Abstract
Cocaine- and amphetamine-regulated transcript (CART) is one of the two known mediators of the leptin regulation of bone mass. Cart is expressed in both the brain and peripheral tissues such as the pituitary gland and the pancreatic islets. Cart−/− mice present a low bone mass phenotype due to an isolated increase in osteoclast number. In an effort to rescue their bone phenotype, we delivered recombinant CART in the third ventricle of the mutant mice but never recorded any improvement of the low bone mass, although this procedure could affect fat pad mass. In contrast, transgenic mice harboring a 2-fold increase in CART circulating level display a high bone mass due to an isolated decrease in osteoclast number and could rescue the low bone mass phenotype of the Cart−/− mice. Thus, our results suggest that in its capacity of a regulator of bone remodeling, CART may act more as a circulating molecule than a neuropeptide.
BONE REMODELING, the physiological process whereby bone tissue is constantly renewed, is regulated by local and systemic signals. One type of regulation whose importance has emerged in the last 10 yr is the neuroendocrine control of bone remodeling (1). This regulation relies either on pituitary hormones such as TSH and FSH or on bona fide neuronal inputs signaling through the leptin receptor, melanocortin 4 receptor, or Y2 receptors among others (2,3,4,5,6,7,8,9,10).
Leptin regulation of bone mass is complex as it involves a hormone made peripherally, signaling of this hormone to hypothalamic neurons and ultimately mediation of its effects on bone remodeling by the sympathetic tone and another molecule called cocaine- and amphetamine-regulated transcript (CART) (5,6,7). Whereas our understanding of the molecular mechanisms used by the sympathetic tone to regulate both arms of bone remodeling (bone formation and bone resorption) has made significant progress, the mechanisms whereby CART regulates bone resorption under the control of leptin are much less clear (7,8,9). There are at least two reasons for this paucity of knowledge: first, a specific CART receptor has not been identified yet, and second, Cart is expressed in both the brain and some peripheral organs (11,12).
Cart, a single gene encoding a 116-amino acid long protein, was discovered in the mid-1990s as a gene whose expression is up-regulated by psychostimulants in the striatum (13,14). Subsequently it was shown that Cart is more highly expressed in the hypothalamus than the striatum (15). In the hypothalamus Cart is highly expressed in lateral hypothalamic area as well as the paraventricular and arcuate nuclei. This cell distribution suggested that CART could be involved in the control of energy homeostasis, a contention supported by the following observations: Cart expression in neurons of the arcuate nuclei is reduced in leptin-deficient mice (16); intracerebroventricular (ICV) CART inhibits normal and starvation-induced feeding, whereas an antiserum against CART increases feeding in normal rats (17,18). Despite these suggestive evidences, Cart-deficient mice have normal appetite and energy expenditure on a chow diet and, instead, present as their only phenotype an osteoporosis secondary to an increase in bone resorption parameters. This increase in bone resorption is itself secondary to an increase in Rankl expression in osteoblasts (7). However, coculture between wild-type (WT) (or Cart−/−) osteoblasts and WT (or Cart−/−) osteoclasts consistently failed to show a direct effect of CART on bone cells, thus implying that CART regulates Rankl expression in an indirect manner, presumably through neural means (7).
In an effort to demonstrate a central action of CART, we tried to rescue Cart−/− low bone mass phenotype using ICV infusion of either recombinant CART or an adenovirus-expressing CART. Surprisingly, these two manipulations failed to affect bone mass. In contrast, we show here that peripheral overexpression of CART increases bone mass by reducing osteoclastogenesis and could correct Cart−/− mice low bone mass phenotype. These data suggest that, in its role as a regulator of bone remodeling, CART may act as a hormone more than as a neuropeptide.
Materials and Methods
Generation and identification of α1(I)collagen-Cart transgenic mice
The 1.7-kb genomic fragment-encoding mouse Cart was cloned downstream of the osteoblast-specific 2.3-kb fragment of α1(I)collagen promoter (19). After microinjections into the pronucleus of fertilized oocytes, founder mice were mated with WT mice to establish individual transgenic lines. PCR genotyping was performed on tail genomic DNA using oligonucleotides 5′-ACTTCCTGAAACATCTCA-3′ and 5′-GGTGGCATTGTTCCTTAGCAG-3′. Expression of the Cart transgene in bone was assessed by Northern blot using CART cDNA as a probe and by quantitative PCR (qPCR) using total RNA extracted from 3-month-old WT and α1(I)collagen-Cart long bones (hemizygous females: n = 6). qPCR analysis was performed on Deoxyribonuclease I-treated total RNA converted to cDNA using primers from SuperArray Bioscience Corp. (Frederick, MD) and the Taq SYBR Green Supermix (Bio-Rad, Hercules, CA) with ROX on an MX3000 (Stratagene, La Jolla, CA) instrument. The institutional animal care committee approved all studies performed.
Bone analyses
Bone histomorphometry was performed as previously described (20,21,22). Briefly, lumbar vertebrae were dissected, fixed for 24 h in 10% formaline, dehydrated in graded ethanol series, and embedded in methyl methacrylate resin according to standard protocols (20). Seven-micrometer sections stained with von Kossa/von Gieson stains were used to determine the bone volume to tissue volume (BV/TV) ratio. Bone formation rate (BFR) was measured by the calcein double-labeling method (21). Calcein (Sigma Chemical Co., St. Louis, MO) was dissolved in calcein buffer (0.15 m NaCl, 2% NaHCO3) and injected twice at 0.125 mg/g body weight on d 1 and 4, and mice were killed on d 6. Unstained 7-μm sections were analyzed for BFR measurements. For osteoclast and osteoblast surfaces, 5-μm sections were stained with tartrate-resistant acid phosphatase and toluidine blue, respectively (23,24). Histomorphometric analyses were performed using the Osteomeasure analysis system (Osteometrics, Atlanta, GA). Six to 10 female mice were analyzed for each group.
ICV administration of CART
ICV infusion was performed as previously described (5,6). Briefly, calvaria were exposed, and a 28-gauge cannula (brain infusion kit II; ALZET, Cupertino, CA) was implanted in the third cerebral ventricle (midline, −0.3 anterior-posterior axis, 3 mm ventral, 0 point bregma). The cannula was fixed to the calvaria with cyanoacrylate and connected to an osmotic pump (Alza) placed on the dorsal side of the animal. After surgery, mice were transferred to individual cages with free access to food and water. ICV infusion of CART (55–102, rat; Bachem, Torrance, CA) or vehicle (PBS) was performed in Cart−/− mice at rate of 0.25 μl/h (1 and 3 ng/h) for 28 d.
Construction of CART recombinant adenovirus (AV)
The 1.7-kb genomic fragment-encoding mouse Cart was cloned between the cytomegalovirus immediate-early promoter and an IRES-green fluorescent protein (GFP)-polyadenylation cassette and ligated into pShuttle vector (CLONTECH, Palo Alto, CA) for the generation of adenoviruses expressing recombinant CART. Expression of this construct was checked by GFP visualization in infected HEK293 cells. After 48 h of infection, cells were lysed, and protein was prepared for Western blot analysis using a CART-specific antibody (RK-003-62; Phoenix Pharmaceuticals Inc., Belmont, CA). The recombinant vector (AV-mCART-eGFP) and control vector (AV-eGFP) were packaged, purified, and tittered (1.8 × 1010 pfu/ml) using standard protocol by the Baylor Vector Development Laboratory (http://vector. bcm.tmc.edu). For in vivo GFP visualization, brains were cryopreserved in 20% sucrose (wt/vol) in 1× PBS and cryoembedded. Thirty-micrometer sections were cut, mounted, and visualized under the microscope (DM 4000 B; Leica Biosystems, St. Louis, MO).
Cell culture experiments
Osteoblasts from calvaria of newborn WT (n = 8), α1(I)collagen-Cart (n = 7), and Cart−/− (n = 6) pups were isolated according to a previously described protocol by serial digestion in digestion medium (α-MEM medium supplemented with 0.1 mg/ml collagenase P and 2.5% trypsin) at 37 C (25). Cells were plated at a density of 12,000 cells/cm2 in α-MEM/10% fetal bovine serum for 2 d. After 2 d medium was changed to differentiation medium (α-MEM/10% fetal bovine serum supplemented with 5 mm β-glycerophosphate and 100 μg/ml ascorbic acid) changed every 2 d. For growth curve analyses, osteoblasts were grown in nondifferentiating conditions (no ascorbic acid) and were counted manually and by crystal violet staining. In addition to growth curve, cell viability in these cultured osteoblasts was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For MTT assay, cells were washed three times with 1× PBS and then incubated with MTT solution (0.5 mg/ml in PBS) at 37 C for 3 h. Thereafter the MTT solution was removed and cells were lysed with dimethyl sulfoxide. The OD was measured at 595 nm wavelength. Osteoblast-specific gene expression for Rankl and Opg (osteoprotegrin) expression was analyzed by qPCR using cDNA from differentiated osteoblast treated with WT, α1(I)collagen-Cart, and Cart−/− serum for 4 h using primers from SuperArray and the Taq SYBR Green Supermix with ROX on an MX3000 instrument.
Biochemistry
Blood samples were collected from 6-month-old female WT and α1(I)collagen-Cart mice by cardiac puncture under isoflurane anesthesia, kept on ice for 5 min, and centrifuged at 3000 rpm for 10 min at 4 C. Serum samples were stored at −80 C until hormonal analyses were performed. Serum levels of CART were measured by RIA (RK-003–62; Phoenix Pharmaceuticals). Measurements of urine deoxypyridinoline (Dpd) cross-links levels were done using the Merta Dpd kit (Quidel Corp. San Diego, CA).
Statistical analyses
Statistical significance was assessed by Student’s two-tailed t test. Values were considered statistically significant at P < 0.05.
Results
ICV infusion of CART does not rescue Cart−/− low bone mass phenotype
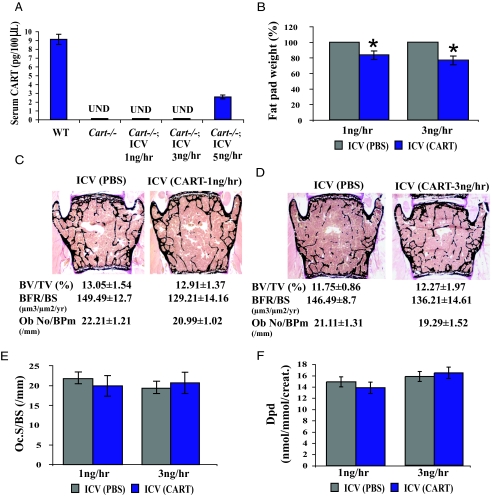
Given the large body of data suggesting that CART is a neuropeptide, we first decided to rescue the low bone mass phenotype of the Cart−/− mice by a long-term ICV infusion of recombinant CART. Before initiation of the experiment, we performed short-term (48 h) ICV infusions of various doses of CART in Cart−/− mice and measured CART serum levels. The goal of this preliminary experiment was to determine the highest possible amount of CART one could infuse ICV without observing any leakage into the general circulation. As shown in Fig. 1A, infusion of CART at either 1 or 3 ng/h did not result in the presence of CART in the general circulation of Cart−/− mice, indicating that we could use these two infusion rates to determine the effect of CART as a neuromediator on bone mass.
Figure 1.
Recombinant CART ICV infusion does not affect bone-remodeling parameters in Cart−/− mice. A, CART ICV infusion at either 1 or 3 ng/h in female Cart−/− mice does not increase serum CART level [undetectable (UND)]. B, Significantly decreased fat pad weight in female Cart−/− mice infused ICV with recombinant CART. C and D, Histological analysis of vertebrae of 6-month-old female Cart−/− mice infused ICV with vehicle (PBS) or CART (1 or 3 ng/h, respectively). Neither 1 nor 3 ng/h CART infusion affects bone mass (BV/TV), BFR, or osteoblast number (N.Ob/BPm), compared with PBS infusion. E and F, Bone resorption parameters (osteoclast surface (Oc.S/BS) and urinary Dpd elimination) are also unaffected by the CART infusions (n ≥ 6/group). *, P < 0.05. Error bars, sd.
Next, we performed ICV infusions of CART either at 1 or 3 ng/hr in 5-month-old Cart−/− female mice for 28 d. We chose this length of treatment because we had shown earlier that ICV infusion of leptin affected bone mass over a 28-d period (26) and 5-month-old mice because Cart−/− mice bone phenotype is best observed in 6-month-old animals (7). This long-term central administration of CART significantly decreased fat pad weight, indicating that recombinant CART was active in vivo and that the infusions were effective (Fig. 1B) (17,18). Surprisingly however, long-term CART ICV infusion, whether it was at a rate of 1 or 3 ng/h, did not decrease the number of osteoclasts nor did it increase bone mass in Cart−/− mice (Fig. 1, C–E). Additionally CART ICV infusion did not affect bone formation parameters (Fig. 1, C and D). Accordingly, there was no significant decrease in urinary elimination of deoxypyridinoline, a collagen degradation byproduct and a biomarker of osteoclast activity (27), in CART-infused Cart−/− mice (Fig. 1F). Taken together these results indicate that, in the conditions of this assay, CART ICV infusion can affect fat pad weight but not bone remodeling parameters.
ICV infusion of AV-mCART-eGFP does not rescue Cart−/− low bone mass phenotype
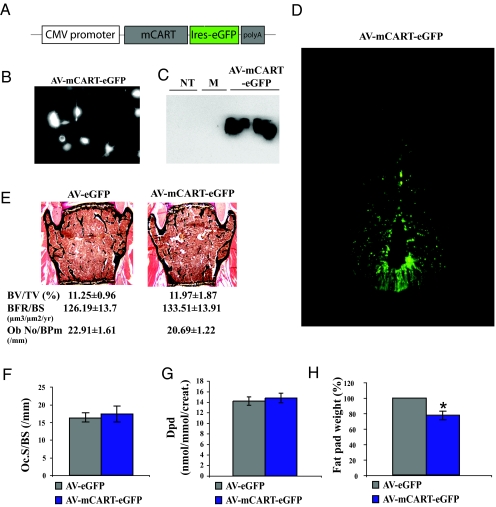
Recombinant adenovirus has been used to introduce genes of interest into various organs including the brain (28,29,30,31,32,33,34,35). Given the lack of effect of recombinant CART, we took advantage of this technique to try to rescue the low bone mass phenotype of Cart−/− mice. The bioactivity of the adenoviral construct (AV-mCART-eGFP) was first demonstrated by GFP visualization in infected HEK293 cells (Fig. 2, A and B). In addition, cells were lysed and protein extracts were analyzed by Western blot analysis using a CART-specific antibody. As shown in Fig. 2C, CART protein was detected in AV-mCART-eGFP-infected HEK293 cells but not mock-infected cells.
Figure 2.
AV-mediated overexpression of CART in the hypothalamic neurons does not affect bone-remodeling parameters in Cart−/− mice. A, Schematic representation of the recombinant AV vector expressing mouse Cart. B, GFP expression in HEK293 cells infected with AV-mCART-eGFP. C, Western blot analysis using specific CART antibody, showing CART protein expression in AV-mCART-eGFP-infected HEK293 cells. NT, No infection; M, mock infection. D, Expression of AV-mCART-eGFP in hypothalamic neurons of Cart−/− mice after 10 d of ICV infusion. E, Histological analysis of vertebrae of 6-month-old Cart−/− mice infused ICV with AV-eGFP or AV-mCART-eGFP. Expression of AV-mCART-eGFP did not affect bone mass (BV/TV) or bone formation parameters [BFR and osteoblast number (N.Ob/BPm)]. F and G, Bone resorption parameters [osteoclast surface (Oc.S/BS) and Dpd] are not affected either. H, Significant decrease in the fat pad weight of AV-mCART-eGFP infused Cart−/− mice (n ≥ 6/group). *, P < 0.05. Error bars, sd.
Next, approximately 9.6 × 109 particles of AV-eGFP or AV-mCART-eGFP were infused ICV for 10 d in 5-month-old Cart−/− mouse (n = 6). Four weeks later, animals were killed for analysis. GFP staining was present in ICV infused animals verifying that CART was indeed delivered in hypothalamic neurons (Fig. 2D). Surprisingly, this long-term overexpression of CART in hypothalamic neurons again failed to correct the bone resorption phenotype of the Cart−/− mice (Fig. 2, E–G). However, it resulted in a significant decrease in fat pad weight, compared with control mice, indicating that ICV infusion of AV-mCART-eGFP was effective on one function controlled by CART (Fig. 2H) (17,18).
Taken together, this adenoviral experiment and the ICV delivery of recombinant CART described above suggest that long-term CART delivery in the hypothalamic neurons of Cart−/− mice has no effect on bone remodeling parameters and cannot rescue the high bone resorption/low bone mass phenotype of the Cart−/− mice.
Cart is expressed outside the central nervous system
The absence of correction of the Cart−/− mice low bone mass phenotype by central administration of CART was unexpected. Together with the presence of a significant amount of CART in the general circulation (9), it raised the hypothesis that CART could regulate bone mass as a circulating molecule rather than as a neuromediator.
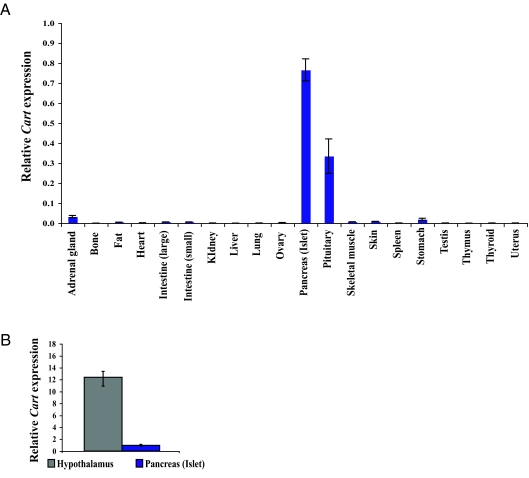
As an initial step to test this hypothesis we checked the level of expression of Cart outside the brain. As shown in Fig. 3A, Cart expression could be detected only in pituitary gland and pancreatic islets but not other peripheral tissues (n = 6). The level of expression of Cart in these tissues was approximately 10 times lower than in hypothalamus (Fig. 3B). Nevertheless, the presence of CART in at least two peripheral tissues as well as blood suggested that CART could regulate bone mass as a circulating molecule.
Figure 3.
Expression of Cart in peripheral tissues by qPCR. A, Cart expression is detected only in pituitary and pancreatic islets. B, Cart expression is about 10-fold less in pancreatic islets, compared with hypothalamus (n ≥ 6/group).
Generation of transgenic mice with increased levels of circulating CART
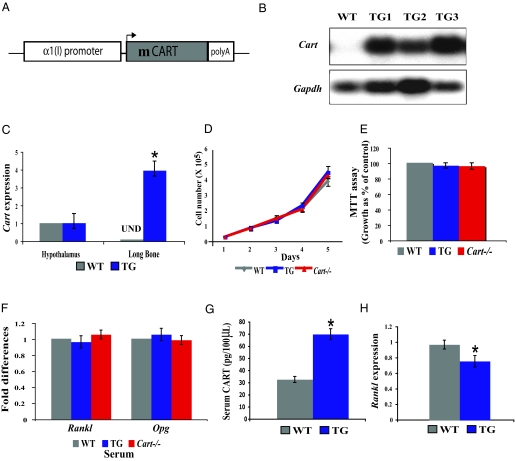
To determine whether CART could regulate bone resorption as a circulating molecule, we analyzed mice in which CART serum levels are increased. Transgenic mice overexpressing mouse Cart under the control of an osteoblast-specific 2.3-kb-long α1(I)collagen promoter fragment were generated (Fig. 4A). Three independent lines that express Cart at a high level in bone (Fig. 4B) were obtained. qPCR analysis showed that Cart is highly expressed in transgenic bone and osteoblasts but not overexpressed in hypothalamus or other peripheral tissues (Fig. 4C and data not shown). This ectopic expression of Cart in osteoblasts did not affect their proliferation or differentiation in vitro (Fig. 4, D and E, and data not shown). Likewise, Rankl and Opg expression was not affected in primary osteoblast culture grown in presence of serum obtained from α1(I)collagen-Cart transgenic or Cart−/− mice (Fig. 4F). These results are consistent with our earlier study that failed to detect any effect of exogenous CART on osteoblasts (7). In contrast, overexpression of Cart in osteoblasts resulted in a 2-fold increase in circulating levels of CART indicating that the α1(I)collagen-Cart transgenic mice are indeed a model of sustained increase in circulating CART level (Fig. 4G).
Figure 4.
Peripheral overexpression of CART does not affect proliferation and differentiation of osteoblasts. A, Schematic representation of the α1(I)collagen-Cart transgene (TG). B, Northern blot analysis showing Cart transgene overexpression in bones of three transgenic lines TG1, TG2, and TG3. C, qPCR expression analysis showing ectopic Cart expression in bone but no increase in hypothalamic Cart expression in the female α1(I)collagen-Cart transgenic mice [undetectable (UND)]. D, Growth curve of WT, α1(I)collagen-Cart, and Cart−/− osteoblasts over 5 d. E, MTT assay showing normal viability of α1(I)collagen-Cart and Cart−/− primary osteoblasts, compared with WT control. F, Rankl and Opg expression is not affected in WT primary osteoblasts treated with WT, α1(I)collagen-Cart, or Cart−/− serum for 4 h after 10 d of differentiation. G, Serum CART level is 2-fold increased in α1(I)collagen-Cart transgenic mice. H, Decreased Rankl expression in α1(I)collagen-Cart transgenic bones. (n ≥ 6/group). *, P < 0.05. Error bars, sd.
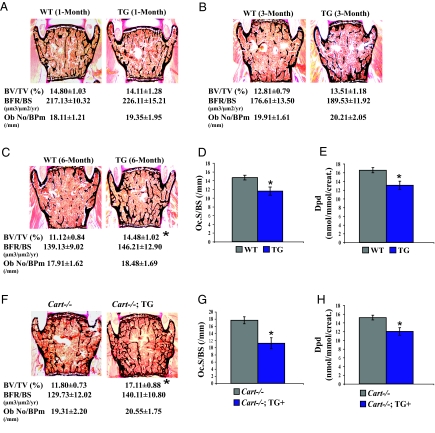
High bone mass in α1(I)collagen-Cart mice and rescue of the Cart−/− low bone mass phenotype
WT and α1(I)collagen-Cart female (hemizygous) mice (n = 8) were analyzed by bone histology and histomorphometry means at 1, 3, and 6 months of age. Whereas α1(I)collagen-Cart and WT mice had similar bone mass at 1 and 3 months of age, 6-month-old α1(I)collagen-Cart transgenic mice showed significant increase in bone mass (Fig. 5, A–C). This increase in bone mass was secondary to a decrease in bone resorption, as measured by osteoclast surface and Dpd elimination, whereas bone formation parameters were not affected (Fig. 5, C–E).
Figure 5.
High bone mass phenotype in α1(I)collagen-Cart transgenic mice and rescue of the low bone mass defect of Cart−/− mice by the α1(I)collagen-Cart transgene. A and B, Normal bone mass (BV/TV) and bone formation parameters [BFR and osteoblast number over bone perimeter (Ob No/BPm)] in 1- and 3-month-old female α1(I)collagen-Cart transgenic mice (hemizygous). C, Increased bone mass (BV/TV) in 6-month-old female α1(I)collagen-Cart mice in presence of normal bone formation parameters (BFR and Ob No/BPm). D and E, Decreased osteoclast surface (Oc.S/BS) and urinary elimination of Dpd in 6-month-old female α1(I)collagen-Cart mice. F, Increased bone mass in 6-month-old female Cart mutant mice harboring the α1(I)collagen-Cart transgene (hemizygous) without modification in bone formation parameters (BFR and Ob No/BPm). G and H, Decreased osteoclast surface (Oc.S/BS) and urinary Dpd elimination in female Cart mutant mice harboring α1(I)collagen-Cart transgene (hemizygous) (n ≥ 6/group). *, P < 0.05. Error bars, sd.
Next we asked whether increased circulating levels of CART in α1(I)collagen-Cart mice could rescue the high bone resorption/low bone mass phenotype of the Cart−/− mice, something that central delivery of CART could not achieve. As shown in Fig. 5F, 6-month-old Cart−/− female mice harboring the α1(I)collagen-Cart transgene (hemizygous) had a significantly higher bone mass than Cart−/− female mice. This increase in bone mass was due to a significant decrease in osteoclast number, whereas bone formation parameters were unaffected (Fig. 5, F and G). Taken together these results show that unlike central overexpression, a moderate peripheral overexpression of Cart can increase bone mass by decreasing bone resorption and thereby can rescue the bone phenotype of Cart−/− mice.
Discussion
The endocrine and neuroendocrine regulation of bone mass is a rapidly evolving field in which new players are constantly identified through genetic means. Among these one can cite FSH, TSH, leptin, ligands of the YY receptor, and neuromedin U. As a result, the boundary, if it exists, between endocrine, neuroendocrine, and neural regulation of bone mass is becoming harder to define (2,3,4,5,6,7,8,9,10,36).
The regulation of bone mass under the control of leptin has been extensively studied (5,6,7,8). Chemical lesioning experiments have identified neurons from the ventromedial hypothalamic nuclei as being necessary for this function of leptin, whereas genetic studies showed that the sympathetic nervous system and CART, a molecule synthesized by neurons of the arcuate hypothalamic nuclei, are regulating bone formation and bone resorption under the control of leptin by acting on osteoblasts (5,6,7,8). Still many questions remain unanswered. For instance, how can we explain that ventromedial hypothalamus neurons are necessary for leptin regulation of bone mass, whereas expression of the leptin receptor on these neurons is apparently not (6,37)? What is the nature of the CART receptor? Where is it expressed? Is CART acting centrally?
Of all the genes whose expression is regulated directly or indirectly by leptin, Cart has been one of the least studied. Aside from its role in bone resorption (7,9), we also know that mice lacking Cart have a normal appetite and are fertile (38,39), two features that are inconsistent with CART being an important effector of leptin in the control of appetite and reproduction. This does not exclude that CART may be involved in energy metabolism as shown by others and by us in this study (17,18). As mentioned above, the signaling pathways triggered by CART or the identity of its receptor are not known. Interestingly, CART is made in not only the brain but also two peripheral organs, the pituitary gland and pancreatic islets (11,12,38). This pattern of expression raises the prospect that CART may not be or may not be acting only as a neuromediator.
In this study, we show that the regulation of fat mass, which was used as an internal control, occurs through a central relay, whereas CART regulation of bone mass does not appear to require this relay. We are aware, however, that in our experiments we affected only a part of all the neurons that could regulate bone mass. For this latter function, it rather appears that CART act as a circulating molecule. The possibility exist that it acts locally on bone cells, although in vitro or in coculture experiments, we failed to see an effect of CART on bone cells (7). Indeed a 2-fold increase in the circulating levels of CART can rescue the bone phenotype of the Cart−/− mice as well as induce a decrease in bone resorption in mice of a WT genetic background. However, as shown in this study as well as our previous study, CART does not appear to affect gene expression in osteoblasts through a direct mechanism (7). The question that remains is how does circulating CART regulate bone resorption if it does not act directly on osteoblasts or osteoclasts? One possibility we cannot rule out at the present time is that CART action on bone cells is below the level of detection in a cell culture assay. A second possibility is that CART acts on neurons in the brain but outside the hypothalamus. A third possibility is that CART regulates the expression of another hormone or cytokine that itself would be regulating bone resorption. To answer these critical questions, we will need to identify the CART receptor and precisely define its pattern of expression.
Acknowledgments
We are thankful to Dr. P. Ducy for critical reading of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant DK58883 (to G.K.) and a sponsored research agreement from Organon. F.E. was supported during these studies by a fellowship of the Children’s Nutrition Research Center (Baylor College of Medicine, Houston, Texas).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: AV, Adenovirus; BFR, bone formation rate; BV/TV, bone volume to tissue volume; CART, cocaine- and amphetamine-regulated transcript; Dpd, deoxypyridinoline; GFP, green fluorescent protein; ICV, intracerebroventricular; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Opg, osteoprotegrin; qPCR, quantitative PCR; WT, wild type.
References
- Karsenty G 2006 Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348 [DOI] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 21:125:247–260 [DOI] [PubMed] [Google Scholar]
- Sun L, Davies TF, Blair HC, Abe E, Zaidi M 2006 TSH and bone loss. Ann NY Acad Sci 1068:309–318 [DOI] [PubMed] [Google Scholar]
- Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M 2003 TSH is a negative regulator of skeletal remodeling. Cell 17:115:151–162 [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G 2000 Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G 2002 Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G 2005 Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G 2005 The molecular clock mediates leptin-regulated bone formation. Cell 122:803–815 [DOI] [PubMed] [Google Scholar]
- Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G 2006 Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4-receptor deficiency. Endocrinology 147:3196–3202 [DOI] [PubMed] [Google Scholar]
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H 2002 Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ 1997 Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol 9:823–833 [DOI] [PubMed] [Google Scholar]
- Wierup N, Sundler F 2006 CART is a novel islet regulatory peptide. Peptides 27:2031–2036 [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P 1995 PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci 2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J, Daoud S 1996 Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene 169:241–245 [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P 2002 Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport 13:1215–1218 [DOI] [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S 1998 Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76 [DOI] [PubMed] [Google Scholar]
- Qing K, Chen Y 2007 Central CART gene delivery by recombinant AAV vector attenuates body weight gain in diet-induced-obese rats. Regul Pept 140:21–26 [DOI] [PubMed] [Google Scholar]
- Adams LD, Gong W, Vechia SD, Hunter RG, Kuhar MJ 1999 CART: from gene to function. Brain Res 848:137–140 [DOI] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G 2002 Mouse α1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn 224:245–251 [DOI] [PubMed] [Google Scholar]
- Chappard D, Palle S, Alexandre C, Vico L, Riffat G 1987 Bone embedding in pure methyl methacrylate at low temperature preserves enzyme activities. Acta Histochem 81:183–190 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1988 Bone histomorphometry: standardization of nomenclature, symbols and units. Summary of proposed system. Bone Miner 4:1–5 [PubMed] [Google Scholar]
- Suda T, Jimi E, Nakamura I, Takahashi N 1997 Role of 1α, 25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol 282:223–235 [DOI] [PubMed] [Google Scholar]
- Baron RVA, Neff L, Silverglate A, Maria AS 1983 Processing of undecalcified bone specimens for bone histomorphometry. Boca Raton, FL: CRC Press [Google Scholar]
- Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G 1999 A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G 2004 Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 101:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Dickson IR, Van Ness K 1988 Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J 252:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Della-Fera MA, Li C, Hartzell DL, Little DE, Kuhar MJ, Baile CA 2004 CART peptide: central mediator of leptin-induced adipose tissue apoptosis? Regul Pept 121:155–162 [DOI] [PubMed] [Google Scholar]
- Muzzin P, Cusin I, Charnay Y, Rohner-Jeanrenaud F 2000 Single intracerebroventricular bolus injection of a recombinant adenovirus expressing leptin results in reduction of food intake and body weight in both lean and obese Zucker fa/fa rats. Regul Pept 92:57–64 [DOI] [PubMed] [Google Scholar]
- Bagnasco M, Dube MG, Kalra PS, Kalra SP 2002 Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology 143:4409–4421 [DOI] [PubMed] [Google Scholar]
- Dube MG, Beretta E, Dhillon H, Ueno N, Kalra PS, Kalra SP 2002 Central leptin gene therapy blocks high-fat diet-induced weight gain, hyperleptinemia, and hyperinsulinemia: increase in serum ghrelin levels. Diabetes 51:1729–1736 [DOI] [PubMed] [Google Scholar]
- Kalra PS, Kalra SP 2002 Obesity and metabolic syndrome: long-term benefits of central leptin gene therapy. Drugs Today (Barc) 38:745–757 [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR 2005 AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem Biophys Res Commun 327:1088–1093 [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, Berns KI, Raisler BJ, Hauswirth WW, Campochiaro PA 1994 AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci 43:1994–2000 [PubMed] [Google Scholar]
- Prasad KM, Yang Z, Bleich D, Nadler JL 2000 Adeno-associated virus vector mediated gene transfer to pancreatic β cells. Gene Ther 7:1553–1561 [DOI] [PubMed] [Google Scholar]
- Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S 2007 Central control of bone remodeling by neuromedin U. Nat Med 13:1234–1240 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahrén B, Sundler F 2005 CART knock out mice have impaired insulin secretion and glucose intolerance, altered β cell morphology and increased body weight. Regul Pept 129:203–211 [DOI] [PubMed] [Google Scholar]
- Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Köster A 2001 Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142:4394–4400 [DOI] [PubMed] [Google Scholar]