Abstract
Decrease of muscle IGF-I plays a critical role in muscle atrophy caused by glucocorticoids (GCs) because IGF-I gene electrotransfer prevents muscle atrophy caused by GCs. The goal of the present study was to identify the intracellular mediators responsible for the IGF-I anti-atrophic action in GC-induced muscle atrophy. We first assessed the IGF-I transduction pathway alterations caused by GC administration and their reversibility by local IGF-I overexpression performed by electrotransfer. Muscle atrophy induced by dexamethasone (dexa) administration occurred with a decrease in Akt (−53%; P <0.01) phosphorylation together with a decrease in β-catenin protein levels (−40%; P <0.001). Prevention of atrophy by IGF-I was associated with restoration of Akt phosphorylation and β-catenin levels. We then investigated whether muscle overexpression of these intracellular mediators could mimic the IGF-I anti-atrophic effects. Overexpression of a constitutively active form of Akt induced a marked fiber hypertrophy in dexa-treated animals (+175% of cross-sectional area; P <0.001) and prevented dexa-induced atrophy. This hypertrophy was associated with an increase in phosphorylated GSK-3β (+17%; P <0.05) and in β-catenin content (+35%; P <0.05). Furthermore, overexpression of a dominant-negative GSK-3β or a stable form of β-catenin increased fiber cross-sectional area by, respectively, 23% (P <0.001) and 29% (P <0.001) in dexa-treated rats, preventing completely the atrophic effect of GC. In conclusion, this work indicates that Akt, GSK-3β, and β-catenin probably contribute together to the IGF-I anti-atrophic effect in GC-induced muscle atrophy.
MANY PATHOLOGICAL states characterized by muscle atrophy (sepsis, cachexia, disuse atrophy, fasting, metabolic acidosis, severe insulinopenia, etc.) are associated with an increase in circulating glucocorticoid (GC) levels (1), suggesting that they could trigger the muscle atrophy observed in these situations. Indeed, adrenalectomy or treatment with an antagonist of GC receptor (RU-486) attenuates and, in some cases, abolishes muscle atrophy (1,2). GC-induced muscle atrophy results from decreased protein synthesis and increased protein degradation rates (3,4,5,6). The inhibitory effect of GC on protein synthesis is thought to result from the inhibition of the p70 ribosomal S6 protein kinase (p70S6K) involved in the regulation of protein synthesis (7,8,9,10). On the other hand, the stimulatory effect of GC on muscle proteolysis results from the activation of two major cellular proteolytic systems (2), namely the ubiquitin proteasome system (11,12,13) and the lysosomal system (cathepsins) (14,15,16).
Several observations indicate that a decrease in the production of IGF-I (17), a growth factor that stimulates the development of muscle mass by increasing protein synthesis and myogenesis while decreasing proteolysis and apoptosis (18,19), could contribute to GC-induced muscle atrophy. Although the down-regulation of IGF-I gene expression by GC has been previously reported (20), the key role of decreased muscle IGF-I in GC-induced muscle atrophy has been only recently demonstrated. First, by activating the phosphatidylinositol-3-kinase (PI3K)/Akt pathway, IGF-I down-regulates the different proteolytic systems (lysosomal and proteasomal) stimulated by GC (16,21,22,23). Second, IGF-I inhibits GC-induced proteolysis in myotubes through PI3K/Akt/GSK-3β and PI3K/Akt/mTOR-dependent mechanisms (24). Third, systemic administration (25,26,27,28) and local overexpression of IGF-I into skeletal muscle, as reported by our group (29), prevent GC-induced muscle atrophy and cause muscle fiber hypertrophy (30). Together, these results indicate that IGF-I has a dominant effect, overriding GC to turn off catabolism. However, the mechanisms by which IGF-I inhibits in vivo muscle atrophy induced by GC are still unclear.
As a direct extension of our previous work, the present study was undertaken to characterize the intracellular signaling pathways responsible for the IGF-I anti-atrophic action in GC-induced muscle atrophy. Although in vitro observations had already assessed the role of Akt, GSK3β, p70S6K in GC-myotube atrophy, we decided to determine whether such factors were also involved in vivo and evaluate the potential role of β-catenin. We then determined whether local IGF-I overexpression could prevent these alterations. Finally, we tested whether muscle overexpression of these intracellular mediators themselves could mimic the IGF-I anti-atrophic effects in GC-induced muscle atrophy.
Materials and Methods
Expression plasmids and DNA preparation
pM1-hIGF-I and pM1-ΔNβ-catenin plasmids were constructed by inserting, respectively, the IGF-I cDNA (gift from Dr. P. Steenbergh, University of Utrecht, Utrecht, The Netherlands) and the ΔNβ-catenin cDNA (gift from Dr. T. Force, Thomas Jefferson University, Philadelphia, PA) into the pM1 Expression Vector (Roche Molecular Biochemicals, Indianapolis, IN). ΔNβ-catenin cDNA codes for a vesicular stomatitis virus-glycoprotein (VSV-G) tagged β-catenin, where the N-terminal 134 amino acids, a region that contains the GSK-3β phosphorylation sites, were deleted (31). pCMV5-(m/p)Akt plasmid (gift from Dr. D. Alessi, University of Dundee, Dundee, Scotland, UK) codes for hemagglutinin (HA)-tagged constitutively active (ca) Akt form, where a Lck myristoylation/palmitylation signal was added to the NH2-terminal (32). pcDNA3-dnGSK-3β plasmid (gift from Dr. J. Woodgett, Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada) codes for a HA-tagged catalytic inactive GSK-3β, where lysine residue at position 85 of the ATP binding site was mutated to alanine (33). Empty pCMV5 (gift from Dr. D. Alessi) and pM1 were used as control (Ctrl) plasmids. Plasmids were amplified in Escherichia coli top 10 F’ (Invitrogen Corp., Carlsbad, CA) and purified with an EndoFree Plasmid Giga Kit (QIAGEN, Inc., Valencia, CA). Plasmids were stocked at −80 C. The day before injection, 100 μg plasmid was lyophilized and resuspended in 100 μl NaCl 0.9% solution.
General in vivo experimental design
Animals.
Six-week-old male Wistar rats (180–220 g) provided by Janvier Breeding (Le Genest St-Isle, France) were used. The animals were housed under controlled conditions of lighting (12-h light, 12-h dark cycle) and temperature (22 ± 2 C). The experiments were conducted and the animals were cared for in accordance with the directives of the institutional animal care and use committee of University of Louvain.
Dexamethasone (dexa) administration.
Rats were divided into three groups: ad libitum, pair-fed, and dexa. The ad libitum and pair-fed groups received daily sc injections of saline solution (vehicle, 0.9% NaCl). The GC-treated group received daily sc injections of a saline solution of dexa (10 μg/100 g body weight; Aacidexam; Organon, Brussels, Belgium). Because this dosage in rats is able to mimic the muscle atrophy observed in catabolic conditions characterized by an increase of endogenous GC, this dose must be considered at the upper limit of the physiological range. Because dexa treatment is known to decrease energy intake, the pair-fed group received the same amount of energy as that consumed by the dexa-treated group during the previous day (−20%; energy intake (kcal/d): 82 ± 2 kcal in ad libitum vs. 66 ± 2 kcal in dexa-treated rats; P < 0.001). The ad libitum and dexa-treated animals were allowed free access to chow and water. Animals were weighed, and food intake was measured every day. After 7-d treatment, animals were killed, and tibialis anterior (TA) muscles were removed.
DNA electrotransfer.
Three days before starting dexa treatment, each rat was anesthetized with a mixture of 75 mg/kg ketamine (Ketalar; Pfizer, Oslo, Norway) and 15 mg/kg xylazine hydrochloride (Rompun; Bayer, Fernwald, Germany) administered by ip injection. Plasmid solutions (1 μg/μl) were injected into 10 different sites (total volume per muscle = 100 μl) in each TA muscle and then muscles were electroporated using the electroporation conditions previously described (29). To assess the IGF-I transduction pathway alterations caused by dexa and their reversibility by local IGF-I overexpression, the two TA muscles of the ad libitum rats were injected with the pM1 plasmid. In pair-fed and dexa-treated animals, one TA muscle was injected with the pM1-hIGF-I plasmid and the contralateral TA muscle with the pM1 plasmid. Previous experiments have shown the absence of exogenous IGF-I overexpression in contralateral muscle together with the absence of increased circulating IGF-I, indicating that IGF-I overexpression occurs only in the TA muscle electroporated with the pM1-hIGF-I plasmid (34). To assess the anti-catabolic effect of caAkt transfection in dexa-treated rats, all animals were injected with the pCMV5-(m/p)Akt plasmid in one TA muscle and with the pCMV5 plasmid in the contralateral TA muscle. To assess the anti-catabolic effect of dnGSK-3β and ΔNβ-catenin DNA transfection in dexa-treated rats, the two TA muscles of all animals were injected either with the pcDNA3-dnGSK-3β plasmid or the pM1-ΔNβ-catenin plasmid. As shown in a previous work (29), dexa treatment did not alter the cytomegalovirus promoter activity driven exogenous expression of IGF-I. Indeed, the levels of exogenous hIGF-I mRNA achieved by pM1-hIGF-I overexpression were similar in GC-treated and pair-fed animals. Because signaling molecules expressed in this work are under control of the same promoter (cytomegalovirus), expression of all these overexpressed molecules is unlikely to be altered by dexa treatment.
Western blots and antibodies
Briefly, 100 mg whole TA muscle, previously pestled in liquid nitrogen, was homogenized with Ultraturrax (IKA-Labortechnik, Staufen, Germany) in 1 ml ice-cold lysis buffer I [50 mm Tris/HCl (pH 7.5), 1 mm EDTA, 1 mm EGTA, 0.5% Nonidet P-40, 1 mm phenylmethylsulfonylfluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 mm β-glycerophosphate, 1 mm K2PO4, 1 mm vanadate, 50 mm NaF, and 10 mm Nappi] or in 1 ml ice-cold lysis buffer II (lysis buffer I with 1% Nonidet P-40 and 150 mm NaCl). Lysis buffer I was used for the assessment of IGF-I transduction pathway alterations caused by dexa administration and their reversibility by local IGF-I overexpression. Lysis buffer II was used in all other experiments. Homogenates were then centrifuged 10 min at 10,000 rpm (Sorvall SS-34 rotor; Thermo Fisher Scientific Inc., Waltham, MA), and the supernatant muscle protein content was determined using Bradford’s protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). Equal amounts of proteins were resolved by a sodium dodecyl sulfate-polyacrylamide gel 10% electrophoresis and transferred to polyvinylidene difluoride membranes (Immobilon P/PVDF; Millipore, Bedford, MA). Membranes were probed with anti-phospho-Akt (Ser 473, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA), anti-Akt (1:1,000; Upstate Biotechnology Inc., Lake Placid, NY), anti-phospho-GSK-3β (Ser 9, 1:3,000; Cell Signaling Technology), anti-GSK-3β (1:10,000; Cell Signaling Technology), anti-β-catenin (1:30,000; Sigma-Aldrich, St. Louis, MO), anti-phospho-p70S6K (Thr 389, 1:1,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-p70S6K (1:1,000; Santa Cruz Biotechnology), anti-HA (1:1,000; Bethyl Laboratories, Inc., Montgomery, TX), and anti-VSV-G (1:5,000; Sigma-Aldrich), followed by horseradish peroxidase-coupled secondary antibody, antimouse or antirabbit (1:40,000; Amersham Biosciences), and developed by a chemiluminescence-based detection system (ECL Plus; Amersham Biosciences). Developed film was scanned (Image Scanner; Amersham Pharmacia Biotech, Uppsala, Sweden) and analyzed using ImageMaster TotalLab software v2.0 (Amersham Pharmacia Biotech). Phospho data blots were normalized for the signal with the cognate pan antibodies.
Immunohistochemistry
For the evaluation of the anti-catabolic effect of caAkt transfection, transversal slices of 0.5-cm thickness made in the middle belly of the muscle were embedded in O.C.T. compound (Tissue-Tek; QIAGEN) and frozen in precooled isopentane. Cryostat serial sections (10 μm thick) were cut and mounted onto glass slides (Superfrost; Menzel-Glaser, Braunschweig, Germany). The sections were fixed 10 min with buffered formol. For immunohistochemistry, sections were incubated at room temperature (RT) with a solution of BSA 5% for 30 min and incubated with a rabbit polyclonal anti-HA antibody (1:400; Bethyl Laboratories) in PBS containing 1% BSA overnight. Primary antibody was detected by applying for 1 h at RT a second antibody, which was a goat antirabbit conjugated to peroxidase-labeled polymer (En Vision; Dako, Heverlee, Belgium). Peroxidase activity was revealed with diaminobenzidine (DAB) substrate (Liquid DAB+ Substrate Chromogen System; Dako), which produces a brown stain. Sections were mounted in Depex (VWR International, Lutterworth, UK). For the evaluation of the anti-catabolic effect of dnGSK-3β and ΔNβ-catenin DNA transfection, transversal slices of 0.5-cm thickness made in the middle belly of the muscle were fixed with buffered formol for 48–72 h and embedded in paraffin. For immunohistochemistry, sections were deparaffinized and pretreated in a microwave oven in Tris-citrate buffer (pH 6.5) for one cycle of 3 min at 750 W and three cycles of 3.5 min at 350 W. Thereafter, sections were blocked in PBS-BSA (5%) containing normal goat serum (4%) for 30 min at RT and incubated with a rabbit polyclonal anti-HA (1:400) for 1 h or with a rabbit polyclonal anti-VSV-G (1:800; Sigma-Aldrich) for 2 h. Primary antibodies were detected by applying for 30 min at RT a biotinylated second antibody (Vector Laboratories, Burlingame, CA), followed by applying for 30 min at RT an avidin/biotinylated peroxidase complex (Vectastain ABC kit Peroxidase Standard; Vector Laboratories). Peroxidase activity was revealed with DAB substrate (CHEMICON International, Inc., Temecula, CA), which produces a brown stain. Sections were counterstained with Mayer’s hematoxylin, rinsed, and mounted in Faramount (Dako). Fiber cross-sectional areas (CSAs) were measured with a microscope (Leitz; Leica Microsystems GmbH, Wetzlar, Germany) coupled to an image analyzer system (MOP-Videoplan; Kontron AG, Eching, Germany). To evaluate muscle fiber CSAs, all the positive muscle fibers were counted. Untransfected (UT) muscle fibers surrounding positive muscles fibers were considered as a Ctrl. The use of insert-less plasmid transfected fibers as a Ctrl would not affect the results because there is no difference between the CSAs of surrounding UT fibers and CSAs of muscle fibers transfected with an insert-less plasmid.
Statistical analysis
Results are presented as means ± sem. Statistical analyses were performed using a Student t test or a one-way ANOVA, followed by a Newman-Keuls multiple comparison test to compare muscles from different animals undergoing different experimental conditions. A paired t test was used to compare muscles undergoing different experimental conditions within the same animal. Fiber CSA distribution statistical analysis was performed using the χ2 Pearson test. Statistical significance was set at P < 0.05.
Results
Inhibition of Akt/p70S6K and GSK-3β/β-catenin signaling pathways by dexa administration is reversed by local IGF-I overexpression
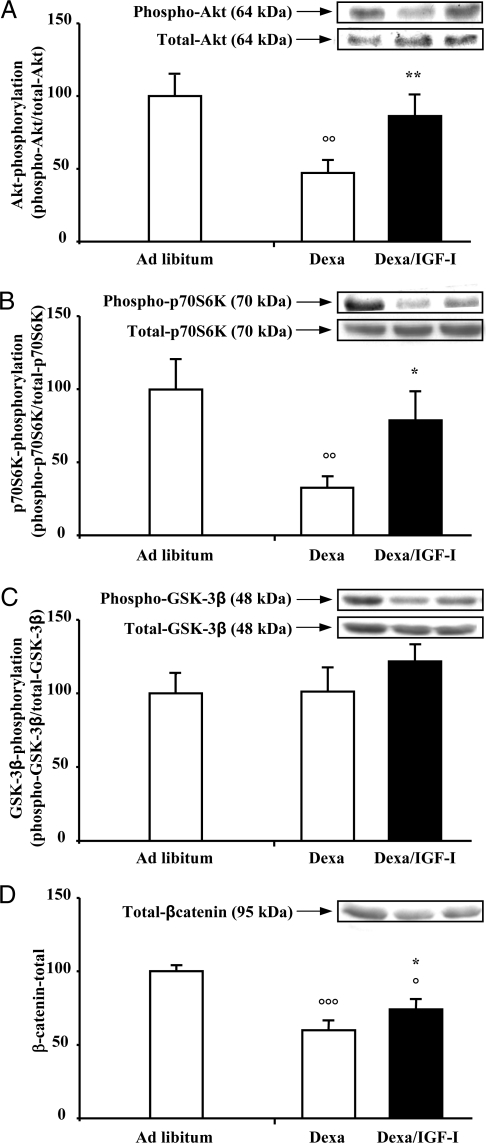
To determine whether local muscle IGF-I decrease observed in dexa-treated rats (29) is associated with the inhibition of IGF-I signaling pathways, we studied the phosphorylation state of several key intracellular mediators of IGF-I action. dexa injection decreased Akt phosphorylation by about 53% [dexa pM1 (n = 6): 47 ± 8% vs. ad libitum pM1 (n = 5): 100 ± 15%; P < 0.01] (Fig. 1A). Interestingly, transfection of pM1-IGF-I plasmid in the muscle of dexa-treated rats restored Akt phosphorylation to the levels observed in ad libitum rats [dexa pM1-hIGF-I (n = 6): 86 ± 15% vs. ad libitum pM1 (n = 5): 100 ± 15%; not significant (ns)]. Changes in Akt phosphorylation occurred without significant modification in Akt protein content (Fig. 1A, inset). Furthermore, the Akt inactivation caused by dexa was associated with decreased phosphorylation of p70S6K [dexa pM1 (n = 6): 33 ± 7% vs. ad libitum pM1 (n = 5): 100 ± 20%; P < 0.01] (Fig. 1B) without any changes of GSK-3β phosphorylation [dexa pM1 (n = 6): 101 ± 16% vs. ad libitum pM1 (n = 5): 100 ± 13%; ns] (Fig. 1C), two downstream targets of Akt. As observed for Akt1, p70S6K [dexa pM1-hIGF-I (n = 6) = 79 ± 20% vs. ad libitum pM1 (n = 5): 100 ± 20%; ns] phosphorylation was restored by IGF-I overexpression in dexa-treated rats. Changes in p70S6K phosphorylation could not be explained by changes in p70S6K protein content (Fig 1B, inset). In contrast, we observed in the muscle of dexa-treated rats a significant decrease in GSK-3β protein content [dexa pM1 (n = 6): 72 ± 5% vs. ad libitum pM1 (n = 5): 100 ± 12%; P < 0.05], which might explain the absence of changes of the phospho-GSK-3β/total-GSK-3β ratio (Fig 1C, inset). We also investigated whether β-catenin protein, a downstream target of GSK-3β, is down-regulated by dexa. dexa decreased by about 40% β-catenin protein levels in the TA muscle [dexa pM1 (n = 6): 60 ± 6% vs. ad libitum pM1 (n = 5): 100 ± 4%; P < 0.001]. IGF-I overexpression in the muscle of dexa-treated rats increased the β-catenin protein levels, which remained, however, significantly lower than in the muscle of ad libitum animals [dexa pM1 hIGF-I (n = 6): 74 ± 7% vs. ad libitum pM1 (n = 5): 100 ± 4%; P < 0.05] (Fig. 1D). Together, the results indicate that Akt, p70S6K, GSK-3β, and β-catenin are potentially involved in the IGF-I anti-catabolic actions in dexa-induced muscle atrophy.
Figure 1.
Decrease of Akt and p70S6K phosphorylation and β-catenin levels by dexa administration is reversed by local IGF-I overexpression. Tibial anterior muscles were injected and electroporated with a total of 100 μg pM1 plasmid at a concentration of 1 μg/μl (white column), whereas contralateral tibial anterior muscles were injected and electroporated with 100 μg pM1-hIGF-I plasmid at a concentration of 1 μg/μl (black column). Inset, Representative Western blot of phosphorylation forms (top) and total forms (bottom). Seven days after dexa administration, Akt phosphorylation (A), p70S6K phosphorylation (B), and β-catenin (D) were decreased without any changes in GSK3-β phosphorylation (C). IGF-I overexpression prevented these transduction pathway alterations observed in dexa-treated rats. Results are expressed as mean ± sem. ○, P < 0.05; ○○, P < 0.01; ○○○, P < 0.001 vs. ad libitum. *, P < 0.05; **, P < 0.01 vs. contralateral muscle.
caAkt muscle overexpression induces a marked fiber hypertrophy and prevents dexa-induced muscle atrophy
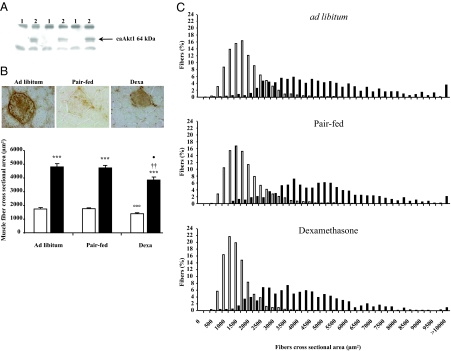
To explore the role of Akt in the anti-catabolic effects of IGF-I, we overexpressed a ca form of Akt in the muscle of dexa-treated rats. Expression of the caAkt transgene was detected by Western blot and immunohistochemistry in TA muscle electroporated with the pCMV5-(m/p)Akt using an antibody raised against HA tag (Fig. 2, A and B). As expected, dexa treatment for 7 d decreased by about 20% the CSA of the UT muscle fibers [dexa UT: 1 394 ± 48 (n = 6) vs. ad libitum UT: 1 738 ± 69 (n = 6) μm2; P < 0.001]. Muscle fiber size was not affected by food restriction (Fig. 2B). As shown by immunohistochemical analysis, caAkt gene transfection induced marked muscle fiber hypertrophy in the three groups of animals. Indeed, we observed an increase in the muscle fiber CSA by about 176% in dexa-injected rats [pCMV5-(m/p)Akt: 3 846 ± 210 vs. UT: 1 394 ± 48 μm2; P < 0.001 (n = 6)], about 169% in pair-fed rats [pCMV5-(m/p)Akt: 4 733 ± 160 vs. UT: 1 760 ± 22 μm2; P < 0.001 (n = 5)], and about 177% in ad libitum rats [pCMV5-(m/p)Akt: 4 813 ± 224 vs. UT: 1 738 ± 69 μm2; P < 0.001 (n = 6)] (Fig. 2B). The increase in the muscle fiber CSA induced by caAkt gene transfection was confirmed by the analysis of fiber size distribution showing a greater proportion of large fibers among the caAkt transfected fibers than among the UT Ctrl fibers in the three groups of rats (P < 0.001) (Fig. 2C).
Figure 2.
caAkt muscle overexpression induces a marked fiber hypertrophy and prevents dexa-induced muscle atrophy. Overexpression of caAkt was detected by Western blot (A) and immunohistochemistry (B, top) in TA muscles electroporated with pCMV5-(m/p)Akt plasmid. caAkt overexpression induced a marked muscle fiber hypertrophy (black column) compared with surrounding UT muscle fibers (white column) (B, bottom). Results are expressed as mean ± sem. ***, P < 0.001 vs. UT muscles fibers of the same muscle. ○○○, P < 0.001 vs. ad libitum UT muscles fibers. ††, P < 0.01 vs. pair-fed caAkt transfected muscles fibers. •, P < 0.05 vs. ad libitum caAkt transfected muscles fibers. C, The proportion of large fibers in the three groups was larger in the caAkt DNA transfected muscle fibers (black column) compared with surrounding UT muscle fibers (white column). Statistical analysis was performed using the χ2 Pearson test. ***, P < 0.001 for the three groups. A total of 1991 positive and 8280 UT muscle fibers were analyzed for measurements of fiber size.
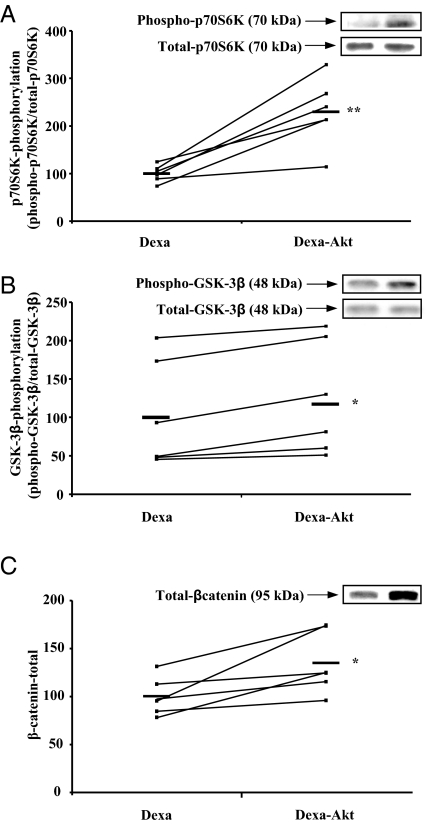
To identify the intracellular mediators involved in the prevention of muscle atrophy by caAkt overexpression in dexa-treated rats, we analyzed the phosphorylation state of several key targets of Akt. As observed for IGF-I overexpression, caAkt transfection increased p70S6K phosphorylation by about 130% in the TA muscle of dexa-treated rats [pCMV5-(m/p)Akt: 230 ± 29% vs. pCMV5: 100 ± 7%; P < 0.01 (n = 6)] (Fig. 3A) without affecting total p70S6K protein levels (Fig 3A, inset). Similarly, caAkt overexpression increased significantly both GSK-3β phosphorylation [pCMV5-(m/p)Akt: 117 ± 30% vs. pCMV5: 100 ± 29%; P < 0.05 (n = 6)] and β-catenin protein levels [pCMV5-(m/p)Akt: 135 ± 13% vs. pCMV5: 100 ± 8%; P < 0.05 (n = 6)] in the TA muscle of dexa-treated rats (Fig. 3, B and C). Total GSK-3β protein levels did not change (Fig 3B, inset). These results indicate that p70S6K, GSK-3β, and β-catenin are potentially implicated in the anti-catabolic action of caAkt overexpression in this model.
Figure 3.
caAkt overexpression stimulates p70S6K and GSK-3β phosphorylation and β-catenin levels in the muscle of dexa-treated rats. Prevention of dexa-induced muscle atrophy by caAkt overexpression was associated with a significant increase of phosphorylated p70S6K (A), GSK-3β (B), and total β-catenin levels (C), indicating that these molecules potentially contribute to the hypertrophic effects of Akt overexpression. Inset, Representative Western blot of phosphorylation forms (top) and total forms (bottom).*, < 0.05; **, P < 0.01 vs. contralateral muscle.
Dominant-negative GSK-3β overexpression induces a modest muscle fiber hypertrophy and prevents dexa-induced muscle atrophy
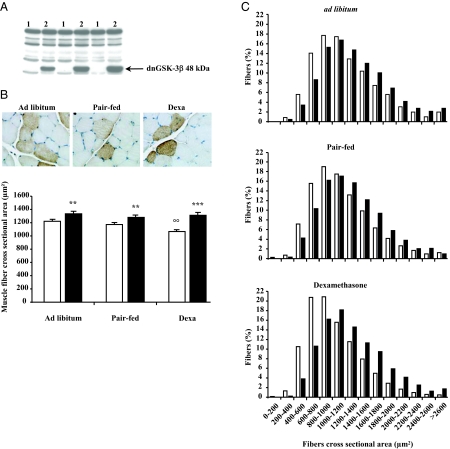
To explore the role of GSK-3β in the anti-catabolic effects of Akt, we overexpressed a dominant-negative form of GSK-3β, dnGSK-3β, in the muscle of dexa-treated rats. Expression of the dnGSK-3β transgene was detected by Western blot and immunohistochemistry in TA muscle electroporated with the pcDNA3-dnGSK-3β using an antibody raised against the tag HA (Fig. 4, A and B). Compared with caAkt overexpression, dnGSK-3β gene transfection induced only a modest fiber muscle hypertrophy in the three groups of animals (Fig. 4B). Indeed, we observed an increase of the muscle fiber CSA by about 23% (P < 0.001, n = 10) in dexa-treated rats, and about 9% in pair-fed and ad libitum rats (P < 0.01, n = 8). The increase in the muscle fiber CSA induced by dnGSK-3β gene transfection was confirmed by the analysis of fiber size distribution showing a greater proportion of large fibers among the dnGSK-3β transfected fibers than among the UT Ctrl fibers in the three groups (P < 0.001) (Fig. 4C).
Figure 4.
Dominant-negative (dn)GSK-3β overexpression induces a modest muscle fiber hypertrophy and prevents dexa-induced muscle atrophy. Overexpression of dnGSK-3β was detected by Western blot (A) and immunohistochemistry (B, top) in TA muscles electroporated with pcDNA3-dnGSK-3β plasmid. dnGSK-3β overexpression induced a modest muscle fiber hypertrophy (black column) compared with surrounding UT muscle fibers (white column) (B, bottom). Results are expressed as mean ± sem. **, P < 0.01; ***, P < 0.001 vs. UT muscles fibers of the same muscle. ○○, P < 0.01 vs. ad libitum UT muscles fibers. C, The proportion of large fibers in the three was greater in the dnGSK-3β DNA transfected muscle fibers (black column) compared with surrounding UT muscle fibers (white column). Statistical analysis was performed using the χ2 Pearson test. ***, P < 0.001 for the three groups. A total of 6,354 positive and 16,431 UT muscle fibers were analyzed for measurements of fiber size.
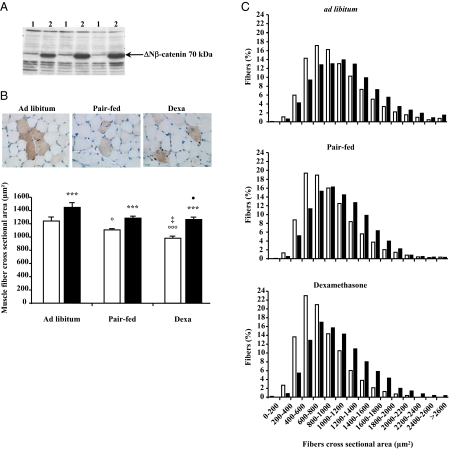
ΔNβ-Catenin overexpression prevents muscle atrophy in dexa-treated rats
To explore the role of β-catenin in the anti-catabolic effects of Akt, we overexpressed a N-terminal deleted form of β-catenin, the ΔNβ-catenin, in the muscle of dexa-treated rats. Expression of the ΔNβ-catenin transgene was detected by Western blot and immunohistochemistry in TA muscle electroporated with the pM1-ΔNβ-catenin using an antibody raised against the VSV-G (Fig. 5, A and B). As dnGSK-3β gene transfection, ΔNβ-catenin overexpression induced a modest increase of muscle fiber CSA by about 29% in the dexa-treated rats (P < 0.001, n = 11), about 16% (P < 0.001, n = 10) in the pair-fed rats, and about 17% (P < 0.001, n = 10) in ad libitum rats (Fig. 5B). The increase in the average muscle fiber CSA induced by ΔNβ-catenin gene transfection was confirmed by the analysis of fiber size distribution showing that the proportion of large fibers was significantly greater among the ΔNβ-catenin transfected fibers than among the UT Ctrl fibers in the three groups (P < 0.001) (Fig. 5C).
Figure 5.
ΔNβ-catenin overexpression prevents muscle atrophy in dexa-treated rats. Overexpression of ΔNβ-catenin was detected by Western blot (A) and immunohistochemistry (B, top) in TA muscles electroporated with pM1-ΔNβ-catenin plasmid. ΔNβ-catenin overexpression increases muscle fiber CSA (black column) compared with surrounding UT (white column) (B, bottom). Results are expressed as mean ± sem. ***, P < 0.001 vs. UT muscles fibers of the same muscle. ○, P < 0.05; ○○○, P < 0.001 vs. ad libitum UT muscles fibers. ‡, P < 0.05 vs. pair-fed UT muscles fibers. •, P < 0.05 vs. ad libitum ΔNβ-catenin transfected muscles fibers. C, The proportion of large fibers in the three groups was greater in the ΔNβ-catenin DNA transfected muscle fibers (black column) compared with surrounding UT muscle fibers (white column). Statistical analysis was performed using the χ2 Pearson test. ***, P < 0.001 for the three groups. A total of 15,312 positive and 31,759 UT muscle fibers were analyzed for measurements of fiber size.
Discussion
The present study demonstrates that muscle atrophy caused by GCs is associated with alterations in the Akt/GSK-3β/β-catenin signaling, a transduction pathway crucial for muscle growth. More interestingly, we show that prevention of the GC-induced muscle atrophy by local IGF-I gene overexpression (29) is associated with the correction of these transduction alterations. Furthermore, muscle overexpression of caAkt, dnGSK-3β, and ΔNβ-catenin themselves is sufficient to alleviate the catabolic effects of GC and, thus, mimic the anti-atrophic action of IGF-I in this model. Together, our results show, for the first time in vivo, the role of the Akt/GSK-3β/β-catenin pathway in the skeletal muscle atrophy caused by GC and its prevention by IGF-I.
It is well established in vitro (33) and in vivo (35,36,37,38) that Akt exerts an anabolic action on skeletal muscle. However, the role of Akt in muscle atrophy caused by GC had not yet been investigated. In the present study, we demonstrate in vivo that muscle atrophy induced by GC is associated with a decrease in Akt phosphorylation levels. Such a decrease in the Akt phosphorylation state has been directly correlated to a large decrease in Akt activity (39), suggesting the role of decreased Akt activity in the atrophic effect of GC. Moreover, our data indicate that prevention of muscle atrophy by IGF-I overexpression is associated with the restoration of Akt phosphorylation levels, a result that extends previous in vitro observations. Indeed, in L6 myotubes, inhibition by IGF-I of GC-induced protein degradation was associated with increased Akt phosphorylation (24). Here, we show for the first time in vivo that Akt overexpression overcomes muscle atrophy caused by GC, supporting the role of Akt in the prevention GC-induced muscle atrophy by IGF-I. These results are in line with a recent report in which evidence was found that the PI3K/Akt pathway mediates the anti-atrophic effect of IGF-I in myotubes. The myristoylation of the Akt form overexpressed in our experiment might explain the dramatic degree of hypertrophy observed. Indeed, the myristoylation maintains Akt attached at the cellular membrane in permanence and so suppresses the negative feed back exerted by PTEN and SHIP-2, two important phosphatases regulating Akt activity (40). In contrast with its anabolic effect, the anti-atrophic effect of Akt has only been demonstrated in a denervation muscle atrophy model (36). The concept that caAkt overexpression is able to prevent muscle atrophy in another condition such as that induced by GCs is supported by our data. Indeed, caAkt overexpression in GC-treated animals totally overcame muscle atrophy. The degree of increase of fiber CSA induced by caAkt overexpression was similar (170%) in GC-treated and ad libitum animals, although the absolute values of CSA achieved were slightly larger in ad libitum than in GC-treated animals.
Inhibition of the phosphorylation of p70S6K, a molecule that plays a key role in the protein synthesis machinery, is thought to be one of the mechanisms by which GCs cause muscle atrophy (41,42). Our data indicate that the inhibition of p70S6K by GC is not transient but persists (7) with time in the skeletal muscles of animals exposed to GC. Moreover, we show for the first time in vivo that prevention of muscle atrophy by IGF-I overexpression in the muscle of GC-treated rats is associated with a restoration of p70S6K phosphorylation to normal levels. These results are in line with a report by Li et al. (24) showing that rapamycin, which inhibits the IGF-I induced phosphorylation of p70S6K, blunts partially the antiproteolytic effects of IGF-I observed in GC-treated L6 myotubes. Together, these observations support a role for p70S6K phosphorylation in the anti-atrophic action of IGF-I in muscle exposed to GC. The decrease of p70S6K phosphorylation may result from decreased Akt activity caused by GC administration. Indeed, as shown by our data, Akt overexpression in skeletal muscle was sufficient to increase p70S6K phosphorylation in GC-treated animals. Alternatively, decreased phosphorylation of p70S6K in response to GC might result from enhanced transcription of REDD1, a repressor of mTOR, the kinase responsible for p70S6K phosphorylation (43). Although muscle REDD1 expression was dramatically increased after GC, its induction was not prevented by IGF-I overexpression (data not shown). This observation indicates that IGF-I can overcome the inhibitory effect of REDD1. Although p70S6K is mandatory for muscle hypertrophy induced by IGF-I and amino acids (44), overexpression of p70S6K alone into muscle seems, however, unable to induce muscle hypertrophy (data not shown).
Several reports, especially in vitro, have demonstrated the role of GSK-3β in muscle atrophy induced by GC. Indeed, stimulation of protein degradation by GC in myotubes was blocked by pharmacological inhibitors (24,45) and small interfering RNA (39) targeting GSK-3β. Therefore, these observations suggest that increased GSK-3β activity may contribute also to the catabolic effects of GC in vivo. The role of GSK-3β in the anti-atrophic action of IGF-I in myotubes exposed to GC has been also suggested. Indeed, inhibition by IGF-I of GC-induced protein degradation was associated with an increase in GSK-3β phosphorylation (24). Our results are the first to demonstrate in vivo the role of GSK-3β in dexa catabolic and IGF-I anti-catabolic effects. Indeed, overexpression of a dominant-negative form of GSK-3β prevented totally muscle atrophy induced by GCs. However, the mechanisms by which GSK-3β inactivation inhibits muscle atrophy caused by GC had not yet been investigated. Evidence has been provided in vitro suggesting that GSK-3β inhibition blocks GC-induced up-regulation of Atrogin-1 and MuRF-1 gene expression, two important molecular markers of muscle atrophy in different wasting conditions (45). However, in our conditions, muscle Atrogin-1 mRNA was not increased after GC treatment (data not shown), suggesting that other mechanisms are operational. Considering the targets of GSK-3β, it is possible that GSK-3β inactivation inhibits muscle atrophy by blocking the degradation of β-catenin, a transcription factor crucial for physiological muscle growth (46). Indeed, muscle atrophy induced by GC is associated with a large decrease in total β-catenin levels, consistent with the fact that, once phosphorylated by GSK-3β, β-catenin is targeted and degraded by the proteasome (47,48). Furthermore, IGF-I overexpression was associated with a partial restoration of β-catenin levels. Interestingly, besides regulating the β-catenin stability, IGF-I is also known to regulate its localization and transcriptional activity (49). Finally, when we overexpressed a stabilized form of β-catenin, resistant to the proteasomal degradation, we blocked totally muscle fiber atrophy induced by GC. To our knowledge, this work provides the first data regarding the role of β-catenin in GC-induced muscle atrophy and its reversibility by IGF-I. The mechanisms by which β-catenin may inhibit muscle atrophy are still unclear. β-Catenin, in tandem with T-cell factor (TCF)/lymphocyte enhancer factor (Lef), is known to activate transcription of several genes (50), including some genes directly implicated in muscle growth. Besides early activation of myogenesis (51,52), β-catenin pathway regulates several genes such as IGFs (53), phosphatase and tensin homolog (54), and Follistatin (55) involved in the regulation of muscle cell size.
In conclusion, our results show that GC-induced muscle atrophy is associated with several alterations in the IGF-I transduction pathway. Given that these mediators play a key role in the anabolic and anti-catabolic action of IGF-I, their alterations may contribute to the muscle atrophy induced by GC. The fact that muscle overexpression of caAkt, dnGSK-3β, and ΔΝβ-catenin is sufficient to alleviate the catabolic effects of GC and, thus, mimic the anti-atrophic action of IGF-I in this model suggests that Akt, GSK-3β, β-catenin, and presumably other mediators (i.e. p70S6K) play a critical role in the anti-atrophic action of IGF-I in skeletal muscle atrophy caused by GC.
Acknowledgments
We thank Professor M. C. Many and M. Senou (Experimental Morphology Unit, School of Medicine, Université Catholique de Louvain) for expert technical assistance.
Footnotes
This work was supported by grants from the Fund for Scientific Medical Research (Belgium), the National Fund for Scientific Research (Belgium), the Association Française contre les Myopathies (France), and the Fonds Spéciaux de Recherche (Université Catholique de Louvain, Belgium).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: ca, Constitutively active; CSA, cross-sectional area; Ctrl, control; DAB, diaminobenzidine; dexa, dexamethasone; GC, glucocorticoid; HA, hemagglutinin; ns, not significant; PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homolog; p70S6K, p70 ribosomal S6 protein kinase; RT, room temperature; SHIP2, SH2-containing phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase; TA, tibialis anterior; UT, untransfected; VSV-G, vesicular stomatitis virus-glycoprotein.
References
- Lecker SH, Solomon V, Mitch WE, Goldberg AL 1999 Muscle protein breakdown and the critical role of the ubiquitin- proteasome pathway in normal and disease states. J Nutr 129(Suppl):227S–237S [DOI] [PubMed] [Google Scholar]
- Hasselgren PO 1999 Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2:201–205 [DOI] [PubMed] [Google Scholar]
- Tomas FM, Munro HN, Young VR 1979 Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N τ-methylhistidine. Biochem J 178:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL, Tischler M, DeMartino G, Griffin G 1980 Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed Proc 39:31–36 [PubMed] [Google Scholar]
- Lofberg E, Gutierrez A, Wernerman J, Anderstam B, Mitch WE, Price SR, Bergstrom J, Alvestrand A 2002 Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur J Clin Invest 32:345–353 [DOI] [PubMed] [Google Scholar]
- Schakman O, Gilson H, and Thissen JP 2008 Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 2008 197:1–10 [DOI] [PubMed] [Google Scholar]
- Shah OJ, Kimball SR, Jefferson LS 2000 Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol Endocrinol Metab 278:E76–E82 [DOI] [PubMed] [Google Scholar]
- Shah OJ, Kimball SR, Jefferson LS 2000 Among translational effectors, p70S6k is uniquely sensitive to inhibition by glucocorticoids. Biochem J 347(Pt 2):389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZQ, Jahn LA, Long W, Fryburg DA, Wei LP, Barrett EJ 2001 Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab 86:2136–2143 [DOI] [PubMed] [Google Scholar]
- Liu Z, Li G, Kimball SR, Jahn LA, Barrett EJ 2004 Glucocorticoids modulate amino acid-induced translation initiation in human skeletal muscle. Am J Physiol Endocrinol Metab 287:E275–E281 [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na EQ, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ 2001 Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708 [DOI] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL 1996 Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335:1897–1905 [DOI] [PubMed] [Google Scholar]
- Combaret L, Adegoke OA, Bedard N, Baracos V, Attaix D, Wing SS 2005 USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am J Physiol Endocrinol Metab 288:E693–E700 [DOI] [PubMed] [Google Scholar]
- Deval D, Mordier S, Obled C, Bechet D, Combaret L, Attaix D, Ferrara M 2001 Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J 360(Pt 1):143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komamura K, Shirotani-Ikejima H, Tatsumi R, Tsujita-Kuroda Y, Kitakaze M, Miyatake K, Sunagawa K, Miyata T 2003 Differential gene expression in the rat skeletal and heart muscle in glucocorticoid-induced myopathy: analysis by microarray. Cardiovasc Drugs Ther 17:303–310 [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL 2004 IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:E591–E601 [DOI] [PubMed] [Google Scholar]
- Gayan-Ramirez G, Vanderhoydonc F, Verhoeven G, Decramer M 1999 Acute treatment with corticosteroids decreases IGF-1 and IGF-2 expression in the rat diaphragm and gastrocnemius. Am J Respir Crit Care Med 159:283–289 [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA 1996 Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517 [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH 2003 Regulation of insulin-like growth factor-I in skeletal muscle and muscle cells. Minerva Endocrinol 28:53–73 [PubMed] [Google Scholar]
- Adamo M, Werner H, Farnsworth W, Roberts Jr CT, Raizada M, LeRoith D 1988 Dexamethasone reduces steady state insulin-like growth factor I messenger ribonucleic acid levels in rat neuronal and glial cells in primary culture. Endocrinology 123:2565–2570 [DOI] [PubMed] [Google Scholar]
- Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP 2004 Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology 145:4806–4812 [DOI] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ 2005 Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280:2737–2744 [DOI] [PubMed] [Google Scholar]
- Li ZF, Shelton GD, Engvall E 2005 Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality. Am J Pathol 166:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BG, Hasselgren PO, Fang CH 2005 Insulin-like growth factor-I inhibits dexamethasone-induced proteolysis in cultured L6 myotubes through PI3K/Akt/GSK-3β and PI3K/Akt/mTOR-dependent mechanisms. Int J Biochem Cell Biol 37:2207–2216 [DOI] [PubMed] [Google Scholar]
- Tomas FM 1998 The anti-catabolic efficacy of insulin-like growth factor-I is enhanced by its early administration to rats receiving dexamethasone. J Endocrinol 157:89–97 [DOI] [PubMed] [Google Scholar]
- Kanda F, Takatani K, Okuda S, Matsushita T, Chihara K 1999 Preventive effects of insulinlike growth factor-I on steroid- induced muscle atrophy. Muscle Nerve 22:213–217 [DOI] [PubMed] [Google Scholar]
- Fournier M, Huang ZS, Li H, Da X, Cercek B, Lewis MI 2003 Insulin-like growth factor-I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol 285:R34–R43 [DOI] [PubMed] [Google Scholar]
- Tomas FM, Knowles SE, Owens PC, Chandler CS, Francis GL, Read LC, Ballard FJ 1992 Insulin-like growth factor-I (IGF-I) and especially IGF-I variants are anabolic in dexamethasone-treated rats. Biochem J 282(Pt 1):91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, Ketelslegers JM, Thissen JP 2005 Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 146:1789–1797 [DOI] [PubMed] [Google Scholar]
- Alzghoul MB, Gerrard D, Watkins BA, Hannon K 2004 Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J 18:221–223 [DOI] [PubMed] [Google Scholar]
- Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T 2003 Stabilization of β-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA 100:4610–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Alessi DR, Hemmings BA, Hundal HS 1998 Constitutive activation of protein kinase B α by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47:1006–1013 [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ 2001 Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013 [DOI] [PubMed] [Google Scholar]
- Schakman O, de Conninck V, Verniers J, Gilson H, Ketelslegers JM, Thissen JP IGF-I gene transfer by electroporation promotes skeletal muscle hypertrophy in hypophysectomized rats. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004 (Abstract P3-187) [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD 2001 Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019 [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S 2002 A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW 2007 Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol 21:215–228 [DOI] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ 2004 Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CH, Li B, James JH, Yahya A, Kadeer N, Guo X, Xiao C, Supp DM, Kagan RJ, Hasselgren PO, Sheriff S 2007 GSK-3β activity is increased in skeletal muscle after burn injury in rats. Am J Physiol Regul Integr Comp Physiol 293:R1545–R1551 [DOI] [PubMed] [Google Scholar]
- Glass DJ 2003 Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90 [DOI] [PubMed] [Google Scholar]
- Shah OJ, Anthony JC, Kimball SR, Jefferson LS 2000 Glucocorticoids oppose translational control by leucine in skeletal muscle. Am J Physiol Endocrinol Metab 279:E1185–E1190 [DOI] [PubMed] [Google Scholar]
- Shah OJ, Anthony JC, Kimball SR, Jefferson LS 2000 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279:E715–E729 [DOI] [PubMed] [Google Scholar]
- Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR 2006 Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281:39128–39134 [DOI] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M 2005 Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7:286–294 [DOI] [PubMed] [Google Scholar]
- Evenson AR, Fareed MU, Menconi MJ, Mitchell JC, Hasselgren PO 2005 GSK-3β inhibitors reduce protein degradation in muscles from septic rats and in dexamethasone-treated myotubes. Int J Biochem Cell Biol 37:2226–2238 [DOI] [PubMed] [Google Scholar]
- Armstrong DD, Wong VL, Esser KA 2006 Expression of β-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol 291:C185–C188 [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R 1997 β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Frame S 2001 The renaissance of GSK3. Nat Rev Mol Cell Biol 2:769–776 [DOI] [PubMed] [Google Scholar]
- Playford MP, Bicknell D, Bodmer WF, Macaulay VM 2000 Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of β-catenin. Proc Natl Acad Sci USA 97:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Kemler R 2000 Curbing the nuclear activities of β-catenin. Control over Wnt target gene expression. EMBO Rep 1:24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jia Y, Wang J, Tao D, Gan X, Tsiokas L, Jing N, Wu D, Li L 2005 β-Catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc Natl Acad Sci USA 102:17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Relaix F, Baesso S, Buckingham M, Cossu G 2007 β-Catenin-independent activation of MyoD in presomitic mesoderm requires PKC and depends on Pax3 transcriptional activity. Dev Biol 304:604–614 [DOI] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA 2002 Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem 277:38239–38244 [DOI] [PubMed] [Google Scholar]
- Huang M, Wang Y, Sun D, Zhu H, Yin Y, Zhang W, Yang S, Quan L, Bai J, Wang S, Chen Q, Li S, Xu N 2006 Identification of genes regulated by Wnt/β-catenin pathway and involved in apoptosis via microarray analysis. BMC Cancer 6:221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R 2002 A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2:8 [DOI] [PMC free article] [PubMed] [Google Scholar]