Abstract
Recent published data have demonstrated elevated levels of human GH (hGH) in endometriosis and endometrial adenocarcinoma. Herein, we demonstrate that autocrine production of hGH can enhance the in vitro and in vivo oncogenic potential of endometrial carcinoma cells. Forced expression of hGH in endometrial carcinoma cell lines RL95-2 and AN3 resulted in an increased total cell number through enhanced cell cycle progression and decreased apoptotic cell death. In addition, autocrine hGH expression in endometrial carcinoma cells promoted anchorage-independent growth and increased cell migration/invasion in vitro. In a xenograft model of human endometrial carcinoma, autocrine hGH enhanced tumor size and progression. Changes in endometrial carcinoma cell gene expression stimulated by autocrine hGH was consistent with the altered in vitro and in vivo behavior. Functional antagonism of hGH in wild-type RL95-2 cells significantly reduced cell proliferation, cell survival, and anchorage-independent cell growth. These studies demonstrate a functional role for autocrine hGH in the development and progression of endometrial carcinoma and indicate potential therapeutic relevance of hGH antagonism in the treatment of endometrial carcinoma.
ENDOMETRIAL CARCINOMA IS the most common gynecological malignancy, and the incidence in developed countries is rising. Increased life expectancies and the rising incidence in obesity have been proposed as contributing to this trend (1). Endometrial cancer is divided into two subtypes. Type I is of endometrial origin, and estrogens play an important role in the development of this class of malignancy, whereas type II endometrial carcinomas are represented largely by serous and clear-cell adenocarcinomas (2). About 70–80% of endometrial carcinomas are detected at early stages, and consequently, the clinical outcome after treatment is usually favorable. However, a significant number of patients will later develop local recurrence and distal metastases. Additionally, tumors identified at late stages are associated with high levels of morbidity and mortality (1). Despite being a common malignancy, the molecular aspects of endometrial carcinoma are poorly understood, and treatment of this disease has remained relatively unchanged over the last few decades (3).
Human GH (hGH) is produced normally by the glandular cells of the human endometrium during the mid to late luteal phase of the female menstrual cycle (4). Recently published data have demonstrated significantly increased levels of hGH in both endometriosis and endometrial adenocarcinoma (5). Elevated levels of serum hGH have also been observed in a study of 115 patients with endometrial adenocarcinoma (6) in which hGH was identified as one of a five-biomarker panel able to discriminate endometrial cancer from ovarian and breast cancers with high sensitivity and specificity. In addition, sporadic cases of ectopic hGH secretion associated with endometrial malignancy have been reported (7). Localization of hGHRH has also been observed in normal, neoplastic, and preneoplastic endometrial tissues (8,9,10). hGHRH antagonists have demonstrated efficacy both in vitro and in xenograft models of human endometrial carcinoma, demonstrating therapeutic potential (11,12).
Recent studies have demonstrated that autocrine hGH is a wild-type orthotopically expressed oncogene for the human mammary epithelial cell (13,14,15). Autocrine hGH increases proliferation and survival of an immortalized human mammary epithelial cell line, thus creating a platform that is sufficient for development of neoplasia (14). In addition, autocrine hGH increases telomerase activity in mammary carcinoma cells through stabilization of the catalytic subunit of telomerase, hTERT mRNA (16), potentially contributing to cell immortalization. Indeed, we have demonstrated that forced expression of hGH in primary human mammary epithelial cells extends the replicative lifespan of this cell line (15). Autocrine hGH may also impact on mammary carcinoma progression as it promotes epitheliomesenchymal transition (EMT) in a mammary carcinoma cell line, resulting in an invasive phenotype (17).
Herein we demonstrate that autocrine hGH concomitantly enhances endometrial carcinoma cell proliferation, survival, anchorage-independent growth, and migration/invasion. In addition, autocrine hGH increases endometrial carcinoma tumor size and progression in an in vivo xenograft model. Functional antagonism of hGH abrogates oncogenicity of endometrial carcinoma cells. Thus, autocrine hGH may be considered a potential therapeutic target in endometrial carcinoma.
Materials and Methods
Cell lines and cell transfection
The human endometrial carcinoma cell lines RL95-2 and AN3 were obtained from the American Type Culture Collection (Rockville, MD). Cell lines were cultured using American Type Culture Collection-recommended conditions. The plasmid pcDNA3-hGH was constructed by cloning a 2.1-kb BamHI fragment containing the entire hGH gene, derived from the vector pMT-hGH (18), into pcDNA3 (Invitrogen, Carlsbad, CA). Stable cell lines, RL95-2-vector, RL95-2-hGH, AN3-vector, and AN3-hGH were generated as previously described (19).
The hGH receptor antagonist, B2036 (Pfizer, New York, NY), was added to medium at a final concentration of 1000 nm for indicated periods of time. An equivalent concentration of BSA (Sigma-Aldrich, Munich, Germany) was added to the control wells.
ELISA
ELISA was performed using a hGH-coated-well ELISA kit (Diagnostic Systems Laboratories Inc., Webster, TX) according to the manufacturer’s instructions on conditioned media as previously described (20).
Cell number and oncogenicity assays
Total cell number.
A total of 5 × 104 cells of RL95-2-vector, RL95-2-hGH, AN3-vector, or AN3-hGH cell lines were seeded into six-well plates in monolayers in complete or serum-reduced (0.2% serum) medium. On indicated days, cells were trypsinized, and the cell number was determined using a hematocytometer as previously described (14).
Cell viability.
Cells (1 × 104 cells per well) were seeded into 96-well microtiter plates in complete or serum reduced (0.2% serum) medium. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine cell viability as previously described (21).
Cell cycle.
Cells were seeded in six-well plates in complete or serum-deficient [0.2% fetal bovine serum (FBS)] medium. 5-Bromo-2-deoxyuridine (BrdU) incorporation and detection was performed as previously described (14) but with a 20 μm BrdU pulse for 45 min.
Apoptosis.
Apoptotic cell death was determined using terminal transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay as described previously (22).
Anchorage-independent growth.
Assays including colony formation, suspension culture, and foci formation were performed as previously described (14,19). For suspension culture, cells (5 × 103) were grown in Poly-HEMA-coated (Sigma) six-well plates.
Cell behavior and morphogenesis assays in Matrigel.
Tissue culture dishes were coated with Matrigel (BD Bioscience, Franklin Lakes, NJ) at 37 C for 30 min before addition of stable cell lines. The behavior of cell lines was assessed and digitally recorded at 24-h intervals using an inverted light microscope (Olympus 1X2-ILL100, Japan).
In vitro cell migration and invasion assays
Assays were performed in BD BioCoat Matrigel invasion chambers according to the manufacturer’s instructions as described previously (17). For wound migration assay, confluent monolayers of stable transfected cell lines were scraped with pipette tips, washed with PBS, and incubated in culture medium supplemented with 10% FBS for 6 d as described (23).
RT-PCR and real-time PCR analysis
Semiquantitative RT-PCR was performed as described previously (22). Genes analyzed and the sequences of the primers used are listed in supplemental data 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
For real-time PCR, total RNA was converted to cDNA using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) as per manufacture’s instructions. Real-time PCR was performed using an ABI 7700 real-time PCR system (Applied Biosystems, Foster City, CA). The ABI 7700 real-time PCR system (Applied Biosystems) was used for analysis. Multiple gene markers distributed around the genome and three housekeeping genes were used for real-time PCR analysis using the SYBR GreenER qPCR SuperMix for ABI PRISM (Invitrogen). Total cDNA (5 ng) isolated from each stable cell line was added to a 20-μl reaction containing SYBR GreenER qPCR SuperMix for ABI PRISM and 200 nm of each primer. Triplicate reactions were performed for each marker in a 384-well plate using a two-step amplification program of initial denaturation at 95 C for 10 min, followed by 40 cycles of 95 C for 20 sec and 60 C for 30 sec. A melting curve analysis step was carried out at the end of the amplification, consisting of denaturation at 95 C for 1 min and reannealing at 55 C for 1 min. Standard curves were generated from each experimental plate using serial 5-fold dilutions of untreated cDNA. The geometric mean of the cycle threshold (Ct) value for each reaction was calculated. Amplification efficiencies were calculated according to the equation E = 10(−1/slope) (24) and ranged from 90–104% for all gene markers; no nonspecific amplification or primer dimer was observed in any of the reactions as confirmed by the melting curve analysis.
Each experiment was performed three or more times with independent samples. Each change in gene expression is expressed as fold change and is the average of three experiments (P value < 0.05). Positive and negative fold change indicates a respective increase or decrease in mRNA levels. To compensate for potential differences between markers, the relative expression was computed, based on the efficiency (E), normalized by a panel of housekeeping genes, β-actin, HPRT, and GAPDH, and the Ct difference (Δ) of sample vs. control (ΔCtsample−control). Relative expression = 2−(Ct,Sample − Ct,HKG) − (Ct,Control − Ct,HKG). Relative expression = 2−ddCt. Changes in relative expression more than 1.5-fold were taken as significant. A list of the genes analyzed by real-time PCR and primer sequences can be found in supplemental data 2.
Xenograft analyses
RL95-2-vector or RL95-2-hGH cells (1.5 × 108) were suspended in 100 μl PBS and injected sc into 3- to 4-wk-old BALBc nu/nu mice (Shanghai Slaccas Co., Shanghai, China). The latency of the tumor from the nude mice in this study is around 1 wk, and tumors were harvested 18 d after inoculation. Tissue samples of the primary tumors were fixed in 4% paraformaldehyde and stained with hematoxylin and eosin for histological assessment. For BrdU analysis, mice were injected ip with BrdU (100 mg/kg) 6 h before euthanasia. Tumors were excised, rinsed with 0.9% saline solution, fixed in 4% paraformaldehyde, and embedded in paraffin wax. Nuclear BrdU was detected in sections using an UltraSensitive S-P Kit for BrdU detection (FuZhou MAIXIN BIO Co., FuZhou, China) according to the manufacturer’s instructions, and slides were counterstained with Gill’s hematoxylin.
For TUNEL analysis, paraffin-embedded tumor tissue sections were deparaffinized and rehydrated, followed by treatment with 15 mg/liter proteinase K for 30 min. Apoptotic nuclei in the section were analyzed with an in situ Cell Death Detection Kit, POD (Roche, Indianapolis, IN) according to the manufacture’s guide. The number of TUNEL-positive cells in tumor sections was determined in at least three independent high-resolution fields selected randomly, and around 800 cells were counted per slide.
Statistics
All data are expressed as means ± se of triplicate determinants. All experiments were performed at least three times. Data were analyzed using an unpaired two-tailed t test or ANOVA.
Results
Autocrine hGH increases endometrial carcinoma cell number, cell proliferation, and cell survival
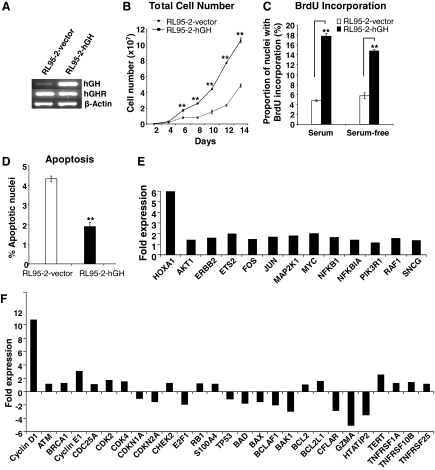
Autocrine hGH has previously been demonstrated to function as a human mammary epithelial oncogene (14,17). To determine whether autocrine hGH modulates endometrial carcinoma cell behavior, we stably transfected RL95-2 cells with an expression plasmid encoding the hGH gene (RL95-2-hGH). As a control, RL95-2 cells stably transfected with the pcDNA3 vector (RL95-2-vector) were established in parallel. Pooled stable transfectants were used to minimize any effect of potential clonal selection. RL95-2-hGH stable cells synthesized hGH mRNA as demonstrated by semiquantitative RT-PCR (Fig. 1A) and secreted hGH protein into the medium [37.5 ng/ml (1.7 nm) over 24 h] as demonstrated by ELISA analysis (data not shown). RL95-2 cells also endogenously express hGH mRNA transcripts (Fig. 1A) and generate hGH protein [0.5 ng/ml (22.73 pm) over 24 h; data not shown]. Real-time PCR analysis also confirmed increased levels of hGH mRNA in the RL95-2-hGH cell line when compared with RL95-2-vector cells (data not shown).
Figure 1.
Autocrine hGH increases total cell number, cell cycle progression, and cell survival and alters the gene expression profile in human endometrial carcinoma cells. RL95-2 cells were stably transfected with an expression vector containing the hGH gene (designated RL95-2-hGH) or pcDNA3 vector alone (RL95-2-vector). A, The level of hGH and hGHR mRNA in RL95-2-hGH and RL95-2-vector cell lines was determined by RT-PCR. B, Growth of RL95-2-vector and RL95-2-hGH cell lines was assessed by total cell number in 10% FBS medium. C, Effect of autocrine hGH on cell cycle progression as assessed by nuclear BrdU incorporation in 10% serum and serum-free conditions as indicated. D, Effect of autocrine hGH on apoptosis induced by serum withdrawal over 48 h as evaluated by TUNEL assay. E, Real-time PCR gene expression analysis of the effect of autocrine hGH expression on the gene expression profile of RL95-2 cells. Results are represented as a fold change in mRNA levels in RL95-2-hGH cells in comparison with RL95-2-vector cells. F, Real-time PCR analysis of the effect of autocrine hGH expression on mRNA levels of several key markers involved in proliferation, cell cycle regulation, and apoptosis. Results are represented as a fold change in mRNA levels in RL95-2-hGH cells in comparison with RL95-2-vector cells. **, P < 0.001.
mRNA expression of the hGH receptor (hGHR) in stably transfected cell lines was demonstrated by semiquantitative RT-PCR (Fig. 1A). These results were again confirmed by real-time PCR (data not shown). Neither RL95-2-vector nor RL95-2-hGH cells exhibited detectable levels of IGF-I mRNA by real-time PCR (data not shown), indicative that the autocrine hGH-mediated responses reported below are not due to IGF-I effects on cell function. Real-time PCR demonstrated, however, that IGF-II mRNA was increased in RL95-2-hGH cells when compared with the RL95-2-vector cell line (data not shown). In addition, Neither RL95-2-vector nor RL95-2-hGH cells exhibited detectable levels of PRL mRNA by real-time PCR (data not shown). mRNA levels of the PRL receptor were detected in both cell lines by real-time PCR. However, no increase in PRL receptor mRNA was observed as a result of forced expression of hGH in RL95-2 cells (data not shown).
RL95-2-hGH total cell number increased dramatically more than RL95-2-vector cell number over a period of 14 d in full (10%) serum conditions (Fig. 1B). Increased cell number results from the net effect of increased cell proliferation and/or a decrease in apoptotic cell death. Autocrine expression of hGH significantly increased cell cycle progression, as determined by BrdU incorporation in RL95-2-hGH cells in both serum and serum-free conditions (Fig. 1C). In addition, autocrine hGH significantly reduced apoptotic cell death consequent to serum deprivation (Fig. 1D) when compared with the control cell line, RL95-2-vector.
Real-time PCR analysis comparing RL95-2-vector and RL95-2-hGH cell lines demonstrated up-regulation of various signal transduction molecules and transcription factors (Fig. 1E). Of note, mRNA levels of JUN and MYC, previously demonstrated to be regulated by hGH (25), were increased by forced expression of autocrine hGH, as was ERBB2. mRNA expression of HOXA1, a potent oncogene previously demonstrated to mediate autocrine hGH-stimulated oncogenesis (19), was increased 6-fold by autocrine hGH in endometrial carcinoma cells (Fig. 1E).
Transcriptional regulation of genes required for cell cycle progression and cell survival is also directly associated with activation of signaling pathways involved in oncogenic transformation of cells (26,27). Forced expression of hGH in RL95-2 cells increased the mRNA level of Cyclin D1, Cyclin E1, Cyclin-dependent kinase 2 (CDK2), and Cyclin-dependent kinase 4 (CDK4) required for cell cycle progression (Fig. 1F). In addition, the mRNA level of CDKN2A (p16), an inhibitor of CDK4, was decreased in RL95-2-hGH cells (Fig. 1F). Forced expression of hGH increased the mRNA level of prosurvival gene BCL-2-related protein long isoform (BCLXL/BCL2L1). Concordantly, the mRNA levels of genes encoding proapoptotic BCL2 antagonist of cell death (BAD), BCL2-associated X protein (BAX), BCL2-associated transcription factor 1 (BTF/BCLAF1), Granzyme A (GZMA), and BCL2 antagonist killer 1 (BAK1) were decreased by expression of autocrine hGH in RL95-2 cells. The mRNA level of the human catalytic subunit of telomerase, hTERT, was also increased in RL95-2 cells by autocrine hGH (Fig. 1F), consistent with the recent demonstration of autocrine hGH-induced stabilization of hTERT mRNA in mammary carcinoma cells (16).
Autocrine hGH enhances anchorage-independent cell growth of endometrial carcinoma cells
One characteristic of oncogenically transformed cells is anchorage-independent cell growth (26,28,29). Autocrine expression of hGH in RL95-2-hGH cells enhanced anchorage-independent growth as indicated by colony formation in soft agar (Fig. 2A), cell growth in suspension culture (Fig. 2B), and foci formation (Fig. 2C). Furthermore, the individual colony size in soft agar was markedly increased by autocrine hGH (Fig. 2A). Oncogenically transformed epithelial cells form large, nonpolarized, undifferentiated colonies without lumina when grown in reconstituted basement membrane (Matrigel) (30,31). Regular three-dimensional spheroid structures were generated by plating RL95-2-vector cells in growth factor-containing and growth factor-reduced Matrigel. In contrast, RL95-2-hGH cells formed disorganized structures of irregular morphology (Fig. 2D).
Figure 2.
Autocrine hGH enhances anchorage-independent growth in RL95-2 cells. A, Soft agar colony formation of RL95-2-vector and RL95-2-hGH cells in 10% FBS medium. Colonies were visualized under ×150 magnification. B, Growth curve of RL95-2-vector and RL95-2-hGH cells in suspension culture in 10% FBS medium. C, Effect of autocrine hGH on colony formation in growth factor (GF) or growth factor-reduced (GFR) Matrigel. Number of colonies indicates the total numbers of colonies with disrupted morphology and filled lumina. Images were captured under ×400 magnification. D, RL95-2-vector and RL95-2-hGH cell foci formation in 10% FBS medium. **, P < 0.001.
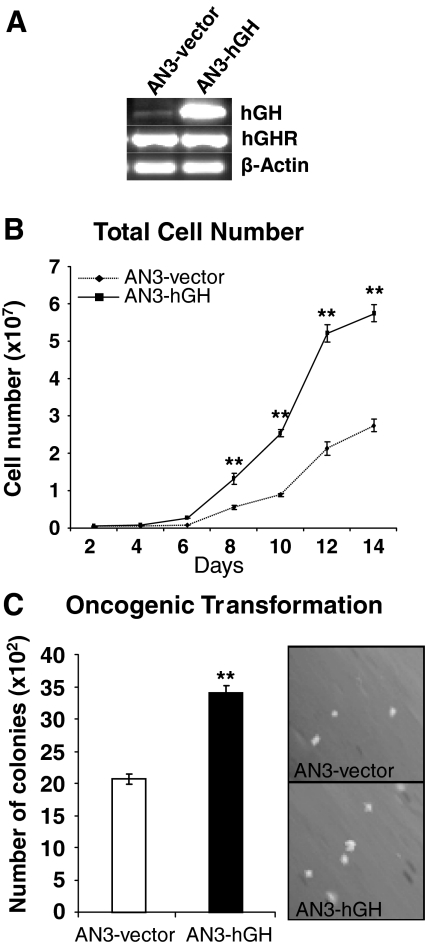
Autocrine hGH increases total cell number and anchorage-independent growth in AN3 endometrial carcinoma cells
We examined the effects of autocrine hGH in a second endometrial carcinoma cell line, AN3. Forced expression of hGH in AN3 cells was verified by semiquantitative RT-PCR and ELISA. AN3-hGH cells possessed increased hGH mRNA levels (Fig. 3A) and secreted increased levels of hGH protein into the medium [65 ng/ml (2.95 nm) over 24 h] (data not shown) when compared with the empty vector-transfected control cell line, AN3-vector. Both cell lines had equivalent levels of hGHR transcript (Fig. 3A). AN3 cells also endogenously expressed hGH mRNA transcripts (Fig. 3A) and hGH protein ]1.2 ng/ml (54.6 pm) over 24 h] (data not shown). Cell growth of the two stable cell lines was assessed in 10% serum-containing medium by counting total cell number. AN3-hGH cells grew significantly faster over a 14 day time period when compared with the control cell line AN3-vector, despite an original identical plating density (Fig. 3B). Autocrine hGH in AN3-hGH cells enhanced anchorage-independent cell growth, as indicated by colony formation in soft agar (Fig. 3C). In addition, the size of the colonies formed by AN3-hGH cells was markedly increased when compared with the control cell line (Fig. 3C).
Figure 3.
Autocrine hGH enhances anchorage-independent growth in AN3 cells. AN3 cells were stably transfected with an expression vector containing the hGH gene (designated AN3-hGH) or pcDNA3 vector alone (AN3-vector). A, The level of hGH and hGHR mRNA in AN3-hGH and AN3-vector cell lines was determined by RT-PCR. B, Growth of the AN3-vector and AN3-hGH cell lines was assessed by total cell number in 10% FBS medium. C, Soft agar colony formation of AN3-vector and AN3-hGH cells in 10% FBS medium. Colonies were visualized under ×150 magnification. **, P < 0.001.
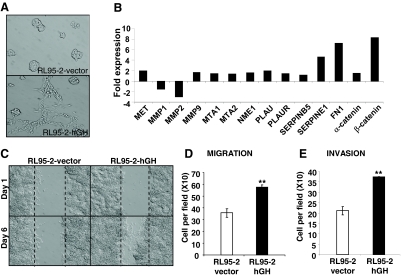
Autocrine hGH promotes the EMT of endometrial carcinoma cells
During carcinoma progression, the phenotypic conversion of cells from an epithelial to a mesenchymal morphology, accompanied by concomitant changes in gene expression, is referred to as EMT (32,33). Autocrine hGH promotes EMT of human mammary carcinoma cells (17). Concordantly, expression of autocrine hGH in RL95-2-hGH cells was associated with altered cell morphology; cells displayed more mesenchymal characteristics such as an elongated fibroblast-like morphology, loss of cell-cell contact, formation of multiple cellular protrusions, and increased cell motility (Fig. 4A). RL95-2-vector cells cultured on plastic exhibited epithelial characteristics and grew as defined grouped colonies with copious cell to cell contact (Fig. 4A) similar to the parental RL95-2 cell line (data not shown).
Figure 4.
Autocrine hGH stimulates mesenchymal transition and results in increased cell motility and acquisition of an invasive phenotype in human endometrial carcinoma cells. A, The morphology of RL95-2-vector and RL95-2-hGH cells cultured in 10% FBS medium on plastic was examined by bright-field microscopy under ×400 magnification. B, Real-time PCR analysis of the effect of autocrine hGH expression on mRNA levels of several key markers involved in EMT. Results are represented as a fold change in mRNA levels in RL95-2-hGH cells in comparison with RL95-2-vector cells. C, Wound-healing assay. Wounded areas were examined under ×100 magnification. D and E, The effect of autocrine hGH on RL95-2 cell migration (D) and invasion (E) was determined by Transwell chamber assay as described in Materials and Methods. **, P < 0.001.
EMT is associated with down-regulation of epithelial marker genes and an induction of mesenchymal markers such as the extracellular matrix protein fibronectin (34). Real-time PCR analysis demonstrated that mRNA levels of fibronectin (FN1), α-catenin, and β-catenin were increased in RL95-2-hGH cells when compared with RL95-2-vector cells (Fig. 4B). In addition, mRNA levels of invasion- and metastasis-promoting genes, MET protooncogene, metalloproteinase 9 (MMP9), urokinase plasminogen activator (PLAU), and Plasminogen activator-1 (PAI-1)/SERPINE1 were also up-regulated in RL95-2 cells with forced expression of hGH (Fig. 4B).
The observed mesenchymal morphology of autocrine hGH-expressing RL95-2 cells was suggestive of an increased potential for migration and invasion. Using a wound-healing assay, we observed that autocrine hGH stimulated RL95-2 cell migration with more rapid closing of the wound than observed in vector-transfected cells (Fig. 4C). Increased migration of autocrine hGH-expressing RL95-2 cells in comparison with the control cell line was demonstrated using a Transwell assay (Fig. 4D). RL95-2-hGH cells also exhibited a significantly increased ability to pass through Matrigel in a standard invasion assay (Fig. 4E).
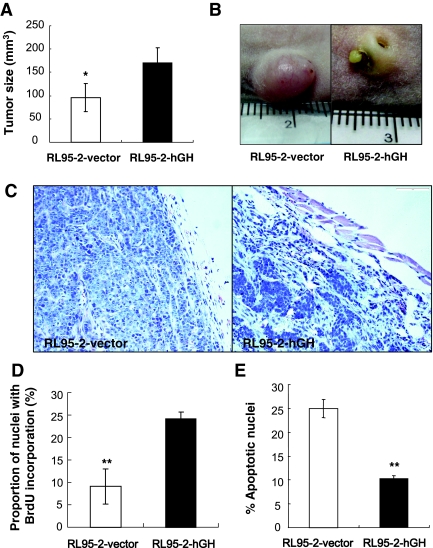
Autocrine hGH production in endometrial carcinoma cells enhances tumorigenic capacity
We implanted both RL95-2-vector and RL95-2-hGH cells sc into female athymic mice using PBS as a vehicle. Both cell lines rapidly formed palpable tumors after less than 1 wk. However, RL95-2-hGH tumors grew significantly faster than the RL95-2-vector tumors for the first 2 wk, resulting in a larger tumor mass at d 18 (Fig. 5A). Strikingly, all of the RL95-2-hGH tumors developed ulceration in the third week (Fig. 5B) accompanied by local tissue spreading with a resultant decrease in tumor mass (data not shown). RL95-2-vector cells grew as a grossly well , circumscribed firm mass loosely attached to the surrounding tissue with no apparent ulceration (Fig, 5B and C). RL95-2-hGH tumors appeared as poorly circumscribed and ulcerated masses with openings of the sinus tract visible on the surface (Fig. 5B). The lesion from the RL95-2-hGH tumors had an ill-defined margin that blended with surrounding tissues and appeared highly invasive, infiltrating the underlying skeletal muscle and fat tissue (Fig. 5C).
Figure 5.
Autocrine hGH enhances endometrial carcinoma cell tumor formation in vivo. RL95-2-vector and RL95-2-hGH cells were injected sc into athymic mice and allowed to grow for 18 d. A, Average tumor mass at 18 d; B, tumor mass visualized on necropsy showing ulceration of RL95-2-hGH-derived tumors after 3 wk; C, histological appearance of the tumors visualized with hematoxylin and eosin; D, evaluation of nuclear BrdU incorporation in RL95-2-vector and RL95-2-hGH tumors; E, evaluation of TUNEL-positive (apoptotic) nuclei in RL95-2-vector and RL95-2-hGH tumors. **, P < 0.001; *, P < 0.05.
To determine whether autocrine hGH has a direct effect on tumor cell proliferation in vivo, we injected 100 mg/kg BrdU ip into athymic mice with d-18 RL95-2-vector and RL95-2-hGH xenografts. Analysis of the paraffin sections with an anti-BrdU antibody demonstrated that the RL95-2-hGH tumors exhibited significantly increased nuclear BrdU incorporation compared with RL95-2-vector-generated tumors (Fig. 5D). In addition, TUNEL staining performed on paraffin-embedded tumor tissue sections demonstrated that RL95-2-hGH tumors contained significantly fewer apoptotic nuclei than RL95-2-vector-generated tumors (Fig. 5E).
Functional antagonism of hGH in RL95-2 cells reduces oncogenic cell characteristics
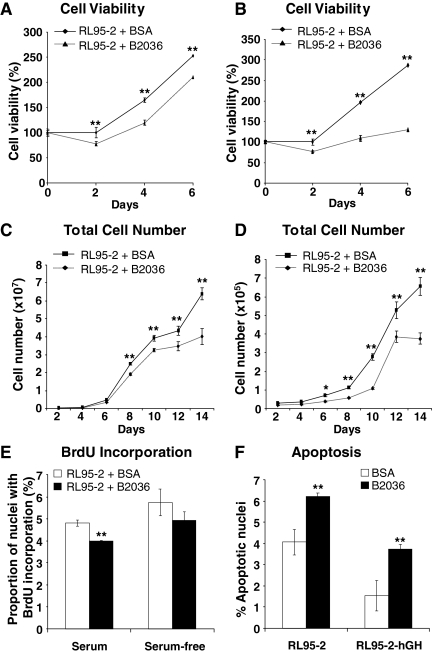
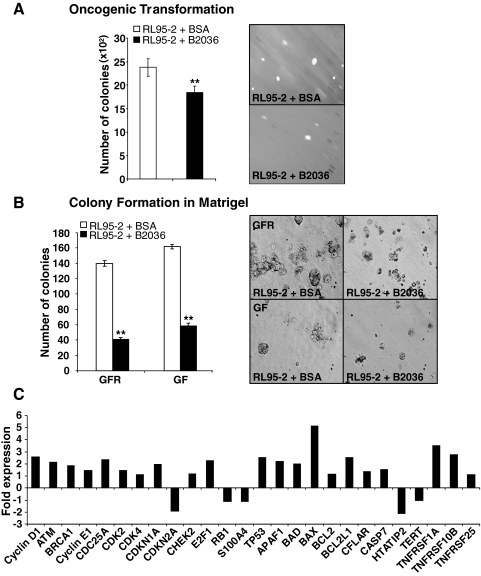
Functional antagonism of hGH was achieved using the hGH-receptor antagonist B2036. B2036 is the protein component of Pegvisomant (Pfizer) used in the treatment of acromegaly (35,36). Treatment of wild-type RL95-2 cells with 1000 nm B2036 reduced cell viability as determined by MTT assay over a 6-d time period in both 10% serum and serum-deficient (0.2%) medium (Fig. 6, A and B). B2036 treatment resulted in a reduction of RL95-2 total cell number in both 10% serum and serum-deficient (0.2%) medium (Fig. 6, C and D). Moreover, treatment of wild-type RL95-2 cells with B2036 decreased cell cycle progression as demonstrated by BrdU incorporation in 10% serum media when compared with untreated cells (Fig. 6E). In addition, B2036 significantly reduced cell cycle progression in stably transfected RL95-2-hGH cells when compared with RL95-2-vector cells (data not shown). B2036 also abrogated cell survival in both wild-type RL95-2 and stably transfected RL95-2-hGH cells in serum-free media (Fig. 6F). B2036 decreased anchorage-independent growth of RL95-2 cells as indicated by decreased colony formation in soft agar (Fig. 7A). Furthermore, the individual colony size was markedly decreased by B2036. Treatment of RL95-2 cells with B2036 also reduced colony formation in Matrigel (Fig. 7B).
Figure 6.
The hGH receptor antagonist, B2036, reduces cell viability, total cell number, cell cycle progression, and cell survival in wild-type RL95-2 cells. A and B, The effect of B2036 on the viability of RL95-2 cells was evaluated by MTT assay in 10% FBS (A) and serum-deficient (0.2% serum) (B) conditions. C and D, The effect of B2036 on the growth of RL95-2 cells was evaluated by total cell number in 10% FBS (C) and serum-deficient (0.2% serum) (D) conditions. E, The effect of B2036 on RL95-2 cell cycle progression was assessed by nuclear incorporation of BrdU in 10% FBS and serum-free medium. F, The effect of treatment of RL95-2 and RL95-2-hGH cells with B2036 on apoptotic cell death induced by serum withdrawal over 48 h was assessed by TUNEL assay in serum-free medium. **, P < 0.001.
Figure 7.
The hGH receptor antagonist, B2036, reduces oncogenic transformation in wild-type RL95-2 cells. A, The effect of B2036 on RL95-2 colony formation in soft agar in 10% FBS medium. Colonies were visualized under ×150 magnification. B, The effect of B2036 on RL95-2 colony formation in growth factor (GF)-containing and reduced growth factor (GFR) Matrigel. Number of colonies indicates the total number of colonies with disrupted morphology and filled lumina. Images were captured under ×400 magnification. C, Real-time PCR analysis of the effect of B2036 on the expression profile of RL95-2 cells. Results are represented as a fold change in mRNA levels in RL95-2 after B2036 treatment. **, P < 0.001.
We determined changes in gene expression in RL95-2 cells resulting from treatment with B2036 using real-time PCR. Real-time PCR analysis demonstrated increased mRNA levels of several proapoptotic genes including TP53, Apoptotic protease activating factor (APAF1), BAD, BAX, and Caspase-7 (CASP7) in RL95-2 cells after treatment with B2036 (Fig. 7D), indicative of an apoptotic effect. Paradoxically, B2036 treatment also increased mRNA levels of several genes involved in cell cycle and proliferation (Cyclin D1, Cyclin E1, CDK2, ataxia-telangiectasia mutated (ATM), and transcription factor E2F (E2F1) (Fig. 7D).
Discussion
Cancer progression is a multistage process involving the deregulation of cell proliferation coupled with suppression of cell death and a subsequent enhanced capacity for anchorage-independent cell growth, local tissue invasion, and metastatic potential (28,29). Initiation and progression of malignancy has been demonstrated to occur under the influence of endocrine/growth factors that when produced locally can act in an autocrine and/or paracrine fashion (37). Recent literature has identified autocrine hGH as a wild-type orthotopically expressed oncogene for the human mammary epithelial cell (13,14,38). Here we demonstrate that autocrine hGH enhances the oncogenicity of endometrial carcinoma cells in vitro and significantly increases tumorigenic capacity in vivo. Furthermore, autocrine hGH stimulated a gene expression profile in endometrial carcinoma cells consistent with the observed autocrine hGH-mediated increase in oncogenicity.
Clinical correlations linking elevated hGH levels and endometrial carcinoma have been reported in the literature. Recently Slater et al. (5), in a retrospective study, found dramatically increased hGH protein in endometrial carcinoma when compared with normal uterine tissue, indicative of the clinical relevance of autocrine hGH in this disease (5). Increased hGH protein expression was also demonstrated to be associated with endometriosis, a proliferative disorder in which the endometrial tissue is present ectopically within the pelvic region (5). Elevated levels of serum hGH have been observed in patients with endometrial adenocarcinoma (6,7). Significantly, hGH was identified as one of a panel of five biomarkers able to discriminate endometrial cancer from ovarian and breast cancers with high sensitivity and specificity (37). Localization of the hGHR has also been identified in endometrial carcinoma, and one study has demonstrated hGHR protein expression in 20 of 27 primary endometrial carcinoma samples by immunohistochemistry (39). Although our studies demonstrate a role for autocrine hGH in endometrial carcinoma development and progression, elevated serum levels of hGH may also be indicative of endocrine hGH-mediated effects. However, it is also possible that local increased hGH production at the tumor site is responsible for the increased serum hGH observed in patients with endometrial carcinoma (6). An analogous situation occurs with hGH-secreting mammary tumors in the dog (40,41). In any case, the role that endocrine hGH, either of pituitary or carcinoma origin, plays in the progression of endometrial carcinoma needs to be further defined. Given that animal and clinical studies definitively implicate endocrine GH in neoplastic progression (13,42), endocrine hGH will presumably also possess important functional effects in endometrial carcinoma.
Ectopic mRNA expression of the hypothalamic hormone hGHRH, which regulates release of hGH from the pituitary, has also been detected in neoplastic endometrial tissues (8,9,10). hGHRH antagonists are currently in development for the treatment of this disease (8,11,12). Such antagonists function through the inhibition of hGH release from the anterior pituitary, thus suppressing hepatic production of IGF-I, or through direct tumor effects (12) including suppression of autocrine hGH production (43). In addition, serum concentrations of prolactin (PRL), a protein hormone closely related to GH, are significantly elevated in patients with endometrial cancer compared with healthy controls (6). In that study, PRL was identified as the strongest discriminative biomarker for endometrial cancer, providing 98.3% sensitivity and 98.0% specificity alone. It should be noted, however, that serum levels of hGH alter temporally, and consequently, measures of patient serum hGH can vary significantly over a 24-h period (44). It should also be noted that hGH binds to and activates the PRL receptor, and autocrine hGH may therefore represent the predominant driver of endometrial carcinoma, despite PRL being a better discriminative marker (45).
Much of the effects of GH on somatic growth are mediated through induction of hepatic IGF-I secretion (46). IGF-I mRNA was not detected in RL95-2 wild-type or stably transfected cells, indicative that the autocrine hGH-mediated oncogenic effects are not mediated through stimulation of IGF-I in our studies. However, it is likely that in a physiological setting, hGH secretion from endometrial carcinoma cells will have both autocrine and paracrine effects on neighboring cells, potentially promoting IGF-I expression and enhancing autocrine hGH-mediated oncogenic effects. It should be noted that autocrine hGH did increase IGF-II mRNA levels in RL95-2. IGF-II can also bind and activate the IGF-I receptor, although with lower affinity, and produce oncogenic activity (47,48), which may be of relevance in endometrial carcinoma.
Autocrine hGH notably up-regulated the transcript level of several oncogenes in endometrial carcinoma cells. c-JUN is a protooncogene that is required for progression through the G1 phase of the cell cycle and has previously been demonstrated to correlate with endometrial carcinoma tumor grade (49). The ERBB2 gene is overexpressed in 9–30% of all endometrial carcinoma, and amplification of the gene has been demonstrated to be of prognostic value in several studies (50). HOXA1 is a member of the homeodomain-containing transcription factor family and has an important role during the normal growth and differentiation of mammalian tissues (51). HOXA1 is itself a powerful oncogene able to induce oncogenic transformation of the human mammary epithelial cell and is implicated as a partial mediator of the oncogenic effects of autocrine hGH in mammary carcinoma (19,52,53).
The acquisition of a migratory and invasive phenotype by cells of epithelial origin is associated with a gradual loss of epithelial phenotype and gain of mesenchymal characteristics in a process referred to as epithelial to mesenchymal transition (EMT). EMT facilitates tumor progression into a metastatic state and is associated with a loss of cell-cell adhesion and degradation of the neighboring extracellular matrix (34). Here we have demonstrated that autocrine hGH promotes endometrial carcinoma cell invasion and migration, thus potentially enhancing metastatic potential. Consistent with this, autocrine hGH promoted tumor progression in a xenograft model of endometrial carcinoma, resulting in tumor ulceration and local invasion. Changes in RL95-2 cell gene expression stimulated by autocrine hGH was consistent with the altered in vitro and in vivo behavior. Autocrine hGH increased mRNA levels of genes involved in EMT and metastasis including the metastasis factor MET and the mesenchymal cell marker fibronectin. In addition, mRNA levels of matrix metalloproteinases MMP-9 was up-regulated in response to autocrine expression of hGH in RL95-2 cells. Increased matrix metalloproteinase-9 protein levels have previously been associated with histological grade and disease stage in endometrial carcinoma (54,55,56). We also found up-regulation of the SERPINE1 (PAI1) gene, which has also been demonstrated to correlate positively with stage I and III endometrial carcinoma (57).
Recent publications have demonstrated that functional antagonism of hGH may be effective in treating tumors that are potentially GH, IGF-I, and/or IGF-II dependent, such as meningiomas and breast and colorectal cancer (5,58,59,60,61,62). In a preclinical study, pegvisomant, the pegylated form of B2036, has been demonstrated to block both mammary gland development and MCF-7 cell-derived tumor growth in a xenograft model (58). In these studies, administration of pegvisomant produced regression of MCF-7 xenografts, with 30% reduction in tumor volume (58). Pegvisomant is FDA approved and used clinically for the treatment of acromegaly (63). Although pegvisomant has not yet been tested in a trial for use in cancer therapy, Pfizer has recently conducted a study demonstrating that pegvisomant treatment in normal subjects is well tolerated (59). Accordingly, we have demonstrated that functional hGH antagonism decreases oncogenicity of endometrial cells, thus indicating the potential utility of pegvisomant in the treatment of endometrial cancer.
In conclusion, we have demonstrated that autocrine hGH increases the oncogenicity of endometrial carcinoma cells with resultant aggressive in vivo behavior. Our results, when combined with available clinical data, indicate autocrine hGH may be a pivotal driver of endometrial carcinoma progression. Antagonism of hGH, especially in late stages of endometrial carcinoma may provide a useful therapeutic strategy to improve patient prognosis.
Supplementary Material
Acknowledgments
We thank Dr. Bing Hu for technical assistance.
Footnotes
This work was funded by the Marsden Fund, Royal Society of New Zealand, The Breast Cancer Research Trust (New Zealand), Foundation for Research, Science and Technology and The National Research Centre for Growth and Development (NRCGD, New Zealand), and The National Basic Research Program of China (2007CB914503).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: BrdU, 5-Bromo-2-deoxyuridine; EMT, epitheliomesenchymal transition; FBS, fetal bovine serum; hGH, human GH; hGHR, hGH receptor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PRL, prolactin; TUNEL, terminal transferase deoxyuridine triphosphate nick end labeling.
References
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I 2005 Endometrial cancer. Lancet 366:491–505 [DOI] [PubMed] [Google Scholar]
- Bokhman JV 1983 Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17 [DOI] [PubMed] [Google Scholar]
- Kitchener H 2006 Management of endometrial cancer. Eur J Surg Oncol 32:838–843 [DOI] [PubMed] [Google Scholar]
- Sbracia M, Scarpellini F, Poverini R, Alo PL, Rossi G, Di Tondo U 2004 Immunohistochemical localization of the growth hormone in human endometrium and decidua. Am J Reprod Immunol 51:112–116 [DOI] [PubMed] [Google Scholar]
- Slater M, Cooper M, Murphy CR 2006 Human growth hormone and interleukin-6 are upregulated in endometriosis and endometrioid adenocarcinoma. Acta Histochem 108:13–18 [DOI] [PubMed] [Google Scholar]
- Yurkovetsky Z, Ta'asan S, Skates S, Rand A, Lomakin A, Linkov F, Marrangoni A, Velikokhatnaya L, Winans M, Gorelik E, Maxwell GL, Lu K, Lokshin A 2007 Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin. Gynecol Oncol 107:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon D, Peyser MR, Limor R, Lessing JB, Ravid R 1982 Paradoxical hypersecretion of growth hormone in patients with endometrial atypical hyperplasia and carcinoma. Effect of hysterectomy. Gynecol Obstet Invest 13:9–16 [DOI] [PubMed] [Google Scholar]
- Chatzistamou I, Schally AV, Kiaris H, Politi E, Varga J, Kanellis G, Kalofoutis A, Pafiti A, Koutselini H 2004 Immunohistochemical detection of GHRH and its receptor splice variant 1 in primary human breast cancers. Eur J Endocrinol 151:391–396 [DOI] [PubMed] [Google Scholar]
- Kahan Z, Arencibia JM, Csernus VJ, Groot K, Kineman RD, Robinson WR, Schally AV 1999 Expression of growth hormone-releasing hormone (GHRH) messenger ribonucleic acid and the presence of biologically active GHRH in human breast, endometrial, and ovarian cancers. J Clin Endocrinol Metab 84:582–589 [DOI] [PubMed] [Google Scholar]
- Khorram O, Garthwaite M, Grosen E, Golos T 2001 Human uterine and ovarian expression of growth hormone-releasing hormone messenger RNA in benign and malignant gynecologic conditions. Fertil Steril 75:174–179 [DOI] [PubMed] [Google Scholar]
- Engel JB, Keller G, Schally AV, Toller GL, Groot K, Havt A, Armatis P, Zarandi M, Varga JL, Halmos G 2005 Inhibition of growth of experimental human endometrial cancer by an antagonist of growth hormone-releasing hormone. J Clin Endocrinol Metab 90:3614–3621 [DOI] [PubMed] [Google Scholar]
- Schally AV, Varga JL 2006 Antagonists of growth hormone-releasing hormone in oncology. Comb Chem High Throughput Screen 9:163–170 [DOI] [PubMed] [Google Scholar]
- Perry JK, Emerald BS, Mertani HC, Lobie PE 2006 The oncogenic potential of growth hormone. Growth Horm IGF Res 16:277–289 [DOI] [PubMed] [Google Scholar]
- Zhu T, Starling-Emerald B, Zhang X, Lee KO, Gluckman PD, Mertani HC, Lobie PE 2005 Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res 65:317–324 [PubMed] [Google Scholar]
- Perry J, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE 2008 The contribution of growth hormone to mammary neoplasia. J Mammary Gland Biol Neoplasia 13:131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerald BS, Chen Y, Zhu T, Zhu Z, Lee KO, Gluckman PD, Lobie PE 2007 αCP1 mediates stabilization of hTERT mRNA by autocrine human growth hormone. J Biol Chem 282:680–690 [DOI] [PubMed] [Google Scholar]
- Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE 2004 Phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc Natl Acad Sci USA 101:15166–15171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL 1983 Metallothionein-human GH fusion genes stimulate growth of mice. Science 222:809–814 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu T, Chen Y, Mertani HC, Lee KO, Lobie PE 2003 Human growth hormone-regulated HOXA1 is a human mammary epithelial oncogene. J Biol Chem 278:7580–7590 [DOI] [PubMed] [Google Scholar]
- Kaulsay KK, Mertani HC, Tornell J, Morel G, Lee KO, Lobie PE 1999 Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp Cell Res 250:35–50 [DOI] [PubMed] [Google Scholar]
- van de Loosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM 1994 A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174:311–320 [DOI] [PubMed] [Google Scholar]
- Mertani HC, Zhu T, Goh EL, Lee KO, Morel G, Lobie PE 2001 Autocrine human growth hormone (hGH) regulation of human mammary carcinoma cell gene expression. Identification of CHOP as a mediator of hGH-stimulated human mammary carcinoma cell survival. J Biol Chem 276:21464–21475 [DOI] [PubMed] [Google Scholar]
- Rahnama F, Shafiei F, Gluckman PD, Mitchell MD, Lobie PE 2006 Epigenetic regulation of human trophoblastic cell migration and invasion. Endocrinology 147:5275–5283 [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM 1996 Real time quantitative PCR. Genome Res 6:986–994 [DOI] [PubMed] [Google Scholar]
- Zhu T, Goh EL, Graichen R, Ling L, Lobie PE 2001 Signal transduction via the growth hormone receptor. Cell Signal 13:599–616 [DOI] [PubMed] [Google Scholar]
- Evan G, Littlewood T 1998 A matter of life and cell death. Science 281:1317–1322 [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Noguchi Y, Mori T, Niki K, Namiki H 1985 Suppression of normal and preneoplastic mammary growth and uterine adenomyosis with reduced growth hormone level in SHN mice given monosodium glutamate neonatally. Eur J Cancer Clin Oncol 21:1547–1551 [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI 2002 A matter of life and death. Cancer Cell 1:19–30 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA 2000 The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ 1992 Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89:9064–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS 2001 ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP 1994 Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 31:325–335 [DOI] [PubMed] [Google Scholar]
- Thiery JP 2002 Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW 2006 The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ 2002 Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 23:623–646 [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Kopchick JJ 2006 Growth hormone receptor antagonists. Neuroendocrinology 83:264–268 [DOI] [PubMed] [Google Scholar]
- Portier CJ 2002 Endocrine dismodulation and cancer. Neuroendocrinol Lett 23(Suppl 2):43–47 [PubMed] [Google Scholar]
- Waters MJ, Barclay JL 2007 Does growth hormone drive breast and other cancers? Endocrinology 148:4533–4535 [DOI] [PubMed] [Google Scholar]
- Lincoln DT, Sinowatz F, Temmim-Baker L, Baker HI, Kolle S, Waters MJ 1998 Growth hormone receptor expression in the nucleus and cytoplasm of normal and neoplastic cells. Histochem Cell Biol 109:141–159 [DOI] [PubMed] [Google Scholar]
- Mol JA, Lantinga-van Leeuwen IS, van Garderen E, Selman PJ, Oosterlaken-Dijksterhuis MA, Schalken JA, Rijnberk A 1999 Mammary growth hormone and tumorigenesis: lessons from the dog. Vet Q 21:111–115 [DOI] [PubMed] [Google Scholar]
- Rijnberk A, Mol JA 1997 Progestin-induced hypersecretion of growth hormone: an introductory review. J Reprod Fertil 51:335–338 [PubMed] [Google Scholar]
- Laban C, Bustin SA, Jenkins PJ 2003 The GH-IGF-I axis and breast cancer. Trends Endocrinol Metab 14:28–34 [DOI] [PubMed] [Google Scholar]
- Szepeshazi K, Schally AV, Armatis P, Groot K, Hebert F, Feil A, Varga JL, Halmos G 2001 Antagonists of GHRH decrease production of GH and IGF-I in MXT mouse mammary cancers and inhibit tumor growth. Endocrinology 142:4371–4378 [DOI] [PubMed] [Google Scholar]
- Markkanen H, Pekkarinen T, Valimaki MJ, Alfthan H, Kauppinen-Makelin R, Sane T, Stenman UH 2006 Effect of sex and assay method on serum concentrations of growth hormone in patients with acromegaly and in healthy controls. Clin Chem 52:468–473 [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Bass S, Fuh G, Wells JA 1990 Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science 250:1709–1712 [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001 The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- Gennigens C, Menetrier-Caux C, Droz JP 2006 Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol 58:124–145 [DOI] [PubMed] [Google Scholar]
- Sachdev D, Yee D 2007 Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 6:1–12 [DOI] [PubMed] [Google Scholar]
- Bircan S, Ensari A, Ozturk S, Erdogan N, Dundar I, Ortac F 2005 Immunohistochemical analysis of c-myc, c-jun and estrogen receptor in normal, hyperplastic and neoplastic endometrium. Pathol Oncol Res 11:32–39 [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Susil B, Jobling TW, Oehler MK 2005 Endometrial cancer. Cell Tissue Res 322:53–61 [DOI] [PubMed] [Google Scholar]
- Barrow JR, Stadler HS, Capecchi MR 2000 Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development 127:933–944 [DOI] [PubMed] [Google Scholar]
- Zhang X, Emerald BS, Mukhina S, Mohankumar KM, Kraemer A, Yap AS, Gluckman PD, Lee KO, Lobie PE 2006 HOXA1 is required for E-cadherin-dependent anchorage-independent survival of human mammary carcinoma cells. J Biol Chem 281:6471–6481 [DOI] [PubMed] [Google Scholar]
- Mohankumar KM, Xu XQ, Zhu T, Kannan N, Miller LD, Liu ET, Gluckman PD, Sukumar S, Emerald BS, Lobie PE 2007 HOXA1-stimulated oncogenicity is mediated by selective upregulation of components of the p44/42 MAP kinase pathway in human mammary carcinoma cells. Oncogene 26:3998–4008 [DOI] [PubMed] [Google Scholar]
- Aglund K, Rauvala M, Puistola U, Angstrom T, Turpeenniemi-Hujanen T, Zackrisson B, Stendahl U 2004 Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol 94:699–704 [DOI] [PubMed] [Google Scholar]
- Guo W, Chen G, Zhu C, Wang H 2002 [Expression of matrix metalloproteinase-2, 9 and it’s tissue inhibitor-1, 2 in endometrial carcinoma]. Zhonghua Fu Chan Ke Za Zhi 37:604–607 (Chinese) [PubMed] [Google Scholar]
- Graesslin O, Cortez A, Fauvet R, Lorenzato M, Birembaut P, Darai E 2006 Metalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation study. Ann Oncol 17:637–645 [DOI] [PubMed] [Google Scholar]
- Li HW, Leung SW, Chan CS, Yu MM, Wong YF 2007 Expression of maspin in endometrioid adenocarcinoma of endometrium. Oncol Rep 17:393–398 [PubMed] [Google Scholar]
- Divisova J, Kuiatse I, Lazard Z, Weiss H, Vreeland F, Hadsell DL, Schiff R, Osborne CK, Lee AV 2006 The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat 98:315–327 [DOI] [PubMed] [Google Scholar]
- Yin D, Vreeland F, Schaaf LJ, Millham R, Duncan BA, Sharma A 2007 Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: implications for cancer therapy. Clin Cancer Res 13:1000–1009 [DOI] [PubMed] [Google Scholar]
- Kaulsay KK, Zhu T, Bennett W, Lee KO, Lobie PE 2001 The effects of autocrine human growth hormone (hGH) on human mammary carcinoma cell behavior are mediated via the hGH receptor. Endocrinology 142:767–777 [DOI] [PubMed] [Google Scholar]
- McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, Friend KE 2001 Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg 94:487–492 [DOI] [PubMed] [Google Scholar]
- Dagnaes-Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A 2004 Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res 24:3735–3742 [PubMed] [Google Scholar]
- Stewart PM 2003 Pegvisomant: an advance in clinical efficacy in acromegaly. Eur J Endocrinol 148(Suppl 2):S27–S32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.