Abstract
Different cellular receptors mediate the biological effects induced by estrogens. In addition to the classical nuclear estrogen receptors (ERs)-α and -β, estrogen also signals through the seven-transmembrane G-protein-coupled receptor (GPR)-30. Using as a model system SkBr3 and BT20 breast cancer cells lacking the classical ER, the regulation of GPR30 expression by 17β-estradiol, the selective GPR30 ligand G-1, IGF-I, and epidermal growth factor (EGF) was evaluated. Transient transfections with an expression plasmid encoding a short 5′-flanking sequence of the GPR30 gene revealed that an activator protein-1 site located within this region is required for the activating potential exhibited only by EGF. Accordingly, EGF up-regulated GPR30 protein levels, which accumulated predominantly in the intracellular compartment. The stimulatory role elicited by EGF on GPR30 expression was triggered through rapid ERK phosphorylation and c-fos induction, which was strongly recruited to the activator protein-1 site found in the short 5′-flanking sequence of the GPR30 gene. Of note, EGF activating the EGF receptor-MAPK transduction pathway stimulated a regulatory loop that subsequently engaged estrogen through GPR30 to boost the proliferation of SkBr3 and BT20 breast tumor cells. The up-regulation of GPR30 by ligand-activated EGF receptor-MAPK signaling provides new insight into the well-known estrogen and EGF cross talk, which, as largely reported, contributes to breast cancer progression. On the basis of our results, the action of EGF may include the up-regulation of GPR30 in facilitating a stimulatory role of estrogen, even in ER-negative breast tumor cells.
GIVEN THE ARRAY of extracellular cues to which they are exposed, cells have developed complex machinery for the reception and interpretation of external stimuli. Multiple intracellular signaling pathways are activated by these signals, which are then translated into changes of cellular functions. A common theme in the arrangement of these pathways is the integration and cross talk between contiguous cascades to fine-tune biological outcomes as diverse as cell proliferation, differentiation, and migration. The transactivation of receptor tyrosine kinases by G protein-coupled receptors (GPCRs) is a nice example of communication and cooperation between different signaling networks. In this regard, agonist binding to GPCRs results in transactivation of the epidermal growth factor (EGF) receptor (EGFR) and activation of the ERK/MAPK cascade in a variety of cellular contexts. Traditionally, the EGF network has been viewed as a direct orchestrator of cell replication under physiological and pathological conditions; nevertheless, the involvement of cross-signaling with steroid hormones such as estrogens has largely been demonstrated, particularly in the regulation of normal mammary development and breast cancer progression (1). Indeed, aberrant expression and activation of EGFR is frequently observed in estrogen-sensitive tumors like breast and ovary, in which it correlates with a poorer patient prognosis (2,3). In addition, up-regulation of EGFR signaling is thought to be an important mechanism, conferring antiestrogen resistance of breast cancer resulting in the failure of endocrine therapy (4).
Several lines of evidence have suggested that the interaction of EGFR with estrogen signaling can occur at different levels. The major estrogen, 17β-estradiol (E2), primarily acts through cognate nuclear receptors [estrogen receptors (ERs)], leading to regulation of gene expression, which has traditionally been deemed as genotropic estrogen activity. Many E2-responsive genes are indeed key signaling molecules that participate in EGFR signaling (1). Alternatively, a cell membrane-associated form of ER has been reported to couple with and activate various G proteins, thereby triggering nongenotropic effects through the transactivation of the EGFR (1,5). More recently our and other studies have shown that an orphan GPCR, named GPR30, is able to mediate rapid E2-dependent signals prompting major biological responses such as gene expression and cancer cell proliferation (6,7,8,9,10,11). Interestingly, it has been shown that GPR30 is involved in the EGFR transactivation by E2 (12) as exhibited by other GPCR ligands (13,14,15,16,17). In addition, different studies including our own have demonstrated that E2 and the mixed ER agonist/antagonist 4-hydroxytamoxifen can signal through GPR30 to activate the EGFR-MAPK cascade, even in cancer cells lacking ERs (8,18).
From all these studies, it is possible to assume that E2 can initiate rapid MAPK signaling in an ER-dependent and ER-independent manner. First, E2 can bind a membrane ER, quite similar or identical with the nuclear receptor, and subsequently activate G proteins; second, E2 can directly activate GPCR at the membrane/intracellular level (see below) in an ER-independent manner, thereby signaling to G protein activation. However, because GPR30 was found to be localized close to the endoplasmic reticulum (6), whether this intracellular receptor coupled with G proteins can directly transactivate EGFR as well as its physiological function(s) remains to be fully understood. ER inhibition has proven to be an effective means of blocking the growth of breast tumors expressing ER, and this modality of treatment still remains the standard endocrine therapy for ER+ tumors. Although there is general concordance between ER expression and responsiveness to ER antagonism, as indicated by greater disease-free survival at 5-yr follow-up for postmenopausal patients with ER+ tumors receiving tamoxifen (19), roughly one in four patients do not respond to tamoxifen therapy from the onset, and after a few years in most patients, treatment with this antiestrogen produces agonist effects.
A variety of explanations have been offered to account for unresponsiveness to ER antagonism, including: 1) intratumoral heterogeneity in ER expression, 2) evolution of mutant ERs with reduced affinity for ER antagonists, 3) drug resistance, 4) partial receptor antagonism, and 5) the presence or absence of trans-acting factors that influence ER functionality. These interpretations have prompted strategies better designed to assess ER activity and have served as the rationale for the discovery and use of new endocrine agents with more complete ER antagonist activity. In this concern, the existence of an alternative ER, such as GPR30, which is potentially stimulated by ER antagonists, may provide a further possible explanation for the 4-hydroxytamoxifen failure. To date, studies in animal and cell models have long indicated that estrogens manifest physiologic actions and biochemical effects inconsistent with its classical genomic mechanism of action (20). For instance, estrogen induces EGF-like activity in female reproductive tissue (21,22) and likewise activates biochemical signals typically associated with the EGFR transduction pathway (23,24). In this regard, it should be noted that GPR30 can act independently from ERs in triggering estrogen-dependent EGFR action. Indeed, GPR30 may play an important role in breast cancer biology because it provides a mechanism through which estrogens promote EGF-like effects. According to this model, ER-negative breast tumors also may remain estrogen responsive through GPR30. This concept should be taken into account, particularly in those patients receiving endocrine therapy because OHT behaves similarly to estradiol, being capable to elicit EGFR activation in breast cancer cells (9,10,18).
Given that GPR30 involves the EGFR pathway in mediating the estrogen signals, in the present study, we evaluated the regulation of GPR30 expression and demonstrate for the first time that EGF is able to induce GPR30 protein levels that accumulate in the intracellular compartment. Consequently, EGF generates a regulatory loop engaging E2 to boost the proliferation of ER-negative breast cancer cells.
Materials and Methods
Reagents
E2, EGF, IGF-I, H89, LY 294,002 (LY), and PD98059 (PD) were purchased from Sigma-Aldrich Corp. (Milan, Italy). AG1478 (AG) was purchased from Biomol Research Laboratories, Inc. (DBA, Milan, Italy), and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2) was obtained from Calbiochem (VWR International, Milan, Italy). 1-[4-(-6-bromobenzol[1, 3]diodo-5-yl)-3a,4,5,9b-tetrahidro-3H-cyclopenta[c]quinolin-8yl]ethanone (G-1) was purchased from Merck KGaA (Frankfurt, Germany). All compounds were solubilized in dimethylsulfoxide, except E2 and PD, which were dissolved in ethanol.
Cell culture
SkBr3 breast cancer cells were maintained in RPMI 1640 without phenol red supplemented with 10% fetal bovine serum (FBS). BT20 breast cancer cells and HEK-293 embryonal kidney cells were cultured in MEM and DMEM, respectively, with phenol red supplemented with 10% FBS. Cells were switched to medium without serum the day before experiments for immunoblots, RT-PCR, and confocal microscopy assessment.
Plasmids
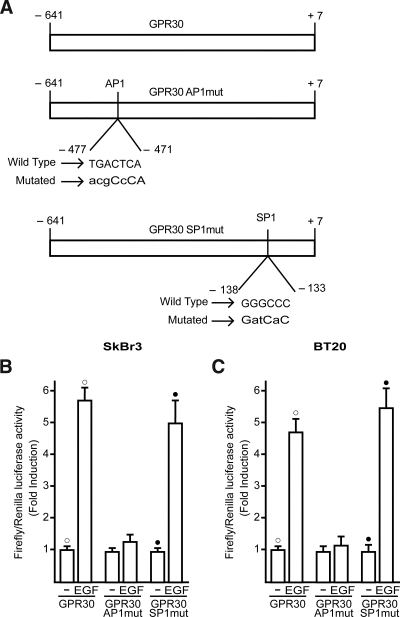
To generate the luciferase expression vector for the GPR30–5′flanking region (GPR30), a 641-bp fragment next to the 5′-flanking region of the GPR30 gene was amplified by PCR using the following primer pairs: 5′-AACACTGGCTTTCCCTTCCTATCT-3′ (forward) and 5′-CTTGAAGTGAGCCTGGCATTTGTC-3′ (reverse) from genomic DNA, which was extracted from SkBr3 cells by Trizol reagent as suggested by the manufacturer (Invitrogen, Milan, Italy). PCR primer pairs were selected analyzing the 5′-flanking region of GPR30 gene in chromosome 7, location 7p22.3. The PCR amplification was performed using 1.25 U GoTaq DNA polymerase according to the manufacturer’s instructions (Promega, Milan, Italy). PCR conditions were 5 min at 95 C followed by 1 min at 94 C, 1 min at 58 C, and 1 min at 72 C for 30 cycles. The fragment was then inserted in the pCR 2.1 plasmid using the TA cloning kit (Invitrogen), sequenced, and cut with HindIII and XhoI. The insert was cloned in the pGL3 basic vector (Promega). Analyses of GPR30–5′ flanking region revealed an activator protein-1 (AP1; −471 to −477) and an specificity protein-1 (SP1; −133 to −138) consensus binding sites. Mutations from position −471 to −477 in the GPR30–5′ flanking sequence corresponding to an AP1 motif and from position −133 to −138 corresponding to the SP1 binding site (Fig. 2A) were generated using QuikChange XL site-directed mutagenesis kit (Stratagene, Milan, Italy).
Figure 2.
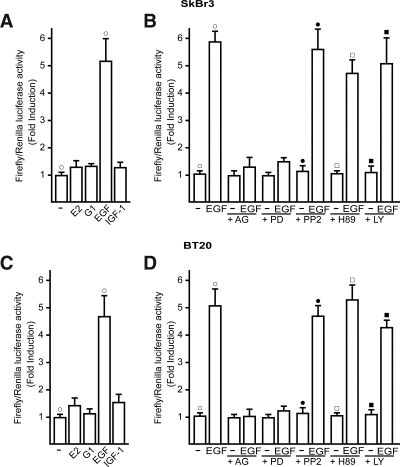
The GPR30–5′-flanking region is transactivated by EGF in SkBr3 and BT20 breast cancer cells. A–D, Cells were transfected with a reporter plasmid encoding the GPR30–5′-flanking region and treated with 100 nm E2, 1 μm G-1, 50 ng/ml IGF-I, or 50 ng/ml EGF, and 10 μm EGFR inhibitor tyrphostin AG 1478 (AG), 10 μm MEK inhibitor PD, 10 μm Src family tyrosine kinase inhibitor PP2, 10 μm PKA inhibitor H89, 10 μm PI3K inhibitor LY, as indicated. The luciferase activities were normalized to the internal transfection control and values of cells receiving vehicle (−) were set as 1-fold induction upon which the activity induced by treatments was calculated. Each data point represents the mean ± sd of three independent experiments performed in triplicate. ○, •, □, ▪, P < 0.05, for cells receiving vehicle (−) vs. treatment.
The following pairs of primers were used to generate the AP1 and Sp1mutants: GPR30AP1mut (forward), 5′-CCCTGCCTGTGGGAGACGCCCACGTCCAGCCTCC-3′ and (reverse) 5′-GGAGGCTGGACGTGGGCGTCTCCCACAGGCAGGG-3′; GPR30SP1mut (forward), 5′-GGACGAGCACGCGGAGATCACTCGCCTCCACGG-3′ and (reverse) 5′-CCGTGGAGGCGAGGTGATCTCCGCGTGCTCGTCC-3′. All plasmids were sequenced before use. Plasmid 3x-FLAG-hGPR30 was constructed using the HindIII/BamHI sites in pCMV10.3x-ratFLAG (25). hGPR30 was amplified with primers CCCCAAGCTTatggatgtgacttcccaag and CAGCGGATCCctacacggcactgctgaac (restriction sites are underlined). Reference plasmid Prl-3x-FLAG is a gift from K. Strub (Department of Cell Biology, University of Genève, Genève, Switzerland), and it expresses an unrelated 26-kDa protein. Short hairpin (sh)RNA constructs against human GPR30 were bought from Openbiosystems (Biocat.de, Heidelberg, Germany) with catalog no. RHS4533-M001505. The targeting strands generated from the shRNA vectors sh1, sh2, sh3, sh4, and unrelated control are complementary to the following sequences, respectively: CGAGTTAAAGAGGAGAAGGAA, CTCCCTCATTGAGGTGTTCAA, CGCTCCCTGCAAGCAGTCTTT, GCAGTACGTGATCGGCCTGTT, and CGAC-ATGAAACCGTCCATGTT.
To evaluate the effectiveness of the different shRNA constructs, HEK-293 cells were seeded at about 50% confluency in 6-cm plates. Six to 8 h later, cells were transfected using the calcium-phosphate method with 1 μg of 3x-FLAG-hGPR30, 10 μg of shRNA construct, and 2 μg of Prl-3x-FLAG. Prl-3xFLAG was used as a transfection control. Forty hours after transfection, cells were harvested and lysed with 20 mm Tris-HCl (pH 8), 100 mm NaCl, 10% glycerol, 0.1% Nonidet P-40, 1 mm monovanadate, 1 mm dithiothreitol, and protease inhibitors. DNA was sheared by several passages through a 25-gauge needle. Lysates were cleared by centrifugation, and protein concentrations were determined by the Bradford method. Thirty micrograms of lysates were subjected to Western blot analysis with the FLAG antibody M2 (Sigma, Lausanne Switzerland). With a 74% knockdown of 3x-FLAG-hGPR30 expression shRNA construct, sh3 showed the highest efficacy. Hereafter sh3 is referred to as shGPR30. The dominant-negative (DN)/c-fos plasmid, a gift from C. Vinson (National Institutes of Health, Bethesda, MD), consists of an acidic amphipathic protein sequence appended onto the N terminus of the fos leucine zipper, replacing the normal basic region critical for DNA binding. The reporter plasmid for 4xAP1-responsive collagen promoter, a gift from H. van Dam (Department of Molecular Cell Biology, Leiden University, Leiden, The Netherlands), contains four AP1 binding sequences (TGAC/GTCA) inserted into a luciferase construct with the minimal promoter sequences from the albumin gene. The GPR30 expression vector was kindly provided by R. Weigel (Department of Surgery, Thomas Jefferson University, Philadelphia, PA) (8).
Transfection and luciferase assays
SkBr3 and BT20 cells (1 × 105) were plated into 24-well dishes with 500 μl of regular growth medium per well the day before transfection. The medium was replaced with that lacking serum on the day of transfection performed using Fugene 6 reagent as recommended by the manufacturer (Roche Diagnostics, Milan, Italy) with a mixture containing 300 ng of GPR30 expression vector and 3 ng of pRL-TK. After 5 h, the serum-free medium containing the indicated treatments was renewed, and then cells were incubated for 18 h. Luciferase activity was measured with the dual luciferase kit (Promega) according to the manufacturer’s recommendations. Firefly luciferase values were normalized to the internal transfection control provided by the Renilla luciferase activity. The normalized relative light unit values obtained from cells treated with vehicle were set as 1-fold induction upon which the activity induced by treatments was calculated.
Western blotting
SkBr3 cells were grown in 10-cm dishes, exposed to ligands, and then lysed in 500 μl of 50 mmol/liter NaCl; 1.5 mmol/liter MgCl2; 1 mmol/liter EGTA; 10% glycerol; 1% Triton X-100; 1% sodium dodecyl sulfate; a mixture of protease inhibitors containing 1 mmol/liter aprotinin, 20 mmol/liter phenylmethylsulfonyl fluoride, and 200 mmol/liter sodium orthovanadate. Protein concentration was determined using Bradford reagent according to the manufacturer recommendations (Sigma-Aldrich). Equal amounts of whole protein extract were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel; transferred to a nitrocellulose membrane (Amersham Biosciences, Milan, Italy); probed overnight at 4 C with the antibody against GPR30 (MBL-Eppendorf, Milan, Italy), c-fos, β-actin, phosphorylated ERK1/2, and ERK2 (all purchased from Santa Cruz Biotechnology, DBA, Milan, Italy); and then revealed using the ECL Western blotting analysis system (Amersham Biosciences).
RT-PCR
SkBr3 cells were grown in 10-cm dishes in regular growth medium and then switched to medium lacking serum for 24 h. Thereafter treatments were added for 1 h, and cells were processed for mRNA extraction using the Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The mRNA expression was evaluated by semiquantitative RT-PCR as previously described (26). For GPR30 and the acid phosphoprotein P0 (36B4), which was used as a control gene, the primers were 5′-CTGGGGAGTTTCCTGCTGA-3′ (GPR30 forward) and 5′-GCTTGGGAAGTCACACCAT-3′ (GPR30 reverse) and 5′-CTCAACATCTCCCCCTTCTC-3′ (36B4 forward) and 5′-CAAATCCCATATCCTCGTCC-3′ (36B4 reverse) to yield products, respectively, of 155 and 408 bp, with 15 PCR cycles for both genes.
Confocal microscopy
Fifty percent confluent cultured SkBr3 and HEK-293 cells grown on coverslips were serum deprived for 24 h and then treated for 2 h with 50 ng/ml EGF and 10 μm AG and PD as indicated. Then cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, washed three times with PBS, and incubated for 1 h with 2 mg/ml primary antibody against GPR30. After incubation with the GPR30 antibody, the slides were washed three times with PBS and incubated with 1 mg/ml rhodamine-conjugated donkey antirabbit IgG (Calbiochem). HEK-293 cells were also stained with propidium iodide. The cellular expression and localization of GPR30 was evaluated by confocal microscope with ×1000 magnification. The optical sections were taken at the central plane. The specificity of the detection was verified by neutralizing the GPR30 antibody with the antigen peptide, which was produced by the TNT quick coupled transcription/translation systems (Promega).
Chromatin immunoprecipitation (ChIP)
Cells grown in 10-cm plates were shifted for 24 h to medium lacking serum and then treated for 2 h with vehicle or 50 ng/ml EGF. ChIP assay was performed as previously described (27). The immuno-cleared chromatin was precipitated with anti-c-fos antibody or nonspecific IgG (Santa Cruz Biotechnology, DBA). A 4-μl volume of each sample was used as template to amplify by PCR two fragments located next the GPR30–5′ flanking region: one fragment of 261 bp containing the AP1 site and the second fragment of 364 bp (from −937 to −1301) not containing the AP1 site. The primer pairs used to amplify the first fragment were: 5′-CGTGCCCATACCTTCATTGCTTCC-3′ (forward) and 5′-CCTGGCCGGGTGTCTGTAG-3′ (reverse), whereas the primer pairs used to amplify the second fragment were: 5′-CCGTGGCCCGCTGCATAGAGAAC-3′ (forward) and 5′-GAGAGGGAGAAGTGGGCTGTC-3′ (reverse). The PCR conditions were 45 sec at 94 C, 40 sec at 58 C, and 90 sec at 72 C. The amplification products obtained in 25 cycles were analyzed in a 2% agarose gel and visualized by ethidium bromide staining. Three microliters of the initial preparations of soluble chromatin were amplified to control input DNA before precipitation.
Cell proliferation assay
Cells (10,000) were seeded in 24-well plates in regular growth medium. Cells were washed once they had attached and then incubated in medium containing 2.5% charcoal-stripped FBS with the indicated treatments; medium was renewed every 2 d (with treatments), and cell growth was monitored by dimethylthiazoldiphenyltetra-zoliumbromide (MTT) assay according to the manufacturer’s protocol (Sigma). A concentration of 200 ng/liter of the shGPR30 or DN/c-fos was transfected using Fugene 6 reagent (Roche Diagnostics) as recommended by the manufacturer the day before treatments and then renewed every 2 d before MTT assay.
Statistical analysis
Statistical analysis was done using ANOVA followed by Newman-Keuls’ testing to determine differences in means. P < 0.05 was considered as statistically significant.
Results
EGF transactivates the 5′ flanking region of GPR30 through an AP1 site in ER-negative breast cancer cells
In our previous studies (8,9,10,11), we demonstrated that GPR30 mediates the stimulatory effects elicited by E2 and other agonists, including the selective GPR30 ligand G-1, in a variety of tumor cells expressing or lacking ERs. The GPR30 activity was clearly coupled to the EGFR-MAPK transduction pathway, which in turn promoted gene expression changes and cell proliferation. Given that no data are currently available regarding the molecular mechanisms involved in the regulation of the GPR30 promoter sequence, we first cloned and characterized the functional response of a 648-bp fragment located at the 5′ flanking region of the human GPR30 gene containing different transcription factor binding sites, such as those for the AP1 and SP1 activating proteins (Fig. 1). Thereafter, we transiently transfected the above construct in ER-negative SkBr3 and BT20 breast cancer cells to evaluate its response to E2 and G-1 as well as the growth factors EGF and IGF-I largely involved in cancer development and progression. As shown in Fig. 2 (A and C), only EGF was able to transactivate the GPR30–5′ flanking region cloned, whereas the other ligands did not exhibit stimulatory activity. Next, the luciferase expression triggered by EGF was no longer observed in presence of the EGFR and ERK inhibitors AG and PD, respectively, whereas the response to EGF was not altered by PP2, H89, and LY, inhibitors of the Src family tyrosine kinase, the protein kinase A (PKA), and phosphatidylinositol 3-kinase (PI3K) transduction pathways, respectively (Fig. 2, B and D). To further assess the activity of the GPR30–5′ flanking region described above, we also cloned two expression vectors mutated in AP1 and SP1 sites, which are potentially involved in the responsiveness to EGF (Fig. 3A). Interestingly, transfection analysis showed that EGF stimulation differentially transactivated these mutants (Fig. 3, B and C). In both SkBr3 and BT20 cells, the construct mutated in the −477 to −471 region (GPR30AP1mut) did not respond to EGF, whereas the construct mutated in the −138 to −133 region (GPR30SP1mut) still maintained the EGF responsiveness. Thus, the AP1 site spanning from −477 to −471 bp within the GPR30–5′ flanking region is required for the transactivation induced by EGF.
Figure 1.
Sequence of the GPR30–5′-flanking region used to generate luciferase reporter constructs.
Figure 3.
An AP1 site is responsible for transactivation of the GPR30–5′-flanking region induced by EGF in SkBr3 and BT20 breast cancer cells. A, AP1 (GPR30AP1mut) and SP1 (GPR30SP1mut) mutations generated within the GPR30–5′-flanking region. B and C, Cells were transfected with the reporter plasmids described in A and treated with 50 ng/ml EGF. The luciferase activities were normalized to the internal transfection control and values of cells receiving vehicle (−) were set as 1-fold induction upon which the activity induced by treatments was calculated. Each data point represents the mean ± sd of three independent experiments performed in triplicate. ○, •, P < 0.05, for cells receiving vehicle (−) vs. treatment.
EGF up-regulates GPR30 expression
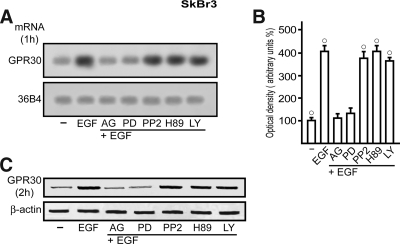
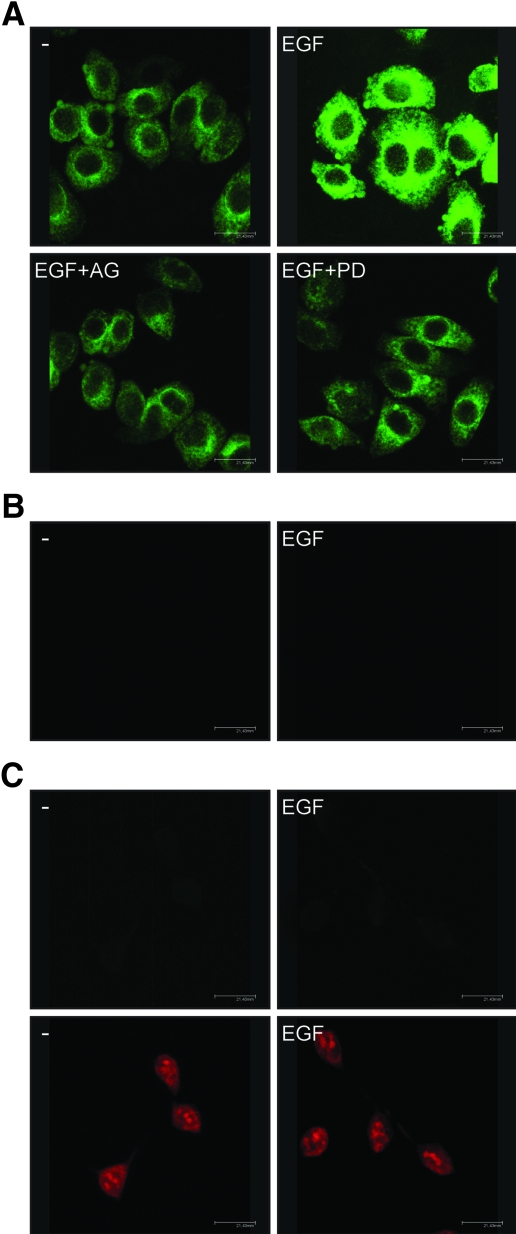
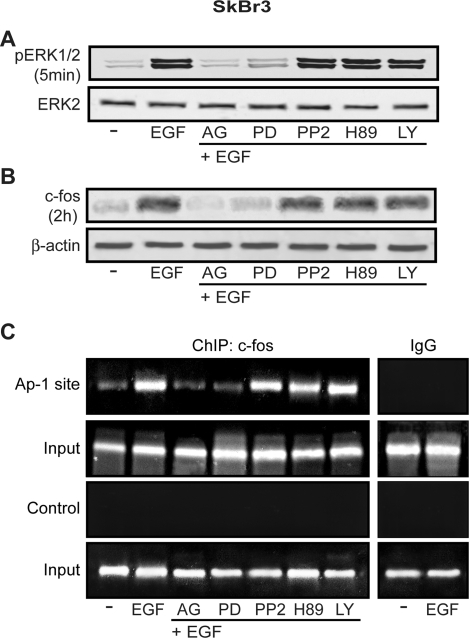
On the basis of the results obtained in transfection experiments, we asked whether EGF regulates GPR30 expression and what transduction pathway(s) could be involved in such ability. To this end, we first performed semiquantitative RT-PCR assays comparing mRNA levels after standardization with a housekeeping gene encoding the ribosomal protein 36B4. Interestingly, a short EGF treatment (1 h) in SkBr3 cells increased GPR30 mRNA expression, yet AG and PD prevented such response, whereas PP2, H89, and LY did not evidence any inhibitory effect (Fig. 4, A and B). The GPR30 protein levels evaluated on a 2-h EGF exposure paralleled the mRNA increase showing a similar signaling regulation (Fig. 4C). To confirm with a different approach the aforementioned findings and evaluate the localization of GPR30 after EGF stimulation, we assessed GPR30 expression by confocal microscopy in SkBr3 cells. GPR30-negative HEK-293 cells were used as controls. Notably, the treatment with EGF (2 h) induced an intracellular GPR30 accumulation, which was no longer evident in presence of AG or PD (Fig. 5A). The specificity of detection in SkBr3 cells was verified by neutralizing the GPR30 antibody by 10-fold molar excess of the antigen peptide (Fig. 5B). Moreover, the GPR30-negative HEK-293 cells showed no immunodetection of GPR30 (Fig. 5C, upper panels), whereas the nuclei were stained with propidium iodide (Fig. 5C, lower panels). Hence, in SkBr3 cells, EGF stimulation triggers GPR30 accumulation at the intracellular level (see Discussion).
Figure 4.
EGF up-regulates GPR30 expression in SkBr3 cells. A, The expression of GPR30 was evaluated by semiquantitative RT-PCR in cells treated for 1 h with vehicle (−) or 50 ng/ml EGF alone and in combination with 10 μm EGFR inhibitor tyrphostin AG, 10 μm MEK inhibitor PD, 10 μm Src family tyrosine kinase inhibitor PP2, 10 μm PKA inhibitor H89, 10 μm PI3K inhibitor LY, as indicated. The housekeeping gene 36B4 was determined as a control. B, Quantitative representation of GPR30 mRNA expression (mean ± sd) of three independent experiments after densitometry and correction for 36B4 expression. ○, P < 0.05, for cells receiving vehicle (−) vs. treatment. C, Immunoblot of GPR30 from SkBr3 cells treated for 2 h with vehicle (−) or 50 ng/ml EGF alone and in combination with 10 μm AG, 10 μm PD, 10 μm PP2, 10 μm H89, and 10 μm LY, as indicated. β-Actin served as a loading control.
Figure 5.
GPR30 localization in SkBr3 cells. A, GPR30 evaluation by confocal microscopy in SkBr3 cells fixed, permeabilized, and stained with anti-GPR30 antibody. Cells were treated for 2 h with vehicle (−) or 50 ng/ml EGF alone and in combination with EGFR inhibitor tyrphostin AG, 10 μm MEK inhibitor PD, as indicated. B, SkBr3 cells were treated for 2 h with vehicle (−) or 50 ng/ml EGF and stained with GPR30 antibody, which was preneutralized with the antigen peptide. C, HEK-293 cells were treated for 2 h with vehicle (−) or 50 ng/ml EGF and stained with GPR30 antibody (upper panels) or propidium iodide (lower panels). The white bars denote 21,43 μm. Data are representative of three independent experiments.
The EGFR-ERK transduction pathway mediates GPR30 induction by EGF
Next, we ascertained that in SkBr3 cells a rapid ERK1/2 phosphorylation induced by EGF is no longer evident in presence of AG and PD but still persists using PP2, H89, and LY at the same time of EGF (Fig. 6A). Given the potential involvement of the PKA transduction pathway in ERK signaling, cells were treated with H89 3, 6, and 12 h before EGF stimulation. Even in these conditions, H89 did not modify the EGF-stimulated ERK1/2 phosphorylation (data not shown), suggesting that PKA does not influence ERK activation in our experimental model. We previously reported (8,9,10,11) that in a variety of hormone-sensitive tumor cells, EGFR/ERK-mediated signals lead to early induction of c-fos, which plays a relevant role in normal cell growth and cellular transformation mainly interacting with diverse members of c-jun family (28). The fos-jun heterodimers form the AP1 transcription factor complex, which binds cognate sites located within promoters of target genes (28). In line with the results obtained on ERK activation, EGF induced a strong c-fos increase, which was abrogated by AG and PD but not in presence of PP2, H89, and LY (Fig. 6B), suggesting that the EGFR-ERK signaling is the key pathway involved in the regulation of c-fos in SkBr3 cells. To evaluate whether the EGF-induced up-regulation of c-fos is involved in GPR30 expression, we performed ChIP analysis immunoprecipitating cell chromatin with an anti-c-fos antibody and amplifying the AP1 site located within the GPR30-5′ flanking region. As shown in Fig. 6C, EGF strongly recruited c-fos at the AP1 site, which was dependent on EGFR-ERK signaling because AG and PD abrogated this association whereas PP2, H89, and LY did not elicit inhibitory activity. Using primer pairs amplifying a control DNA sequence that does not contain the AP1 site, we did not visualize any ethidium bromide staining (Fig. 6C).
Figure 6.
EGF induces ERK1/2 phosphorylation and c-fos expression, which is recruited at the AP1 site located in the GPR30–5′-flanking region. A, The rapid ERK1/2 phosphorylation induced by 50 ng/ml EGF in SkBr3 cells is abrogated in presence of 10 μm EGFR inhibitor tyrphostin AG and 10 μm MEK inhibitor PD but not in presence of 10 μm Src family tyrosine kinase inhibitor PP2, 10 μm PKA inhibitor H89, or 10 μm PI3K inhibitor LY. B, The up-regulation of c-fos induced by 50 ng/ml EGF in SkBr3 cells is abrogated in presence of 10 μm AG and 10 μm PD but not in presence of 10 μm PP2, 10 μm H89, or 10 μm LY. C, EGF treatment (50 ng/ml) induces in SkBr3 cells the recruitment of c-fos at the AP1 site located in the GPR30–5′-flanking region. This recruitment is abrogated by 10 μm AG or 10 μm PD but persists in presence of 10 μm PP2, 10 μm H89, or 10 μm LY. The amplification of a region lacking the AP1 site (control) does not show the recruitment following the same experimental conditions described above. In control samples, nonspecific IgG was used instead of the primary antibody.
The up-regulation of GPR30 by EGF engages E2 to boost the proliferation of breast cancer cells
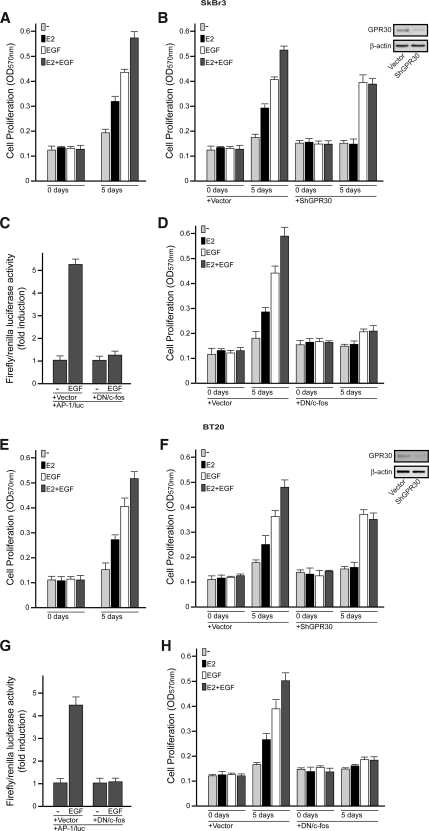
The biological counterpart of the aforementioned findings was ascertained evaluating cell proliferation by MTT assay. In SkBr3 and BT20 cells, the growth effects, stimulated by E2 and EGF alone, further increased in presence of both mitogens (Fig. 7, A and E). The role of GPR30 in the biological activity elicited by E2 was clearly evidenced silencing GPR30 in both breast cancer cells. As shown in Fig. 7 (B and F), the growth effects of E2 alone or in combination with EGF were prevented transfecting cells with shGPR30, which knocked down GPR30 expression. Engineering cells to express the DN/c-fos, which effectively blocked the AP1-mediated transcriptional activity (Fig. 7, C and G), we did not observe the proliferative effects induced by either E2 or those triggered by EGF (Fig. 7, D and H). Hence, the c-fos/AP1 signaling exerts a key role in the growth stimulation of both mitogens in SkBr3 and BT20 cells. Taken together, the up-regulation of GPR30 after exposure to EGF may represent a molecular mechanism through which EGF engages E2 to boost the proliferative effects elicited in these ER-negative breast cancer cells.
Figure 7.
In SkBr3 and BT20 breast cancer cells, EGF engages E2 through GPR30 to boost the growth effects, which were monitored by MTT assay. A and E, The combination of E2 and EGF treatments enhances the proliferation of SkBr3 and BT20 cells stimulated by each mitogen used alone. Cells were treated with vehicle or 100 nm E2 and/or 50 ng/ml EGF in medium containing 2.5% charcoal-stripped FBS (medium was refreshed and treatments were renewed every 2 d). B and F, The growth effects induced by E2 alone or in combination with EGF were abolished by GPR30 silencing in both SkBr3 and BT20 cells. Cells were transfected with an empty vector or shGPR30 and the next day were treated with vehicle (−), 100 nm E2, and/or 50 ng/ml EGF. Transfections and treatments were renewed every 2 d. Efficacy of GPR30 silencing was evaluated by immunoblots, as indicated. C and G, The DN/c-fos construct effectively blocked the AP1 mediated transcriptional activity in SkBr3 and BT20 cells. The luciferase activities were normalized to the internal transfection control and values of cells receiving vehicle (−) were set as 1-fold induction upon which the activity induced by 50 ng/ml EGF was calculated. D and H, The growth effects induced by E2 and EGF used alone or in combination were abolished transfecting the SkBr3 and BT20 cells with the DN/c-fos expression vector. Cells were transfected with an empty vector or the DN/c-fos construct and the next day were treated with vehicle (−), 100 nm E2, and/or 50 ng/ml EGF. Each data point is the mean ± sd of three independent experiments performed in triplicate.
Discussion
Positive feedback loops enhance the amplitude and prolong the active state of transduction pathways to convey robustness in the face of variable inputs (29). In the case of EGFR, the output of the main switch, as can occur through receptor activation by ligand binding, is fine-tuned by the MAPK pathway involved in the expression of GPCRs, which in turn is coupled to EGFR signaling in diverse cell types (5,30). In this regard, agonist-stimulated GPCRs lead to intracellular activation of diverse metalloproteinases, release of EGFR ligands at the cell surface, and subsequent activation of EGFR-MAPK signaling (5,30).
The EGFR transduction pathway has been implicated in estrogen action (31). Intrauterine E2 administration increased EGF concentrations (21) and EGFR autophosphorylation (32), whereas neutralizing antibodies against EGF inhibited estrogen-induced uterine growth (22). In addition, in vitro experiments proved that E2 stimulates various EGFR-associated cascades, including MAPK activation, dependent on rapid release of heparin-binding EGF and activation of matrix metalloproteinases-2 and -9 (5,12,18,20,33).
It is worth noting that GPR30-mediated, estrogen-induced ERK activation occurs via Gβγ-subunit protein signaling and downstream Src involvement because pertussis toxin and Src inhibitors blunt ERK activity by E2 but not EGF. Moreover, E2-induced ERK signaling is prevented by: 1) specific inhibitors of EGFR tyrosine kinase; 2) neutralizing heparin-binding EGF antibodies; and 3) down-regulation of pro-heparin-binding EGF by a diphtheria toxin mutant (12). Therefore, to trigger MAPK activity, GPR30 couples membrane-associated enzymes along with a familiar regulatory circuit controlled by independent G protein signaling pathways.
In this concern, one important aspect regarding GPR30-mediated signals is that ER antagonists binding GPR30 may behave as estrogen agonists in stimulating HB-EGF release from tumor cells (18,20). This observation has relevant implications on the possible role elicited by GPR30 in human cancer biology including the potential agonist activity exerted by ER antagonists in tumor progression (8,9,10,11,20,33).
Indeed, evidence that GPR30 can act in an ER-independent manner in mediating estrogen action is provided by diverse experimental observations. First, rapid E2 stimulation does not correlate with ER expression because it occurs in human ER-negative cancer cells as shown in our and other studies (8,9,10,11,12,18,20,33). Second, ER antagonists promote rapid estrogen action in breast cancer cells expressing GPR30 independently of whether they express estrogen receptor-α gene or estrogen receptor-β gene, the genes encoding ERα and ERβ, respectively (12,18). These findings suggest that ER or ER-related proteins are not required in GPR30-dependent EGFR activation; however, it should be noted that GPR30 and ERα cooperate in mediating the effects of E2 and even those exerted by the selective GPR30 ligand G-1, as we recently demonstrated in ovarian cancer cells (11).
The present study provides novel evidence regarding the regulation and activity of GPR30 in ER-negative SkBr3 and BT20 breast cancer cells. For the first time, we have demonstrated that EGF through the EGFR transduction pathway transactivates the GPR30-5′flanking region and up-regulates the expression of GPR30 protein, which localizes intracellularly as demonstrated by confocal microscopy.
In accordance with our results, GPR30 was visualized predominantly in the endoplasmic reticulum (6,34), and even functional GPCRs (35,36,37) and receptor tyrosine kinases like EGFR (38) were found in the intracellular compartment. These observations raise the question of how ligand binding to a GPCR within cells could initiate signaling events, particularly those involving transactivation of EGFR. Given that G protein βγ-subunits are initially targeted to the endoplasmic reticulum in which they subsequently associate with G protein α-subunits (39), the basic machinery for a GPCR to initiate signaling may also be located close to the endoplasmic reticulum. Although the transduction cascade initiated by GPR30 remains to be completely elucidated, often the GPCR-mediated transactivation of EGFR occurs through shuttle cytosolic molecules, which activate metalloproteinases leading, in turn, to the release of EGF-like ligands (40). Of note, recent investigations in endometrial and breast tumors (41,42) corroborate the aforementioned results because GPR30 staining yielded uniform density throughout the cell, consistent with a primarily intracellular location. A similar expression pattern of GPR30 has been observed in neurons (43), although contradictory results have also been reported (25,44). In this regard, the regulation and activity of a distinct subcellular distribution of GPR30 in both normal and cancer cell contexts is still an open question.
The c-fos represents a prototypical early gene because its expression is rapidly induced by different extracellular stimuli including mitogens and hormones (45). The nuclear protein encoded by c-fos interacting with c-jun family members form the heterodimeric AP1 transcription factor complex (28), which regulates the expression of genes involved in proliferation, invasion, differentiation, and cell survival (46). The transcription of c-fos is controlled by multiple cis-elements such as the serum-response element mediating growth factor-induced c-fos expression, which leads to the activation of the MAPK transduction pathway (47).
Several studies have shown that ERα is also involved in the regulation of c-fos (48), although E2 and other compounds stimulate c-fos expression and cell proliferation through GPR30-EGFR-MAPK signaling in ER-negative breast tumor cells as we previously demonstrated (8,11). Here we provide novel insight into the molecular mechanisms by which EGF can further convey robustness to this pathway because it induces consecutive events such as rapid ERK activation and induction of c-fos, which in turn is recruited to an AP1 site located next to the GPR30-5′ flanking region. Interestingly, the biological action exerted by E2 and EGF recapitulated the aforementioned effects in ER-negative breast cancer cells. Indeed, the growth stimulation induced by each compound was boosted by the exposure to E2 in combination with EGF, whereas GPR30 silencing abrogated the proliferation stimulated by E2 alone and that additionally induced by E2 used in combination with EGF. Hence, the present data suggest that EGF triggers a positive feedback loop engaging GPR30-mediated signals, such as those elicited by E2, to enhance the potential of the EGFR signaling network.
The possible binding and activation of GPR30 by ER antagonists should be taken into account when considering either the failure of their inhibitory activity in breast cancer or the agonist effects observed in other tissues like the endometrium. Thus, our findings point toward the need of new endocrine agents able to block widespread estrogen action without exerting any stimulatory outcome through transduction pathways shared by the steroid and growth factor signaling network. From the data currently available, the potential of GPR30-mediated signals should be considered in estrogen-sensitive tumors to discover innovative antiestrogens. GPR30 overexpression was recently associated with lower survival rates in endometrial cancer patients (41) and higher risk of developing metastatic disease in patients with breast tumor (42). Therefore, the expression levels of GPR30 may characterize not only estrogen sensitivity and the potential response to endocrine pharmacological intervention in these tumors but could also be predictive of biologically aggressive phenotypes consistent with adverse outcome and survival. In addition, the up-regulation of GPR30 expression by the ligand-activated EGFR further extends our knowledge regarding the cross talk between EGF and E2 signaling in breast cancer progression. Likewise, our results indicate that the action of EGF may include the up-regulation of GPR30 in facilitating a stimulatory role of estrogen even in ER-negative breast tumor cells.
Footnotes
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro and Ministero dell’Università e Ricerca Scientifica e Tecnologica and Regione Calabria.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: AG, AG1478; AP1, activator protein-1; ChIP, chromatin immunoprecipitation; DN, dominant negative; E2, 17β-estradiol; EGF, epidermal growth factor; EGFR, EGF receptor; ER, estrogen receptor; FBS, fetal bovine serum; G-1, 1-[4-(-6-bromobenzol[1, 3]diodo-5-yl)-3a,4,5,9b-tetrahidro-3H-cyclopenta[c]quinolin-8yl]ethanone; GPCR, G protein-coupled receptor; LY, LY 294,002; MTT, dimethylthiazoldiphenyltetra-zoliumbromide; PD, PD98059; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine; sh, short hairpin; SP1, specificity protein-1.
References
- Levin ER 2003 Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17:309–317 [DOI] [PubMed] [Google Scholar]
- Keen JC, Davidson NE 2003 The biology of breast carcinoma. Cancer 97:825–833 [DOI] [PubMed] [Google Scholar]
- Roskoski Jr R 2004 The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun 319:1–11 [DOI] [PubMed] [Google Scholar]
- Ali S, Coombes RC 2002 Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER 2003 Proximal events in signaling by plasma membrane estrogen receptor. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17-β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17-β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Andò S, Maggiolini M 2006 17-β-Estradiol, genistein and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol Pharmacol 70:1414–1423 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M 2007 G-protein coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17-β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30 and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A 1999 EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888 [DOI] [PubMed] [Google Scholar]
- Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A 2001 Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20:1594–1600 [DOI] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ 2001 Lysophospholipids-receptor revelations. Science 294:1875–1878 [DOI] [PubMed] [Google Scholar]
- Tanimoto T, Lungu AO, Berk BC 2004 Sphingosine 1-phosphate transactivates the platelet-derived growth factor β receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res 94:1050–1058 [DOI] [PubMed] [Google Scholar]
- Greco S, Muscella A, Elia MG, Salvatore P, Storelli C, Mazzotta A, Manca C, Marsigliante S 2003 Angiotensin II activates extracellular signal regulated kinases via protein kinase C and epidermal growth factor receptor in breast cancer cells. J Cell Physiol 196:370–377 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialist’ Collaborative Group 2005 Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-years survival: an overview of the randomized trials. Lancet 365:1687–1700 [DOI] [PubMed] [Google Scholar]
- Filardo EJ 2002 Epidermal Growth Factor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signalling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80:231–238 [DOI] [PubMed] [Google Scholar]
- DiAugustine RP, Petrusz P, Bell GI, Brown CF, Korach KS, McLachlan JA, Teng CT 1988 Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 122:2355–2363 [DOI] [PubMed] [Google Scholar]
- Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA 1991 Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci USA 88:21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F 1996 Tyrosine kinase/p21ras/MAPK kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15:1292–1300 [PMC free article] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A 2000 A role for AKT in mediating the estrogenic functions of epidermal growth factor and insuline-like growth factor I. Endocrinology 141:4503–4511 [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y 2006 G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Donzè O, Picard D 1999 A nonradioactive method for inexpensive quantitative RT-PCR. J Biol Chem 380:695–697 [DOI] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Sisci D, del Rincon S, Cascio S, Tu X, Vecchione A, Sauter ER, Miller Jr WH, Surmacz E 2004 Nuclear insulin receptor substrate 1 interacts with estrogen receptor α at ERE promoters. Oncogene 23:7517–7526 [DOI] [PubMed] [Google Scholar]
- Curran T, Franza BR J 1988 Fos and Jun: the AP1 connection. Cell 55:395–397 [DOI] [PubMed] [Google Scholar]
- Freeman M 2000 Feedback control of intercellular signalling in development. Nature 408:313–319 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Conti M 2005 G-protein-coupled receptor signaling and the EGF network in endocrine systems. Trends Endocrinol Metab 16:320–326 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wiesen JF, Werb Z, Young P, Hom YK, Cooke PS, Lubahn DB 2000 Paracrine mechanisms of mouse mammary ductal growth. Adv Exp Med Biol 480:93–97 [DOI] [PubMed] [Google Scholar]
- Mukku VR, Stancel GM 1985 Regulation of epidermal growth factor receptor by estrogen. J Biol Chem 260:9820–9824 [PubMed] [Google Scholar]
- Filardo EJ, Thomas P 2005 GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–370 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER 2007 Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol 2:536–544 [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D 2006 G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84:287–297 [DOI] [PubMed] [Google Scholar]
- Marrache AM, Gobeil F, Zhu T, Chemtob S 2005 Intracellular signaling of lipid mediators via cognate nuclear G-protein-coupled receptors. Endothelium 12:63–72 [DOI] [PubMed] [Google Scholar]
- Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, Chemtob S 2006 Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF and LPA1 receptors. Can J Physiol Pharmacol 84:377–391 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjorberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR 2005 A G protein-coupled receptor for estrogen: the end of the search? Mol Interv 5:158–161 [DOI] [PubMed] [Google Scholar]
- Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER 2007 GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol 196:386.e1–386.e9, 386.e9–386.e11 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E 2006 Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ 2007 Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the novel estrogen receptor (GPR30) at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- Hill CS, Treisman R 1995 Differential activation of c-fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors. EMBO J 14:5037–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M 2002 AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136 [DOI] [PubMed] [Google Scholar]
- Karin M, Hunter T 1995 Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol 5:747–757 [DOI] [PubMed] [Google Scholar]
- Bonapace IM, Addeo R, Altucci L, Cicatiello L, Bifulco M, Laezza C, Salzano S, Sica V, Bresciani F, Weisz A 1996 17β-Estradiol overcomes a G1 block induced by HMG-CoA reductase inhibitors and fosters cell cycle progression without inducing ERK-1 and -2 MAP kinases activation. Oncogene 12:753–763 [PubMed] [Google Scholar]