Abstract
Thyroid hormone (TH) is essential for normal brain development, and polychlorinated biphenyls (PCBs) are known to interfere with TH action in the developing brain. Thus, it is possible that the observed neurotoxic effects of PCB exposure in experimental animals and humans are mediated in part by their ability to interfere with TH signaling. PCBs may interfere with TH signaling by reducing circulating levels of TH, acting as TH receptor analogs, or both. If PCBs act primarily by reducing serum TH levels, then their effects should mimic those of low TH. In contrast, if PCBs act primarily as TH agonists in the developing brain, then they should mimic the effect of T4 in hypothyroid animals. We used a two-factor design to test these predictions. Both hypothyroidism (Htx) and/or PCB treatment reduced serum free and total T4 on postnatal d 15. However, only Htx increased pituitary TSHβ expression. RC3/neurogranin expression was decreased by Htx and increased by PCB treatment. In contrast, Purkinje cell protein-2 expression was reduced in hypothyroid animals and restored by PCB treatment. Finally, PCB treatment partially ameliorated the effect of Htx on the thickness of the external granule layer of the cerebellum. These studies demonstrate clearly that PCB exposure does not mimic the effect of low TH on several important TH-sensitive measures in the developing brain. However, neither did PCBs mimic T4 in hypothyroid animals on all end points measured. Thus, PCBs exert a complex action on TH signaling in the developing brain.
POLYCHLORINATED BIPHENYLS (PCBS) are a family of industrial compounds consisting of two linked phenyl rings and varying degrees of chlorination, resulting in 209 different congeners (1,2). They remain ubiquitous environmental contaminants found in high concentrations in both human and animal tissues (3) despite their production being banned in the United States in the 1970s. Two types of observations about PCBs are central to the current study. First, PCBs are developmental neurotoxins. Schantz et al. (4) reviewed this literature for humans and concluded that, on balance, there is strong evidence indicating that PCB exposure is associated with negative effects on cognitive development. Interestingly, many different neuropsychological strategies have been used to identify effects of PCB exposure on cognitive function, providing additional strength for this conclusion.
These correlative studies in humans are consistent with controlled experiments in animals. Developmental exposure to PCBs can affect hearing (5,6), motor activity in an open field test, and operant behavior (7,8). In addition, PCBs can affect neurite outgrowth in culture (9). Thus, PCBs are developmental neurotoxins in both humans and experimental animals.
A second type of observation documents human exposures and body burdens. In general, these exposures to PCBs are high enough to be associated with adverse effects on brain development in humans as described above, and they provide a context for animal exposure studies. For example, a recent study by Lackmann et al. (10) found that 6-wk-old breast-fed infants had serum PCB levels of 1.19 μg/liter, which was significantly higher than the 0.29 μg/liter found in the serum of bottle-fed infants. Furthermore, Kalantzi et al. (11) reported that total PCB levels in breast milk in the United Kingdom ranged from 26 to 530 ng/g lipid, translating to a daily infant intake of 6.24–2067 ng/kg·d. In addition, She et al. (12) recently reported PCB levels in milk samples collected from women in the Pacific Northwest of the United States. On average, these milk samples had PCB levels of 147 ng/g lipid, which translates to an approximate daily intake of 1 μg/kg·d. Considering that the half-life of PCB congeners in the human body ranges from years to decades (e.g. Ref. 13), PCBs clearly accumulate, accounting for PCB levels in brain tissues of adult humans at autopsy in the micromolar range (14,15). Thus, PCBs remain important contaminants in human tissues and are associated with adverse effects on brain development.
Some investigators speculate that one mechanism by which PCBs may produce neurotoxic affects is by interfering with normal thyroid hormone (TH) action during development (16,17). Early findings showed that accidental exposure to PCBs reduced serum TH levels in humans (18); these findings are supported in more recent studies (19). Findings in animal studies almost uniformly show that PCBs reduce serum TH (20,21,22). Moreover, Crofton (5) reported a correlation between the PCB-induced reduction in serum T4 during development and hearing loss in rats. Therefore, it is possible that PCBs can exert adverse effects on the developing brain by causing a reduction in serum thyroid hormones.
Although PCBs reduce circulating TH levels in animals, they do not produce effects on the developing brain that are fully consistent with the effects of low T4. For example, developmental PCB exposure increases the expression of several known TH-responsive genes, including HES1 in the fetal rat brain, Malic enzyme in the maternal rat liver, and RC3/neurogranin in the neonatal cerebral cortex and hippocampus (23,24,25), despite causing a severe reduction in serum total and free T4. Furthermore, PCB exposure does not recapitulate the effect of hypothyroidism on the cellular composition of white matter in rat pups, despite causing a reduction in circulating T4 (26). Thus, PCBs may exert agonistic effects on TH signaling, perhaps by acting directly on the TH receptor (TR). This concept is supported by the observation that specific PCB congeners can produce TH-like effects on oligodendrocyte differentiation in vitro (27) and on GH3 cell proliferation and GH secretion (28). In addition, individual PCB metabolites can bind to the human TR (29) and can activate the rat TR using a reporter assay (30).
However, other studies report that PCBs can antagonize the TR. Specific PCB congeners inhibit T3-mediated dendritic growth in cerebellar Purkinje cells in vitro (31). In addition, PCBs can inhibit T3-induced gene activation in transient transfection assays (32,33,34). Taken together, these findings indicate that the actions of PCBs on TH signaling are complex but that they could account, at least in part, for their neurotoxic effects.
To discriminate between PCB effects on TH signaling in the developing brain by their ability to reduce circulating TH or by their ability to interact directly with the TR, we performed a two-factor experiment in which the effects of PCB treatment was tested on TH signaling in euthyroid animals and hypothyroid animals. We reasoned that if PCBs can exert a direct action on TH signaling in the developing brain, then this effect should be observable in animals deprived of TH. We used a PCB formulation, Aroclor 1254 (A1254), known to reduce serum TH levels and produce thyroid hormone-like effects (25). These TH-like effects appear to be mediated by specific metabolites of PCB congeners enriched in A1254 (36). We evaluated two kinds of TH-dependent end points as a way of monitoring TH signaling in the developing brain. First, we evaluated the effect of PCBs on the expression of two genes known to be direct targets of TH, including RC3/neurogranin in the hippocampus (25,37) and Purkinje cell protein (PCP)-2 in the cerebellum (38). RC3 appears to be regulated by the α-isoform of TR, whereas PCP-2 appears to be regulated by the β-isoform (39). Second, we evaluated the effect of PCBs on cerebellar histogenesis. The cerebellum is particularly sensitive to TH insufficiency during development (40), exhibiting a delay in granule cell proliferation in and migration from the external granule layer (EGL) (41). The effect of TH on cerebellar granule cell migration appears to be mediated by the α-isoform of TR (42).
Materials and Methods
Experimental animals
All animal procedures were performed according to the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the University of Massachusetts-Amherst Institutional Animal Care and Use Committee. Timed pregnant Sprague Dawley rats (n = 32; Zivic Miller Inc., Pittsburgh, PA) arrived at our facility on gestational day (G) 2 and were housed individually in polycarbonate plastic cages with pine-wood shavings for bedding. Water in glass bottles and food were provided continuously and animals were maintained on a 12-h light, 12-h dark cycle (0600–1800 h lights on). Briefly, animals were randomly assigned to one of four treatment groups: control, hypothyroid (HTx), PCB, or HTx+PCB. Starting on G3, animals were weighed in the morning and provided with a single untreated wafer (mini-vanilla wafer, Keebler Co., Battle Creek, MI) 2 h before lights off. This initial period (G3-G6) trains animals to consume the wafer quickly and in its entirety during experimental treatment. Beginning on G7, wafers were dosed with A1254 (lot 124–191; Accustandard Inc., New Haven, CT) dissolved in methanol and calibrated to deliver 5 mg/kg·d body weight. Animals in control and HTx groups received wafers dosed with methanol alone. All wafers were dosed in the morning and allowed to dry in a fume hood through the day. Dams in the HTx and HTx+PCB groups were treated with a combination of potassium perchlorate (Sigma, St. Louis, MO) and methimazole (MMI; Sigma) in their drinking water as described previously (26). This treatment was initiated incrementally as follows: from G7 to G9, 0.1% MMI plus 0.5% perchlorate; from G9 to G11, 0.1% MMI plus 0.1% perchlorate; and from G11 to postnatal day (P) 15, 0.02% MMI plus 0.5% perchlorate.
Male pups were weighed and killed on P15. Trunk blood was collected for serum hormone analysis, which have been reported previously (26). Brains were removed from the skull, frozen on pulverized dry ice, and stored at −80 C until sectioned for in situ hybridization.
RIA
Total T4 was measured in 5 μl of rat serum as previously described (24). Briefly, each assay tube contained 100 μl barbital buffer [0.11 m sodium barbital, 0.1% (wt/vol) 8 anilino-1-napthalen-sulfonic acid sodium salt, 15% (wt/vol) bovine γ-globulin, Cohn fraction II, 0.1% (wt/vol) gelatin (pH 8.6)], 100 μl anti-T4 (rabbit; Sigma) diluted to a final concentration of 1:30,000, and 100 μl 125I-labeled T4 (12,000–15,000 cpm; PerkinElmer/NEN Life Science Products, Waltham, MA). Standards were prepared from T4 (Sigma) measured using a Cahn electrobalance; standards were run in triplicate, whereas samples were run in duplicate. Standards were calibrated to measure serum T4 levels from 0.4 to 25.6 μg/dl. Tubes were incubated at 37 C for 30 min and then chilled on wet ice for 30 min. Bound counts were precipitated by adding 300 μl ice-cold polyethylene glycol 8000 [20% (wt/vol); Sigma]. Tubes were centrifuged at 1800 × g for 20 min at 4 C, the supernatant aspirated, and the pellet counted in a γ-counter (Cobra II; PerkinElmer). The assay was run at 40–50% binding; nonspecific binding was generally less than 8%.
Free T4 was measured in 50 μl of serum using a RIA kit (MP Biomedicals, Solon, OH) according to the manufacturer’s instructions. We previously determined that parent PCBs did not interfere in the RIA for T4 (our unpublished data).
Quantitative real-time PCR
Total RNA was extracted from frozen pituitary using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The resultant RNA pellet was resuspended in nuclease free water and quantified using UV spectrophotometry. Gel electrophoresis confirmed RNA integrity. Relative levels of TSHβ were determined by quantitative real-time PCR using the QuantiTect SYBR Green RT-PCR kit (QIAGEN, Valencia, CA) and the Mx3000P real-time PCR machine (Stratagene, La Jolla, CA). Each 10-μl reaction contained 1× QuantiTect SYBR Green RT-PCR master mix, 0.5 μm forward primer (TSHβ, 5′-AAT GCT GTC GTT CTC TTT TCC-3′, or β-actin, 5′-TGA ACC CTA AGG CCA ACC GTG AAA-3′), 0.5 μm reverse primer (TSHβ, 5′-AGA ATG TCT GTG GCT TGG TGC-3′, or β-actin, 5′-ATA CAG GGA CAA CAC AGC CTG GAT-3′), 0.1 μl reverse transcriptase and 200 ng total RNA. The conditions for cDNA synthesis and target amplification were as follows: one cycle of 50 C for 30 min; one cycle of 95 C for 15 min; and 40 cycles each of 95 C for 15 sec, 60 C for 30 sec, and 72 C for 30 sec. All samples were run in duplicate. Amplification values were normalized to β-actin mRNA, which was run in parallel duplicate wells. Although TH has been reported to increase β-actin expression in the cortex (43), we have not found previously that β-actin expression is affected by TH (44). Moreover, cycle threshold values were not affected by TH or PCB treatment in the current experiment. Thus, we used β-actin expression as a fiducial for our TSHβ mRNA measures.
In situ hybridization
We used in situ hybridization to measure the effects of treatments on RC3 and PCP-2 gene expression because this semiquantitative approach has a very high degree of neuroanatomical resolution. Specifically, RC3 expression in the hippocampus is responsive to TH only in the dentate gyrus, not in Ammon’s horn (37); therefore, more quantitative approaches to measure RC3 mRNA (e.g. real-time PCR) would be revealing only if coupled to a method of RNA isolation that has a high degree of neuroanatomical resolution (e.g. laser capture microdissection). Coronal and sagittal sections of frozen brain tissues were taken at 12 μm in a cryostat (Reichert-Jung Frigocut 2800N; Leica Corp., Deerfield, IL). Two adjacent sections were thaw mounted onto each microscope slide twice coated with gelatin and stored at −80 C until hybridization. The rostrocaudal placement of the section was matched using internal landmarks when slides were chosen for the in situ hybridization.
RC3/neurogranin in the rostral hippocampus was measured using a 3′-end-labeled 45-base oligonucleotide (IDT, Coralville, IA) directed against bp 830–786 of the RC3/neurogranin mRNA (5′-ACC TGT CCA CGC GCC CAG CAT GCA GCT CTG CCT CCG CAG CCT CGG-3′). End labeling was carried out using terminal transferase (Roche Applied Sciences, Indianapolis, IN) and 33P-dATP according to the manufacturer’s instructions. PCP-2 mRNA was measured using a 33P-cRNA probe that was in vitro transcribed from a cDNA kindly provided by Dr. Grant Anderson (University of Minnesota, Minneapolis, MN). Two slides from each brain, four sections total, were thawed at room temperature and hybridized as previously described (24,45). Sections from the same brain region were matched across treatment groups using specific internal landmarks. In the case of the hippocampus, we matched sections using the shape and size of the dentate gyrus and the lateral ventricles. In the case of the cerebellum, we collected midsagittal sections. Although it is difficult to be assured that the section in one brain exactly matched that of another, the brains were sectioned in random order and potential variation in the placement of the section would not represent a systematic error. After in situ hybridization, slides were exposed to BioMax film (Eastman Kodak, Rochester, NY) in x-ray cassettes along with 14C-labeled standards (American Radiolabeled Chemicals Inc., St. Louis, MO) to control for overexposure. The hybridization signal was analyzed using an imaging system described previously (23,46). Film density was measured in the upper and lower leaflets of the dentate gyrus of P15 brains for RC3/neurogranin as previously described (25) except that we used a SPOT Insight 2 camera and a Macintosh G5 computer. Film density was measured over the dentate gyrus by an operator who did not know the identity of the image. Film density values of the dentate gyrus were averaged over the four sections for each brain, with one brain representing each litter. PCP-2 mRNA hybridized slides were dipped in NTB photographic emulsion (Eastman Kodak), allowed to air dry, and stored at 4 C for 72 h. Slides were then developed with Dektol developer, rinsed in water, and fixed. After rinsing in cold water, slides were counterstained with 0.5% methyl green and coverslipped using Permount. Silver grain reflectance was measured over the perikarya and dendrites of labeled Purkinje cells using dark-field optics.
Histological analysis
Sagittal sections of frozen P15 cerebellum were taken at 12 μm in a cryostat (Reichert-Jung Frigocut 2800N; Leica). Two adjacent sections were thaw mounted onto microscope slides twice coated with gelatin and stored at −80 C.
Two slides per animal were thawed, fixed with 4% formaldehyde, and stained with hematoxylin and eosin (Sigma), dehydrated in ethanol, and coverslipped using Permount. Images were magnified using a Dage-72 series video camera equipped with a Nikon macrolens mounted on a bellows and captured using a Scion AG-5 capture board interfaced with NIH Image version 1.61 (W. Rashband, National Institute of Mental Health, Bethesda, MD) run on Macintosh G4. For each cerebellum, the deepest sulcus was located and a 1-mm grid was placed over the image. The area of each layer was measured over a 1-mm length using NIH image calibrated with a stage micrometer (Fig. 1). Four sections were measured from each brain, with a single measurement made for each layer taken in a single section.
Figure 1.
Measurement of cerebellar layers. Midsagittal sections of P15 rat cerebellum were fixed, stained with hematoxylin and eosin, and coverslipped. The deepest sulcus for each individual brain was identified (A) and a 1-mm grid was placed over the magnified image (B). The area of each layer was measured over a 1-mm length using NIH image calibrated with a stage micrometer.
Statistical analysis
Results were analyzed using a two-way ANOVA. Post hoc tests, where appropriate, were performed by Fisher’s protected least significant differences test (SuperAnova software; Abacus Concepts Inc., Berkeley, CA). Total T4 and free T4 RIA data were analyzed using a two-tailed unpaired t test. Statistical outliers were identified using the Grubb’s test (GraphPad Prism, San Diego, CA; http://www.graphpad.com/quickcalcs). Sample loss during processing accounts for differences in sample size between groups. Only one male pup was used from each litter; thus, the statistical n represents the number of independent litters used in these studies.
Results
Serum hormone levels
Serum hormone levels are shown in Table 1. Serum total T4 levels for these animals have been published in a separate manuscript (26) and are provided here for completeness. Briefly, total T4 levels were significantly lower in pups derived from all treatment groups, compared with control animals; total T4 levels were not detectable in pups derived from dams treated with MMI+perchlorate but were reduced to about 23% of control values in pups derived from dams exposed to A1254 alone. Likewise, serum-free T4 levels were significantly lower in pups derived from all treatment groups, compared with control animals; free T4 levels were not detectable in pups derived from dams treated with MMI+perchlorate but were reduced to about 38% of control values in pups derived from dams exposed to A1254 alone. Values for control and PCB-treated animals were evaluated using unpaired two-tailed t test, which showed that free T4 was significantly reduced in the PCB-treated animals (t = 5.835, P = 0.0001).
Table 1.
Serum hormone levels in pups on P15
| Treatment | Total T4 (μg/dl) | Free T4 (ng/dl) |
|---|---|---|
| Control | 5.851 ± 0.38 | 1.416 ± 0.07 |
| HTx | 0.4a | 0.32a |
| PCB | 1.365 ± 0.42b | 0.542 ± 0.13b |
| HTx+PCB | 0.4a | 0.32a |
Values below the lowest standard (0.4 μg/dl) for total T4 and (0.32 ng/dl) for free T4.
Significantly different from control (P < 0.0001).
TSHβ expression in the pituitary
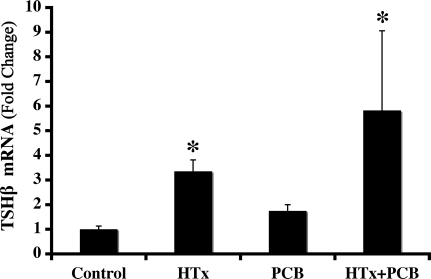
Real-time PCR analysis of TSHβ expression in the pituitary showed a significant effect of HTx (F1,16 = 15.662; P = 0.0011; Fig. 2) by two-way ANOVA. In contrast, TSHβ expression was not affected by PCB treatment (F1,16 = 0599; P > 0.05), and there was no significant interaction between these two treatments (F1,16 = 0.106; P > 0.05). Due to unequal variance across groups, normalized values were log transformed before statistical analysis was performed. For the purpose of presentation, untransformed values are shown in Fig. 2. Post hoc analysis of these data demonstrated that TSHβ mRNA levels were significant higher in the pituitaries of Htx animals and those Htx animals treated with PCBs, compared with controls. Interestingly, despite PCB treatment causing a significant reduction in serum total and free T4, pituitary TSHβ mRNA levels were not affected by PCB treatment. However, it is important to note that because these animals were exposed to treatments during development, the TSHβ mRNA levels we observed may reflect changes in cellular levels of TSHβ mRNA, may reflect changes in the numbers of thyrotropes, or both. The use of a thyrotrope-specific fiducial in the real-time PCR would obviate caution in this interpretation, but none was available.
Figure 2.
Treatment effects on TSHβ mRNA expression in the P15 rat pituitary TSHβ mRNA was significantly elevated in the pituitary of HTx-treated animals, measured by real-time PCR (see Materials and Methods). There was no significant effect of PCB treatment and no significant interaction. Bars represent the mean TSHβ/β-actin ± sem. *, P < 0.01, compared with control (control, n = 8; HTx, n = 4; PCB, n = 4; HTx+PCB, n = 3).
RC3 expression in the dentate gyrus
Figure 3A shows the expression pattern of RC3/neurogranin in sections containing the upper (DG-upper) and lower (DG-lower) leaflets of the dentate gyrus; brain regions in which this gene is known to be affected by TH (37). We obtained relative levels of RC3 mRNA using in situ hybridization because RC3 expression is regulated by TH only in very specific brain regions (e.g. Ref. 39); therefore, even microdissection would include brain tissue in which RC3 expression is not affected by thyroid status. The two-way ANOVA of RC3 expression in the dentate gyrus in P15 pups revealed a significant effect of HTx (F1,22 = 780; P = 0.0001) and PCB (F1,22 = 6.786; P = 0.0162) treatment in the lower leaflet; however, no significant interaction was observed (F1,22 = 1.047; P > 0.05). Similarly in the upper leaflet, we observe a significant effect of HTx (F1,22 = 568; P = 0.001) and PCB (F1,22 = 7.955; P = 0.01) treatment but no significant interaction between the two main effects. Post hoc analysis using Fisher’s protected least significant differences test revealed that RC3 mRNA levels in HTx- and HTx+PCB-treated animals were significantly lower than RC3 mRNA in controls and that RC3 expression in animals treated with PCB was significantly higher than in controls (Fig. 3B).
Figure 3.
Treatment effects on RC3/neurogranin mRNA expression in the upper and lower leaflet of the dentate gyrus (DG) of P15 rat pups. A, Film autoradiograms after in situ hybridization, representing the mean density in the dentate gyrus for each treatment group as graphically displayed in B. B, Graphic representation of film analysis as described in the text. The density of the film over the dentate gyrus was measured on digitized images using NIH Image J. Pups exposed to HTx and HTx+PCB treatment exhibited significantly lower RC3/neurogranin mRNA in both the upper and lower leaflet of the dentate gyrus. In contrast, RC3/neurogranin mRNA levels in pups exposed to PCB was significantly higher than that observed in control animals in both the upper and lower leaflet. Bars represent the mean ± sem. *, P < 0.01; ***, P < 0.0001, compared with control (control, n = 7; HTx, n = 5; PCB, n = 7; HTx+PCB, n = 7).
PCP-2 expression in the cerebellum
PCP-2 expression was measured separately in the Purkinje cell perikarya and dendritic field (Fig. 4A). Two-way ANOVA on PCP-2 expression in Purkinje perikarya revealed that there was no significant effect of HTx (F1,21 = 0.467; P > 0.05) or PCB exposure (F1,21 = 1.364; P > 0.05) but that there was a significant interaction (F1,21 = 4.523; P = 0.0455). In the dendritic field, there was again a significant interaction of the two main effects (F1,21 = 8.202; P = 0.0093) but no effect of HTx (F1,21 = 0.014; P > 0.05) or PCB treatment (F1,21 = 2.026; P > 0.05). In the Purkinje cell body, post hoc analysis revealed a significant increase in PCP-2 signal density in the HTx+PCB group, compared with HTx alone. Likewise, in the dendritic field, signal density in the HTx+PCB group was significantly increased, compared with both PCB and HTx alone (Fig. 4B).
Figure 4.
Treatment effects on PCP-2 expression in the cerebellum of P15 rats. A, Dark-field image of PCP-2 expression in the cerebellum using radioactive in situ hybridization. B, The signal density was measured using dark-field optics. In the Purkinje cell perikarya, signal density was increased in the HTx+PCB group, compared with HTx-only treatment. In the dendritic field, the signal density was significantly increased in the HTx+PCB group, compared with both PCB and HTx-only treatment. Bars represent the mean ± sem. *, P < 0.05; **, P < 0.001 (control, n = 7; HTx, n = 4; PCB, n = 7; HTx+PCB, n = 7).
Cerebellar histogenesis
TH insufficiency is known to delay granule cell migration from the EGL to the internal granule layer (IGL). Therefore, we measured thickness of these layers in hematoxylin and eosin stained sagittal sections of cerebellum as an additional measure of TH action in the developing cerebellum in this experiment. Two-way ANOVA showed significant treatment effects of HTx (F1,23 = 79.782; P = 0.0001), PCB (F1,23 = 6.106; P = 0.0213), and the interaction of HTx and PCB (F1,23 = 7.085, P = 0.0139) (Fig. 5). Post hoc analysis showed that both HTx and HTx+PCB-treated pups had a thicker EGL, compared with controls. There was no difference in EGL thickness between control and PCB-treated pups. However, HTx animals that were also treated with PCBs exhibited an EGL that was significantly thinner than that of HTx animals not treated with PCBs.
Figure 5.
Treatment effects on the thickness of cerebellar layers in P15 rat pups. A, Hypothyroidism was associated with a significant increase in the thickness of the EGL, compared with control. Interestingly, the thickness of the EGL in HTx animals also treated with PCBs was significantly less than that of animals receiving HTx alone. In contrast, the thickness of the ML was reduced in HTx animals, whether or not they also received PCBs. No difference was observed in the thickness of the IGL. Bars represent the mean ± sem. *, P < 0.01; ***, P < 0.0001. B, Hematoxylin and eosin-stained sagittal sections of P15 rat cerebella. Scale bar, 100 μm (control, n = 9; HTx, n = 4; PCB, n = 7; HTx+PCB, n = 7).
There was a significant effect of HTx (F1,23 = 54.224; P = 0.0001) on thickness of the mitral layer (ML) but no effect of PCB (F1,23 = 0.006; P > 0.05) or an interaction between the two main effects (F1,23 = 3.583; P > 0.05). Post hoc analysis showed that the thickness of the ML was significantly reduced in HTx-treated animals, compared with control. There were no treatment effects on the thickness of the IGL; HTx (F1,23 = 0.072; P > 0.05), PCB (F1,23 = 0.744; P > 0.05); or the interaction of HTx and PCB (F1,23 = 1.078; P > 0.05) (Fig. 5).
Discussion
Our current findings demonstrate that developmental exposure to A1254, which produces a significant decrease in serum total and free T4, does not produce effects on end points of TH action in the developing brain that are produced when TH levels are brought to similar values with other goitrogens. Our PCB treatment resulted in an approximately 75% reduction in serum total T4 and a 60% reduction in serum-free T4 (Table 1). This degree of T4 deficit, produced by treatment with propylthiouracil (PTU), is sufficient to cause an increase in serum TSH (47), which changes in parallel to pituitary TSHβ (48). Moreover, as little as a 28% decrease in serum total T4 produced by PTU is associated with significant changes in the cellular makeup of the corpus callosum (49). Finally, treatment with PTU at 3 ppm, producing a 75% reduction in serum total T4, also produces the development of a large neuronal heterotopia in the corpus callosum (50). Thus, it is enigmatic that PCBs can reduce serum total and free T4 without affecting serum TSH (51) or TSHβ mRNA in the pituitary gland or other downstream markers of TH action in the developing brain.
This finding is difficult to reconcile with an idealized model of the hypothalamic-pituitary-thyroid axis in which low free T4 regulates serum TSH by negative feedback (e.g. Ref. 52). One possible explanation is that there are specific PCB congeners in A1254 that exerts a TH agonistic effect that can ameliorate the effects of low TH. As we reported previously (25), PCB exposure increased RC3 expression in the dentate gyrus. However, PCB exposure did not affect RC3 expression in hypothyroid animals. This observation is not likely to be an artifact of the experimental measurements. We confirmed that low serum total and free T4, produced by treatment with MMI is associated with significantly reduced RC3 mRNA expression in the dentate gyrus. The degree of this decrement in RC3 was much greater than the increase in RC3 expression observed in response to PCB exposure; thus, we expected to observe at least a small increase in RC3 expression in response to PCB treatment in hypothyroid animals if PCBs act as a direct TR agonist in vivo. The present observations remain perplexing because we previously observed that PCB effects on RC3 expression were likely to be transcriptional both because PCB exposure increased RC3 mRNA levels only in those brain areas in which RC3 expression is increased by thyroid hormone (37) and because single-cell levels of RC3 mRNA were increased by PCB exposure.
Likewise, we expected to observe that PCB exposure would produce a TH-like effect on PCP2 expression in Purkinje cells. We observed that PCP2 mRNA is localized in the Purkinje cell body and dendritic field, as had been previously reported (53), but the effect of hypothyroidism on PCP2 mRNA expression was not robust. Several authors have shown that PCP-2 expression is suppressed in hypothyroid pups on P15 (e.g. Ref. 54) and that there are functional thyroid response elements in the PCP-2 gene (38), indicating a direct action of TH on PCP2 expression. Others have failed to observe changes in PCP-2 expression in the cerebellum of hypothyroid animals (e.g. Ref. 55). We observed a strong trend for PCP-2 mRNA reduction in hypothyroid animals as reported by Dong et al. (55), but PCB exposure failed to increase its expression as it had done for RC3 in the hippocampus. PCB exposure did increase the expression of PCP-2 in hypothyroid animals; thus, PCB exposure exerted an action similar to that of TH, but only in hypothyroid animals.
Finally, we tested the effect of hypothyroidism and/or PCB exposure on a developmental event, namely cerebellar histogenesis. We confirmed that the external granule layer of the cerebellum is retained on P15 in hypothyroid animals, which further confirmed that our experimental treatments were effective. However, PCB exposure alone had no effect on cerebellar histogenesis but diminished the thickness of the EGL in hypothyroid animals. Thus, PCB exposure exerted a TH-like effect on cerebellar histogenesis but only in hypothyroid animals. This profile of effects of PCBs is similar to that observed on PCP2 expression but dissimilar from that observed with RC3 expression in the hippocampus.
These studies are the first to evaluate the interaction of thyroid status in combination with PCB exposure on molecular targets of TH action in the developing rat brain. The results indicate clearly that the effect of PCB exposure on TH signaling in the developing brain differs among specific end points of TH signaling. We considered the possibility that differences may reflect differences in TR isoform mediating TH action on different end points. Effects of TH on TSHβ (56) and PCP-2 (39) mRNAs are mediated by the TRβ receptor, whereas TH action on RC3 (39) expression and granule cell migration (42) are mediated by the TRα receptor. However, the different effects of PCB exposure on these various endpoints do not appear to be associated with the TR isoform. For example, PCB exposure increased RC3 expression (TRα) only in euthyroid animals but affected cerebellar histogenesis only in animals made hypothyroid with MMI (TRα).
Differences in PCB effects on these end points may also be related to the cellular context; for example, specific TR isoforms may interact with different cellular proteins that in some way influence PCB action on the TR. Moreover, TH may itself regulate some of this machinery, which could influence the specific pattern of PCB action on these end points. It is clear that some PCB congeners can bind to TRs and regulate gene expression. We showed previously that 4-OH-2′,3,3′,4′,5′-pentachlorobiphenyl can bind to the rat TR and can increase GH mRNA in GH3 cells as well as activate luciferase driven by a canonical thyroid response element (29). Likewise, others have similarly shown the ability of hydroxylated PCBs to interact directly with the TR (35).
It is also important to consider the potential role of PCB metabolism in its action on TH signaling. Parent PCB congeners do not appear to bind to TRs (24); rather, hydroxylated PCBs appear to be more capable of interacting with TRs. We recently reported that the metabolism of specific PCB congeners is required for their ability to drive TR action in rat pituitary cells in vitro (36). Because this metabolism was dependent on induction of the cytochrome P450 enzyme Cyp1A1 by the aryl hydrocarbon receptor, it required a combination of PCB congeners that included those that activate the aryl hydrocarbon receptor and those that would be metabolized by Cyp1A1 to form TH agonists. Thus, it is possible that the ability of different cells or brain regions to metabolize PCBs may differ, depending on the degree to which Cyp1A1 is induced. In addition, it is possible that TH itself may influence Cyp1A1 induction such that euthyroid animals differ in their sensitivity to the agonist action of PCBs, compared with hypothyroid animals. These observations are germane in the present study because the PCB mixture used (A1254) has the same PCB congeners at the same concentration that we previously used to demonstrate the role of metabolism in PCB action on TH signaling (36).
In conclusion, the current data demonstrate that PCB exposure produces different effects on a variety of end points of TH action in the developing brain. Although there are differences in the specific profile of effects, in no case do these end points behave in a manner predicted by total and free T4 levels in PCB-treated animals. That is, the endocrine profile of low total and free T4 induced by PCB exposure is not associated with any of the downstream consequences that occur when total and free T4 are brought to the same level by PTU. This enigma has considerable implications for interpreting hormone levels in a clinical, regulatory, or epidemiological setting.
Footnotes
This work was supported by Grant ES/HD10026-01A1 from the National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 17, 2008
Abbreviations: A1254, Aroclor 1254; EGL, external granule layer; G, gestational day; HTx, hypothyroid; IGL, internal granule layer; ML, mitral layer; MMI, methimazole; P, postnatal day; PCB, polychlorinated biphenyl; PCP, Purkinje cell protein; PTU, propylthiouracil; TH, thyroid hormone; TR, TH receptor.
References
- Tilson HA, Kodavanti PRS 1997 Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology 13:727–744 [PubMed] [Google Scholar]
- Erickson MD 2001 PCB properties, uses, occurrence, and regulatory history. In: Robertson LW, Hansen LG, eds. PCBs: recent advances in environmental toxicology and health effects. Lexington, KY: University Press of Kentucky; xii–xxx [Google Scholar]
- Tilson HA, Jacobson JL, Rogan WJ 1990 Polychlorinated biphenyls and the developing nervous system: cross-species comparison. Neurotoxicology 13:139–148 [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC 2003 Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111:357–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM 2004 Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Anal 24:1665–1671 [DOI] [PubMed] [Google Scholar]
- Lasky RE, Widholm JJ, Crofton KM, Schantz SL 2002 Perinatal exposure to Aroclor 1254 impairs distortion product otoacoustic emissions (DPOAEs) in rats. Toxicol Sci 68:458–464 [DOI] [PubMed] [Google Scholar]
- Sugawara N, Nakai K, Nakamura T, Ohba T, Suzuki K, Kameo S, Satoh C, Satoh H 2006 Developmental and neurobehavioral effects of perinatal exposure to polychlorinated biphenyls in mice. Arch Toxicol 80:286–292 [DOI] [PubMed] [Google Scholar]
- Sable HJ, Powers BE, Wang VC, Widholm JJ, Schantz SL 2006 Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol Teratol 28:548–556 [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, Kuroda Y 2007 Disrupting effects of hydroxy-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: a possible causal factor for developmental brain disorders? Chemosphere 67:S412–S420 [DOI] [PubMed] [Google Scholar]
- Lackmann GM, Schaller KH, Angerer J 2004 Organochlorine compounds in breast-fed vs. bottle-fed infants: preliminary results at six weeks of age. Sci Total Environ 329:289–293 [DOI] [PubMed] [Google Scholar]
- Kalantzi OI, Martin FL, Thomas GO, Alcock RE, Tang HR, Drury SC, Carmichael PL, Nicholson JK, Jones KC 2004 Different levels of polybrominated diphenyl ethers (PBDEs) and chlorinated compounds in breast milk from two U.K. regions. Environ Health Perspect 112:1085–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K 2007 Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere 67:S307–S317 [DOI] [PubMed] [Google Scholar]
- Ogura I 2004 Half-life of each dioxin and PCB congener in the human body. Organohalogen Compounds 66:3376–3384 [Google Scholar]
- Dewailly E, Hansen JC, Pedersen HS, Mulvad G, Ayotte P, Weber JP, Lebel G 1995 Concentration of PCBs in various tissues from autopsies in Greenland. Organohalogen Compounds 26:175–180 [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC 1999 Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect 107:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP, Hendry LB 1998 Impact of PCBs on thyroid hormone directed brain development. Toxicol Ind Health 14:103–120 [DOI] [PubMed] [Google Scholar]
- Porterfield SP 2000 Thyroidal dysfunction and environmental chemicals—potential impact on brain development. Environ Health Perspect 108(Suppl 3): 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsano CP 1981 Environmental factors altering thyroid function and their assessment. Environ Health Perspect 38:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Petrik J, Chovancova J, Drobna B, Jursa S, Pavuk M, Trnovec T, Sebokova E, Klimes I 2005 Human thyroid in the population exposed to high environmental pollution by organochlorinated pollutants for several decades. Endocr Regul 39:13–20 [PubMed] [Google Scholar]
- Bowers WJ, Nakai JS, Chu I, Wada M, Moir D, Yagminas A, Gill S, Pulido O, Meuller R 2004 Early developmental neurotoxicity of a PCB/organochlorine mixture in rodents after gestational and lactational exposure. Toxicol Sci 77:51–62 [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM 1995 Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol 135:77–88 [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A 1996 Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol 136:269–279 [DOI] [PubMed] [Google Scholar]
- Bansal R, You SH, Herzig CT, Zoeller RT 2005 Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs). Brain Res 156:13–22 [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT 2004 Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Perspect 112:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Dowling AL, Vas AA 2000 Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinol 141:181–189 [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Bansal R, Zoeller RT 2006 Polychlorinated biphenyls exert selective effects on cellular composition of white matter in a manner inconsistent with thyroid hormone insufficiency. Endocrinology 147:846–858 [DOI] [PubMed] [Google Scholar]
- Fritsche E, Cline JE, Nguyen NH, Scanlan TS, Abel J 2005 Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect 113:871–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Jinno N, Suzuki T, Sugihara K, Ohta S, Kuroki H, Fujimoto N 2005 Thyroid hormone-like and estrogenic activity of hydroxylated PCBs in cell culture. Toxicology 208:377–387 [DOI] [PubMed] [Google Scholar]
- You SH, Gauger KJ, Bansal R, Zoeller RT 2006 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol 257–258:26–34 [DOI] [PubMed] [Google Scholar]
- Gauger K, Giera S, Sharlin D, Bansal R, Iannacone E, Zoeller RT 2007 Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists following cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect 115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, Kuroda Y 2005 Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Brain Res 154:259–263 [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Kuroda Y, Koibuchi N 2004 Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J Biol Chem 279:18195–18202 [DOI] [PubMed] [Google Scholar]
- Bogazzi F, Raggi F, Ultimieri F, Russo D, Campomori A, McKinney JD, Pinchera A, Bartalena L, Martino E 2003 Effects of a mixture of polychlorinated biphenyls (Aroclor 1254) on the transcriptional activity of thyroid hormone receptor. J Endocrinol Invest 26:972–978 [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Miyazaki W, Takeshita A, Kuroda Y, Koibuchi N 2002 Polychlorinated biphenyls suppress thyroid hormone-induced transactivation. Biochem Biophys Res Commun 299:384–388 [DOI] [PubMed] [Google Scholar]
- Zoeller RT 2005 Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol 242:10–15 [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT 2007 Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect 115:1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez MA, DeLecea L, Guadano-Ferraz A, Morte B, Gerendasy D, Sutcliffe JG, Bernal J 1996 Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinol 137:1032–1041 [DOI] [PubMed] [Google Scholar]
- Zou L, Hagen SG, Strait KA, Oppenheimer JH 1994 Identification of thyroid hormone response elements in rodent Pcp-2, a developmentally regulated gene of cerebellar Purkinje cells. J Biol Chem 269:13346–13352 [PubMed] [Google Scholar]
- Manzano J, Morte B, Scanlan TS, Bernal J 2003 Differential effects of triiodothyronine and the thyroid hormone receptor β-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144:5480–5487 [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Chin WW 2000 Thyroid hormone action and brain development. Trends Endocrinol Metab 11:123–128 [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J 1972 The effects of early hypo- and hyperthyroidism on the development of rat cerebellar cortex. I. Cell proliferation and differentiation. Brain Res 44:13–23 [DOI] [PubMed] [Google Scholar]
- Morte B, Manzano J, Scanlan T, Vennstrom B, Bernal J 2002 Deletion of the thyroid hormone receptor α1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R, Paul S, Chaudhury S, Sarkar PK 1996 Regulation of actin and tubulin gene expression by thyroid hormone during rat brain development. Brain Res Mol Brain Res 35:111–118 [DOI] [PubMed] [Google Scholar]
- Iannacone EA, Yan AW, Gauger KJ, Dowling AL, Zoeller RT 2002 Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Mol Cell Endocrinol 186:49–59 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Fletcher DL, Butnariu O, Lowry C, Moore FL 1997 N-ethylmaleimide (NEM) can significantly improve in situ hybridization results using 35S-labeled oligodeoxynucleotide or complementary RNA probes. J Histochem Cytochem 45:1035–1041 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C 2005 Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612 [DOI] [PubMed] [Google Scholar]
- Sui L, Gilbert ME 2003 Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology 144:4195–4203 [DOI] [PubMed] [Google Scholar]
- Franklyn JA, Wood DF, Balfour NJ, Ramsden DB, Docherty K, Chin WW, Sheppard MC 1987 Effect of hypothyroidism and thyroid hormone replacement in vivo on pituitary cytoplasmic concentrations of thyrotropin-β and α-subunit messenger ribonucleic acids. Endocrinology 120:2279–2288 [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, Zoeller RT 2008 The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology 149:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, Gilbert ME 2007 Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: a model of cortical dysplasia. Endocrinology 148:2593–2597 [DOI] [PubMed] [Google Scholar]
- Hood A, Hashmi R, Klaassen CD 1999 Effects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol Appl Pharmacol 160:163–170 [DOI] [PubMed] [Google Scholar]
- Connors JM, Hedge GA 1981 Feedback regulation of thyrotropin by thyroxine under physiological conditions. Am J Physiol 240:E308–E313 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Oberdick J 2002 Conservation of the developmentally regulated dendritic localization of a Purkinje cell-specific mRNA that encodes a G-protein modulator: comparison of rodent and human Pcp2(L7) gene structure and expression. Brain Res Mol Brain Res 105:1–10 [DOI] [PubMed] [Google Scholar]
- Strait KA, Zou L, Oppenheimer JH 1992 β1 isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development. Mol Endocrinol 6:1874–1880 [DOI] [PubMed] [Google Scholar]
- Dong H, Wade M, Williams A, Lee A, Douglas GR, Yauk C 2005 Molecular insight into the effects of hypothyroidism on the developing cerebellum. Biochem Biophys Res Commun 330:1182–1193 [DOI] [PubMed] [Google Scholar]
- Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J 1999 Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J 18:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]