LPS at a dose of 1 μg/g body weight. The animals were anesthetized with 1% pentobarbital sodium (40 mg/kg body weight, ip) and decapitated 0, 2, 4, 6, 12, 24, or 36 h after the administration of LPS. Blood samples were then collected into heparinized syringes, and aliquots were taken immediately for the determination of hemoglobin (Hb) concentration and hematocrit (Hct). The remaining blood was cooled to 4 C and centrifuged at 3000 × g for 10 min at 4 C, and the serum samples were analyzed for serum iron, TIBC, and transferrin (Tf) saturation (serum iron/TIBC). After perfusion with ice-cold PBS [Milli-Q water (Millipore Corp., Billerica, MA) prepared and diethylpyrocarbonate treated (pH 7.4)] through the left ventricle, the brain was rapidly removed and immediately dissected into the four regions of cortex, hippocampus, striatum, and substantia nigra, according to Ke (18) and Chang (19) et al., and used immediately for total RNA extraction. The liver was also rapidly removed, and aliquots were rinsed with cold saline to remove blood and used immediately for total RNA extraction. The remaining tissues were wrapped with aluminum and immediately frozen below −70 C for storage after treatment with fluid nitrogen.
Hematological measurements
The Hb concentration was determined by the cyanmethemoglobin method, and Hct was measured using a micro-Hct centrifuge. Serum iron and TIBC were determined using a commercial kit (Stanbio Laboratory), and the Tf saturation (serum iron/TIBC) was calculated as described previously (20). Statistical analysis was performed using one-way ANOVA (SPSS, Inc., Chicago, IL), followed by Dunnett’s multiple comparison test.
Real-time PCR
Total RNA was isolated from the liver or different brain regions using TRIzol Reagent for real-time RT-PCR (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s instructions. Any genomic DNA potentially present in the RNA processing was removed by incubating the RNA with ribonuclease-free deoxyribonuclease I. The relative purity of the isolated RNA was assessed spectrophotometrically, and the ratio of A260–A280 nm exceeded 1.8 for all preparations, or 28S RNA bands equal twice the amounts of the 18S RNA. Total RNA (1 μg) was reversely transcribed in a 20-μl reaction using the RT-for-PCR kit with oligo deoxythymidine primers according to the manufacturer’s instructions. The primers for real-time PCR of hepcidin were based on the cDNA sequence reported by Pigeon et al. (9). The forward primer sequence is 5′-acagaaggcaagatggcact-3′ (nucleotides 13–32); the reverse primer sequence is 5′-gaagttggtgtctcgcttcc-3′ (nucleotides 194–213). Amplification was performed using the SmartCycler with initial denaturation at 95 C for 5 min, followed by 40 cycles at 95 C (15 sec), 59 C (15 sec), and 72 C (30 sec). The β-actin cDNA (5′-primer, 5′-gtcgtaccactggcattgtg-3′; 3′-primer, 5′-ctctcagctgtggtggtgaa-3′) was amplified simultaneously as the internal control. The gene relative expression levels were calculated using method arithmetic formulas. The control (nontreated) sample was used as a calibrator. The amount of target, normalized to an endogenous housekeeping gene (β-actin) and relative to the calibrator, is then given by ΔΔCT, where ΔΔCT = ΔCT (sample) − ΔCT (calibrator), and ΔCT is the CT of the target gene subtracted from the CT of the housekeeping gene. For the untreated control sample, ΔΔCT equals zero, and 20 equals one, so that the fold change in gene expression relative to the untreated control equals one, by definition. For the treated samples, evaluation of ΔΔCT indicates the fold change in gene expression relative to the untreated control. Statistical analysis was performed using one-way ANOVA (SPSS), followed by Dunnett’s multiple comparison test.
Immunofluorescence analysis
The male Sprague Dawley rats were iv injected with sterile saline (the control, n = 1) or LPS (n = 2) at a dose of 1 μg/g body weight. At 6 h after the administration, the rats were deeply anesthetized with ether and transcardially perfused with 0.1 m PBS, followed by 4% paraformaldehyde in 0.1 m PBS. Brains were removed and postfixed in 4% paraformaldehyde for 24 h, then transferred into 30% sucrose in 0.1 m PBS, and stored at 4 C overnight. Cryosections (15 μm) were made using the Leica CM3050S cryostat (Leica Microsystems, Wetzlar, Germany). After mounting on Superfrost slides (Langenbrick, Bauhaus, Germany), sections were blocked with 3% normal goat serum (diluted in PBS containing 0.3% Triton X-100) for 1 h. Sections were immunostained for hepcidin by incubating with an affinity purified antibody raised against murine hepcidin protein (Alpha Diagnostics International Inc., San Antonio, TX) for 24 h at 4 C. The antibody was diluted in PBS (1:100) containing 1% goat serum and 0.1% Triton X-100. After washing three times with PBS plus 1% normal goat serum and 0.1% Triton X-100, sections were incubated with Rhodamine-labeled goat antirabbit IgG (1:2000) (Invitrogen) as the secondary antibody for 30 min. After further washing in PBS, sections were coverslipped in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) for fluorescence. In a set of control (negative) experiments, the primary antibody was omitted. Fluorescent images were captured using a Nikon Eclipse TE2000-U microscope (Nikon UK Ltd., Kingston upon Thames, Surrey, UK) equipped with T-FL Epi-FI attachment and a SPOT cooled CCD camera (Diagnostic Instruments, Sterling Heights, MI). SPOT software version 4.6 software was used for image acquisition (Diagnostic Instruments). The intensities of fluorescence were calculated by using ImageJ software (from National Institutes of Health and available at http://rsb.info.nih.gov/ij/). Statistical analysis was performed using a Student’s t test.
Results
Time-course analysis of hematological parameters in rats injected with LPS
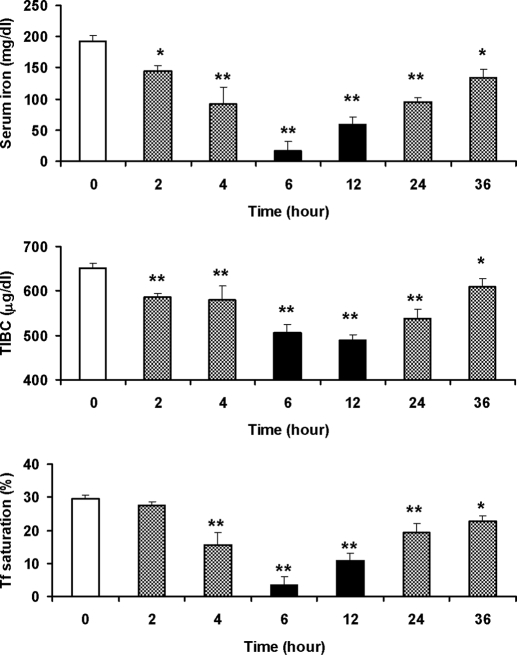
The administration of LPS induced a significant decrease in serum iron, TIBC, and Tf saturation in the rats (Fig. 1). The serum iron decreased to 75.29 and 47.56% of the control level 2 and 4 h after LPS administration, respectively. Six hours after LPS administration, which was 2 h after the maximal level of liver hepcidin had been reached (Fig. 2), serum iron decreased to its nadir value, 16.94 ± 14.20 μg/dl, which was significantly lower than that (192.83 ± 8.78 μg/dl) of the control (P < 0.001 vs. the control). At this time point, Tf saturation also decreased to the lowest value (P < 0.001 vs. the control). Both serum iron and Tf saturation began to increase 6 h after the LPS injection, but they were still lower than the control values 36 h after the LPS injection. TIBC decreased progressively with time of LPS injection and reached the lowest 12 h after the LPS treatment. After this time point, TIBC gradually increased, but it still did not reach the control level 36 h after the LPS injection. To determine whether the hypoferremic effect of LPS would result in anemia, Hb concentration and Hct were determined. No significant differences were found between the control and measurements of Hb and Hct at the different time points after the LPS administration.
Figure 1.
Time-course analysis of hematological parameters in rats injected with LPS. The male Sprague Dawley rats (n = 4 rats per group) were iv injected with LPS at a dose of 1 μg/g body weight. Zero (the control), 2, 4, 6, 12, 24, or 36 h after the administration of LPS, blood samples were collected and centrifuged, serum iron (top panel) and TIBC (middle panel) were determined, and the Tf saturation (serum iron/TIBC; bottom panel) was calculated as described in Materials and Methods. The data are presented as the mean ± sem of four independent measurements from four rats. *, P < 0.005; **, P < 0.001 (compared with the control, by ANOVA, followed by Dunnett’s multiple comparison test). Serum iron: F6,21 = 57.299; P < 0.001. TIBC: F6,21 = 38.658; P < 0.001). Tf saturation: F6,21 = 65.033; P < 0.001.
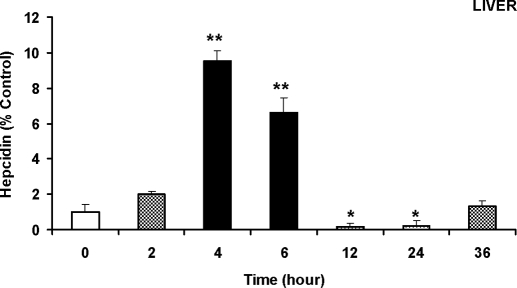
Figure 2.
A significant increase in the expression of hepcidin mRNA in the liver induced by LPS administration. The male Sprague Dawley rats (n = 3 rats per group) were iv injected with LPS at a dose of 1 μg/g body weight. The animals were anesthetized and decapitated 0 (the control), 2, 4, 6, 12, 24, or 36 h after the administration of LPS. After perfusion with ice-cold PBS through the left ventricle, the liver was rapidly removed, aliquots were rinsed with cold saline to remove blood, and total RNA extraction was immediately conducted for quantitative real-time RT-PCR analysis of hepcidin mRNA expression, as described in Materials and Methods. The data are presented as the mean ± sem [percentage (%) of the control] of six independent experiments from three rats. *, P < 0.005; **, P < 0.001 (compared with the control, by ANOVA, followed by Dunnett’s multiple comparison test). F6,14 = 5.672; P < 0.005 (= 0.004).
A significant increase in expression of hepcidin mRNA in the liver induced by LPS administration
LPS injection induced a rapid increase in hepatic hepcidin mRNA expression. By 2 h after the LPS administration, expression of hepcidin mRNA increased to about twice of the control. The increase continued with a peak of about 10 times (9.54 ± 0.54) of the control 4 h after the administration and then the expression subsided (Fig. 2). From 12–24 h after the LPS administration, the hepcidin mRNA even decreased to a level lower than the control. An expansion of the study to 36 h showed that there was a second increase of hepcidin mRNA expression, but it was lower than the one 4 h after the LPS injection.
LPS induces a significant increase of hepcidin mRNA expression in the cortex and substantia nigra
The major objective of this study was to investigate the effects of LPS on the expression of hepcidin mRNA in different brain regions of rats, including the cortex, hippocampus, striatum, and substantia nigra, and to find out whether hepcidin corresponded to LPS treatment like the peripheral organs such as the liver. Our data provided further evidence for the existence of hepcidin in all the regions we examined and demonstrated for the first time that the administration of LPS by iv injection can regulate the expression of hepcidin mRNA not only in the peripheral organs such as the liver, but also in the brain.
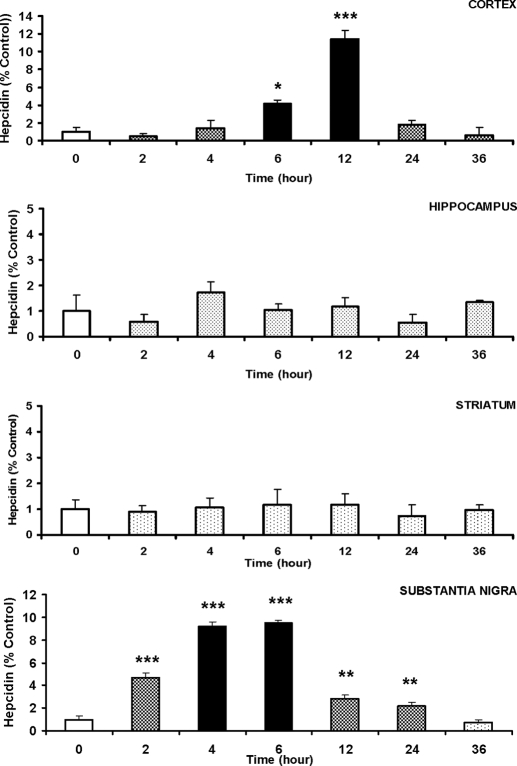
Real-time RT-PCR analysis showed that hepcidin mRNA in the cortex began to increase 6 h after the LPS treatment and reached the highest level, which was about 11.45 times of the control group (P < 0.001) (Fig. 3), 12 h after the LPS treatment (4 h after the maximal level of liver hepcidin was reached). The expression of hepcidin mRNA then subsided. By 36 h after the LPS injection, hepcidin mRNA decreased to lower than the control (0.59 times; P > 0.05). A very similar response of the expression of hepcidin mRNA to LPS treatment was also found in the substantia nigra (Fig. 3). However, a significant increase in the expression of hepcidin was found 2 h after the administration of LPS in the substantia nigra. This was about 4 h earlier than the significant increase in hepcidin expression found in the cortex 6 h after the administration. By 2 h after the LPS treatment, hepcidin expression in the substantia nigra increased to about 4.64 times of the control (P < 0.001). The increase continued with a peak expression 6 h after the treatment (9.51 times of the control). The expression then decreased progressively and decreased to the minimal level (0.69 times of control) 36 h after the treatment. Unlike in the cortex and substantia nigra, peripheral administration of LPS did not induce any significant changes in the expression of hepcidin mRNA in the hippocampus and striatum. There were no significant differences in the expression of hepcidin mRNA between the control values and all measurements at different time points after the LPS administration in the hippocampus and striatum (Fig. 3).
Figure 3.
Quantitive real-time RT-PCR analysis of hepcidin mRNA expression in the cortex, hippocampus, striatum, and substantia nigra of rat brains. The male Sprague Dawley rats (n = 3 rats per group) were iv injected with LPS at a dose of 1 μg/g body weight. The animals were anesthetized and decapitated 0 (the control), 2, 4, 6, 12, 24, or 36 h after the administration of LPS. After perfusion with ice-cold PBS through the left ventricle, the brain was rapidly removed and immediately dissected into four regions, cortex, hippocampus, striatum, and substantia nigra, and total RNA extraction was immediately conducted for quantitative real-time RT-PCR analysis of hepcidin mRNA expression, as described in Materials and Methods. The data are presented as the mean ± sem [percentage (%) of the control] of six independent experiments from three rats. *, P < 0.05; **, P < 0.005; ***, P < 0.001 (compared with the control, by ANOVA, followed by Dunnett’s multiple comparison test). Cortex: F6,14 = 16.534; P < 0.001. Hippocampus: F6,14 = 5.672; P = 0.04. Striatum: F6,14 = 0.334; P = 0.908. Substantia nigra: F6,14 = 60.398; P < 0.001.
LPS induces a significant increase in the expression of hepcidin protein in the cortex and substantia nigra
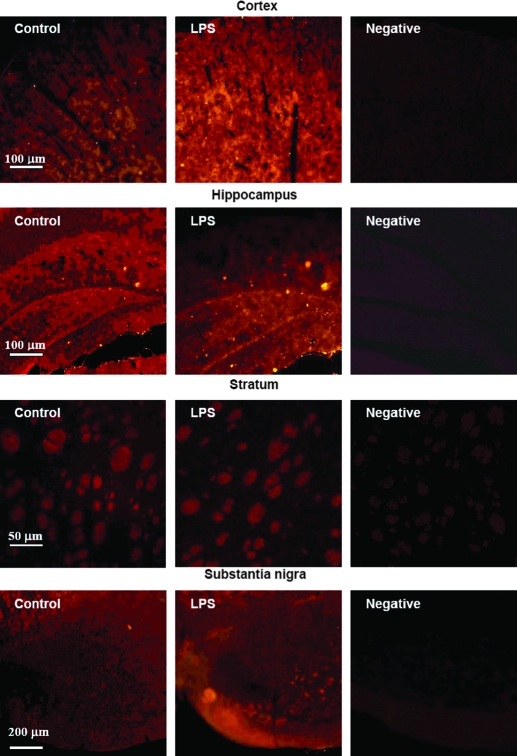
To confirm the regionally specific effects of LPS on hepcidin in the brain, the effects of LPS on the expression of hepcidin protein in the different brain regions were investigated using immunofluorescence analysis. The expression of hepcidin mRNA in the cortex and substantia nigra was the highest 6–12 and 4–6 h after the administration of LPS, respectively. Therefore, the adult Sprague Dawley rats were pretreated with or without LPS for 6 h, and immunofluorescence analysis was then conducted as described in Materials and Methods. Figure 4A presents representative immunofluorescence photomicrographs of hepcidin expression in different brain regions. Quantitative analysis of fluorescence density of hepcidin expression (Fig. 4B) demonstrated that LPS treatment for 6 h induces a significant increase in the expression of hepcidin protein in the cortex (P < 0.005 vs. the control) and substantia nigra (P < 0.001 vs. the control), but not in the hippocampus or striatum. There were no significant differences in the expression of hepcidin protein of the hippocampus (P = 0.107) or striatum (P = 0.078) between the rats treated with and without LPS for 6 h. The findings show that the response of hepcidin protein expression to LPS treatment is very similar to that of hepcidin mRNA expression.
Figure 4.
Immunofluorescence analysis of the expression of hepcidin protein in the cortex, hippocampus, striatum, and substantia nigra of rat brains. The male Sprague Dawley rats were iv injected with sterile saline (the control, n = 1 rat) or LPS at a dose of 1 μg/g body weight (n = 2 rats). The animals were anesthetized and decapitated 6 h after the administration of LPS. After perfusion with ice-cold PBS through the left ventricle, the brain was rapidly removed and immediately dissected into four regions: cortex, hippocampus, striatum, and substantia nigra. Immunofluorescence analysis was immediately conducted as described in Materials and Methods. A, Representative immunofluorescence photomicrographs of hepcidin expression in different brain regions. B, Quantitative analysis of fluorescence density of hepcidin expression in different brain regions. The data are presented as the mean ± sem [percentage (%) of the control] of eight cryosections from one (the control group) or two rats (LPS group). *, P < 0.005; **, P < 0.001 (compared with the control by a Student’s t test). No significant differences in the expression of hepcidin protein of the hippocampus (P = 0.107) or striatum (P = 0.078) were found between the rats treated with and without LPS.
Discussion
Hepcidin is an iron regulation hormone as well as an antimicrobial peptide (8,9,10,11,12). It plays an essential role in iron homeostasis and a potential role in the host defense outside the brain (5,6). In peripheral organs such as the liver and heart, the expression of hepcidin is regulated by iron, hypoxia, and infection inflammatory factors (5,21). However, very little is known about the mechanisms involved in the regulation of hepcidin expression in the brain, although the presence of this peptide in the brain has already been demonstrated (9,16,17). Thus, in this study we investigated the effects of bacterial LPS on the expression of hepcidin in the different brain regions, including the cortex, hippocampus, striatum, and substantia nigra. To our knowledge, this is the first attempt to determine the relationship between inflammation and hepcidin in the brain. Quantitative real-time RT-PCR and immunofluorescence analysis clearly revealed that hepcidin is not only expressed in rat liver but also in rat brain. There is the expression of hepcidin mRNA and protein in all the brain regions we examined. Our data also demonstrated for the first time that iv LPS injection can regulate the expression of hepcidin mRNA and protein not only in peripheral organs such as the liver, but also in the brain in the cortex and substantia nigra.
It is unknown what the mediator is that induces the observed up-regulation of hepcidin in the cortex and substantia nigra in response to the administration of LPS in the periphery. The brain has a specialized barrier system that excludes plasma proteins, metals, and polar substances from gaining free access to the brain interior. LPS is a macromolecule that is unable to cross the blood-brain barrier (BBB) to regulate the hepcidin expression in these two brain regions directly. Based on available information, we speculate that IL-6 might be one of the key mediators in the up-regulation of hepcidin in the brain found in this study. During inflammation induced by sc injections of turpentine, normal mice show a marked decrease in serum iron (12,22). This response is completely ablated in the hepcidin-deficient mice as well as in the IL-6-deficient mice. In humans the increase in hepcidin elicited by IL-6 infusion is accompanied by a decrease in serum iron (22). It appears that the IL-6-hepcidin axis is critically important for this response and that the IL-6 mediated up-regulation of hepcidin is the main reason for the hypoferremia of inflammation. In addition, recent studies have demonstrated that infection in the periphery can stimulate the immune cells to produce inflammatory cytokines, including IL-6. These cytokines can activate the neural and humoral communication pathways that induce glial cells in discrete brain regions to produce the same inflammatory cytokines (23,24). Within the brain, cytokines provide a sensory representation of the conditions in the periphery and induce neurochemical changes that elicit an adaptive behavioral response (25,26). This may explain why administration of LPS in the periphery could regulate the expression of hepcidin mRNA and protein in the two brain regions. This hypothesis is worth further investigation.
Although a significant increase in hepcidin expression was found in the cortex and substantia nigra, our data also showed that LPS did not induce any changes in the expression of hepcidin in the hippocampus and striatum. Currently, it is unknown what leads to this regionally specific regulation of LPS on hepcidin in the brain. The relevant mechanisms remain to be elucidated. In addition, the function and functional mechanisms of hepcidin and the linkage between hepcidin and iron metabolism, as well as the host defense in the brain, are not clear. However, the presence and wide distribution of hepcidin in the brain and the role of hepcidin in the peripheral organs (11,12) imply that this peptide might also have a key role in iron homeostasis and function as an antimicrobial peptide in the brain. Hepcidin might play a central role to control the amount of iron entering into the brain by regulating the expression of iron transport proteins, including Tf receptor, divalent metal transporter 1, and ferroportin 1, in the BBB as this peptide does in the intestine. It is also possible that this iron hormone operates as a regulator to keep iron balance in brain cells, including neuron and glial cells, by controlling the expression of these iron uptake and release proteins. The existence of these iron transport proteins in the BBB and neuronal cells has been well documented (18,27,28,29). In addition, the observed up-regulation of hepcidin in the brain might be particularly valuable in the earliest phases of infection, before other components of the innate and adaptive immunity are fully mobilized (15).
In summary, we have demonstrated that the iron regulatory hormone hepcidin is expressed in all the brain regions we examined. Its expression, not only in the liver but also the brain, is regulated in response to inflammation induced by LPS injection. Up-regulation of hepcidin in the liver and the brain in response to LPS injection may be an important component in local and systemic responses against infection. Further studies are needed to define the molecular mechanisms by which LPS or inflammation induces up-expression of hepcidin in the brain, and the effects of the up-regulation of hepcidin on the expression of iron metabolism proteins and iron distribution in different brain regions.
Footnotes
The studies in our laboratories were supported by the Competitive Earmarked Grants of The Hong Kong Research Grants Council (CUHK466907-KY), Direct Grant of The Chinese University of Hong Kong Faculty of Medicine (A/C: 4450226-KY), Jiangsu Natural Science Foundation (05-BK2005430), research grants from The Hong Kong Polytechnic University (Niche Area-I-BB8L, GU-384, and G-YG11), and National Key Laboratory of Chinese Medicine and Molecular Pharmacology (Shenzhen), and Shenzhen-Hong Kong Innovation Circle Program.
Disclosure Statement: The authors have nothing to declare.
First Published Online May 1, 2008
Abbreviations: BBB, Blood-brain barrier; CNS, central nervous system; Hb, hemoglobin; Hct, hematocrit; LPS, lipopolysaccharide; Tf, transferrin; TIBC, total iron binding capacity.
References
- Qian ZM, Wang Q 1998 Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Res Rev 27:257–267 [DOI] [PubMed] [Google Scholar]
- Andrews NC 1999 Disorders of iron metabolism. N Engl J Med 341:1986–1995 [DOI] [PubMed] [Google Scholar]
- Fleming RE, Sly WS 2002 Mechanisms of iron accumulation in hereditary hemochromatosis. Annu Rev Physiol 64:663–680 [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthale MU, Andrews NC 2004 Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T 2006 Regulation of iron metabolism by hepcidin. Annu Rev Nutr 26:323–342 [DOI] [PubMed] [Google Scholar]
- Ganz T 2006 Hepcidin–a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol 306:183–198 [DOI] [PubMed] [Google Scholar]
- Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K 2000 LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480:147–150 [DOI] [PubMed] [Google Scholar]
- Park CH, Valore EV, Waring AJ, Ganz T 2001 Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810 [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O 2001 A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276:7811–7819 [DOI] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S 2001 Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98:8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S 2002 Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 99:4596–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S 2002 The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler M, Roy CN, Custodio AO, Minana B, deGraaf J, Montross LK, Andrews NC, Hentze MW 2003 Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet 34:102–107 [DOI] [PubMed] [Google Scholar]
- Andrews NC 2004 Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest 113:1251–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo, Schiller G, Lichtenstein A, Ganz T 2003 Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101:2461–2463 [DOI] [PubMed] [Google Scholar]
- Clardy SL, Wang X, Boyer PJ, Earley CJ, Allen RP, Connor JR 2006 Is ferroportin-hepcidin signaling altered in restless legs syndrome? J Neurol Sci 247:173–179 [DOI] [PubMed] [Google Scholar]
- Zechel S, Huber-Wittmer K, von Bohlen und Halbach O 2006 Distribution of the iron-regulating protein hepcidin in the murine central nervous system. J Neurosci Res 84:790–800 [DOI] [PubMed] [Google Scholar]
- Ke Y, ChangYZ, Duan XL, Du JR, Zhu L, Wang K, Yang X, Ho KP, Qian ZM 2005 Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol Aging 26:739–748 [DOI] [PubMed] [Google Scholar]
- Chang YZ, Qian ZM, Wang K, Zhu L, Yang X, Du JR, Jiang L, Ho KP, Wang Q, Ke Y 2005 Effects of development and iron status on ceruloplasmin expression in rat brain. J Cell Physiol 204:623–631 [DOI] [PubMed] [Google Scholar]
- Qian ZM, Xiao DS, Ke Y, Liao QK 2001 The increased nitric oxide is one of the causes for the changes in iron metabolism in the exercised rats. Am J Physiol Regul Integr Comp Physiol 280:R739–R743 [DOI] [PubMed] [Google Scholar]
- Merle U, Fein E, Gehrke SG, Stremmel W, Kulaksiz H 2007 The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology 148:2663–2668 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J 2004 Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093 [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M 1998 Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 83:281–293 [DOI] [PubMed] [Google Scholar]
- Rivest S 2003 Molecular insights on the cerebral innate immune system. Brain Behav Immun 17:13–19 [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR 2003 Cytokine-induced sickness behavior. Brain Behav Immun 17(Suppl 1):S112–S118 [DOI] [PubMed] [Google Scholar]
- Dantzer R 2004 Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500:399–411 [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR 2001 Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66:1198–1207 [DOI] [PubMed] [Google Scholar]
- Jeong SY, David S 2003 Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem 278:27144–27148 [DOI] [PubMed] [Google Scholar]
- Jiang DH, Ke Y, Cheng YZ, Ho KP, Qian ZM 2002 Distribution of ferroportin1 protein in different regions of developing rat brain. Dev Neurosci 24:94–98 [DOI] [PubMed] [Google Scholar]