Abstract
Gonadal steroids induce synaptic plasticity in several areas of the adult nervous system. In the arcuate nucleus of adult female rats, 17β-estradiol triggers synaptic remodeling, resulting in a decrease in the number of inhibitory synaptic inputs, an increase in the number of excitatory synapses, and an enhancement of the frequency of neuronal firing. In the present paper, we studied the specificity of hormonal effects by determining the changes in synaptic connectivity of tyrosine hydroxylase (TH) immunoreactive (IR) neurons in the arcuate nucleus. We combined pre-embedding TH and post-embedding γ-aminobutyric acid (GABA) immunostaining, and performed unbiased stereological measurements in gonadectomized and 17β-estradiol-treated rats. We conclude that the synaptic connectivity of the TH-IR neurons is different from the other, nonlabeled population, and the response to estradiol is not uniform. TH-IR (dopaminergic) arcuate neurons of both male and female rats have more GABAergic (inhibitory) axosomatic inputs than the nondopaminergic population. Our study shows that the effect of 17β-estradiol is sex and cell specific in the sense that not all arcuate neurons are affected by the structural synaptic remodeling. In ovariectomized females hormone treatment decreased the numerical density of GABAergic axosomatic synapses on TH-IR, but not on nondopaminergic, neurons, whereas in orchidectomized males, 17β-estradiol treatment increased inhibitory synapses onto nondopaminergic neurons but did not affect the number of inhibitory terminals onto TH-IR neurons. The hormone-induced plastic changes in synaptic connectivity of TH-IR neurons may serve as the morphological basis for the cyclical regulation of the anterior pituitary.
THE HYPOTHALAMIC ARCUATE nucleus, which is important for regulation of reproductive functions, growth, and energy balance, is sexually dimorphic; it exhibits sexually differentiated astrocyte morphology (1,2) and synaptic connectivity. In rats the number of axosomatic synapses is higher in females by 20 d of age, and this difference is maintained throughout adult life (3). Apart from these differences, a natural phasic synaptic remodeling can be observed in females that is linked to hormonal variations during the ovarian cycle (4). The number of axosomatic synapses on arcuate neurons changes across the estrous cycle, with the highest numbers occurring on metestrus and diestrus; after the peak of estradiol that occurs on the morning of proestrus, the number of axosomatic synapses decreases and remains low on the day of estrus. Experimental data show that these synaptic changes are driven by 17β-estradiol because in ovariectomized (OVX) rats, the hormone replacement resulted in a reversible decline of axosomatic synapses (5) and increased activity of arcuate neurons (6). Quantitative immunocytochemical analysis revealed that the majority of axosomatic terminals are γ-aminobutyric acid (GABA)ergic, and this population of synapses is affected by estradiol (7).
The fact that the hormone treatment specifically affects only GABAergic synapses raises the question of whether there is specificity at the level of postsynaptic cells as well. Previous studies had reported that GABAergic nerve terminals in the arcuate nucleus make extensive synaptic contact with cells containing tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine (DA) synthesis (8,9). This anatomical organization demonstrates that GABAergic neurons have the capacity to influence directly the activity of these tuberoinfundibular dopaminergic (TIDA) neurons and the release of DA from TIDA axon terminals. DA is transported via the portal system of the median eminence to the anterior pituitary gland where it binds to receptors on lactotrophs, ultimately resulting in inhibition of prolactin (PRL) secretion. It is known that the pattern of PRL release and the basal TIDA neuron activity is different in female and male rats (10), although according to Shieh and Pan (11), the effect of estrogen is not sex specific, i.e. in gonadectomized animals the hormone treatment increased the activity in both sexes. This observation clearly shows that the TIDA neurons are targets for estrogens in both female and male animals. For an understanding of the possible cellular mechanisms involved in regulation of TIDA neuronal activity by estrogens, it is important to know if the synaptic remodeling induced by estradiol, as described previously (7,12), is similar in females and males. With the combination of pre-embedding TH immunostaining and the post-embedding immunogold method, we have identified two different neuronal populations within the arcuate nucleus and quantified GABAergic and non-GABAergic axosomatic synapses in gonadectomized adult male and female rats, with or without 17β-estradiol treatment.
Materials and Methods
Animals and surgical procedures
CFY albino rats were used. Animals were raised and maintained in standard laboratory conditions, with tap water and regular rat chow available ad libitum on a 12-h dark, 12-h light cycle. All efforts were made to minimize the number of animals and stress to the animals. The Principles of Laboratory Animal Care (National Institutes of Health publication no. 85–23) and the European Communities Council Directive of November 24, 1986 (86/609/EEC) were followed, and the protocols for animal care were approved by the Hungarian Health Committee (1998) and the Biological Research Center (XVI/1999). Two-month-old rats were anesthetized with Nembutal (Hospira, Inc., Lake Forest, IL for OVATION Pharmaceuticals, Inc., Deerfield, IL) and were OVX or orchidectomized (ORCH). One month later the rats received sc injections of either a single dose (45 μg/kg body weight) of 17β-estradiol (Sigma Chemical Co., St. Louis, MO) dissolved in sesame oil (13) or vehicle.
Immunocytochemistry
Twenty-four hours after the estradiol injection, all animals were anesthetized with Nembutal and perfused through the left cardiac ventricle, first with 50 ml 0.9% NaCl, then with 250 ml 1% paraformaldehyde and 1% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4). After perfusion, the brains were removed and placed at 4 C in glutaraldehyde-free fixative for an additional 3 h. After washing in Tris-buffered saline, 50-μm thick coronal Vibratome sections (Vibratome, St. Louis, MO) were cut through the middle of the arcuate nucleus (bregma −2.30 to −3.60) and processed for TH immunostaining performed according to the routine peroxidase-antiperoxidase procedure using diaminobenzidine as the chromogen. Sections were analyzed by light microscopy before embedding.
For electron microscopy, sections were osmicated (1% OsO4 in phosphate buffer) for 30 min, dehydrated in ethanol, and flat embedded in araldite between liquid release-coated slides (Electron Microscopic Sciences, Fort Washington, PA) and coverslips. After capsule embedding, blocks were trimmed, and ribbons of serial ultrathin sections were collected on Formvar-coated single-slot gold grids. Post-embedding immunostaining for GABA was performed using a modification of the immunogold method of Somogyi and Hodgson (14), described in detail by Parducz et al. (7).
Quantitative analysis
The numerical density of axosomatic synapses was determined by the unbiased dissector method (15). On consecutive 90-nm thick sections, we determined the average projected height of the synapses and used about 30% of this value as the distance between the dissectors. On the basis of this calculation, the number of axosomatic synapses was counted in two consecutive serial sections about 270-nm apart (“reference” and “look-up” sections) for 12–15 perikaryal profiles of TH-IR and TH-immunonegative neurons in each block. To increase the sampling, the procedure was repeated in such a way that the reference and look-up sections were reversed. Section thickness was determined using the Small’s minimal-fold method.
There were six rats in each experimental group, and three tissue blocks per animal were counted. The data from the same animals were pooled because no variations were detected in the three blocks from any group of rats. The Mann-Whitney U test was used to determine the significance of differences between the mean values of data groups. A level of confidence of P < 0.05 was adopted for statistical significance.
Results
In agreement with published reports (16), we observed a sexually dimorphic pattern of TH-IR neurons in the arcuate nucleus; males had more labeled cells in the ventrolateral region. Most of these ventrolateral neurons are nondopaminergic monoenzymatic neurons, expressing individual complementary enzymes of the DA synthetic pathway: only TH or aromatic l-amino acid decarboxylase (17,18,19). For that reason we performed the morphometric measurements in the dorsomedial region of the arcuate nucleus (between 2, 3–3, and 6-mm caudal from bregma), where the two sexes do not differ with respect to the number of dopaminergic neurons.
The electron micrographs revealed clear diaminobenzidine reaction product indicative of TH-immunoreactive (IR) neurons; the post-embedding immunogold staining was highly specific and clearly labeled the GABAergic terminals (Figs. 1 and 2).
Figure 1.
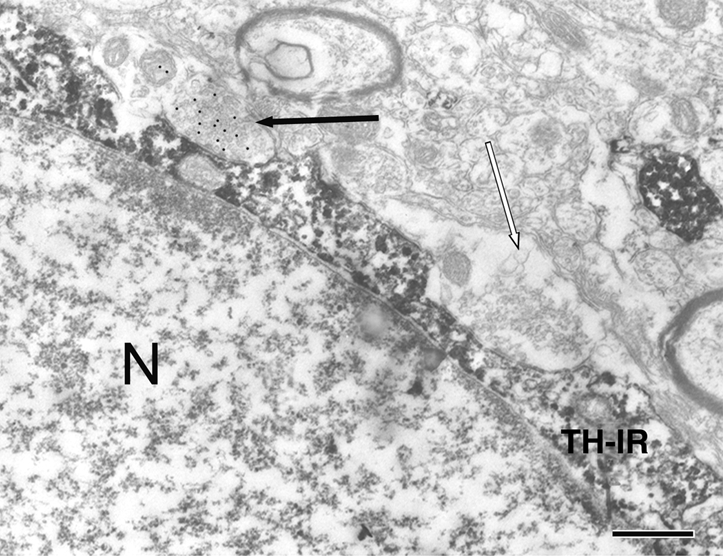
GABAergic (black arrow) and non-GABAergic (white arrow) nerve terminals synapsing on TH-IR neuron in the arcuate nucleus (N) of ORCH male rat. Bar, 0.5 μm. TH-IR, TH-IR neuron.
Figure 2.
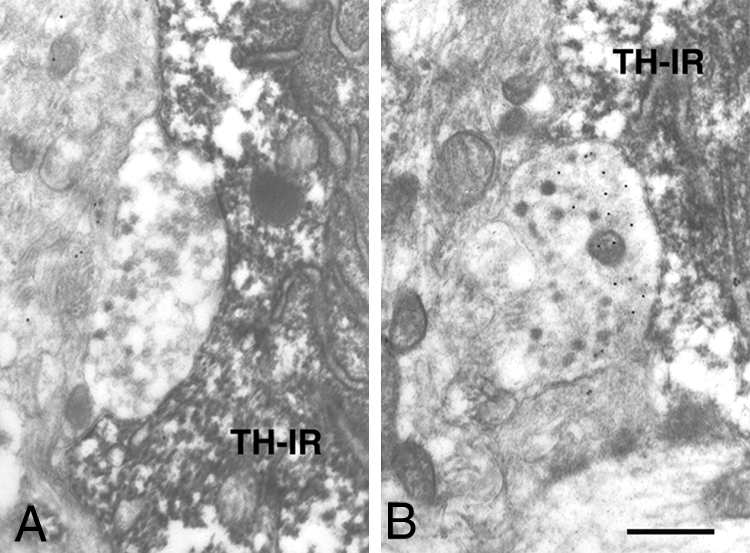
Non-GABAergic (A) and GABAergic (B) axosomatic synapses on TH-IR arcuate neurons of OVX and 17β-estradiol treated adult rats. Bar, 0.5 μm. TH-IR, TH-IR neuron.
Morphometric analysis demonstrated a sex difference in the total number of axosomatic synapses; arcuate neurons receive more synaptic terminals in females than males (Table 1).
Table 1.
Number of axosomatic synapses in the arcuate nucleus of female and male rats
The number of synapses in female and male animals is statistically different.
P < 0.01.
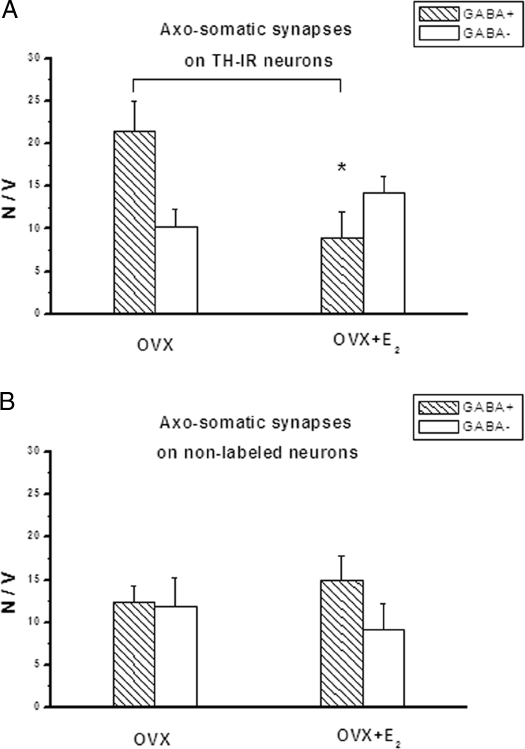
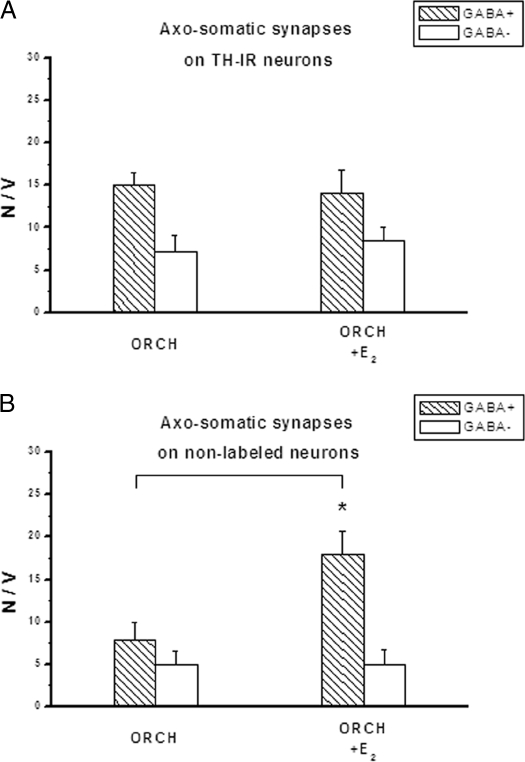
Although the total number of axosomatic synapses was higher overall in females, the pattern of synaptic connectivity that is the ratio of GABAergic to non-GABAergic synapses was similar in the gonadectomized males and females. TH-IR neurons had significantly more GABAergic than non-GABAergic axosomatic synapses (P < 0.05), with a ratio of about 2:1 (Fig. 3A, OVX, and Fig. 4A, ORCH), whereas the nondopaminergic arcuate neurons received about equal numbers of these two types of synapses (Fig. 3B, OVX, and Fig. 4B, ORCH).
Figure 3.
The effect of 17β-estradiol (E2) on the number (N) of GABAergic and non-GABAergic axosomatic synapses on TH-IR (A) and nonlabeled (B) arcuate neurons of OVX rats. The number of synapses was counted by the Sterio method (15), and the data are expressed as the number of synaptic contacts per unit volume (V) of perikaryon (1000 μm3). There is a significant decrease of GABAergic terminals synapsing on TH-IR neurons. *, P < 0.05.
Figure 4.
Numerical density of axosomatic synapses in the male arcuate nucleus on TH immunopositive (TH+) (A) and nonlabeled (TH-) neurons (B). 17β-Estradiol (E2) treatment results in an increase of GABAergic axosomatic synapses on nonlabeled neurons. *, P < 0.05. N, Number; V, volume.
The effect of 17β-estradiol was specific because not all arcuate neurons were affected by the structural synaptic remodeling. In the OVX females, 17β-estradiol significantly reduced the numerical density of GABAergic synapses onto TH-IR neurons alone (Fig. 3, A and B) In contrast, in ORCH males the synaptic connectivity of TH-IR neurons was not affected by 17β-estradiol, whereas 17β-estradiol increased the density of GABAergic terminals onto nondopaminergic neurons (Fig. 4, A and B).
Discussion
The anatomical organization and the afferent and efferent connections of the arcuate nucleus indicate that this structure is an important integrative endocrine part of the hypothalamus. Among many other functions, including regulation of energy balance, it is involved in the mediation of PRL and GH secretion by the pituitary, and in the regulation of the estrous cycle and sexual behaviors. The many complex functions of the arcuate nucleus are reflected in the high degree of complexity in the internal organization and connectivity of the nucleus (20). The presence of many neurochemically distinct neuron populations within the arcuate nucleus raises the question of whether the synaptic remodeling that is known to occur within this nucleus, i.e. hormonally induced changes in number of synapses, is a general phenomenon or has some specificity, and does not affect all neurons and synapses equally. In this study we used double immunolabeling to localize and separately analyze the synapses on neurons of the TIDA system and demonstrate that the response to the hormonal treatment is different in the dopaminergic vs. nondopaminergic neurons.
Our results provide an additional example of the morphological sexual dimorphism in the arcuate nucleus and confirm earlier data by Perez et al. (3), who found that the number of axosomatic synapses in this brain area was higher in females than males. In the present paper, we were able to identify specifically the dopaminergic neurons at the ultrastructural level, and the morphometric data show that the sex difference in synaptic connectivity exists in the TIDA neuron population. This observation corresponds well with the functionally dimorphic character of the arcuate dopaminergic system, which has already been demonstrated by several authors. Demarest et al. (21) reported differences in basal TH activity and in the DA concentration of the TIDA neurons, and according to Arbogast and Voogt (22), TH mRNA levels as assessed by in situ hybridization are higher in females. The same authors found that the TH immunostaining of the TIDA perikarya in the arcuate nucleus and of the nerve terminals in the median eminence was qualitatively more intense in females than males.
According to our present data, 17β-estradiol induces synaptic remodeling not only in females (7,12), but also in males, and in both sexes the GABAergic synapses are involved in the plastic changes. However, the hormonally triggered synaptic plasticity is sexually dimorphic in the sense that in females, inhibitory synapses onto the TH-IR neurons, whereas in males, inhibitory synapses onto nondopaminergic neurons are affected by the hormone. In OVX females 17β-estradiol induced synaptic remodeling in the TIDA neurons. The number of GABAergic axosomatic synapses was significantly lower 24 h after the hormone treatment. The nonlabeled arcuate neurons were not affected by 17β-estradiol because the synaptic connectivity of those neurons remained unchanged. This observation is in agreement with our earlier results obtained using systemic application of the tracer Fluoro-Gold (Fluorochrome, LLC, Denver, CO) to label the subpopulation of arcuate neurons that project to the median eminence (12). We found that the synaptic connectivity of these “hypophysiotropic neurons” was different from the other neurons that did not take up the tracer and that the neurons projecting to the median eminence underwent synaptic remodeling in response to 17β-estradiol. The present data confirm, by inference, that the retrograde labeling by Fluoro-Gold identifies TIDA neurons.
The physiological relevance of these observations can be understood by considering the role of TIDA neurons in the regulation of PRL secretion. It is generally accepted that this process is paced by a light-entrained circadian rhythm, and modulated by a very complex and sensitive balance of stimulatory and inhibitory inputs (10). Exogenous estrogen stimulates the secretion of PRL in OVX rats (23,24) and may influence PRL homeostasis at several anatomical sites, including the hypothalamus and pituitary (10). It is also well documented that the TIDA neurons play the predominant role in the control of PRL release. On the basis of measurements of DA in portal blood and its concentration and turnover rates in both the arcuate nucleus and median eminence, it has been established that the activity of the TIDA neurons is altered under many physiological conditions known to affect PRL release (10). Although the exact mechanisms are not completely understood, DA is considered the main inhibitory factor for PRL secretion (25).
The specificity of the estrogen effect on the dopaminergic neurons shown in the present studies supports the notion that the hormonally induced synaptic remodeling has functional consequences. The decrease in the number of GABAergic inhibitory inputs onto TH-IR neurons in females that was observed after 24-h estradiol treatment may result in an increase in their activity, i.e. elevated DA release. This hypothesis is supported by numerous reports showing that basal TIDA neuronal activity is decreased by ovariectomy and is restored by estrogen (10,26,27). The estradiol-induced synaptic changes can be paralleled by the physiological observations too; they correspond well with the termination phase of the PRL surge (28). We interpret these data to mean that estrogen plays a complex role in the process, in that estradiol: 1) initially increases PRL secretion (23,24); and 2) over time, induces morphological synaptic remodeling in the arcuate nucleus, which eventually results in increased activity of TIDA neurons and inhibits PRL secretion.
The observation that estradiol-induced remodeling of GABAergic synapses occurs not only in females but also in males suggests a central role of this hormone in the structural synaptic plasticity of the arcuate nucleus in both sexes. There is a certain specificity in the hormone action because dopaminergic neurons are affected in females, but nondopaminergic neurons are affected in males. However, despite the lack of an effect of estradiol on synaptic remodeling of dopaminergic neurons in males, the arcuate dopaminergic system in male animals is clearly a target for estrogen because in ORCH animals, estradiol increases basal activity TIDA neurons (11). More detailed studies are needed to determine the exact mechanism of hormone action on the TIDA system in males.
Footnotes
This study was supported by grants from National Institutes of Health and Fogarty International Center (1RO3-TW00933-01), the Hungarian Research Fund (OTKA T-075954), and the Hungarian National Office for Research and Technology RET 08/2004, GVOP 0052/3.0-2004, ETT 476/2006, E-438/2006.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 17, 2008
Abbreviations: DA, Dopamine; GABA, γ-aminobutyric acid; IR, immunoreactive; ORCH, orchidectomized; OVX, ovariectomized; PRL, prolactin; TH, tyrosine hydroxylase; TIDA, tuberoinfundibular dopaminergic.
References
- McCarthy MM, Amateau SK, Mong JA 2002 Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod 67:691–698 [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM 2002 Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res 139:151–158 [DOI] [PubMed] [Google Scholar]
- Perez J, Naftolin F, Garcia Segura LM 1990 Sexual differentiation of synaptic connectivity and neuronal plasma membrane in the arcuate nucleus of the rat hypothalamus. Brain Res 527:116–122 [DOI] [PubMed] [Google Scholar]
- Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM 1989 Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience 32:663–667 [DOI] [PubMed] [Google Scholar]
- Perez J, Luquin S, Naftolin F, Garcia-Segura LM 1993 The role of estradiol and progesterone in phased synaptic remodelling of the rat arcuate nucleus. Brain Res 608:38–44 [DOI] [PubMed] [Google Scholar]
- Parducz A, Hoyk Z, Kis Z, Garcia-Segura LM 2002 Hormonal enhancement of neuronal firing is linked to structural remodelling of excitatory and inhibitory synapses. Eur J Neurosci 16:665–670 [DOI] [PubMed] [Google Scholar]
- Parducz A, Perez J, Garcia-Segura LM 1993 Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience 53:395–401 [DOI] [PubMed] [Google Scholar]
- Decavel C, van den Pol AN 1992 Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol 316:104–116 [DOI] [PubMed] [Google Scholar]
- van den Pol AN 1986 Tyrosine hydroxylase immunoreactive neurons throughout the hypothalamus receive glutamate decarboxylase immunoreactive synapses: a double pre-embedding immunocytochemical study with particulate silver and HRP. J Neurosci 6:877–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Shieh KR, Pan JT 1996 Sexual differences in the diurnal changes of tuberoinfundibular dopaminergic neuron activity in the rat: role of cholinergic control. Biol Reprod 54:987–992 [DOI] [PubMed] [Google Scholar]
- Parducz A, Zsarnovszky A, Naftolin F, Horvath TL 2003 Estradiol affects axo-somatic contacts of neuroendocrine cells in the arcuate nucleus of adult rats. Neuroscience 117:791–794 [DOI] [PubMed] [Google Scholar]
- Maclusky NJ, Luine VN, Hajszan T, Leranth C 2005 The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146:287–293 [DOI] [PubMed] [Google Scholar]
- Somogyi P, Hodgson AJ 1985 Antisera to γ-aminobutyric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections of cat striate cortex. J Histochem Cytochem 33:249–257 [DOI] [PubMed] [Google Scholar]
- Sterio DC 1984 The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134(Pt 2):127–136 [DOI] [PubMed] [Google Scholar]
- Cheung S, Will YM, Hentschel K, Moore KE, Lookingland KJ 1997 Role of gonadal steroids in determining sexual differences in expression of Fos-related antigens in tyrosine hydroxylase-immunoreactive neurons in subdivisions of the hypothalamic arcuate nucleus. Endocrinology 138:3804–3810 [DOI] [PubMed] [Google Scholar]
- Meister B, Hokfelt T, Steinbusch HW, Skagerberg G, Lindvall O, Geffard M, Joh TH, Cuello AC, Goldstein M 1988 Do tyrosine hydroxylase-immunoreactive neurons in the ventrolateral arcuate nucleus produce dopamine or only L-dopa? J Chem Neuroanat 1:59–64 [PubMed] [Google Scholar]
- Okamura H, Kitahama K, Nagatsu I, Geffard M 1988 Comparative topography of dopamine- and tyrosine hydroxylase-immunoreactive neurons in the rat arcuate nucleus. Neurosci Lett 95:347–353 [DOI] [PubMed] [Google Scholar]
- Ershov PV, Ugrumov MV, Calas A, Krieger M, Thibault J 2005 Degeneration of dopaminergic neurons triggers an expression of individual enzymes of dopamine synthesis in non-dopaminergic neurons of the arcuate nucleus in adult rats. J Chem Neuroanat 30:27–33 [DOI] [PubMed] [Google Scholar]
- Chronwall BM 1985 Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6(Suppl 2):1–11 [DOI] [PubMed] [Google Scholar]
- Demarest KT, McKay DW, Riegle GD, Moore KE 1981 Sexual differences in tuberoinfundibular dopamine nerve activity induced by neonatal androgen exposure. Neuroendocrinology 32:108–113 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1991 Ontogeny of tyrosine hydroxylase mRNA signal levels in central dopaminergic neurons: development of a gender difference in the arcuate nuclei. Brain Res Dev Brain Res 63:151–161 [DOI] [PubMed] [Google Scholar]
- Caligaris L, Astrada JJ, Taleisnik S 1974 Oestrogen and progesterone influence on the release of prolactin in ovariectomized rats. J Endocrinol 60:205–215 [DOI] [PubMed] [Google Scholar]
- Chen CL, Meites J 1970 Effects of estrogen and progesterone on serum and pituitary prolactin levels in ovariectomized rats. Endocrinology 86:503–505 [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N 1985 Dopamine: a prolactin-inhibiting hormone. Endocr Rev 6:564–589 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 1998 Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67:377–383 [DOI] [PubMed] [Google Scholar]
- Pan J 1996 Neuroendocrine functions of dopamine. In: Stone T, ed. CNS neurotransmitters and neuromodulators: dopamine. Boca Raton, FL: CRC Press; 213–231 [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 2000 Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Res 879:139–147 [DOI] [PubMed] [Google Scholar]