Abstract
Androgen induces androgen receptor (AR) nuclear import, which allows AR to act as a transcriptional factor and ultimately leads to biological activity. However, the mechanism of AR translocation to the nucleus is still unclear. In the present study, we assessed the nuclear import abilities of each domain of AR and their mechanisms related to Ran and importin α/β using green fluorescent protein real-time imaging. The localization of AR to the nucleus in the absence and presence of ligands was dependent upon a complex interplay of the amino terminal transactivation domain (NTD), the DNA binding domain (DBD), and the ligand binding domain (LBD). NTD and DBD showed ligand-independent nuclear import ability, whereas LBD had ligand-dependent transport. In addition, AR deletion mutant lacking DBD was distributed in the cytoplasm regardless of ligand existence, suggesting that the remaining domains, NTD and LBD, are responsible for AR cytoplasmic localization. Cotransfection with a dominant negative form of Ran dramatically inhibited the nuclear import of all AR domains, and a dominant negative form of importin α prevented AR and DBD import. Importin β-knockdown strongly blocked DBD import. These results indicate that there are two additional nuclear localization signals (NLSs) in the NTD and LBD, and there are distinct pathways used to attain domain-specific AR nuclear import: the NLS of DBD is Ran and importin α/β-dependent, whereas the NLSs of NTD and LBD are Ran dependent but importin α/β-independent. Our data suggest that the nuclear import of AR is regulated by the interplay between each domain of the AR.
THE ANDROGEN RECEPTOR (AR) is a member of a steroid receptor superfamily that includes the glucocorticoid receptor (GR), mineralocorticoid receptor (MR), estrogen receptor α/β, and progesterone receptor A/B. Common features of this group include not only their sequence homology, but also their ability to activate target gene transcription via the hormone response element; the hormone-receptor complexes interact directly with their target genes to regulate transcription (1).
The AR, like other members of the steroid receptor superfamily, is composed of four principal domains: a large amino terminal transactivation domain (NTD), a DNA binding domain (DBD), a hinge region (H), and a carboxy-terminal ligand binding domain (LBD). Unliganded AR is located in the cytoplasm and forms a complex with heat shock protein 90 and other chaperone proteins (2). Androgen binding induces the dissociation of AR from the complex, followed by a conformational change that facilitates nuclear transport. Liganded AR interacts with the androgen responsive element as a dimer that triggers transcriptional activity, ultimately leading to the biological response to androgen (3,4). Thus, nuclear translocation is a prerequisite for AR’s function as a transcriptional factor, and the failure of the receptor to activate its target genes in the presence of ligand results in target organ resistance to the hormone (5). Many studies have revealed that AR gene mutations from single nucleotide mutation to complete gene failure contribute to diseases that include spinobulbar muscular atrophy (SBMA), androgen insensitivity syndrome (AIS), and prostate cancer (1,6,7,8).
The McGill database contains core mutation maps and a variety of mutation types for AIS phenotypes and prostate cancer (9). According to this database, although mutations are spread throughout the AR gene, there are hot spots, especially at exon 3 and exon 5, which encode the DBD and LBD, respectively. Several reports have revealed that mutant ARs obtained from both AIS and prostate cancer patients can be characterized by their abnormal intracellular localization and lower capacity for ligand-dependent translocation when compared with wild-type AR (6,7,8,10): AR mutants fail to achieve normal nuclear import and exhibit a peculiar intracellular aggregation profile. From these findings, it is conceivable that the characteristics of these mutant ARs could be involved in the pathogenesis of AR-related diseases (8,11). The elucidation of the nuclear import mechanism of AR not only provides us with a target for the endocrinological regulation of androgens but could also contribute to an understanding of androgen’s influence on AR-related diseases.
Among the potential eukaryotic nuclear import mechanisms, active import pathways mediated by the nuclear localization signal (NLS) are the most likely because molecules larger than approximately 40 kDa cannot pass through the nuclear pore complexes (NPCs) on the nuclear envelope. The classical NLS (cNLS) was isolated from the SV40 large T antigen, and it binds to importin α. After complexation with importin β, the cNLS-containing protein/importin α/β trimer crosses the nuclear envelope from the cytoplasm into the nucleus (12). Inside the nucleus, the trimer is dissociated by binding of Ran-GTP to importin β, allowing the isolated cNLS-containing protein to function. Nuclear protein export from the nucleus is analogous to nuclear import, except that the exportins, distinct importin β-family members are involved. Dependent on Ran-GTP, exportins specifically recognize nuclear export signals (NESs) and the trimeric complex of exportin/NES-containing protein/Ran-GTP moving through the NPC. Ran-GTP hydrolysis is then catalyzed by cytoplasmic RanGTPase-activating protein (12). In such a manner, molecules containing NLS and NES are considered to shuttle between the nucleus and cytoplasm.
With regard to nuclear import, one region of the AR has gained attention as a regulator of receptor trafficking. The DNA-binding zinc finger region (DBD) together with the flanking H constitutes a tri-partite type NLS consisting of three clusters of Lys and Arg residues (13) that are similar in sequence to the SV40 cNLS. AR NLS acts constitutively and participates in rapid nuclear import that is less than 60 min (13,14) after a specific ligand treatment, and Zhou et al. (15) found that the amino acid substitutions of 632, 633 are sufficient to prevent the nuclear import of AR. Recent reports demonstrated the existence of additional NLSs in the LBD of GR (16,17) and the NTD of MR (18).
In this study, to resolve the mechanism mediating the nuclear import of AR, we observed AR domain-specific trafficking between the cytoplasm and the nucleus with the use of the green fluorescent protein real-time imaging technique. We report here that the localization of AR to the nucleus in the absence or presence of the ligand was dependent upon the complex interplay of at least three NLSs of different compositions located in the NTD, DBD, and LBD of the AR. Furthermore, the nuclear transfer of potent NLSs located in AR DBD works via the importin α/β and Ran-GTP pathways, and importin-independent pathways are also involved in the nuclear import of NLS sequences in the NTD and LBD.
Materials and Methods
All experiments were conducted according to the guidelines of the Experimental Committee of Kyoto Prefectural University of Medicine.
Plasmid construction
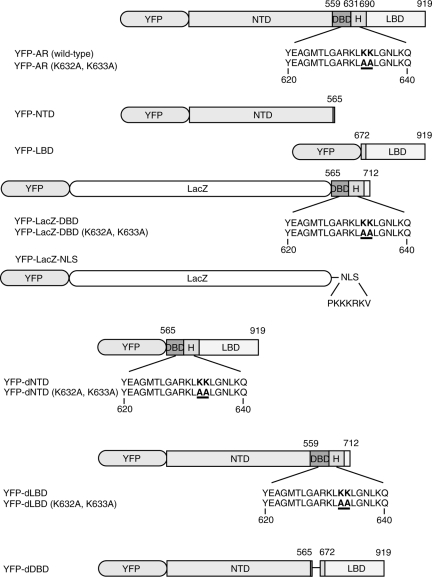
Human full-length wild-type AR (19) was inserted into plasmid pEYFP-C1 from Clontech Laboratories Inc. (Mountain View, CA), generating the pEYFP-AR plasmid. pEYFP-AR (K632A, K633A) was derived from pEYFP-AR by site-directed mutagenesis using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA) to introduce alanine substitutions at amino acids 632 and 633 (K632A, K633A). pEYFP-NTD was derived from pEYFP-AR by subcloning respective fragments of the amino terminus (amino acids 1–565) into pEYFP-C1. The LBD sequence, corresponding to amino acids 672–919, was PCR amplified and subcloned into pEYFP-C1 to generate pEYFP-LBD. pEYFP-LacZ-DBD encodes the β-galactosidase (LacZ) coding sequence as well as AR DBD, including the identified AR NLS sequence, encompassing residues 565–712. pEYFP-LacZ-DBD (K632A, K633A) is a derivative of pEYFP-LacZ-DBD that contains alanine substitutions at 632 and 633. pEYFP-NTD deletion (dNTD) and pEYFP-dNTD (K632A, K633A) were generated to delete the NTD regions (amino acids 1–564) from pEYFP-AR and pEYFP-AR (K632A, K633A), respectively. pEYFP-LBD deletion (dLBD) and pEYFP-dLBD (K632A, K633A) were also generated to delete the LBD regions (amino acids 713–919) from pEYFP-AR and pEYFP-AR (K632A, K633A), respectively. pEYFP-DBD deletion (dDBD) was constructed by the fusion of both NTD (amino acids 1–565) and LBD (amino acids 672–919) fragments into pEYFP-C1. To obtain a positive control cargo molecule, the pEYFP-LacZ-NLS plasmid encoding LacZ and the cNLS sequence (amino acids: PKKKRKV) from the SV40 large T antigen was constructed.
pET-3d-RanQ69L encoding full-length human Ran with a leucine substitution at Gln69 was kindly provided by Dr. Y. Yoneda (Osaka University, Osaka, Japan). To generate pECFP-RanQ69L, the coding sequence for RanQ69L was subcloned into pECFP-C1 plasmid from Clontech Laboratories. pECFP-importin α-dIBB was constructed from pECFP-importin α, which codes full-length wild-type mouse importin α, by generating a truncated mutant encoding residues 54–529.
Cell culture and transfections
COS-1 cells were cultured in DMEM (Life Technologies, Inc., New York, NY) supplemented with 10% fetal bovine serum, and 0.1 mg/ml streptomycin and 100 U/ml penicillin. In coexpression studies with dominant negative mutants of import factors, 1.0 × 104 cells were seeded on glass-bottom dishes with a 10-mm diameter. Transfections were conducted using LipofectAmine Plus reagent (Invitrogen Corp., Carlsbad, CA) with 25 ng of the cargo protein expression vector and 75 ng of the import factor expression vector. After overnight incubation after transfection, the cells were incubated in Opti-MEM reduced serum medium (Life Technologies) with or without 1 μm testosterone. Real-time images were captured by a LSM510META confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with an argon ion laser.
For RNA interference experiments, 1.0 × 104 cells were seeded on glass-bottom dishes and transfected with 20 pmol importin β-small interfering RNA (siRNA) using LipofectAmine 2000 reagent (Invitrogen) according to the manufacturer’s procedure. On the following day, 100 ng of the cargo protein expression vector were introduced to importin β-suppressed cells using LipofectAmine Plus reagent. At least 12 h after the second transfection, the intracellular distribution of each import molecule was visualized using an LSM510 confocal microscope in the absence or presence of 1 μm testosterone.
Western blotting
There were 2 × 105 cells seeded on 35-mm dishes and transfected with or without the respective plasmids and siRNA. The cells were washed with PBS and collected in 250 μl PBS using cell scrapers. After treatment with 0.1% Triton X-100 and 10 U deoxyribonuclease I (Roche, Basel, Switzerland), a portion of the lysate was used to determine protein content by BCA (bicinchoninic acid) protein assay (Pierce, Rockford, IL). Remaining lysates were denatured in parallel by addition of 1 vol 2× sodium dodecyl sulfate sample buffer and boiled at 95 C for 5 min for PAGE, followed by immunoblotting. Proteins were transferred onto polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA) and incubated with the optimized antibodies. Bands were ultimately visualized with a streptavidin-biotin reaction followed by development with diaminobenzidine.
Quantification of subcellular distribution
Transfected cells were classified into five categories according to a scoring system widely used to examine the subcellular localization of steroid receptors (16,20,21). The line scanning values of fluorescence intensity ratio between the cytoplasm and the nucleus in cells that could be classified were characterized as such (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org): N, nuclear fluorescence was relatively high, and cytoplasmic fluorescence was not detected; N>C, nuclear fluorescence showed maximum intensity, and cytoplasmic fluorescence was low; N=C, nuclear and cytoplasmic fluorescence was equal; N<C, cytoplasmic fluorescence was higher than nuclear fluorescence; and C, cytoplasmic fluorescence was relatively high, and nuclear fluorescence was not detected. To semiquantify the aforementioned classification, each score was expressed with a numerical order corresponding to its subcellular distribution: N, N>C, N=C, N<C, and C were 5, 4, 3, 2, and 1, respectively (supplemental Fig. 1). Each experiment was repeated at least three times, and we assessed more than 100 cells in each treatment, as described in the legend of Table 1. One-way ANOVA was used for the double-transfection experiments with RanQ69L or dIBB compared with single transfection, and the Student’s t test was used for siRNA transfection experiments compared with the control transfection for statistical analysis.
Table 1.
Import inhibition indexes by cotransfection
| LacZ-NLS | AR + T | NTD | LacZ-DBD | LBD + T | |
|---|---|---|---|---|---|
| Single | 4.87 ± 0.09 | 4.81 ± 0.06 | 3.97 ± 0.03 | 4.27 ± 0.07 | 3.88 ± 0.06 |
| + RanQ69 liter | 2.39 ± 0.20a (51% reduction) | 2.03 ± 0.09a (58% reduction) | 3.03 ± 0.03a (24% reduction) | 1.82 ± 0.13a (57% reduction) | 3.21 ± 0.08a (17% reduction) |
| + dIBB | 4.09 ± 0.09a (16% reduction) | 4.35 ± 0.13a (10% reduction) | 4.03 ± 0.02 | 3.93 ± 0.15 | 3.76 ± 0.07 |
| + Control siRNA | 4.91 ± 0.03 | 4.58 ± 0.33 | 3.97 ± 0.03 | 4.14 ± 0.09 | 3.71 ± 0.08 |
| + Importin β siRNA | 3.16 ± 0.04a (36% reduction) | 4.47 ± 0.02a (2% reduction) | 3.90 ± 0.12 | 3.46 ± 0.09a (16% reduction) | 3.67 ± 0.11 |
Each index value is displayed with se. Double transfections with RanQ69L or dIBB were compared with single transfections of each cargo molecule and analyzed statistically by ANOVA. Importin β -siRNA transfection was compared with control siRNA transfection and analyzed statistically by the t test. T, Testosterone.
Significant differences (P < 0.05).
Antibodies and reagents
Testosterone was purchased from Nacalai Tesque (Kyoto, Japan). Anti-AR antibody (N-20), anti-importin α (C-20), importin β siRNA and, control siRNA were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal 3E9 against importin β and 6C5 against glyceraldehyde-3-phosphate dehydrogenase were purchased from Abcam (Cambridge, UK).
Results
Plasmid construction of AR chimera expression vectors
AR and several truncated mutants were generated to verify their functions and relationship with regard to nuclear translocation (Fig. 1). YFP-AR contains the entire wild-type human AR sequence, and YFP-AR (K632A, K633A) has a mutant NLS that is inactivated. YFP-NTD, -LBD, and -LacZ-DBD contain each functional domain in the whole AR. YFP-dNTD, -dLBD, and -dDBD contain combinations of DBD and LBD, NTD and DBD, and NTD and LBD, respectively. The cNLS derived from SV40 large T antigen was used as a positive control. In the cases of DBD, DBD (K632A, K633A), and NLS, the LacZ coding gene was placed between the YFP marker and each signal domain to obtain sufficient molecular mass.
Figure 1.
Plasmid construction of YFP-full length AR and YFP-AR deletion mutant fusion proteins. AR contains four domains: NTD, DBD, H, and LDB. Mutants were created for each domain: YFP-NTD, YFP-LBD, and YFP-LacZ-DBD. The domain deletion mutants YFP-dNTD, YFP-dLBD, and YFP-dDBD were also created. A DBD mutant was also created with K632A and K633A to disrupt the already known NLS of AR. YFP-LacZ-NLS containing the SV40 large T-antigen NLS was also created as the positive control for nuclear import.
Subcellular distribution of full-length and mutant AR
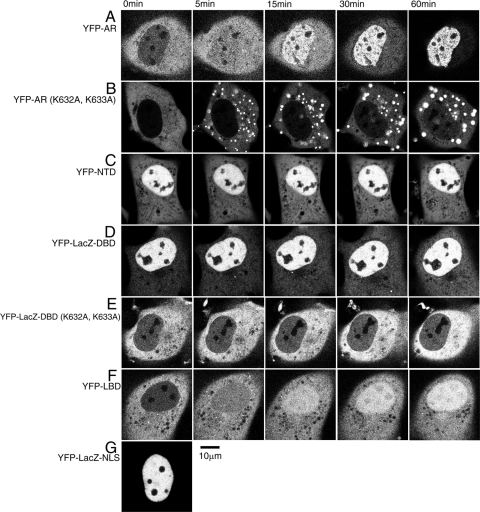
Wild-type full-length AR labeled with YFP existed predominantly in the cytoplasm in the absence of ligand. Testosterone stimulation caused rapid nuclear import of YFP-tagged AR. At 5 min after stimulation, the boundary between the cytoplasm and nucleus became equal in fluorescence intensity, with the nuclear fluorescence gradually becoming stronger than that of the cytoplasm by 15–30 min. Almost all YFP-ARs accumulated in the nucleus within 60 min, with typical punctuate distribution previously shown by our group (4) (Fig. 2A). YFP-AR (K632A, K633A), a YFP-full-length AR with a two amino acid substitution, was also localized to the cytoplasm in the absence of ligand, however, testosterone treatment caused cytoplasmic aggregation by 60 min after the ligand addition instead of rapid nuclear import (Fig. 2B). Because these two amino acids located in the DBD region have a great influence on YFP-AR nuclear import, it is likely that this region serves as an NLS for AR. In addition, it was found that the cytoplasmic aggregation was ligand dependent and transient because ligand withdrawal dissolved the aggregation (supplemental Fig. 2). From these observations, it is likely that ligand-activated but import-deficient AR (K632A, K633A) tends to accumulate in the cytoplasm.
Figure 2.
Subcellular distribution of full-length AR and each domain mutant. YFP-fused forms of full-length AR and each domain mutant were transiently expressed in COS-1 cells a day before observation. Time-lapse analysis of nuclear trafficking of each protein was performed after addition of 10 μm testosterone. YFP-full-length AR migrated into the nucleus completely within 60 min (A), and the amino acid substitutions K632A and K633A dramatically reduced the nuclear import of AR (B), and LacZ-DBD (E). Observation of the domain mutants NTD (C), LacZ-DBD (D), and LBD (F) revealed that they have nuclear import abilities with different characteristics. G, LacZ-NLS was a positive control for nuclear import via the importin α/β and Ran-GTP pathways.
To investigate the nuclear import properties of each AR domain, three domains of NTD, DBD, and LBD were examined by real-time imaging. The amino terminal domain YFP-NTD that lacked DBD and LBD was distributed predominantly in the nucleus in the absence of ligand. YFP-NTD was constitutively nuclear (Fig. 2C). Because DBD is less than 20 kDa, and it has been previously revealed that molecules smaller than 40 kDa can passively diffuse through the NPC, the LacZ protein was added between YFP and DBD to increase the molecular size. YFP-LacZ-DBD was more likely constitutively nuclear and was not affected by hormone (Fig. 2D). Similarly to YFP-full-length AR (K632A, K633A), YFP-LacZ-DBD (K632A, K633A) was distributed as constitutively cytoplasm, and testosterone did not stimulate its cytoplasmic aggregation (Fig. 2E). The difference of the aggregation pattern between YFP-AR (K632A, K633A) and YFP-LacZ-DBD (K632A, K633A) may be due to the existence of NTD and LBD. The LBD YFP-LBD was distributed predominantly in the cytoplasm before ligand treatment. The addition of testosterone triggered the nuclear translocation of YFP-LBD, but the import speed was slower than that of YFP-full-length wild-type AR (Fig. 2F); at 5 min after testosterone addition, cytoplasmic YFP-LBD fluorescence was still dominant and gradually moved into the nucleus. At 60 min it showed a nuclear as well as a cytoplasmic distribution. In addition, within the nucleus the other mutants showed a specific localization that excluded the nucleoli (Fig. 2, A, C, and D), whereas liganded intranuclear YFP-LBD showed a homogeneous distribution pattern (Fig. 2F).
These observations indicate that AR, NTD, DBD, and LBD each contain individual NLSs with varying degrees of ligand dependency. In addition, to clarify which direction of protein translocation, from the cytoplasm to the nucleus or from the nucleus to the cytoplasm, decides this distribution, we performed whole nuclear fluorescence recovery after photobleaching analysis, and, as a result, these three NLSs were imported from the cytoplasm to the nucleus (supplemental Fig. 3). All three functional domains showed different patterns of trafficking from the movement of YFP-full-length AR. Thus, it is likely that interaction within domains may mediate full-length AR nuclear import. In the next study, the intracellular dynamics of each AR deletion mutant with two functional domain combinations were analyzed.
Subcellular distribution of domain deletion AR mutants
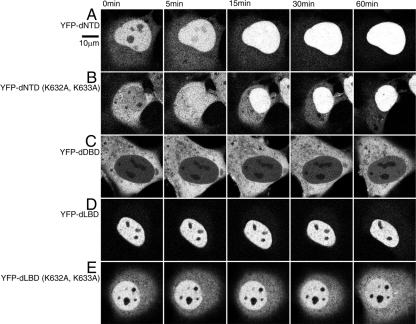
To evaluate the effect of interdomain relationships on nuclear import capacity, deletion mutants that lacked one of three functional domains, NTD, DBD, or LBD, were prepared (Fig. 1).
YFP-dNTD, which has a functional DBD and LBD, was localized predominantly in the nucleus but not the nucleoli in the absence of the ligand. After testosterone treatment the remaining YFP-dNTD in the cytoplasm migrated into the nucleus, where YFP-dNTD occupied the whole nuclear area at 15 min. Finally, almost all YFP-dNTD was imported into the nucleus at 60 min (Fig. 3A). YFP-dNTD (K632A, K633A), which has inactivated DBD and LBD NLSs, was distributed in not only the cytoplasm but also the nucleus. The distribution pattern of both YFP-dNTD and YFP-dNTD (K632A, K633A) was opposite in the absence of ligand; YFP-dNTD was predominant in the nucleus compared with the cytoplasm, whereas YFP-dNTD (K632A, K633A) was more prominent in the cytoplasm than in the nucleus. The addition of testosterone caused time-dependent nuclear import of YFP-dNTD (K632A, K633A) with final accumulation in the nucleus (Fig. 3B).
Figure 3.
Subcellular distribution of each domain deletion AR mutant. YFP-fused forms of domain deletion mutants were transiently transfected in COS-1 cells a day before observation. Time-lapse analysis of the nuclear trafficking of each protein was performed after addition of 10 μm testosterone. At 0 min, YFP-dNTD (A) was distributed predominantly in the nucleus in a manner similar to YFP-LacZ-DBD, and ligand treatment further enhanced nuclear import. Amino acid substitutions K632A and K633A changed the ligand-free distribution of YFP-dNTD such that it resembled that of YFP-LBD (B). YFP-dLBD showed nuclear localization regardless of the presence of ligand (D), and amino acid substitutions K632A and K633A caused a slight redistribution toward the cytoplasm similar to that observed for YFP-NTD (E). Interestingly, YFP-dDBD was obviously localized to the cytoplasm (C).
YFP-dDBD, which has NTD and LBD, remained in the cytoplasm regardless of the presence of the ligand. No nuclear YFP-dDBD signals were noticed even at 60 min (Fig. 3C). To clarify the possible existence of anchor molecules involved in YFP-dDBD ligand insensitivity, cytoplasmic mobility was examined with fluorescence recovery after photobleaching analysis (supplemental Fig. 4). According to the result that there is no difference in cytoplasmic mobility between YFP-full-length AR and YFP-dDBD, the cytoplasmic distribution of YFP-dDBD is not due to its hormone insensitivity. These results indicate that NTD and LBD might cooperate to achieve cytoplasmic localization even in the presence of ligand.
YFP-dLBD, which has NTD and DBD, was transported into the nucleus with or without the presence of ligand (Fig. 3D). YFP-dLBD did not show any change of its distribution on ligand stimulation, and it remained in the nucleus at 60 min after testosterone treatment. Substitution of two amino acids in the NLS site of YFP-dLBD (K632A, K633A) resulted in predominant distribution in the nucleus in both the absence and presence of the ligand throughout the investigated time course (Fig. 3E).
From these distribution patterns, we identified a hierarchical relationship between these domains: the distribution of dNTD tended to match the DBD localization (Figs. 3A and 2D), and the distribution of dDBD tended to match LBD localization without the ligand (Figs. 3C and 2F). In addition, the intracellular distribution of dLBD was similar to that of DBD (Figs. 3D and 2D). Thus, among these three domains, the following order is estimated for nuclear import potency: DBD > NTD > LBD.
Import inhibition assays using a cotransfection technique
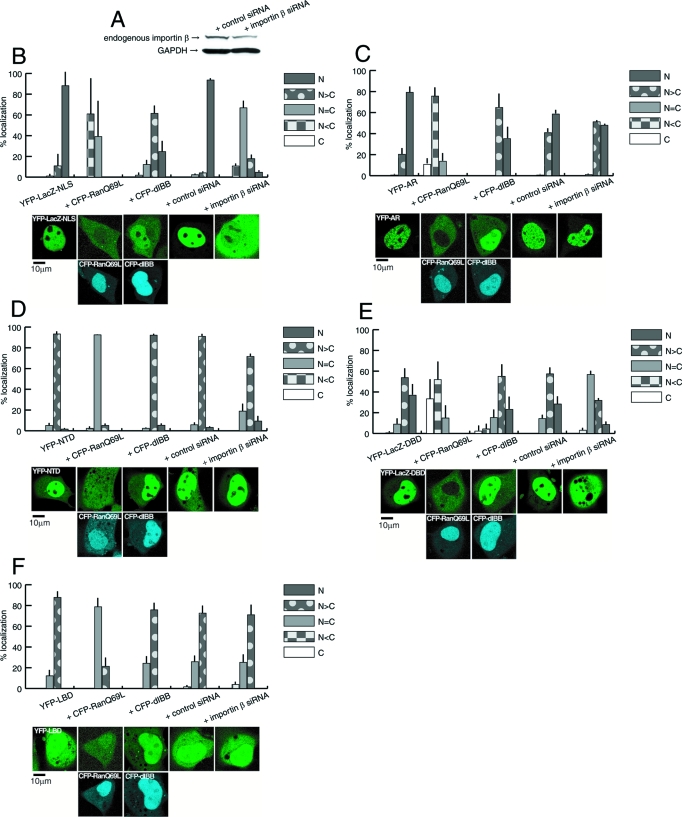
It has been revealed that the cNLS is imported into the nucleus via the importin α/β and Ran-GTP system. Consistent with previous reports, the NLS-tag dramatically introduced the LacZ protein to the nucleus, indicating that LacZ-NLS could be a positive control for nuclear import by the importin α/β and Ran-mediated pathways.
We aimed to resolve whether the nuclear transport factors importin α/β and Ran-GTP that mediate the nuclear import of the cNLS are also involved in the nuclear import of AR functional domains. A transfection technique was used to inhibit endogenous transport factors. Ran-GTP contributes to intracellular recycling of importin β between the nucleus and cytoplasm, therefore, the dominant negative expression of the GTP hydrolysis-deficient form of Ran-GTP, RanQ69L, was hypothesized to break the normal import pathway. Importin α initially recognizes a NLS containing protein, and it acts as an adapter to bind importin β, therefore, the dominant negative expression of the importin β-binding domain-truncated importin α, dIBB, was predicted to prevent the nuclear import of dIBB-binding NLS protein. Importin β binds the importin α-recognized NLS protein heterodimer, then brings it to the nucleus through the NPC in an energy dependent manner. Thus, we speculated that RNA interference knockdown of endogenous importin β could prevent the import of α/NLS protein into the nucleus. By setting up five criteria on the distribution (supplemental Fig. 1), we studied changes in the localization of each molecule.
Inhibition of the cNLS was tested to make sure that these conditions prevent the endogenous nuclear transport pathway. Single transfection of YFP-LacZ-NLS showed nuclear localization (Fig. 4B). Quantitative analysis of subcellular distribution revealed that CFP-RanQ69L expression dramatically inhibited YFP-LacZ-NLS nuclear import. The import inhibition index showed a 51% reduction of the subcellular distribution of YFP-LacZ-NLS by CFP-RanQ69L. CFP-dIBB induced a moderate but significant inhibition of import (16% reduction). Importin β-RNA interference by siRNA transfection strongly affected the nuclear translocation of YFP-LacZ-NLS with a 36% reduction compared with the control (Fig. 4B). Thus, these cotransfection conditions were demonstrated to be useful in determining whether specific import factors play an important role in the nuclear import of a targeting molecule.
Figure 4.
Import inhibition assays by cotransfection. A, Western blotting analysis of the whole cell lysate of COS-1 cells. COS-1 cells were transfected with either control siRNA or importin β-siRNA, and the amounts of endogenous importin β and glyceraldehyde-3-phosphate dehydrogenase were identified as described in Materials and Methods. B–F, Single transfections of each cargo molecule, and double transfections of each cargo molecule with RanQ69L, dIBB, control siRNA, or importin β siRNA. B, LacZ-NLS displayed the nuclear localization, whereas simultaneous expression of RanQ69L or dIBB diminished its nuclear movement. In addition, importin β-knockdown prevented the nuclear localization of Lac-NLS when compared with control siRNA cotransfection. C, Wild-type full-length AR normally showed nuclear localization with a punctuate pattern at 60 min after ligand treatment. Double transfection with RanQ69L and dIBB both suppressed AR nuclear localization, and importin β-knockdown moderately decreased its nuclear trafficking. D, NTD showed relative nuclear localization without the ligand. The cotransfection of RanQ69L prevented nuclear translocation, but dIBB did not affect the general distribution. Moreover, importin β-knockdown caused little change in NTD subcellular localization. E, LacZ-DBD was predominantly distributed in the nucleus, and coexpression with RanQ69L and dIBB greatly or moderately affected its localization, respectively. Importin β-knockdown prevented the nuclear trafficking of LacZ-DBD. F, At 60 min after ligand treatment, LBD displayed a predominantly nuclear pattern. Both dIBB and importin β-knockdown did not change the distribution patterns of LBD when compared with the influence of the simultaneous existence of RanQ69L on nuclear localization. All distribution properties were divided into five categories according to supplemental Fig. 1. and indicated as line charts. Representative images of each treatment are shown below. Upper panels are the fluorescence images of YFP-tagged import molecules, and bottom panels are the fluorescence images of CFP-tagged RanQ69L or dIBB. The columns show double expression in a single cell.
As shown previously, YFP-AR migrated into the nucleus in a ligand-dependent manner with a resulting punctuate distribution (Fig. 4C). When CFP-RanQ69L was cotransfected, the nuclear import of YFP-AR was obviously inhibited (58% reduction). CFP-dIBB expression prevented YFP-AR nuclear import partially but significantly (10% reduction). Importin β-siRNA treatment caused a slight decrease (2% reduction) in the nuclear import of YFP-AR when compared with the control treatment (Fig. 4C).
Single transfection of YFP-NTD displayed a predominant nuclear distribution (Fig. 4D). Coexpression of CFP-RanQ69L diminished the nuclear import activity (24% reduction), whereas cotransfection of CFP-dIBB and importin β-siRNA did not cause any significant changes in YFP-NTD intracellular localization: localization pattern of + CFP-dIBB, + control siRNA, and + importin β-siRNA is the same (Fig. 4D).
YFP-LacZ-DBD exhibited almost complete nuclear localization. CFP-RanQ69L coexpression greatly decreased this effect (57% reduction), but CFP-dIBB only tended to shift slightly the distribution from the nucleus to the cytoplasm (8% reduction). Interestingly, cells transfected with importin β-siRNA showed a significant inhibition of YFP-LacZ-DBD nuclear import similar to that of YFP-LacZ-NLS (Fig. 4, B and E).
YFP-LBD migrated slowly in response to ligand addition (Fig. 4F). Similarly to YFP-AR and the other truncated domains, YFP-LBD nuclear translocation was significantly reduced by CFP-RanQ69L coexpression (17% reduction). However, cotransfection of CFP-dIBB and importin β-siRNA did not affect the nuclear import activity of YFP-LBD (Fig. 4F).
From these results the presence of RanQ69L prevented the nuclear import of all cargo molecules, and greater than 50% reductions were observed for LacZ-NLS, AR, and LacZ-DBD. RNA interference of importin β did not affect the other mutants or strongly inhibit AR import; only LacZ-DBD showed a significant inhibition similar to LacZ-NLS (Fig. 4, B and E). Although dIBB did not have much influence on LacZ-DBD nuclear import, the nuclear translocation of liganded AR was significantly prevented (Fig. 4C). Accordingly, it is possible that LacZ-DBD has a potent role in the nuclear transport of full-length ARs mediated by the NLS located in DBD, which may use the importin α/β and Ran-GTP pathway for nuclear localization. However, the NLS in the NTD and LBDs showed importin-independent translocation pathways. Semiquantification of subcellular distribution in the import inhibition assay is summarized in Table 1.
Discussion
We have investigated the mechanism of nuclear trafficking of AR in living cells by characterizing the specific features of each domain in the AR molecule. Our observations revealed that AR has at least three NLSs. The first NLS in the DBD has been well characterized (15), and even though the second NLS in the LBD has not been characterized to a specific amino acid sequence (16), its nuclear import ability has been confirmed (14,22). Thus far, no attention has been given to the third NLS in the NTD. In the case of MR, Walther et al. (18) recently showed that the amino terminal domain of MR contains a third NLS named NL0 that has a capacity for nuclear import activity in the absence of ligand. The distribution pattern of the AR NTD is similar to that of MR NL0, but there is no significant homology in the serine-threonine motifs of MR NL0 and AR NTD.
In the present study, the distribution of all three NLSs was not consistent with that of full-length AR, particularly in the cytoplasmic localization in the absence of ligand (Fig. 2, A, C, D, and F). These results are consistent with previous studies (13,22,23). Our studies showed that cytoplasmic localization of unliganded AR requires cooperation by NTD and LBD (Fig. 3C), and the nuclear import of liganded AR requires DBD (Fig. 2, B and E). The major function of AR is the regulation of specific gene expression in a ligand-dependent fashion, thus, the regulation of AR nuclear import and export represents an essential step in androgen action. Saporita et al. (24) found a ligand-regulated NES in AR LBD. Therefore, it is logical that unliganded LBD would be localized in the cytoplasm and that the LBD NES might take part in the cytoplasmic localization of full-length AR. The authors have suggested that the LBD NES is dominant over the DBD NLS in the absence of ligand and that it is repressed by ligand binding (24). However, our data indicated the opposite; we found that the DBD NLS is dominant over the LBD NES (Fig. 3, A and B). This discrepancy may have occurred because Saporita et al. (24) excluded other NLSs in NTD and LBD, whereas our data gave some evidence that AR functional domains have various capabilities for nuclear import and cytoplasmic retention. Henceforth, it is expected that the accumulation of such detailed information could elucidate the regulatory mechanism for AR intracellular trafficking.
Our study indicated that Ran is required for the nuclear import of all three domains; however, importins are only for that of the DBD. We speculate that some compensational nuclear import systems prevent androgen insensitivity caused by mutation in the AR gene. Ran is an abundant GTP-binding protein that is required for NLS-mediated accumulation of nuclear targeting proteins. The expression of the Ran mutant G19V, a constitutively GTP-bound isoform, dramatically reduces the accumulation of GR in the nucleus (25). In the present study, expression of RanQ69L, which is also a constitutively GTP-bound form mutant, markedly inhibited the nuclear import of AR. Among steroid receptors, AR particularly resembles GR in terms of its intracellular movement in response to ligands (4,26), therefore, it is plausible that AR uses the same pathway as GR for nuclear translocation. The nuclear import mechanism of GR has been well investigated and involves two NLSs (NL1 and NL2). NL1 located in the DBD is similar in sequence to the SV40 large T-antigen NLS, whereas NL2 is poorly defined and resides in the LBD (17). An in vitro import assay using digitonin-permeabilized cells revealed that the GR DBD can bind importin α, and be imported by importin α/β and Ran to the nucleus. The present study showed that the nuclear import of the AR DBD depended on importin α/β, and that the importin α/β (and Ran)-mediated pathway also regulated the nuclear import of full-length AR. In addition, Freedman and Yamamoto (17) showed that importin 7 is necessary and sufficient for the nuclear import of the GR DBD. Importin 7 is a member of the importin β-family, so we cannot exclude the possibility that other importins can interact with the AR DBD and other AR NLSs. In humans there are three subfamilies of importin α (27), and in mammals 20 proteins of the importin β/Ran-binding families have been identified (28). This diversity may be the cause of the slight reduction of AR DBD nuclear import in the dIBB importin α-deletion mutant.
There are other molecules that are likely to participate in the intracellular movement of AR. The actin-binding protein filamin, a 280-kDa component of the cytoskeleton, might be involved in the nuclear translocation of liganded AR because nuclear import of AR failed in filamin-deficient cells (29). Another candidate is the multifunctional protein β-catenin. It is well established that β-catenin plays a central role in cell adhesion and is also a major component of the Wnt/Wingless signaling pathway (30). When the Wnt/Wingless pathway is stimulated, stabilized β-catenin can translocate to the nucleus to achieve specific gene expression. When coexpressed, both AR and β-catenin migrated into the nucleus with the same time progression (31). Direct interaction between the AR LBD and β-catenin may occur based on a report that β-catenin itself can act as a nuclear import receptor for its partner transcription factor Lef-1 (32). This potential interaction may allow β-catenin to be involved directly in the nuclear import of AR. More than 90% of colorectal cancers contain mutations that result in aberrant activation of the Wnt signaling pathway. Stable β-catenin accumulates in the nucleus where it can complex with the Lef-1/Tcf family of transcription factors, and activate that transcription leads to cell proliferation and transformation (33). Detailed investigation of the effects of filamin or β-catenin on each specific domain of AR could help to confirm this mechanism.
Our studies showed that strong import inhibition after the loss of the DBD NLS by amino acid substitution caused the intracellular accumulation of exogenous AR. This accumulation phenomenon was often observed in transiently expressed wild-type AR (data not shown), and was always ligand-dependent and reversible upon withdrawal of the ligand (supplemental Fig. 1). Therefore, it is possible that excess liganded AR could aggregate, particularly when nuclear import is prevented. The cytoplasmic aggregation of DBD NLS-deficient AR may result in pathological AR accumulation in SBMA patients (6,7). Because the genomic locus of AR is on the X chromosome, AR mutations in males have a dominant effect. The expansion of a glutamine tract in the AR amino terminus results in the formation of nuclear inclusions and inactivation of the receptor, which subsequently causes adult onset SBMA (34,35). In AIS, mutations and deletions in the AR gene cause minimal to complete loss of AR activity that results in various phenotypical manifestations ranging from fertile but undervirilized males to complete testicular feminization (5). AR is also involved in the development and progression of prostate cancer, which is one of the most frequency diagnosed cancers in males. Somatic mutations of the AR gene have been found in prostate tumors that contribute to androgen-independent growth of cancer cells (36). AR activation by androgens also plays an important role in both the development and progression of prostate cancer (37). Because nuclear AR is common in most prostate cancers, targeting AR for export out of the nucleus might reverse or prevent cell transformation.
Nucleocytoplasmic transport of oncogenes and tumor suppressors is disrupted in many different types of cancer cells. In some cases, mutation or altered expression of key nuclear receptors such as importin α or disruption of the Ran-GTP/GDP gradient could alter the nuclear/cytoplasmic distribution of nuclear factors, including oncoproteins and tumor suppressors. A truncated form of importin α that lacks the NLS binding domain was discovered in a breast cancer cell line (38). In these cells the nuclear import of the p53 tumor suppressor was reduced, resulting in accumulation of p53 in the cytoplasm. In addition, nuclear mislocalization of both nuclear factor-κB and β-catenin has been observed in many cancer cells (39). Nuclear factor-κB in the nucleus plays a role in cell proliferation and inhibition of apoptosis; consequently, its nuclear mislocalization can lead to up-regulation of target genes and promote tumorigenesis (40). Considering these findings together with the results presented in this study, abnormality in nuclear import machinery as well as AR itself could be a cause of AR-related cancers.
In summary, we have identified an intramolecular relationship important for AR subcellular localization. NTD and LBD cooperatively act to enhance cytoplasmic retention, and the NLS in the DBD is active and dominant over the other NLSs. The nuclear import pathway of the potent NLS located in DBD is mediated by importin α/β and Ran. Our findings provide a mechanistic explanation for the nuclear localization of AR at the molecular level.
Supplementary Material
Acknowledgments
We thank Dr. Y. Yoneda (Osaka University, Osaka, Japan) for the kind gift of RanQ69L cDNA and Dr. H. Nawata (Kyusyu University, Fukuoka, Japan) for the kind supply of human androgen receptor cDNA. We have great respect for the work of Zhou et al. (15) in creating important deficient mutants of androgen receptor.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research from Ministry of Education, Science, Sports, Culture and Technology, Japan (to M.K. and K.-i.M.), and a Special Coordination Fund for Promoting Science and Technology, Japan (to M.K.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 17, 2008
Abbreviations: AIS, Androgen insensitivity syndrome; AR, androgen receptor; cNLS, classical nuclear localization signal; DBD, DNA binding domain; dDBD, DNA binding domain deletion; diBB, importin β-binding-domain deletion mutant of importin α; dLBD, ligand binding domain deletion; dNTD, amino terminal transactivation domain deletion; GR, glucocorticoid receptor; H, hinge region; LBD, ligand binding domain; MR, mineralocorticoid receptor; NES, nuclear export signal; NLS, nuclear localization signal; NPC, nuclear pore complex; NTD, amino terminal transactivation domain; SBMA, spinobulbar muscular atrophy; siRNA, small interfering RNA.
References
- Quigley CA, Bellis A, Marschke KB, El-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev [Erratum (1995) 16:546] 16:271–321 [DOI] [PubMed] [Google Scholar]
- Prescott J, Coetzee GA 2006 Molecular chaperons throughout the life cycle of the androgen receptor. Cancer Lett 231:12–19 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai I, Matsuda K, Nishi M, Ozawa H, Kawata M 2004 Imaging analysis of subcellular correlation of androgen receptor and estrogen receptor α in single living cells using green fluorescent protein color variants. Mol Endocrinol 18:26–42 [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Pinsky L, Beitel LK, Trifiro M 1999 Androgen insensitivity. Am J Med Genet 89:210–217 [DOI] [PubMed] [Google Scholar]
- Becker M, Martin E, Schneikert J, Krug HF, Cato ACB 2000 Cytoplasmic localization and the choice of ligand determine aggregate formation by androgen receptor with amplified polyglutamine stretch. J Cell Biol 149:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Dadgar N, Aphale A, Harrell JM, Kunkel R, Pratt WB, Lieberman AP 2004 Androgen receptor acetylation site mutations cause trafficking defects, misfolding, and aggregation similar to expanded glutamine tracts. J Biol Chem 279:8389–8395 [DOI] [PubMed] [Google Scholar]
- Kawate H, Wu Y, Ohnaka K, Tao R, Nakamura K, Okabe T, Yanase T, Nawata H, Takayanagi R 2005 Impaired nuclear translocation, nuclear matrix targeting, and intranuclear mobility of mutant androgen receptors carrying amino acid substitutions in the deoxyribonucleic acid-binding domain derived from androgen insensitivity syndrome patients. J Clin Endocrinol Metab 90:6162–6169 [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Lehvaslaiho H, Beitel LK, Lumbroso R, Pinsky L, Trifiro M 1998 The Androgen Receptor Gene Mutations Database. Nucleic Acids Res 26:234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi H, Matsuda K, Ochiai I, Kawauchi A, Mizutani Y, Miki T, Kawata M 2007 A differential ligand-mediated response of green fluorescent protein-tagged androgen receptor in living prostate cancer and non-prostate cancer cell lines. J Histochem Cytochem 55:535–544 [DOI] [PubMed] [Google Scholar]
- Georget V, Terouanne B, Lumbroso S, Nicolas J, Sultan C 1998 Trafficking of androgen receptor mutants fused to green fluorescent protein: a new investigation of partial androgen insensitivity syndrome. J Clin Endocrinol Metab 83:3597–3603 [DOI] [PubMed] [Google Scholar]
- Poon IK, Jans DA 2005 Regulation of nuclear transport: central role in development and transformation? Traffic 6:173–186 [DOI] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM 1991 Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem 266:510–518 [PubMed] [Google Scholar]
- Poukka H, Karvonen U, Yoshikawa N, Tanaka H, Palvimo JJ, Janne OA 2000 The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J Cell Sci 113(Pt 17):2991–3001 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sar M, Simental JA, Lane MV, Wilson EM 1994 Ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem 269:13115–13123 [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA 1999 Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol 19:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ND, Yamamoto KR 2004 Importin 7 and importin α/importin β are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell 15:2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther RF, Atlas E, Carrigan A, Rouleau Y, Edgecombe A, Visentin L, Lamprecht C, Addicks GC, Hache RJ, Lefebvre YA 2005 A serine/threonine-rich motif is one of three nuclear localization signals that determine unidirectional transport of the mineralocorticoid receptor to the nucleus. J Biol Chem 280:17549–17561 [DOI] [PubMed] [Google Scholar]
- Tomura A, Goto K, Morinaga H, Nomura M, Okabe T, Yanase T, Takayanagi R, Nawata H 2001 The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J Biol Chem 276:28395–28401 [DOI] [PubMed] [Google Scholar]
- Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P 1992 Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J 11:3681–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackey FN, Hache RJ, Reich T, Kwast-Welfeld J, Lefebvre YA 1996 Determinants of subcellular distribution of the glucocorticoid receptor. Mol Endocrinol 10:1191–1205 [DOI] [PubMed] [Google Scholar]
- Jenster G, Van Der Korput JA, Trapman J, Brinkmann AO 1992 Functional domains of the human androgen receptor. J Steroid Biochem Mol Biol 41:671–675 [DOI] [PubMed] [Google Scholar]
- Jenster G, Trapman J, Brinkmann AO 1993 Nuclear import of the human androgen receptor. Biochem J 293(Pt 3):761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z 2003 Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem 278:41998–42005 [DOI] [PubMed] [Google Scholar]
- Carey KL, Richards SA, Lounsbury KM, Macara IG 1996 Evidence using a green fluorescent protein-glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J Cell Biol 133:985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Ogawa H, Ito T, Matsuda K, Kawata M 2001 Dynamic changes in subcellular localization of mineralocorticoid receptor in living cells: in comparison with glucocorticoid receptor using dual-color labeling with green fluorescent protein spectral variants. Mol Endocrinol 15:1077–1092 [DOI] [PubMed] [Google Scholar]
- Goldfarb DS, Corbett A H, Mason DA, Harreman MT, Adam SA 2004 Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol 14:505–514 [DOI] [PubMed] [Google Scholar]
- Mosammaparast N, Pemberton LF 2004 Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol 14:547–556 [DOI] [PubMed] [Google Scholar]
- Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN 2000 Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol Endocrinol 14:1618–1626 [DOI] [PubMed] [Google Scholar]
- Clevers H 2006 Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC 2002 The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 277:17933–17943 [DOI] [PubMed] [Google Scholar]
- Asally M, Yoneda Y 2005 β-Catenin can act as a nuclear import receptor for its partner transcription factor, lymphocyte enhancer factor-1 (lef-1). Exp Cell Res 308:357–363 [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H 2003 Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653:1–24 [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH 1991 Androgen receptor gene mutations in X-linked spinal and bulbar muscle atrophy. Nature 352:77–79 [DOI] [PubMed] [Google Scholar]
- Adachi H, Katsuno M, Minamiyama M, Waza M, Sang C, Nakagomi Y, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Yoshida M, Hashizume Y, Sobue G 2005 Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 128(Pt 3):659–670 [DOI] [PubMed] [Google Scholar]
- Liao G, Chen L, Zhang A, Godavarthy A, Xia F, Ghosh JC, Li H, Chen JD 2003 Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J Biol Chem 278:5052–5061 [DOI] [PubMed] [Google Scholar]
- Reddy GPV, Barrack ER, Dou QP, Menon M, Pelley R, Sarkar F, Sheng S 2006 Regulatory processes affecting androgen receptor expression, stability, and function: potential targets to treat hormone-refractory prostate cancer. J Cell Biochem 98:1408–1423 [DOI] [PubMed] [Google Scholar]
- Kim I, Kim D, Han S, Chin M, Nam H, Cho H, Choi S, Song B, Kim E, Bae Y, Moon Y 2000 Truncated form of importin α identified in breast cancer cell inhibits nuclear import of p. J Biol Chem 275:23139–23145 [DOI] [PubMed] [Google Scholar]
- Kau TR, Way JC, Silver PA 2004 Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 4:106–117 [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li Z 2002 NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.