Abstract
The past few years have seen an increase in the reported incidence of endometrial carcinoma, one of the most frequently diagnosed malignancies of the female genital tract. Estrogen production is vital for the mitogenesis of endometrial tumors. Inhibition of steroid sulfatase (STS), an enzyme responsible for the synthesis of steroids with estrogenic properties, may represent a novel therapeutic target for this type of cancer. This study investigates the effects of STX64 (also known as 667Coumate and BN83495) and STX213, two potent STS inhibitors, on hormone-dependent endometrial cancer cell growth in vivo. When tested in intact mice with endometrial cancer xenografts, STX64 had limited effect on tumor growth. In contrast, the microtubule disruptor STX140 reduced tumor growth by 55%. In a hormone-dependent endometrial xenograft model in ovariectomized mice, both STX64 and STX213 given orally, daily at 1 mg/kg significantly inhibited tumor growth by 48 and 67%, respectively. However, when given orally at 1 mg/kg once weekly, only STX213 still inhibited tumor proliferation. At a higher dose of STX64 (10 mg/kg, orally, daily), a greater tumor growth inhibition of 59% was observed. Liver and tumor STS activity was completely inhibited in all daily treatment groups. Plasma estradiol (E2) levels were also significantly decreased. A significant correlation was observed between plasma E2 concentrations and STS activity, indicating the importance of circulating E2 on tumor growth. This novel study demonstrates for the first time that STS inhibitors are potent inhibitors of endometrial cancer growth in nude mice.
THE PRODUCTION OF both estrogen and progesterone is vital for the healthy proliferation of endometrial cells. Clinical, biological, and epidemiological investigations have succinctly demonstrated that a prolonged or excessive exposure to estrogens increases the risk of developing endometrial cancer (1,2,3,4). Paradoxically, evidence also suggests that there is an increased incidence of developing estrogen-dependent endometrial cancer in postmenopausal women, i.e. when the ovaries have ceased to produce any active estrogens. This suggests that in situ estrogen metabolism and synthesis plays an important role in the maturation and subsequent progression of a range of endometrial carcinomas (5). Consequently, the inhibition of the production of these sex steroids provides a potential therapeutic target to reduce biologically available estrogens, leading to an improved prognosis (6,7).
There has been much interest in the importance of in situ estrogen synthesis and metabolism on a range of human hormone-dependent epithelial neoplasms, including breast and endometrial carcinomas (6,7,8,9). Several studies have indicated that human serum and breast tissue have elevated estrogen levels compared with noncancerous tissues (10,11). Importantly, it is the biologically active estradiol (E2), its metabolic precursor estrone (E1), and their sulfates (E2S and E1S, respectively) that are found in significantly high concentrations (12,13). This strongly suggests that it is the intratumoral biosynthesis of these hormones that plays a cardinal role in the development of neoplastic tissue.
Unfortunately, the information on estrogen-dependent endometrial cancer is not so straightforward. Various studies have attempted to measure estrogen concentrations in endometrial carcinoma tissues with varying degrees of success (14,15). However, the general consensus is that the overproduction of E2, E1, and testosterone in endometrial tissue significantly encourages tumor growth (16,17). Therefore, the importance of inhibiting the synthesis of these bioactive steroids is desirable in future therapies.
The production of these steroids is via three primary enzymes: aromatase, 17β-hydroxysteroid dehydrogenase (17β-HSD) type-1, and steroid sulfatase (STS). The role of aromatase inhibitors in endometrial cancer patients has been recently well documented (18). Some studies have indicated the poor clinical effects of letrozole (19), anastrozole (20), and a combination of anastrozole and gefitinib in patients with endometrial cancer (21). Limited improvement was only seen in patients with estrogen receptor-positive tumors. There is limited evidence for a role of 17β-HSD type-1 in endometrial cancer: studies suggest that this enzyme is not up-regulated in this malignancy (22). There have been no reported attempts to inhibit 17β-HSD type-1 in preclinical endometrial cancer models. However, new inhibitors of this enzyme have recently been effective against hormone-dependent breast cancer (23).
Therefore, the involvement of the STS enzyme in the formation of E2 is a potential target for the treatment of endometrial cancer. There is growing evidence that supports this hypothesis. Evidence suggests that malignant endometrial tissue has a 12-fold higher STS activity compared with normal disease free endometrium (24). Immunohistochemical techniques have also demonstrated that 86% of human endometrial carcinoma is immunoreactive for STS, which significantly correlates with the enzyme activity and RNA STS expression of the tissue (25). This information indicates that the use of a STS inhibitor may be beneficial in treating patients with endometrial cancer.
We have previously demonstrated the development and effects of STS inhibitors on STS activity in a nitrosomethylurea (NMU)-induced mammary tumor (26) and models of hormone-dependent breast cancer (27,28). Estrone-3-O-sulfamate was identified as the first irreversible STS inhibitor (29) but unexpectedly proved to be estrogenic in rodents (30). In the pursuit of alternative nonestrogenic mimics of estrone-3-O-sulfamate, a large number of steroidal and nonsteroidal sulfamates was then synthesized and tested as STS inhibitors. STX64, also known as 667Coumate or BN83495, (30,31) a tricyclic coumarin sulfamate, resulted from such a study, and this compound has been selected for further clinical development. In the NMU-induced mammary tumor model, STX64 caused significant regression of E1S-stimulated tumor growth at 10 mg/kg (85 ± 5%) and at 2 mg/kg (56 ± 13%). Recent Phase I clinical studies testing STX64 in female breast cancer patients have shown very promising results (32,33). STX213, a second-generation STS inhibitor, has recently been preclinically tested against E2S-stimulated breast tumors and has had a different pharmacokinetic profile than STX64.
Because these STS inhibitors have shown potential against hormone-dependent breast cancer, it was of significant interest to determine their efficacy against endometrial cancer. There have been no previous studies on the effect of STS inhibitors on endometrial cancer growth. Therefore, STX64 and STX213 were tested in mice bearing Ishikawa xenograft tumors, an endometrial cancer cell line. In all studies, liver and tumor STS activity and plasma E2 levels were measured. The critical importance of circulating E2 levels on tumor growth was also investigated.
Materials and Methods
Compounds
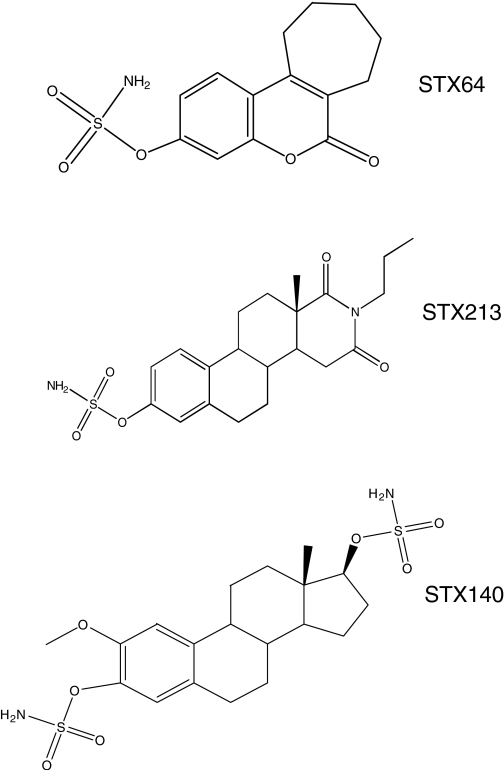
Compounds were produced as micronized formulations after they were synthesized according to the methods described by Fischer (34,35) (STX213) and Woo (30) et al. (STX64). STX140 was synthesized as previously described (36). All compounds exhibited spectroscopic and analytical data in accordance with their structures (Fig. 1) and were pure, as determined by HPLC. Previous work has shown that in placental microsomes, STX64, STX213, and STX140 have a STS inhibitory IC50 of 8, 1, and 39 nm, respectively (27,28,37). Medroxyprogesterone acetate (MPA), the standard clinical treatment for endometrial cancer, was purchased from Sigma (Poole, UK). Taxol was obtained from Bristol-Myers Squibb Co. (New York, NY).
Figure 1.
The chemical structures of STX64, STX213, and STX140.
Cell culture
Ishikawa cells, a human endometrial cancer estrogen receptor-positive cell line, was obtained from the American Type Culture Collection (LGC Promochem, Teddington, UK) and grown in 10% serum supplemented RPMI 1640 medium (Sigma). Cells were cultured at 37 C under 5% CO2 in a humidified incubator.
In vivo tumor growth model
Intact, athymic, female MF-1 nude mice (ν−/ν−) were purchased from Harlan (Bicester, Oxon, UK) at 5–6 wk of age (∼20–25 g in weight). All experiments were performed under conditions that complied with institutional guidelines and conform to the United Kingdom Home Office regulations. Animals were kept in a 12-h light, 12-h dark cycle, and given food and water ad libitum. Monolayers of Ishikawa cells were removed by trypsinization, the resultant cell suspension centrifuged for 5 min at 1000 g, and then resuspended in ice-cold Matrigel (BD Bioscience, Oxford, UK). Two million Ishikawa cells in 100 μl Matrigel were injected sc into the right flank of the animal. When tumors reached 90–100 mm3, mice were randomly divided into treatment groups of six mice: vehicle (10% tetrahydrofuran:90% propylene glycol); STX64 [10 mg/kg, per os (po), daily]; STX140 (20 mg/kg, po, daily); or Taxol (15 mg/kg, iv, once weekly). STX140 (also known as 2-methoxyestradiol-bis-sulfamate) is a well-documented microtubule disruptor with good oral bioavailability and tumor efficacy (38,39,40,41,42). In the current study, it was chosen to treat Ishikawa xenografts in intact animals with this compound because, along with microtubule disruption, it has potent STS inhibitory activity (37). Throughout this tumor study, mice were weighed and tumor measurements taken on a weekly basis. Tumor volumes were calculated using the formula: (length × width2)/2.
Hormone-dependent in vivo tumor growth model
Ovariectomized athymic, female MF-1 nude mice (ν-/ν-) were purchased from Harlan at 5–6 wk of age (∼20–25 g in weight). Ovariectomized animals were chosen because they have had similar serum estrogen levels to those of postmenopausal women (43,44). Mice received sc injections of E2S at 50 μg/50 μl per mouse 24 h before cell injection. E2S was chosen to stimulate tumor growth rather than E1S because this avoids the need for the liberated E1 to be converted to E2 by the Ishikawa cells. Monolayers of Ishikawa cells were removed by trypsinization, the resultant cell suspension centrifuged for 5 min at 1000 g, and then resuspended in ice-cold Matrigel. Two million Ishikawa cells in 100 μl Matrigel were injected sc into the right flank of the animal. E2S (50 μg/50 μl) was injected sc thrice weekly until the end of the study. When tumors reached 80–100 mm3, mice were randomly divided into treatment groups of eight mice: vehicle (10% tetrahydrofuran:90% propylene glycol); STX64 (10 or 1 mg/kg, po, daily or weekly); STX213 (1 mg/kg, po, daily or weekly); STX140 (20 mg/kg, po, daily); or MPA (2 mg, ip, twice weekly). This dose of MPA has had an inhibitory effect on the growth of metastatic tumors of the lung in nude mice (45). At the end of this study, 1 h before cardiac punctures and collection of liver and tumor tissue, animals received a final compound dose and E2S injection. Throughout these tumor studies, mice were weighed and tumor measurements taken on a weekly basis. Tumor volumes were calculated using the formula: (length × width2)/2.
STS biochemical assay
STS activities of tissue samples obtained from animals were measured as previously described (46). Briefly, tissues were homogenized in ice-cold PBS (pH 7.4) containing 250 mm sucrose, and supernatants were prepared by centrifugation (2000 g, 4 C for 10 min). Aliquots of tissue supernatants were incubated with [6,7-3H] E1S (4 × 105 dpm; PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA) and adjusted to a final concentration of 20 μm with unlabeled E1S (Sigma). [4-14C] E1 (1 × 104 dpm; PerkinElmer Life And Analytical Sciences) was included in the reaction mixture to monitor procedural loses. Samples were incubated for 60 min at 37 C after which the product, E1, was separated from E1S by partition with toluene. An aliquot of toluene was removed, and 3H and 14C radioactivity was measured by liquid scintillation spectrometry. The mass of E1S hydrolyzed was calculated from the 3H counts detected corrected for procedural losses. Protein measurements were also obtained for the tissue samples using the Bradford assay method. Results are determined as nmol product formed per hour per mg protein, and expressed as the percent inhibition or activity compared with the vehicle-treated control.
Plasma and tumor E2 measurement
Blood taken from mice was collected into heparinized tubes and centrifuged at 13,000 rpm for 1 min to remove erythrocytes. The plasma was collected and stored at −20 C until assayed. Plasma E2 levels were measured using a Coat-A-Count Estradiol RIA kit (Diagnostic Products Corp., Los Angeles, CA) according to the manufacturer’s instructions. The intraassay coefficient of variation for these measurements of plasma E2 concentrations by this method was less than 10%.
Statistical analysis
An ANOVA followed by Bonferroni’s posttest was performed to determine tumor growth differences. Where only two groups are compared, a Student’s t test was applied. A linear regression curve was calculated to compare liver STS activity against plasma E2 levels. All values are represented as the mean ± sem.
Results
Effect of STX64, STX140, and Taxol on Ishikawa xenograft tumors grown in intact animals
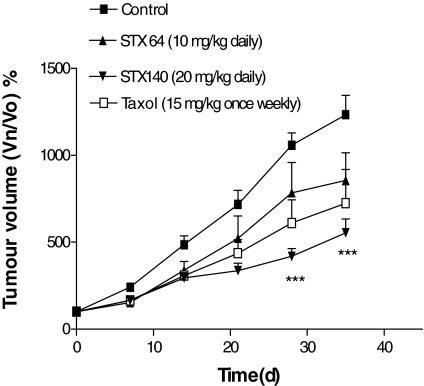
Initial xenograft studies were undertaken to examine the tumorigenicity of the Ishikawa cells. In addition, these experiments, using intact female nude mice, investigated the effect of STS inhibition on hormone-independent endometrial cancer. Consequently, 2 million cells in 100 μl Matrigel were inoculated into the flanks of MF-1 female nude mice. All of these injections resulted in a single, rapidly growing tumor. These animals, once their tumors had reached approximately 100 mm3, were then split into four groups of six and treated with daily oral doses of either vehicle control, STX64 (10 mg/kg), the microtubule disruptor STX140 (20 mg/kg), or iv, once weekly, with Taxol (15 mg/kg). The results for this study are shown in Fig. 2. The tumors in the vehicle control-treated group increased in size by 1233 ± 111% after 35 d compared with d 0, the start of the dosing regime. Treatment with STX64 and Taxol did not result in a significant reduction in tumor proliferation; however, both did cause a modest slowing of tumor growth. In contrast, STX140 significantly (P < 0.001) attenuated Ishikawa xenograft growth, reducing the growth by 55% compared with controls after 35-d treatment. These drug treatments had no effect on animal weights throughout the study (data not shown).
Figure 2.
Effect of daily po dosing of STX64 (10 mg/kg), or STX140 (20 mg/kg) and weekly iv dosing of Taxol (15 mg/kg) on Ishikawa xenograft tumor growth in MF-1 female nude mice. Results represent mean ± sem (n = 6). ***, P < 0.001 compared with control. Vn/V0, Volume at day n/volume at d 0.
Effect of STX64, STX213, and MPA on E2S-stimulated Ishikawa xenograft tumors in ovariectomized mice
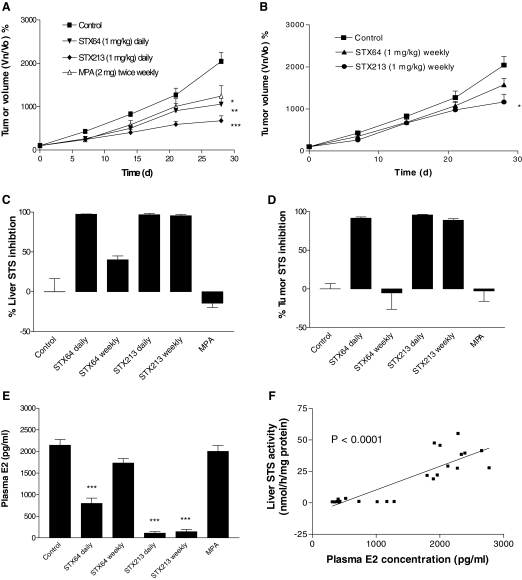
Because STS inhibition, using oral dosing of STX64, had a slight inhibitory effect on Ishikawa tumor growth in intact animals, it was decided to examine the effect of this compound in a hormone-dependent endometrial cancer model. STX140 was not used in these studies because along with STS inhibitory properties, this compound is a potent microtubule disruptor (38,39,40,41,42). Therefore, interpretation of any results obtained with STX140 in this hormone-dependent endometrial cancer model would be difficult. Therefore, Ishikawa cells were sc injected into the flanks of ovariectomized MF-1 nude mice stimulated with E2S. The Ishikawa cells were unable to develop into growing tumors without the addition of E2S (data not shown), indicating their reliance on E2 for tumorigenicity. E2S-stimulated animals developed fast-growing tumors, indicating the mitogenic effect of the STS-liberated E2. Six dosing regimes were used in these studies: the vehicle control group treated orally with 0.5% methyl cellulose; the MPA (2 mg, ip, once weekly) group; and four groups comprising the use of a low-dose, 1 mg/kg STX64 and STX213 dosed daily or weekly. In Fig. 3, A and B, the results from these studies demonstrate that a daily dose of STX64 and STX213 was able to significantly (P < 0.01 and P < 0.001, respectively) inhibit endometrial xenograft tumor growth: STX64-treated animals, at d 28, had a 1056 ± 135% increase in tumor growth compared with d 0; and STX213-treated animals, over the same time period, had a 675 ± 112% increase. In contrast, control vehicle-treated animal tumors had grown by 2042 ± 203%. MPA treatment significantly (P < 0.05) decreased tumor development by 39%. When these compounds were given weekly at 1 mg/kg, STX213 was the only compound that significantly inhibited tumor growth.
Figure 3.
Effect of daily and weekly po dosing of STX64 (1 mg/kg), or STX213 (1 mg/kg) and twice weekly ip dosing of MPA (2 mg) on Ishikawa xenograft tumor growth in MF-1 OVX female nude mice. Daily dosing regime tumor growth curves are seen in panel A, and the weekly dosing regime is seen in panel B. At the end of the study, tissue samples were collected for STS activity measurements in liver (C) and tumor (D). E, Circulating plasma E2 levels were also determined. F, The animals did not lose any weight throughout the study. All results show mean ± sem (n = 8–9). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with control. Vn/V0, Volume at day n/volume at d 0.
The STS activity of tissue samples taken from these tumor-bearing animals was determined. These studies demonstrate that both STX64 and STX213 were capable of completely inhibiting liver and tumor STS activity when dosed daily (Fig. 3, C and D). However, when these compounds were given on a weekly regime, then only STX213 maintained an almost complete inhibition of STS activity. Circulating plasma E2 concentrations measured for these animals showed that daily or weekly dosing of STX213 was capable of almost complete (>90%) E2 ablation. In contrast, STX64, at 1 mg/kg, failed to block E2 production when given as a weekly dose and only reduced circulating E2 levels by 63% when given daily. This suggests that a 1 mg/kg daily dose of STX64 was not sufficient to inhibit tumor growth driven by E2. In all these studies, MPA had no effect on STS activity or plasma E2 levels. Furthermore, the compounds tested in this model did not have a detrimental effect on animal weights and are, therefore, unlikely to have a toxic profile (data not shown).
Data were also reviewed to assess the correlation between liver STS activity and plasma E2 concentrations (Fig. 3F). These results graphically represent all data points regardless of treatment given to the animals. This shows a significant correlation between inhibition of liver STS activity and circulating E2 levels. This indicates the importance of maintaining STS inhibition to limit E2 production, which can be used by the tumor to develop.
Effect of high-dose STX64 on E2S-stimulated Ishikawa xenograft tumors in ovariectomized mice
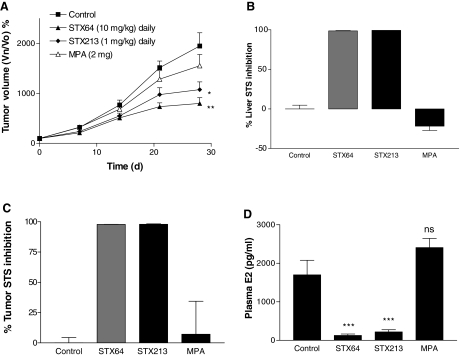
Because a 1 mg/kg dose of STX64 had limited effect on Ishikawa tumor growth, it was decided to increase this dose 10-fold. Consequently, MF-1 nude mice were inoculated with Ishikawa cells and then treated with STX64 (10 mg/kg, po, daily), STX213 (1 mg/kg, po, daily), or MPA (2 mg, ip, weekly). At this elevated dose, STX64 inhibited Ishikawa xenograft growth by 59% at d 28 (Fig. 4A). STX213 and MPA were able to decrease tumor growth by 45 and 20%, respectively. In this study, STX213 and MPA gave similar results to those shown in Fig. 3A.
Figure 4.
Effect of daily po dosing of STX64 (10 mg/kg), or STX213 (1 mg/kg) and twice weekly ip dosing of MPA (2 mg) on Ishikawa xenograft tumor growth in MF-1 OVX female nude mice. Tumor growth curves are shown in panel A. At the end of the study, tissue samples were collected for STS activity measurements in the liver (B) and tumor (C). D, Circulating plasma E2 levels were also determined. All results show mean ± sem (n = 8–9). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with control. Vn/V0, Volume at day n/volume at d 0.
A liver and tumor STS tissue assay confirmed that STX64 and STX213 were capable of completely inhibiting STS activity (Fig. 4, B and C). Circulating plasma E2 concentrations were also significantly reduced by both these compounds (Fig. 4D). Importantly, 10 mg/kg STX64 was able to reduce the E2 levels by 93%, in contrast to the previous study in which a 75% reduction was observed by 1 mg/kg STX64. MPA had no effect on STS activity or plasma E2 levels.
Discussion
Currently, the treatments for endometrial cancer are limited. The majority of experimental therapeutics are at the Phase I or Phase II clinical trial stage. This includes investigations on tamoxifen (47) and progesterone (48). For more advanced and aggressive endometrial cancers, erlotinib, an epidermal growth factor receptor inhibitor, and flavopiridol, a kinase inhibitor, are also undergoing testing (49). Aromatase inhibitors, such as letrozole, that are now used routinely for hormone-dependent breast cancer, have shown only limited clinical success against endometrial cancers (18).
STS inhibitors have successfully been tested in various hormone-dependent breast in vivo cancer models and in a Phase I clinical trial on postmenopausal women with breast carcinoma (27,28,32,33). However, there is significant potential for these types of compounds to be effective in other hormone-dependent malignancies. Normal endometrial tissue is reliant on the in situ production of estrogens, and this dependency is transferred when the tissue becomes cancerous. Therefore, STS inhibitors, which block the production of biologically active E2, should affect hormone-dependent endometrial cancer growth. Consequently, this study aimed to examine the daily and weekly dose efficacy of STX64 and STX213 on hormone-dependent, Ishikawa endometrial xenografts in vivo. Furthermore, liver and tumor STS activity and plasma E2 measurements were determined. Finally, correlations of STS activity against liver STS activity and circulating plasma E2 levels were determined indicating the importance of STS activity in maintaining high-plasma E2 concentrations.
STX64 is a tricyclic coumarin sulfamate that was identified as a potent STS inhibitor in placental microsomes (30). Since then it has shown efficacy in an NMU-induced, E1S driven mammary tumor model and in MCF-7 breast xenograft models stimulated with E2S (26,27,28). In the latter studies, STX213, a D-ring modified steroid-like inhibitor, demonstrated a greater therapeutic potential compared with STX64. This is mainly because STX213 has a more efficacious pharmacokinetic profile and a longer plasma half-life in mice (28). Because both these agents are effective at inhibiting breast cancer in vivo, it was logical to examine them against hormone-dependent endometrial cancer.
Initial studies determined the STS inhibitory activity of STX64 and STX213 in Ishikawa cells in vitro. STS activity in Ishikawa cells was completely (<99%) inhibited by both compounds. Previous studies, using a JEG-3 cell assay, have shown similar IC50 results (1.5 and 0.5 nm, respectively) (27). Consequently, because these compounds are potent STS inhibitors, they were further tested in vivo to determine their efficacy against endometrial cancer.
Therefore, STX64, with Taxol, a microtubule stabilizer, and STX140, a microtubule disruptor, were tested using intact female nude mice bearing Ishikawa xenograft tumors. These tumors developed rapidly, and despite STX64 having some effect on the growth rate, this was not significant when compared with the control. However, STX140, a compound that has shown promising anticancer effects against a range of cancer types (38,39,40,41,42) not including endometrial cancer, did cause a significant reduction in the cancerous growth. Although STX140 is an effective STS inhibitor, in this model it is hypothesized that the majority of action of STX140 is via antiproliferative activity resulting in a greater inhibition of tumor growth compared with STX64, a STS inhibitor. Therefore, STX140 has potential as an effective agent against endometrial cancer, however, further studies still need to be undertaken to explore fully these possibilities.
Taxol, dosed at 12 mg/kg, iv, once weekly, has previously been effective at attenuating endometrial cancer in intact mice (50). However, these studies used the poorly differentiated Hec50co endometrial cancer cell line to generate xenografts. In the current study, Taxol, dosed at 15 mg/kg, iv, once weekly, did not significantly reduce Ishikawa tumor growth, suggesting that this cell line is more resistant to microtubule stabilizer treatment.
The limited effect of STX64 on Ishikawa xenograft growth in intact animals is not surprising. Intact animals mimic premenopausal women in whom a range of biological active estrogens are synthesized in the ovaries. This allows for the production of estrogenic steroids via different routes other than STS activity. Among these other pathways are the aromatization of androstenedione to E1 and the subsequent reduction of E1 to E2. Therefore, further studies were undertaken in ovariectomized nude mice bearing Ishikawa xenograft tumors. These mice were given a sc injection of E2S thrice weekly to maintain tumor growth. In ovariectomized animals that did not receive E2S stimulation, Ishikawa xenografts failed to develop. This suggests the absolute necessity of estrogenic steroids for the maturation of this tumor type and, furthermore, indicates its hormone-dependent nature.
Animals with growing tumors were dosed with STX64 or STX213 at 1 mg/kg on a daily or weekly regime. In previous studies a daily oral dose of STX213 at 1 mg/kg was able to almost completely inhibit breast cancer tumor growth (28). In the present study, the endometrial cancer was much more aggressive than the MCF-7 breast cancer xenografts. Daily dosing of STX64 and STX213 did significantly inhibit tumor growth, as did twice weekly dosing of MPA (2 mg/kg). In contrast, weekly dosing of STX64 had no effect on tumor development, and only STX213 showed a significant effect. Liver and tumor STS activity measurements, as well as plasma E2 concentrations, in these animals demonstrate why STX64 given as a weekly dose had limited effect on tumor growth. This dosing schedule did not completely inhibit tissue STS activity or significantly ablate plasma E2 levels. Consequently, endometrial cancer was able to develop. This suggests that it is absolutely vital for the total inhibition of STS activity to reduce hormone-dependent endometrial tumor growth in vivo. This hypothesis is supported by the fact that when the dose of STX64 was increased to 10 mg/kg given daily, a more effective response was observed regarding tumor growth inhibition and E2 plasma concentration reduction.
There is growing interest in the use of MPA for the treatment of hormone-dependent endometrial cancer. In the current work, MPA had a significant effect in one study and only a limited effect in the other on the growth of endometrial tumors. Work by other groups has shown that MPA inhibits the development of a range of hormone-dependent cancers in vivo (51) and is routinely used in the clinical against endometrial cancer. However, in patients with advanced or recurrent endometrial carcinomas, it is only effective in a quarter of those patients (52). There have been no studies on how MPA affects hormone-dependent Ishikawa xenografts driven by E2S stimulation. It is possible that the Ishikawa xenografts that developed in vivo were to some extent progestin resistant, although this was not examined in the present study.
To conclude, the work presented here demonstrates, for the first time, the successful use of STS inhibitors for the treatment of hormone-dependent endometrial cancer in mice. To treat this malignancy, the importance of complete inhibition of the STS enzyme and the significant reduction of circulating E2 was ascertained. STX213 had a superior treatment profile compared with STX64 because it inhibited tumor growth at a dose of 1 mg/kg, even when administered weekly. Therefore, the use of STS inhibitors for the treatment of hormone-dependent endometrial cancer is a novel therapy.
Footnotes
This work was funded by Ipsen Limited.
Disclosure Statement: P.A.F. and L.W.L.W. have nothing to declare. B.V.L.P., M.J.R., and A.P. are consultants for Ipsen Limited.
First Published Online May 1, 2008
Abbreviations: E2, Estradiol; E1, estrone; E2S, estradiol sulfate; E1S, estrone sulfate; 17β-HSD, 17β-hydroxysteroid dehydrogenase; MPA, medroxyprogesterone acetate; NMU, nitrosomethylurea; po, per os; STS, steroid sulfatase.
References
- Sherman ME, Sturgeon S, Brinton L, Kurman RJ 1995 Endometrial cancer chemoprevention: implications of diverse pathways of carcinogenesis. J Cell Biochem Suppl 23:160–164 [DOI] [PubMed] [Google Scholar]
- Sherman ME 2000 Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol 13:295–308 [DOI] [PubMed] [Google Scholar]
- Thoma DB 1984 Do hormones cause cancer? Cancer 53:595–604 [DOI] [PubMed] [Google Scholar]
- Kelsey JL 1982 A case control study of cancer of the endometrium. Am J Epidemiol 116:333–342 [DOI] [PubMed] [Google Scholar]
- Ryan KJ 1975 Cancer risk and estrogen use in the menopause. N Engl J Med 293:1199–1200 [DOI] [PubMed] [Google Scholar]
- Reed MJ, Purohit A, Woo LWL, Newman SP, Potter BVL Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 26:171–202 [DOI] [PubMed] [Google Scholar]
- Reed MJ, Purohit A, Woo LWL, Potter BVL 1996 The development of steroid sulphatase inhibitors. Endocr Relat Cancer 3:9–23 [Google Scholar]
- Dickson RB, Thompson EW, Lippman ME 1990 Regulation of proliferation, invasion and growth factor synthesis in breast cancer by steroids. J Steroid Biochem Mol Biol 37:305–316 [DOI] [PubMed] [Google Scholar]
- Reed MJ, Beranek PA, Ghilchik MW, James VH 1986 Estrogen production and metabolism in normal postmenopausal women and postmenopausal women with breast or endometrial cancer. Eur J Cancer Clin Oncol 22:1395–1400 [DOI] [PubMed] [Google Scholar]
- van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH 1985 Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res 45:2900–2906 [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite GS 1999 Estrone sulfatase versus estrone sulfotransferase in human breast cancer: potential clinical applications. J Steroid Biochem Mol Biol 69:287–292 [DOI] [PubMed] [Google Scholar]
- Falany JL, Falany CN 1996 Expression of cytosolic sulfotransferases in normal mammary epithelial cells and breast cancer cell lines. Cancer Res 56:1551–1555 [PubMed] [Google Scholar]
- Falany JL, Macrina N, Falany CN 2002 Regulation of MCF-7 breast cancer cell growth by β-estradiol sulfonation. Breast Cancer Res Treat 74:167–176 [DOI] [PubMed] [Google Scholar]
- Bonney RC, Scanlon MJ, Jones DL, Reed MJ, Anderson MC, James VH 1986 The relationship between oestradiol metabolism and adrenal steroids in the endometrium of postmenopausal women with and without endometrial cancer. Eur J Cancer Clin Oncol 22:953–961 [DOI] [PubMed] [Google Scholar]
- Vermeulen-Meiners C, Poortman J, Haspels AA, Thijssen JH 1986 The endogenous concentration of estradiol and estrone in pathological human postmenopausal endometrium. J Steroid Biochem 24:1073–1078 [DOI] [PubMed] [Google Scholar]
- Berstein LM, Tchernobrovkina AE, Gamajunova VB, Kovalevskij AJ, Vasilyev DA, Chepik OF, Turkevitch EA, Tsyrlina EV, Maximov SJ, Ashrafian LA, Thijssen JH 2003 Tumor estrogen content and clinico-morphological and endocrine features of endometrial cancer. J Cancer Res Clin Oncol 129:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Utsunomiya H, Yaegashi N, Sasano H 2007 Biological roles of estrogen and progesterone in human endometrial carcinoma: new developments in potential endocrine therapy for endometrial cancer. Endocr J 54:667–679 [DOI] [PubMed] [Google Scholar]
- Krasner C 2007 Aromatase inhibitors in gynecologic cancers. J Steroid Biochem Mol Biol 106:76–80 [DOI] [PubMed] [Google Scholar]
- Ma BB, Oza A, Eisenhauer E, Stanimir G, Carey M, Chapman W, Latta E, Sidhu K, Powers J, Walsh W, Fyles A 2004 The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers–a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer 14:650–658 [DOI] [PubMed] [Google Scholar]
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee RB 2000 A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 78:212–216 [DOI] [PubMed] [Google Scholar]
- Krasner C, Debernardo R, Findley M, Penson R, Matulonis U, Atkinson T, Roche M, Seiden MV 2005 Phase II trial of anastrozole in combination with gefitinib in women with asymptomatic mullerian cancer: ASCO Annual Meeting Proceedings. J Clin Oncol 23:5063 [Google Scholar]
- Ito K, Utsunomiya H, Suzuki T, Saitou S, Akahira J-I, Okamura K, Yaegashi N, Sasano H 2006 17β-Hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol Cell Endocrinol 248:136–140 [DOI] [PubMed] [Google Scholar]
- Day JM, Foster PA, Tutill HJ, Parsons MF, Newman SP, Chander SK, Allan GM, Lawrence HR, Vicker N, Potter BVL, Reed MJ, Purohit A 2008 17β-Hydroxysteroid dehydrogenase type 1, and not type 12, is a target for endocrine therapy of hormone-dependent breast cancer. Int J Cancer 122:1931–1940 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kitawaki J, Urabe M, Honjo H, Tamura T, Noguchi T, Okada H, Sasaki H, Tada A, Terashima Y 1993 Estrogen productivity of endometrium and endometrial cancer tissue; influence of aromatase on proliferation of endometrial cancer cells. J Steroid Biochem Mol Biol 44:463–468 [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Ito K, Suzuki T, Kitamura T, Kaneko C, Nakata T, Niikura H, Okamura K, Yaegashi N, Sasano H 2004 Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin Cancer Res 10:5850–5856 [DOI] [PubMed] [Google Scholar]
- Purohit A, Woo LWL, Potter BVL, Reed MJ 2000 In vivo inhibition of estrone sulfatase activity and growth of nitrosomethylurea-induced mammary tumors by 667 COUMATE. Cancer Res 60:3394–3396 [PubMed] [Google Scholar]
- Foster PA, Newman SP, Chander SK, Stengel C, Jhalli R, Woo LWL, Potter BVL, Reed MJ, Purohit A 2006 In vivo efficacy of STX213, a second-generation steroid sulfatase inhibitor, for hormone-dependent breast cancer therapy. Clin Cancer Res 12:5543–5549 [DOI] [PubMed] [Google Scholar]
- Foster PA, Chander SK, Parsons MF, Newman SP, Woo LWL, Potter BVL, Reed MJ, Purohit A 4 October 2007 Efficacy of three potent steroid sulfatase inhibitors: pre-clinical investigations for their use in the treatment of hormone-dependent breast cancer. Breast Cancer Res Treat [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Howarth NM, Purohit A, Reed MJ, Potter BVL 1994 Estrone sulfamates: potent inhibitors of estrone sulfatase with therapeutic potential. J Med Chem 37:219–221 [DOI] [PubMed] [Google Scholar]
- Woo LWL, Purohit A, Malini B, Reed MJ, Potter BVL 2000 Potent active site-directed inhibition of steroid sulphatase by tricyclic coumarin-based sulphamates. Chem Biol 7:773–791 [DOI] [PubMed] [Google Scholar]
- Malini B, Purohit A, Ganeshapillai D, Woo LWL, Potter BVL, Reed MJ 2000 Inhibition of steroid sulphatase activity by tricyclic coumarin sulphamates. J Steroid Biochem Mol Biol 75:253–258 [DOI] [PubMed] [Google Scholar]
- Stanway SJ, Purohit A, Woo LWL, Sufi S, Vigushin D, Ward R, Wilson RH, Stanczyk FZ, Dobbs N, Kulinskaya E, Elliott M, Potter BVL, Reed MJ, Coombes RC 2006 Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res 12:1585–1592 [DOI] [PubMed] [Google Scholar]
- Stanway SJ, Delavault P, Purohit A, Woo LWL, Thurieau C, Potter BVL, Reed MJ 2007 Steroid sulfatase: a new target for the endocrine therapy of breast cancer. Oncologist 12:370–374 [DOI] [PubMed] [Google Scholar]
- Fischer DS, Chander SK, Woo LWL, Fenton JC, Purohit A, Reed MJ, Potter BVL 2003 Novel D-ring modified steroid derivatives as potent, non-estrogenic, steroid sulfatase inhibitors with in vivo activity. J Steroid Biochem Mol Biol 84:343–349 [DOI] [PubMed] [Google Scholar]
- Fischer DS, Woo LWL, Mahon MF, Purohit A, Reed MJ, Potter BVL 2003 D-ring modified estrone derivatives as novel potent inhibitors of steroid sulfatase. Bioorg Med Chem 11:1685–1700 [DOI] [PubMed] [Google Scholar]
- Leese MP, Leblond B, Smith A, Newman SP, Di Fiore A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BVL 2006 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem 49:7683–7696 [DOI] [PubMed] [Google Scholar]
- Raobaikady B, Purohit A, Chander SK, Woo LW, Leese MP, Potter BVL, Reed MJ 2003 Inhibition of MCF-7 breast cancer cell proliferation and in vivo steroid sulphatase activity by 2-methoxyestradiol-bis-sulphamate. J Steroid Biochem Mol Biol 84:351–358 [DOI] [PubMed] [Google Scholar]
- Raobaikady B, Reed MJ, Leese MP, Potter BVL, Purohit A 2005 Inhibition of MDA-MB-231 cell cycle progression and cell proliferation by C-2-substituted oestradiol mono- and bis-3-O-sulphamates. Int J Cancer 117:150–159 [DOI] [PubMed] [Google Scholar]
- Newman SP, Leese MP, Purohit A, James DR, Rennie CE, Potter BVL, Reed MJ 2004 Inhibition of in vitro angiogenesis by 2-methoxy- and 2-ethyl-estrogen sulfamates. Int J Cancer 109:533–540 [DOI] [PubMed] [Google Scholar]
- Chander SK, Foster PA, Leese MP, Newman SP, Potter BVL, Purohit A, Reed MJ 2007 In vivo inhibition of angiogenesis by sulphamoylated derivatives of 2-methoxyoestradiol. Br J Cancer 96:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Leese MP, Smith AC, Potter BVL, Reed MJ 2004 Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br J Cancer 90:932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PA, Ho YT, Newman SP, Kasprzyk PG, Leese MP, Potter BVL, Reed MJ, Purohit A 24 October 2007 2-MeOE2bisMATE and 2-EtE2bisMATE induce cell cycle arrest and apoptosis in breast cancer xenografts as shown by a novel ex vivo technique. Breast Cancer Res Treat [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Seibert K, Shafie SM, Triche TJ, Whang-Peng JJ, O'Brien SJ, Toney JH, Huff KK, Lippman ME 1983 Clonal variation of MCF-7 breast cancer cells in vitro and in athymic nude mice. Cancer Res 43:2223–2239 [PubMed] [Google Scholar]
- Brunner N, Spang-Thomsen M, Vindelov L, Wolff J, Engelholm SA 1985 Effect of tamoxifen on the receptor-positive T61 and the receptor-negative T60 human breast carcinomas grown in nude mice. Eur J Cancer Clin Oncol 21:1349–1354 [DOI] [PubMed] [Google Scholar]
- Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, Figg WD, Hollingshead M, Hursting S, Berrigan D, Steinberg SM, Merino MJ, Steeg PS 2005 Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst 2005 97:632–642 [DOI] [PubMed] [Google Scholar]
- Purohit A, Williams GJ, Roberts CJ, Potter BVL, Reed MJ 1995 In vivo inhibition of oestrone sulphatase and dehydroepiandrosterone sulphatase by oestrone-3-O-sulphamate. Int J Cancer 63:106–111 [DOI] [PubMed] [Google Scholar]
- Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W, Gynecologic Oncology Group study 2004 Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 92:10–14 [DOI] [PubMed] [Google Scholar]
- Decruze SB, Green JA 2007 Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 17:964–978 [DOI] [PubMed] [Google Scholar]
- Gadducci A, Cosio S, Genazzani AR 2006 Old and new perspectives in the pharmacological treatment of advanced or recurrent endometrial cancer: Hormonal therapy, chemotherapy and molecularly targeted therapies. Crit Rev Oncol Hematol 58:242–256 [DOI] [PubMed] [Google Scholar]
- Dai A, Holmes AM, Nguyen T, Davies S, Theele DP, Verschraegen C, Leslie KK 2005 A potential synergistic anticancer effect of paclitaxel and amifostine on endometrial cancer. Cancer Res 65:9517–9524 [DOI] [PubMed] [Google Scholar]
- Carey MS, Gawlik C, Fung-Kee-Fung M, Chambers A, Oliver T 2006 Systematic review of systemic therapy for advanced or recurrent endometrial cancer. Gynecol Oncol 101:158–167 [DOI] [PubMed] [Google Scholar]
- Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, Soper JT, Given FT 1999 Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol 17:1736–1744 [DOI] [PubMed] [Google Scholar]