Abstract
The objective of this study was to identify genes regulated by thyroid hormone (T3) and associated with tumor invasion. The gene encoding furin, as previously identified by cDNA microarray, is known to be up-regulated by T3 treatment, and stimulated furin production occurs in thyroidectomized rats after administration of T3. Presently, by using serial deletion of the promoter and EMSAs, the T3 response element on the furin promoter was localized to the −6317/−6302 region. T3-mediated furin up-regulation was cooperative with TGF-β because T3 induction increased after Smad3/4 addition. Furthermore, the invasiveness of HepG2-thyroid hormone receptor (TR) cells was significantly increased by T3 treatment, perhaps due to furin processing of matrix metalloproteinase-2 and -9. In addition, furin up-regulation either by stable overexpression or T3 and/or TGF-β induction was evident in severe-combined immune-deficient mice inoculated with HepG2-TRα1 cells. The HepG2-furin mice displayed a higher metastasis index and tumor size than HepG2-neo mice. Notably, the increased liver and lung tumor number or size in the hyperthyroid severe-combined immune-deficient mice as well as TGF-β mice was attributed specifically to furin overexpression in the HepG2-TRα1 cells. Furthermore, this study demonstrated that furin overexpression in some types of hepatocellular carcinomas is TR dependent and might play a crucial role in the development of hepatocellular carcinoma. Thus, T3 regulates furin gene expression via a novel mechanism or in cooperation with TGF-β to enhance tumor metastasis in vitro and in vivo.
THYROID HORMONE (TH) receptors (TRs) regulate cellular growth, development, and metabolic homeostasis by binding to thyroid hormone response elements (TREs) in the regulatory regions of target genes, both in the presence and absence of their cognate ligands T3 or T4 (1). Ligand-bound TRs tend to activate target gene expression, whereas expression is repressed by nonligand-bound TRs.
Two TR genes, TRα and TRβ, have been identified and mapped to human chromosomes 17 and 3, respectively (2,3). Each gene encodes at least two TR isoforms (TRα1 and 2, and TRβ1 and 2) generated by alternative splicing and alternative promoter usage, respectively (4). The liver is a typical target organ for THs. Equal amounts of TRα1 and TRβ1 proteins are expressed in human hepatocytes (5). However, the mechanisms involved in the regulation of liver-specific genes by TRα1 and TRβ1 have not been clearly elucidated. Normal human hepatocytes are difficult to maintain in vitro and quickly become senescent after isolation. Therefore, hepatocytes derived from hepatocarcinoma are most typically studied. As a well-differentiated hepatocellular carcinoma (HCC) cell line, HepG2 has been characterized and shown to secrete all 15 known liver-specific plasma proteins (6). Therefore, HepG2 cells are widely used as a model for studying hepatocyte physiology and liver-specific gene regulation. These cells have been the genesis of isogenic cell lines expressing high levels of wild-type (wt) TRα1 and β1 (HepG2-TRα1 and -TRβ1 cells) (7). These lines are useful tools for investigating target genes of THs.
Using cDNA microarrays, we have previously identified 148 genes positively regulated by T3 in a TRα1 overexpressed hepatoma cell line (HepG2-TRα1) (8,9,10,11). These genes include fibrinogen (8), fibronectin (9), transferrin (10), SULT2A1 (11), and several other coagulation factor system components. Regulation of these genes by TR in the HepG2-TRα1 cells has been confirmed by real-time PCR and promoter assays in vitro, and has also been observed in thyroidectomized (Tx) rats (8). Therefore, to clarify the mechanisms of TR-mediated transcriptional regulation of hepatocyte genes, additional study is needed to determine the regulatory effect of TR on more of these 148 genes. The present study elucidates the transcriptional regulation of the furin gene by TR.
Furin, a member of the protease family of pro-protein convertases (PCs), activates precursor proteins via cleavage at the specific carboxy terminus of a sequence of basic paired amino acids, RXKyRR (12). PCs are expressed ubiquitously, and are involved in numerous physiological and pathological processes (12,13). PC-mediated processing of latent precursor proteins into their biologically active products is a vital mechanism in many important biological functions, including activation of matrix metalloproteinase (MMP), a critical factor in tumor malignancy. Moreover, numerous proteins produced in the liver contain a furin-specific cleavage site such as platelet-derived growth factor, IGF-I, and oncoprotein c-Met, all of which are involved in liver regeneration (14). Therefore, the liver is an important target organ for furin function. In addition, furin is overexpressed in lung and breast malignant tumors (15,16,17), as well as in head and neck tumors (18).
The present results indicate that T3 up-regulates furin gene expression in HepG2-TRα1 and -TRβ1 cells. This T3-mediated induction in TRα1 and β1 overexpressing cells is observed at both mRNA and protein levels. Similar findings were obtained in Tx rats after administration of T3. The results most likely indicate that T3 cooperates with TGF-β signaling and its components, Smad3/4, in controlling furin expression. Finally, the possible physiological function of such regulation is elucidated.
Materials and Methods
Cell culture
The human hepatoma cell lines HepG2, Huh7, Mahlavu, J7, and CV-1 (African green monkey kidney fibroblast) were routinely grown in DMEM supplemented with 10% (vol/vol) fetal bovine serum. The HepG2 cell lines used in this study were stably transfected with TRα1 (HepG2-TRα1#1, HepG2-TRα1#2), TRβ1 (HepG2-TRβ1), and the control cell line (HepG2-Neo) (7). HepG2-furin indicates the HepG2 cells stably expressing furin. Serum was depleted of T3 (Td) as described previously (19). Cells were cultured at 37 C in a humidified atmosphere of 95% air and 5% CO2.
Immunoblotting
Total cell lysates, including nuclear and cytosolic components, were fractionated by 10% SDS-PAGE. The separated proteins were then transferred to a nitrocellulose membrane (Amersham Biosciences Inc., Piscataway, NJ) as described previously (11).
Northern blotting
Total RNA was extracted from cells using TRIzol reagent (Invitrogen Corp., Carlsbad, CA). Equal amounts of total RNA (20 μg) were examined on a 1.2% agarose-formaldehyde gel as described previously (20). The gel was then blotted onto a nitrocellulose membrane and subjected to Northern blot analysis as described previously (11).
Effect of exogenous Smad on furin expression
HepG2-TRα1#1 cells (2 × 105 per 60-mm diameter dish) were transfected with wt or dominant-negative (dn) Smad expression plasmid using Lipofectamine (Life Technologies, Inc., Gaithersburg, MD). Cells were lysed, and Western blot analysis was performed 24 h after transfection.
Cloning the furin promoter fragments and assay of their activities
Fragments of the furin promoter (P1A) (21) were inserted into the pA3TK vector (Promega Corp., Madison, WI). Serial deletion mutants of the promoter were PCR amplified. Promoter construct sequences were confirmed via automated DNA sequencing. To determine the transactivation activity of TREs on the furin promoter, HepG2-TRα1#1 cells (5 × 104 cells per 12-well dish) were transfected with 0.66 μg DNA of pA3TK vector containing furin promoter sequences and Lipofectamine. Cells were also transfected with 0.33 μg of the pSVβ plasmid (Promega, Madison, WI), which is a β-galactosidase expression vector. Twenty-four hours after transfection, some cells received 10 nm T3; these and untreated cells were incubated for a further 24 h and then lysed to measure luciferase and β-galactosidase activities (22). The activity of luciferase was assayed by a luminometer (LMax II 384; Molecular Devices, Sunnyvale, CA) and normalized against β-galactosidase activity.
EMSA
The wt furin gene probe, which encompassed −6328 to −6288 of the P1A promoter, was used for EMSA. The normal sequence 5′-TCATAAACGATA-(AAAAGA)cccg(ACTGGA)-AAGAAGATCCGG-3′ and mutant sequence 5′-TCATAAACGATA-(GGGGGG)cccg(GGGGGG)-AAGAAGATCCGG-3′ were annealed with their partially complementary sequences (data not shown) and were fill-in labeled with [α-32P]deoxycytidine triphosphate by Klenow reaction after gel purification. For each EMSA, an equal amount of TNT Quick (Promega) in vitro-translated TRs or retinoid X receptor (RXR) α-proteins were incubated with α-32P-labeled furin gene fragments. EMSAs used C4 mouse monoclonal antibody to TR (23) and, as a negative control, MOPC21 mouse monoclonal antibody (ICN Biomedicals, Aurora, OH). Protein-oligonucleotide complexes were detected by SDS-PAGE and autoradiography as described previously (7).
Animals
There were 20 male Sprague Dawley rats that underwent thyroidectomies, and 10 additional rats were sham operated at 6 wk of age as previously described (8). Briefly, 2 wk after thyroidectomies, each rat was injected peritoneally with T3 at 10 μg/100 g body weight or a control vehicle (2.5 mm NaOH in PBS) daily for an additional 2 wk.
Alternatively, to investigate whether T3 and/or TGF-β induced furin expression was evident in vivo and its physiological consequence(s), severe combined immunodeficiency (SCID) mice were segregated into five groups, each of which consisted of six animals (See Fig. 11F). Group A consisted of animals that were given PBS orally for 7 wk, then were inoculated iv with 1 × 107 HepG2-TRα1 tumor cells. To induce hypothyroid SCID mice, propylthiouracil (PTU) was given orally (24). Group B consisted of animals that orally received 0.4 mg PTU/150 g body weight (Sigma-Aldrich, St. Louis, MO) for 7 wk before inoculation with HepG2-TRα1 cells, and that continued to receive PTU for the 7 wk after inoculation. Group C consisted of six animals that had been given PTU for 4 wk and a 3-wk simultaneous administration of PTU/T3 (10 μg/100 g) before inoculation with HepG2-TRα1 cells, and that continuously received PTU/T3 for an additional 7 wk after inoculation. Group D consisted of six animals that had been given PTU for 4 wk and PTU/TGF-β (1 μg/mouse) simultaneously for an additional 3 wk before inoculation with HepG2-TRα1 cells, and that continuously received PTU/TGF-β for an additional 7 wk after inoculation. Finally, Group E consisted of six animals that received PTU for 4 wk, followed by PTU/T3/TGF-β for an additional 3 wk before inoculation with HepG2-TRα1 cells, and that continued receiving PTU/T3/TGF-β throughout the experiment.
Figure 11.
Furin overexpression induced by T3 or TGF-β enhances HepG2-TRα1 cell invasion in SCID mice. SCID mice were divided into five groups (n = 6 per group): group A (control, panels A1–A6); group B (PTU, panels B1–B6); group C (PTU plus T3, panels C1–C6); group D (PTU plus TGF-β, panels D1–D6); and group E (PTU plus T3 plus TGF-β, panels E1–E6). All SCID mice were iv inoculated with 1 × 107 HepG2-TRα cells. At wk 7, after tumor (T) cell injection, grossly visible metastases were present on the surface of the organ. Livers (panels A1–E1 to A3–E3) or lungs (panels A4–E4 to A6–E6) were fixed in Bouin fixative and examined under a low-magnification microscope for tumor foci on their surfaces. H&E stained sections from the liver or lungs were examined histologically. Staining of metastasized HepG2-TRα1 cells in liver (panels A1–E1 to A2–E2) or lung sections (A4–E4 to A5–E5). Panels A2–E2 and A5–E5 are the higher magnification (×200) of liver or lung sections from the corresponding white squares shown in panels A1–E1 or A4–E4 (×100). The tumor region is circled by the dotted line (A1–E1 to A3–E3) or indicated by the arrowhead (A4–E4 to A6–E6). Panels A3–E3 and A6–E6 show furin expression detected by immunohistochemistry. The experimental protocol is shown in F. Each block (▩) in F indicates 1 wk. Average of the metastasis index (G) (fold increase, density of tumor numbers in group C, D, E/group A per cm2 area) or relative tumor size (H) (fold-increase, average of tumor size in group C, D, E/group A per cm2 area) in liver or lung is shown. Scale bar in panels is 200 μm. Tumor is indicated by arrowheads. Student’s t test. *, P < 0.05; **, P < 0.01. The statistical significance is being compared with PTU or control mice. N, Normal.
SCID mice were used to determine the invasive ability of the furin overexpressing HepG2 cells. HepG2-furin and HepG2-neo cells (each 1 × 107) were injected iv. All animals were killed at wk 7 after tumor inoculation, whereupon the liver and lungs were removed. All procedures were performed under sterile conditions in a laminar flow hood. All animal experiments were performed in accordance with U.S. National Institutes of Health guidelines, and the Chang-Gung Institutional Animal Care and Use Committee Guide for Care and Use of Laboratory Animals.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues from the liver or lung of SCID mice were examined by immunohistochemistry using polyclonal antibody to furin (Affinity Bioreagents, San Francisco, CA) after the avidin-biotin complex method, as described previously (25). The positive staining consisted of cancer cells with dark brown of furin immunoreactivity.
Quantitative RT-PCR (Q-RT-PCR)
Total RNA was extracted from cells using TRIzol as previously described (26). Subsequently, cDNA was synthesized using the Superscript II kit for RT-PCR (Life Technologies) as described previously. Real-time Q-RT-PCR was conducted in a 15-μl reaction mixture containing 50 nm forward and reverse primers, 1× Syber Green reaction mix (Applied Biosystems, Foster City, CA), and varying quantities of template, as described previously (26). SYBR Green fluorescence was measured with the ABI PRISM 7000 sequence detection system (Applied Biosystems), as described previously (11,26). Rat furin PCR sequences used were: forward primer 5′-TGACAACTCATTCCTGGGATGA-3′ and reverse primer 5′-GCCTCGCTGGTATTTTCAATCT-3′.
Zymography assay for MMP-2 and -9
Cells (3 × 106) were plated in DMEM medium with 10% fetal bovine serum. After 24-h incubation, cells were washed, and incubation was continued in serum-free medium containing T3 or TGF-β. The medium was collected 24 h later and concentrated using an Amicon ultra-4 membrane (Millipore, Billerica, MA) to roughly 500 ng/μl. Forty micrograms of concentrated medium were diluted in 50 mm Tris-HCl (pH 7.4) without a reducing agent and separated by 10% SDS-PAGE in the presence of 1 mg/ml gelatin. After electrophoresis, the gels were washed in 2.5% Triton X-100 for 30 min and incubated for 16 h at 37 C in buffer with 50 mm Tris-HCl (pH 7.4), 200 mm NaCl, and 10 mm CaCl2. Gels were stained with Coomassie brilliant blue R-250, and destained in 40% methanol and 10% acetic acid until clear bands appeared.
In vitro assay of invasive activity
The influence of T3 on the effect of furin-mediated invasive activity of HepG2-TRα1 or HepG2-furin cell lines was assessed with a Transwell rapid in vitro assay as described previously (7). Briefly, cell density was adjusted to 1 × 105 per ml, and 200 μl of this suspension was added to each well coating with Matrigel (BD, Franklin Lakes, NJ) in triplicate. The medium in the upper chamber was serum-free DMEM and that in the lower chamber was supplemented with 10% fetal bovine serum. After incubation for 20 h at 37 C, the number of viable cells that had traversed the filter to the lower chamber was determined.
Human HCC specimens
With informed consent, 64 patients with HCC diagnosed between 2000 and 2003 were consecutively selected for this study. The study protocol was approved by the Medical Ethics and Human Clinical Trial Committee at Chang-Gung Memorial Hospital.
Statistical analysis
Values are expressed as mean ± se of at least three observations. Statistical analysis of data was performed using the Student’s t test. P < 0.05 was considered statistically significant.
Results
Effects of T3 treatment on furin gene expression at both protein and mRNA levels
The four HepG2 cell lines used in this study were HepG2-TRα1#1, HepG2-TRα1#2, HepG2-TRβ1, and HepG2-Neo. The TR protein was overexpressed approximately 11.3-, 7.1-, and 3.3-fold, respectively, compared with the HepG2-Neo control cell line (Fig. 1A). The effect of TRs on furin protein expression was assessed when HepG2 isogenic cell lines were incubated in medium containing various levels of T3 at different time points (Fig. 1, B–E). Two furin proteins (pro-furin, 100 kDa; active furin, 94 kDa) (27) were detected. In HepG2-TRα1#1 cells, furin protein levels were increased approximately 2.3-fold after treatment with 10 nm T3 for 12 h. Expression continued to increase with incubation time (3.5-fold at 24 h and 5.7-fold at 48 h). Surprisingly, increasing the concentration of T3 from 10–100 nm did not significantly enhance the induced production of furin (Fig. 1B), indicating that 10 nm T3 was the saturating concentration. Similar results with lower induction levels were observed using HepG2-TRα1#2 cells (Fig. 1C). In HepG2-TRβ1 cells, furin expression induced by T3 was about 2-fold at both 10 and 100 nm concentrations; this may be due to lower TR expression in these cells (Fig. 1, A and D). Moreover, exposure of control HepG2-Neo cells to 100 nm T3 for 48 h did not significantly affect furin protein expression (Fig. 1E). One interpretation is that the effect of T3 on furin expression in TR overexpressed cells depends on the level of TR proteins in these cells. However, it is also possible that posttranscriptional modification of furin mRNA enhanced translational efficiency induced by T3.
Figure 1.
Additive activation of furin by T3 and TGF-β in HepG2 cell lines analyzed at the protein level. A, Expression of TR protein in four cell lines. The HepG2-TRα1#1 (B), -TRα1#2 (C), -TRβ1 (D), and -Neo (E) cells were also treated with TGF-β (5 ng/ml) in the absence or presence of 10–100 nm T3 for 12, 24, and 48 h. Cell lysates (100 μg protein) were then subjected to immunoblot analysis with polyclonal antibodies against furin (affinity bioreagents). The position of the 100- or 94-kDa furin protein is indicated. The intensities of furin bands in B–E were quantified and normalized to that of actin. Data are means ± se of values from three independent experiments. Values are shown as fold induction of 12-h Td control (T3-depleted medium, 0 nm T3), and differences were analyzed by the Student’s t test. *, P < 0.05; **, P < 0.01. The statistical significance is being compared with the 12-h Td control.
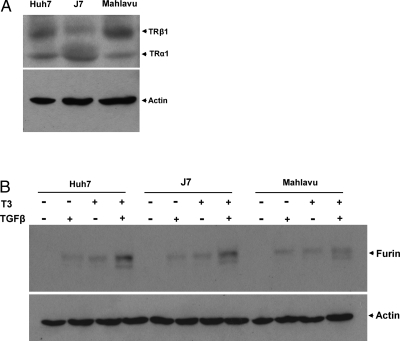
The TGF-β signaling pathway is critical in the regulation of furin expression (28). Thus, it was of interest to determine whether T3 acted additively with TGF-β to regulate furin expression. Cells treated solely with TGF-β (5 ng/ml) induced furin protein expression 2.0, 2.3, and 7.0-fold in HepG2-TRα1#1 cells at 12, 24, and 48 h, respectively (Fig. 1B). Furin expression was further induced 6.4-, 8.7-, and 8.6-fold at 12, 24, and 48 h, respectively, after simultaneous treatment with T3 and TGF-β. A similar induction was observed in HepG2-TRα1 no. 2 and HepG2-TRβ1 cells (Fig. 1, C and D). Notably, in HepG2-Neo cells treated for 48 h, a 1.7-fold furin increase was observed only by both T3 and TGF-β treatment (Fig. 1E). Thus, the data suggest that T3 and TGF-β additively affected furin expression. Similar results were observed in the three other HCC cell lines (Huh7, J7, and Mahlavu), which expressed detectable endogenous TR proteins (Fig. 2A). T3 (10 nm) or TGF-β (5 ng/ml) induced furin protein expression roughly 1.5- to 2-fold in the three cell lines at 24 h (Fig. 2B). Furthermore, furin protein production was additively induced (1.6- to 2.8-fold) in cells simultaneously treated with T3 and TGF-β.
Figure 2.
Induction of furin in three HCC cell lines expressing endogenous TRs. Huh7, J7, and Mahlavu cells, which expressed detectable endogenous TR (A) or furin proteins (B), as determined by Western blot analysis.
To determine whether the same effect of T3 and TGF-β was exerted on furin expression at the mRNA level, Northern blot analysis was used to investigate the treatment with T3 and TGF-β. A 4.5-kb gene transcript was detected in all three cell lines (Fig. 3). Exposure to 10 and 100 nm T3 for 12–48 h significantly (P < 0.01) induced expression of the furin gene in HepG2-TRα1#1, -TRα1#2, and -TRβ1 cells from 2.2- to 5.7-fold (Fig. 3, A–C). Applying TGF-β alone to the three cell lines for 48 h elevated gene expression levels by 5.0, 5.4, and 2.9-fold, respectively. Simultaneous exposure of cells to T3 and TGF-β for 48 h further enhanced gene activity by 11.9-fold (HepG2-TRα1#1), 12.2-fold (HepG2-TRα1#2), and 12.4-fold (HepG2-TRβ1), indicative of T3 and TGF-β additive on furin expression. The effect of T3 or TGF-β alone on the degree of furin gene expression in HepG2-Neo cells was not detected. However, only a 2-fold increase was observed when the cells were treated with both T3 and TGF-β (Fig. 3D). The results imply that the effect of T3 and TGF-β on furin protein expression is mediated, at least in part, at the transcription level.
Figure 3.
Effects of T3 and TGF-β on the furin mRNA level in HepG2 cell lines. HepG2-TRα1#1 (A), HepG2-TRα1#2 (B), HepG2-TRβ1 (C), and HepG2-Neo (D) cell lines were incubated for 12, 24, or 48 h in the absence or presence of T3 (10, 100 nm) and TGF-β (5 ng/ml). Total RNA was isolated and sequentially analyzed (20 μg/lane) by Northern blot with 32P-labeled cDNA probes of the genes encoding furin and 18S ribosomal RNA. The positions of the 4.5-kb furin mRNA and the 1.9-kb 18S ribosomal RNA are indicated. The intensities of the furin mRNA bands shown in A–D were quantified and normalized to that of 18S ribosomal RNA. The extent of the T3- or TGF-β-induced elevation of furin transcripts was plotted against that of T3 and TGF-β free control cells for each time point (shown on right). Values (means ± se) are shown as fold induction of Td control. Differences were examined by the Student’s t test. **, P < 0.01. The statistical significance is being compared with the 12-h Td control.
Stimulation of furin by TGF-β is mediated by the Smad 3/4-dependent pathway
Other studies have shown that TGF-β regulates furin expression (28,29). TGF-β signaling is mediated by phosphorylation and activation of either Smad2 or Smad3, which then allows Smad2/3 to form a heterodimeric complex with Smad4. This complex subsequently translocates into the nucleus, where it acts on target gene promoters (30). A Smad3/4 binding site exists in the furin gene promoter (P1A, −5095 upstream of transcriptional initiation site) (31), making it likely that Smad3 and Smad4 are involved in T3- or TGF-β-induced furin activation in HepG2 cells. Appropriately, we investigated the possible role of Smad3/4 and whether the dn mutants of Smad3 and 4 mediated activation of furin protein. Both wt and dn forms of Smad3 and Smad4 were transiently overexpressed in HepG2-TRα1#1 cells to determine their effect on T3- or TGF-β-induced furin activation. The up-regulated expression of wt Smad3/4 further increased furin expression by 6.41- to 6.72-fold (Fig. 4A, lanes 4 and 7) and 5.07- to 5.12-fold (Fig. 4B, lanes 3 and 5), when treated simultaneously with either T3 (Fig. 4A) or TGF-β (Fig. 4B). However, in the absence of T3, wt Smad3/4 only increased furin expression by 1.21- to 1.69-fold (Fig. 4A, lanes 3 and 6). In addition, dn Smad3 and Smad4 blocked activation of furin by T3 or TGF-β (Fig. 4), suggesting that these components of the TGF-β signaling pathway are involved in T3-mediated transcriptional activation of furin.
Figure 4.
Involvement of Smad3 and Smad4 in T3- and TGF-β-induced furin expression. To elucidate the involvement of Smad proteins in T3- and TGF-β-induced furin expression, dn and wt Smad3 or Smad4 expression vectors were transfected into HepG2-TRα1#1 in the presence or absence of T3 (10 nm) (A) or TGF-β (5 ng/ml) (B). Western blot analysis was subsequently performed to determine the amount of furin protein in these treatment groups. The relative intensity of furin protein in each treatment group is shown under the furin blot.
Modulation of T3 and TGF-β pathways mediate activation of furin expression by MAPKs
Both T3 and TGF-β pathways are critically modulated by MAPKs in the cytoplasm (32). Thus, we were interested to know how these two signaling pathways were modulated when both were involved in the activation of furin expression.
To clarify the modulating effect of MAPKs on furin induction by T3 or TGF-β, several MAPK inhibitors were used in the treatment. Both PD98059 and U0126 are selective inhibitors of MAPK kinase (MEK) 1/2 that targets ERK, SB203580 inhibits Jun N-terminal kinase, and SB202190 inhibits p38 MAPKs. These inhibitors were applied individually to HepG2-TRα1#1 cells for 3 h before adding 10 nm T3. After incubation for an additional 12 or 24 h, the induction of furin protein expression in the absence of inhibitor was significantly induced by both TGF-β (Fig. 5A, lane 3) or T3 (Fig. 5B, lane 3). However, activation of furin expression by TGF-β was blocked by adding either SB203580 or SB202190 (Fig. 5A, lanes 6 and 7), but furin activation by T3 was sensitive to PD98059 and U0126 (Fig. 5B, lanes 4 and 5, 12 or 24 h), and there was no effect to SB203580 and SB202190 (Fig. 5B, lanes 6 and 7, 12 or 24 h). In cells treated with both T3 and TGF-β, only PD98059 and U0126 suppressed activation of furin expression (Fig. 5B, lanes 9 and 10). These findings demonstrate that activation of furin expression by either T3 or TGF-β is modulated by different MAPKs, namely ERK or p38 MAPKs. However, ERK signaling was the principal modulator when both TGF-β and T3 were applied together (Fig. 5B, lanes 9 and 10). These findings also imply that ligand-bound TR might have a more critical role than TGF-β signaling pathway in the activation of furin gene expression because its expression was no longer sensitive to the presence of TGF-β-specific MAPK inhibitors.
Figure 5.
Modulation of T3 and TGF-β pathways mediate activation of furin expression by MAPKs. HepG2-TRα1#1 cells were maintained in Td and treated with MAPK inhibitors PD98059 (10 μm), U0126 (10 μm), SB203580 (15 μm), and SB202190 (15 μm) 3 h before treatment with TGF-β (5 ng/ml) alone (A) or T3 (10 nm) combined with TGF-β (B). After 12 or 24 h hormone treatment, cells were lysed, and their proteins isolated and blotted. DMSO, Dimethyl sulfoxide.
In vivo expression of furin protein induced by T3
To determine the in vivo response of furin gene to T3 treatment, two groups of 6-wk-old male Sprague Dawley rats (n = 10 in each group) were Tx. Subsequently, the first group was injected with T3 daily for 2 wk (Tx plus T3), and a second control group (Tx) received no T3 injections. The third group received sham operations. After 2-wk Tx, the rats were killed, and their serum was collected for analysis of T3 and TSH. The livers were dissected, and protein was extracted for Western blot analysis. The T3 serum levels in the Tx group were approximately 0.022-fold (12.3 vs. 548.1 ng/dl) those in the group that underwent T3 treatment (Tx plus T3). Levels of TSH in the Tx group were approximately 67.2-fold (2.42 vs. 0.036 ng/ml) those in the T3-treated group. The T3 and TSH serum levels in the sham group were approximately 48.1 ng/dl and 0.197 ng/ml, respectively (11). Western blot analysis demonstrated that liver furin protein levels were elevated approximately 7.8-fold in the T3-treated group (Tx plus T3), higher than in the Tx group (Fig. 6A). Furthermore, furin mRNA levels in the Tx plus T3 rat were approximately 22.1-fold higher than that in the Tx group (Fig. 6B). Furin gene expression in the three rat groups was analyzed by agarose gel electrophoresis after Q-RT-PCR. The RNA and protein levels in sham-operated rats were 10.2 and 5.2-fold, respectively, higher than that in the Tx rats (Fig. 6).
Figure 6.
Induction of furin expression by TH in rat liver. A, Expression of furin protein in sham, Tx, or Tx plus T3 male Sprague Dawley rat liver was determined by Western blot analysis. Intensities of furin and actin bands on blots were quantified. B, Expression of furin in Tx, Tx plus T3, and sham male Sprague Dawley rat liver was determined by Q-RT-PCR and analyzed by the agarose gel. Values (means ± se) are shown as fold induction of control (Tx) group, and differences were examined by the Student’s t test. Data are means ± se of values from two independent experiments (n = 10 rats per group). **, P < 0.01.
T3 induces furin expression at the transcriptional level
CV-1 cells were chosen because of their high transfection efficiency and because many TR-regulated mechanisms have been well characterized using this cell line (33,34,35). The expression of the furin gene is reportedly regulated by a region −6408 to −4531 bp upstream to its transcriptional start site (P1A). This region was inserted upstream to a minimal thymidine kinase promoter to create a luciferase-based reporter plasmid, P1A reporter. Increases of approximately 3.86-, 2.06-, and 4.66-fold in P1A promoter activity were stimulated by T3, TGF-β, and T3 plus TGF-β, respectively (Fig. 7A). A bioinformatics search revealed three putative TREs and one Smad3/4 binding site (Fig. 7A) (31) in the P1A promoter. Figure 7, B and C, displays their location and sequences.
Figure 7.
T3-dependent activation of furin promoter by TR. The CV-1 cells were transfected with luciferase (Luc) reporter plasmid driven by the furin 5′-flanking region (−6408 to −4531) containing a minimal thymidine kinase (TK) promoter along with vectors expressing TR-β and β-galactosidase (as a transfection efficiency control). Cells were then incubated for 24 h in the presence or absence of T3 (100 nm) or TGF-β (5 ng/ml), or T3 plus TGF-β before harvesting to determine luciferase activity. The activity of luciferase was normalized to the activity of β-galactosidase. A, Various deletion mutants of furin 5′-flanking region were also generated and transfected. The region contained in these mutants is shown. Data are means ± se of values from three independent experiments, each performed in triplicate. B, Sequence and location of three putative TREs in the 5′-flanking region of the furin promoter. C, Sequence and location of the Smad3/4 binding site in the 5′-flanking region of the furin promoter.
Therefore, serially deleted mutants were also constructed from the P1A fragment to identify the region targeted by TR. Treatment with T3 resulted in a 3-fold increase in the activity of P1A deletion mutants (−6394/−6120, −6394/−6276, −6342/−6120, and −6342/−6276) compared with the T3 free condition (Fig. 7A). However, this was evident only when the putative TRE located at −6317/-6302 (Fig. 7B, fragment a or TREa) was deleted and the promoter activities were lacking (Fig. 7A) in T3-induced transactivation ability. Conversely, the activity of fragments −6159/−4531 and −5606/−4531 (Fig. 7A) was not induced by T3. Together, these experimental results suggest not only that T3 induces furin gene expression at transcriptional level but also clearly demonstrates that the fragment located between −6317 and −6302 (TREa) confers T3 responsiveness to the furin gene. Furthermore, the sequence AAAAGAcccgACTGGA revealed an atypical direct repeated TRE with four spacing (DR4).
Binding of TR to a −6328/−6288 fragment of the furin promoter containing TREa
To verify that TREa is directly targeted by TR proteins, this fragment was used as an EMSA probe. Reactions performed with α-32P-labeled −6328/−6288 fragment and TRα1 protein produced two predominant bands consisting of a TRα1/TRα1 homodimer and TRα1/RXR heterodimer (Fig. 8A, lane 2). These bands were competed out by adding five or 10 times molar excess of cold probe (data not shown) but could not be competed off by nonspecific cold probe (data not shown). Moreover, these two bands could be specifically supershifted (SS) by the C4 antibody against TR (Fig. 8A, lanes 3 and 6), but not the control antibody (MOPC21, Fig. 8A, lanes 4 and 7). Similar data were obtained using TRβ1 (Fig. 8A, lanes 8–13). Furthermore, if the TREa sequence had been mutated to (GGGGGG)cccg(GGGGGG), TR binding activity was lost (Fig. 8B). These results suggest that the binding of TR to the −6317/−6302 (TREa) on the furin gene promoter was specific. The analytical findings of promoter assay and EMSA suggest that T3-bound TRs activate furin gene transcription by directly binding to the TREa located between −6328 and −6288 bp (Figs. 7 and 8).
Figure 8.
Direct binding of TR proteins to the TRE spanning −6328 to −6288 fragment of the furin gene promoter. In vitro-translated TRα1, TRβ1 incubated with wt TRE (A), or mutated TRE (B) along with TNT in vitro-translated RXRα in a final volume of 20 μl. The mixture was reacted with approximately 100,000 cpm of α-32P- labeled oligonucleotide probes of the furin promoter for 40 min at room temperature. Antibody C4 is a mouse monoclonal antibody against TR proteins, and MOPC21 is a nonspecific monoclonal antibody. The position of probe complexed with TR and RXR is indicated by the arrows. SS, TR complexes SS by C4 antibody.
Furin overexpression promotes cell invasion in vitro and in vivo
MMPs are important zinc- and calcium-dependent proteinases that degrade extracellular matrix components and numerous other proteins (36). However, these MMPs are produced as proenzymes that require cleaving by other proteases before becoming enzymatically active. Furin can activate MMPs and cleave pro-MMP-2 or -9 to MMP-2 or -9 (37,38). Both pro-MMP-9 (97 kDa) and pro-MMP-2 (72 kDa) were observed in HepG2-TRα1 cells (Fig. 9A). Notably, active MMP-2 and MMP-9 were detectable in cells treated with either T3 (Fig. 9A, lane 2) or TGF-β (Fig. 9A, lane 3) alone. Similar results were obtained when cells were simultaneously treated with both T3 and TGF-β (Fig. 9A, lane 4). Without T3 treatment, no MMP-9 and MMP-2 activities were detected (Fig. 9A, lane 1). However, the additive activation of MMP activity was not observed in the T3 plus TGF-β condition (Fig. 9A, lane 4), perhaps because the amount of furin induced by T3 or TGFβ1 was sufficient to induce maximal MMP activity. Moreover, active MMP-2 and MMP-9 were also observed in the furin-overexpressed HepG2 (Fig. 9A, lanes 5 and 6), but not in HepG2-Neo cells (Fig. 9A, lane 7). Figure 9B presents the overexpression of furin in two HepG2-furin sublines (nos. 1 and 2). The invasiveness of HepG2 cells under various conditions clearly corresponded with the appearance of active MMP-2 and MMP-9. Results from the Transwell assay indicated that the invasive ability of HepG2-TRα1 cells was increased approximately 4 and 2.75-fold, respectively, when treated with either T3 or TGF-β. A roughly 5.5-fold increased invasiveness was observed in cells treated with both ligands. Similarly, the invasive ability was increased 4.14- to 4.43-fold in cells overexpressing furin (Fig. 9C). However, the invasive ability of HepG2 was not changed after T3, TGF-β, or T3 plus TGF-β treatment. To determine whether the in vitro results extended to the in vivo situation, SCID mice were used. The control HepG2-Neo and HepG2-furin SCID mice developed multiple macroscopic tumor nodules in the liver or lung (Fig. 10, B, C, K, and L) by hematoxylin and eosin (H&E) staining, with a metastasis index (average tumor number per cm2 liver or lung section in HepG2-furin/HepG2-Neo) of 4.3- and 2.6-fold, respectively, compared with the HepG2-Neo cells (Fig. 10S). In addition, the relative tumor size (average tumor size per cm2 liver or lung section in HepG2-furin/HepG2-Neo) of HepG2-furin mice was about 8.6- or 4.6-fold larger than those in the HepG2-Neo mice (Fig. 10T). Figure 10, D–F, is at a higher magnification (×200) of the liver sections shown in Fig. 10, A–C (×100), whereas Fig. 10, M–O, displays the higher magnification (×200) of lung sections shown in Fig. 10, J–L (×100). The normal morphology of liver or lung sections from the control group (inoculated with PBS only) was evident (Fig. 10, A, D, J, and M). Moreover, furin protein expression, which was evident as a dark-brown color analyzed by immunohistochemical staining, was markedly stronger in HepG2-furin liver tumors (Fig. 10, I vs. H) or lung tumors (Fig. 10, R vs. Q) than that in the HepG2-Neo tumor.
Figure 9.
Mediated overexpression of furin by TH promotes hepatocyte invasion. A, Overexpression of furin promotes activation of MMP proteins. HepG2 derived cells (3 × 106) were plated in DMEM with 10% fetal bovine serum. After 24-h incubation and subsequent washing, the medium was replaced with serum-free medium supplemented with or without T3 (10 nm) or TGF-β (5 ng/ml). Medium was collected for MMP detection. Preparation and zymography were performed; the position of proenzyme and active form of MMPs is shown to the left. B, Furin protein expression levels in the two furin gene overexpressed HepG2 sublines. Neo is an empty vector control line. C, Effect of T3 or TGF-β treatment on invasive activity in HepG2 and its derived cell lines. The cell lines were added to the upper chamber of Transwell units and incubated in the absence or presence of T3 (10 nm) or TGF-β (5 ng/ml) for 24 h. The number of cells that transversed the filter to the lower chamber was then determined and expressed as the total number of cells to provide an index of invasive activity. Data are means ± se of values from three independent experiments. The numbers above each column indicate the fold induction compared with T3 and TGF-β free culture medium or HepG2-Neo cells. Values are shown as transversed cell numbers in various conditions of medium or in HepG2, HepG2-Neo, and furin overexpressed HepG2 cells. Their difference was examined by the Student’s t test. **, P < 0.01.
Figure 10.
Furin overexpression in HepG2 cells enhances tumor (T) invasion in SCID mice. HepG2-furin or HepG2-Neo SCID mice (n = 6 per group) were established. All analyses were performed 7 wk after inoculation of tumor cells. H&E staining of metastasized HepG2-furin or HepG2-Neo cells in liver (A–I) or lung sections (J–R) are displayed. Panels D–F and M–O are the higher magnification (×200) of liver or lung sections coming from the corresponding white squares shown in panels A–C or J–L. The tumor region is circled by the dotted line (B, C, E, F, H, and I) or indicated by the arrowhead (K, L, N, Q, and R). The normal (N) morphology of liver or lung sections is shown in panels A and D (liver) and panels J and M (lung). Panels G–I and P–R show furin expression detected by immunohistochemistry. The tumor size from the HepG2-Neo cell is smaller than that from the HepG2-furin cell in liver (B vs. C) or lung (K vs. L). Average of the metastasis index (S) (fold, density of tumor numbers in HepG2-furin/HepG2-Neo per cm2 area) or relative tumor size (T) (fold increase) in liver or lung (average of tumor size in HepG2-furin/HepG2-Neo per cm2 area) is shown. Scale bar in panels is 200 μm. Tumor is indicated by arrowheads. Student’s t test. **, P < 0.01. The statistical significance is being compared with each HepG2-Neo cell. The control group was inoculated with PBS only without tumor cells.
Furin overexpression induced by T3 or TGF-β enhances HepG2-TRα1 cell invasion in vivo
To investigate whether the T3 and/or TGF-β induced furin expression could be observed in vivo and its physiological significance, SCID mice were divided into five groups and used as summarized in Fig. 11F. All groups of mice were inoculated with 1 × 107 HepG2-TRα1 cells for 7 wk before being killed. T3 serum levels (ng/dl) were 45.28 ± 2.28 (control), 9.38 ± 2.26 (PTU), 407 ± 8.31 (PTU plus T3), 8.33 ± 2.17 (PTU plus TGF-β), and 392 ± 6.72 (PTU plus T3 plus TGF-β), indicative of the development of euthyroid-, hypothyroid-, and hyperthyroid-SCID mice. Although tumor formation could be observed in all groups of mice, the number or size of the tumors differed. All SCID mice developed multiple macroscopic tumor nodules in the liver or lung (Fig. 11, A1–E1 and A4–E4), with an average of 1.89, 2.21, and 3.32-fold metastatic indexes in the liver from PTU plus T3 or PTU plus TGF-β, or PTU plus T3 plus TGF-β mice, respectively, compared with the control or PTU mice (Fig. 11G). Similarly, the metastatic index from lung of PTU plus T3 or PTU plus TGF-β, or PTU plus T3 plus TGF-β mice was 1.83, 1.81, and 2.94-fold, respectively, higher than that from the control or PTU mice (Fig. 11G). In addition, the relative tumor size in the liver or lung of PTU plus T3, PTU plus TGF-β, or PTU plus T3 plus TGF-β mice was 2.21-, 2.61-, and 4.94-fold, respectively, for liver, and 2.65-, 3.07-, and 6.67-fold, respectively, for lung, higher than those in the control or PTU mice (Fig. 11H). Figure 11, A2–E2 and A5–E5, is the higher magnification (×200) of liver or lung sections from the corresponding white squares shown in Fig. 11, A1–E1 and A4-E4 (×100). This increased tumor formation (number or size) could be attributed specifically to furin expression in the HepG2-TRα1 cells in liver or lung. Notably, furin protein expression (dark brown color) was much stronger in the PTU plus T3, PTU plus TGF-β, or PTU plus T3 plus TGF-β tumors in liver (Fig. 11, C3–E3 vs. A3 and B3) or lung (Fig. 11, C6–E6 vs. A6 and B6) than those in the control or PTU mice. The data indicated that furin expression was up-regulated by T3 and TGF-β treatment in HepG2-TRα1 cells, which significantly enhanced their invasiveness in vitro as well as in vivo.
Furin is up-regulated in human HCC
Furthermore, the clinicopathological significance of furin expression in HCC was also investigated. In total, 106 patients with HCC were consecutively selected for this study. An equal amount (100 μg) of protein from each specimen was loaded for electrophoresis and Western blot analysis. Equal loading was confirmed by Coomassie blue staining after SDS-PAGE (data not shown). The furin proteins were detected in most of the cancerous tissues. The results from 24 representative paired-HCC specimens are shown in Fig. 12, indicating the increased furin expression and the concomitantly increased TR protein expression in HCC tissues. The percentage of all paired samples with up-regulated furin was 70.8% (75 of 106) in the cancerous tissues, relative to the matched noncancerous adjacent tissues. In addition, both TRα1 and TRβ 1 were increased in 31.1% (33 of 106) of the cancerous tissues.
Figure 12.
Overexpression of furin in human HCC. The furin and TR proteins were overexpressed in the 24 representative tumor tissues (T) compared with matched noncancerous adjacent tissue (N) by Western blot analysis. Equal loading amount was confirmed by the Coomassie blue staining after SDS-PAGE (data not shown). The figure shows a compilation of separate blots, pieced together.
Discussion
This study characterized furin gene up-regulation, previously identified in cDNA microarray screening for T3-responsive genes in HepG2-TRα1 cells. Blanchette et al. (28) documented a significant positive correlation between furin expression and TGF-β. However, the significance of furin regulation by T3 has not been described. The liver is one of the principal target organs of THs (1) and is the predominant site of furin activity (14). We attempted to elucidate the molecular mechanism of furin regulation by T3 in isogenic HepG2 cell lines. The results also revealed that T3-induced furin expression in human HCC cell lines resembles that observed in animal model studies. Moreover, the experimental data presented here clearly demonstrate not only that expression of furin mRNA is induced by T3 but also identify a DR4-like TRE located between −6317 and −6302 on the furin promoter. In the current study, three animal models were established to determine the functional role of furin in vivo. The first model was the Tx rat, in which furin expression levels in the Tx plus T3 rat were much higher than that in the Tx group. The second model was HepG2-furin overexpressing mice, which displayed a higher metastasis index as well as larger tumor size than HepG2-Neo mice. Finally, euthyroid, hypothyroid, and hyperthyroid SCID mice were established to investigate furin expression and its functional consequence. Similarly, the increased tumor number or size in the hyperthyroid SCID mice as well as in the TGF-β mice can be attributed specifically to furin expression in the HepG2-TRα cells in liver or lung. The experimental results indicate the T3 and/or TGF-β solely induced furin expression occurs in vitro and in vivo.
The expression of furin, which is a powerful convertase, is very low in normal cells. However, transformed and metastasized cells are characterized by up-regulated furin expression and concomitantly enhanced ability to degrade the extracellular matrix. This enhanced invasiveness results from increased MMP activation by processing enzymes such as furin or PCs. Moreover, furin activity can be blocked by a selective furin inhibitor, α1-antitrypsin Portland (α1-PDX), which subsequently reduces the invasiveness of tumor cells (39). Apart from T3, furin expression is markedly enhanced by TGF-β (28). In this study the invasive ability of hepatocytes was substantially enhanced after treatment with T3 and TGF-β. In addition, simultaneous treatment with both T3 and TGF-β additively enhanced invasiveness in comparison with separate treatments. However, MMP activity in cells treated with both ligands resembled that of cells treated with either T3 or TGF-β alone. These results suggest that enhanced invasiveness is only partly mediated by MMP activation. Conversely, previous studies indicated that T3, acting through TRs, inhibits expression of Nm23-H1 and promotes tumor metastasis (7). The function of Nm23-H1 is associated with antimetastasis (40). Moreover, the altered PC profile in liver metastases has been noted in certain primary colon cancers, indicating possible selection processes involving PCs during metastasis and a critical role of PCs in liver metastasis (41). Notably, furin has been associated with enhanced invasion and proliferation in head, neck, breast, and lung cancers (42). Numerous PC substrates such as growth factors, growth factor receptors, integrins, and MMPs are cancer-associated proteins; their activation by furin may account for the enhanced cell invasiveness in furin up-regulated tumor cells. In this study, furin is reported to be up-regulated in HCC. However, its significance requires further study.
This study examined the principal role of T3 and TGF-β in regulating the expression of the furin gene. The results reveal a close relationship between T3 and TGF-β in the previous work of this laboratory (43). Previous studies have also demonstrated that 12 h or more T3 treatment induces minimal TGF-β expression (43,44). Furthermore, increased TGF-β plasma concentration is associated with high-plasma T3 levels in elderly patients with nonthyroidal illnesses (34). In addition, TGF-β signaling can be mediated by members of the MAPK family (45). The MAPK family incorporates the ERK pathway (46) and two stress-activated pathways, the Jun N-terminal kinase and p38 pathways (47,48). Data presently obtained suggest that MEK/ERK is the principal pathway modulating T3-induced expression of furin evident by the specific inhibitors used. However, a previous report has shown that TGF-β-induced expression of furin is suppressed by PD98059 and U0126 in HepG2 cell lines (28). The latter study disagrees with the present results. The different cell culture conditions may account for the discrepancy. Moreover, furin protein expression level was not measured previously (28). This work found that TGF-β-induced furin expression in Td medium is limited by SB203580 and SB202190, suggesting that the p38 pathway modulates activation of furin expression by TGF-β. However, in the presence of T3, furin activation is modulated by MEK/ERK. In the previous study, the culture medium was supplemented with normal serum possibly containing sufficient T3 to activate endogenous TR; therefore, a reasonable conjecture is that furin activation was modulated by the MEK/ERK pathway. From this perspective, the data obtained by Blanchette et al. (28) are entirely consistent with the current findings. In addition, MAPKs are the primary kinases in nuclear phosphorylation of TR, and are crucial factors for modulating transcriptional activity and protein stability of TR (32). Experimental results in this study extend previous conclusions (29) by demonstrating the link between the action of TRs, TGF-β, and MAPK pathways and furin activation. Notably, T3-induced furin activation is mediated by the MEK/ERK pathway. Subsequently, MEK/ERK phosphorylates and stabilizes TR (32). Furthermore, TR binds to its cognate TRE on the furin promoter region, thereby activating furin expression and enhancing tumor metastasis (Fig. 13).
Figure 13.
Cooperative model for T3- and TGF-β-activated furin gene expression. Adding TGF-β to cells activates TGF-β-specific Smad3/4 or p38 pathways. In parallel, T3 or TGF-β activation induces a rapid and sustained phosphorylation of endogenous MEK1/2. Cross talk with activated MEK1/2 or downstream MAPK cascade elements enhances Smad3/4 nuclear translocation where it interacts with DNA-binding proteins and directs transcription of the furin gene. Increased intracellular levels of furin influence the bioactivation of multiple growth/cell differentiation-related factors and enhance tumor metastasis. P, Phosphorylated protein.
Furin induction can also be mediated by T3 indirect pathway and/or via other TR-independent pathways. TGF-α stimulates furin promoter activity highly in a rat gastric surface mucosal cell line (49), and the transcription factor GATA1 regulates furin gene transcription in megakaryocytes (50). In HepG2 cells the transcriptional activity of the furin promoter is under the control of endogenous C/EBP-β and Smad protein (21,51).
The present study reveals the important role of T3 and TGF-β in furin mRNA expression and the translational protein levels. In addition, the TGF-β pathway, particularly Smad3 and Smad4, is involved in furin induction by T3. Regulation of furin expression involves cross talk in the T3, TGF-β, and MEK/ERK pathways. In conclusion, this investigation demonstrates that ligand-activated TR directly transactivates furin gene expression cooperatively with TGF-β and is modulated by the MEK/ERK pathway. The TRE is localized between −6317 and −6302 of the furin promoter region. In addition, T3 directly regulates furin gene expression, thereby enhancing its invasiveness. Although this phenomenon has been documented in a human tumor cell line, this regulation is similarly observed in SCID mice models.
Acknowledgments
Smad expression plasmids were generously provided by Dr. P. T. Dijke (Ludwig Institute, Uppsala, Sweden). P1A was a gift from Wim J. M. Van de Ven (University of Leuven, Leuven, Belgium). The C4 mouse monoclonal antibody to thyroid hormone receptor was kindly provided by S.-Y. Cheng (National Cancer Institute, Bethesda, MD).
Footnotes
This work was supported by grants from Chang-Gung University, Taoyuan, Taiwan (CMRPD 34013, NMRP 140511), Chang-Gung Molecular Medicine Research Center Taoyuan, Taiwan (CMRP 140041), and the National Science Council of the Republic of China (NSC 94-2320-B-182-052).
Disclosure Statement: The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
First Published Online May 8, 2008
Abbreviations: dn, Dominant-negative; H&E, hematoxylin and eosin; HCC, hepatocellular carcinoma; MEK, MAPK kinase; MMP, matrix metalloproteinase; PC, pro-protein convertase; PTU, propylthiouracil; Q-RT-PCR, quantitative RT-PCR; RXR, retinoid X receptor; SCID, severe combined immunodeficiency; SS, supershifted; Td, depleted of T3; TH, thyroid hormone; TR, TH receptor; TRE, thyroid hormone response element; Tx, thyroidectomized; wt, wild type.
References
- Cheng SY 2000 Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord 1:9–18 [DOI] [PubMed] [Google Scholar]
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Wood WM, Dowding JM, Bright TM, McDermott MT, Haugen BR, Gordon DF, Ridgway EC 1996 Thyroid hormone receptor β2 promoter activity in pituitary cells is regulated by Pit-1. J Biol Chem 271:24213–24220 [DOI] [PubMed] [Google Scholar]
- Chamba A, Neuberger J, Strain A, Hopkins J, Sheppard MC, Franklyn JA 1996 Expression and function of thyroid hormone receptor variants in normal and chronically diseased human liver. J Clin Endocrinol Metab 81:360–367 [DOI] [PubMed] [Google Scholar]
- Chang C, Lin Y, O-Lee TW, Chou CK, Lee TS, Liu TJ, P’Eng F K, Chen TY, Hu CP 1983 Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol Cell Biol 3:1133–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Shieh HY, Hsu HC 2000 Negative regulation of the antimetastatic gene Nm23–H1 by thyroid hormone receptors. Endocrinology 141:2540–2547 [DOI] [PubMed] [Google Scholar]
- Shih CH, Chen SL, Yen CC, Huang YH, Chen CD, Lee YS, Lin KH 2004 Thyroid hormone receptor-dependent transcriptional regulation of fibrinogen and coagulation proteins. Endocrinology 145:2804–2814 [DOI] [PubMed] [Google Scholar]
- Lin KH, Chen CY, Chen SL, Yen CC, Huang YH, Shih CH, Shen JJ, Yang RC, Wang CS 2004 Regulation of fibronectin by thyroid hormone receptors. J Mol Endocrinol 33:445–458 [DOI] [PubMed] [Google Scholar]
- Lin KH, Lee HY, Shih CH, Yen CC, Chen SL, Yang RC, Wang CS 2003 Plasma protein regulation by thyroid hormone. J Endocrinol 179:367–377 [DOI] [PubMed] [Google Scholar]
- Huang YH, Lee CY, Tai PJ, Yen CC, Liao CY, Chen WJ, Liao CJ, Cheng WL, Chen RN, Wu SM, Wang CS, Lin KH 2006 Indirect regulation of human dehydroepiandrosterone sulfotransferase family 1A member 2 by thyroid hormones. Endocrinology 147:2481–2489 [DOI] [PubMed] [Google Scholar]
- Molloy SS, Anderson ED, Jean F, Thomas G 1999 Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol 9:28–35 [DOI] [PubMed] [Google Scholar]
- Bassi DE, Mahloogi H, Klein-Szanto AJ 2000 The proprotein convertases furin and PACE4 play a significant role in tumor progression. Mol Carcinog 28:63–69 [PubMed] [Google Scholar]
- Hoshino H, Konda Y, Takeuchi T 1997 Co-expression of the proprotein-processing endoprotease furin and its substrate transforming growth factor β1 and the differentiation of rat hepatocytes. FEBS Lett 419:9–12 [DOI] [PubMed] [Google Scholar]
- Cheng M, Watson PH, Paterson JA, Seidah N, Chretien M, Shiu RP 1997 Pro-protein convertase gene expression in human breast cancer. Int J Cancer 71:966–971 [DOI] [PubMed] [Google Scholar]
- Schalken JA, Roebroek AJ, Oomen PP, Wagenaar SS, Debruyne FM, Bloemers HP, Van de Ven WJ 1987 fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest 80:1545–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M, Sirois F, Yao J, Seidah NG, Chretien M 1997 Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br J Cancer 75:1509–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ 2001 Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog 31:224–232 [DOI] [PubMed] [Google Scholar]
- Samuels HH, Stanley F, Casanova J 1979 Depletion of l-3,5,3′-triiodothyronine and l-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80–85 [DOI] [PubMed] [Google Scholar]
- Lin KH, Wang WJ, Wu YH, Cheng SY 2002 Activation of antimetastatic Nm23–H1 gene expression by estrogen and its α-receptor. Endocrinology 143:467–475 [DOI] [PubMed] [Google Scholar]
- Ayoubi TA, Creemers JW, Roebroek AJ, Van de Ven WJ 1994 Expression of the dibasic proprotein processing enzyme furin is directed by multiple promoters. J Biol Chem 269:9298–9303 [PubMed] [Google Scholar]
- Sambrook J, Russell DW 2001 Molecular cloning: a laboratory manual. In: Russell S, ed. Extraction, purification, and analysis of mRNA from eukaryotic cells. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 7.4–7.75 [Google Scholar]
- Bhat MK, Yu C, Yap N, Zhan Q, Hayashi Y, Seth P, Cheng S 1997 Tumor suppressor p53 is a negative regulator in thyroid hormone receptor signaling pathways. J Biol Chem 272:28989–28993 [DOI] [PubMed] [Google Scholar]
- Theodossiou C, Skrepnik N, Robert EG, Prasad C, Axelrad TW, Schapira DV, Hunt JD 1999 Propylthiouracil-induced hypothyroidism reduces xenograft tumor growth in athymic nude mice. Cancer 86:1596–1601 [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H 1981 Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580 [DOI] [PubMed] [Google Scholar]
- Tai PJ, Huang YH, Shih CH, Chen RN, Chen CD, Chen WJ, Wang CS, Lin KH 2007 Direct regulation of androgen receptor-associated protein 70 by thyroid hormone and its receptors. Endocrinology 148:3485–3495 [DOI] [PubMed] [Google Scholar]
- Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G 1997 Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO J 16:1508–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette F, Day R, Dong W, Laprise MH, Dubois CM 1997 TGFβ1 regulates gene expression of its own converting enzyme furin. J Clin Invest 99:1974–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM 2001 Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor β1-induced furin gene transactivation. J Biol Chem 276:33986–33994 [DOI] [PubMed] [Google Scholar]
- Itoh S, Itoh F, Goumans MJ, Ten Dijke P 2000 Signaling of transforming growth factor-β family members through Smad proteins. Eur J Biochem 267:6954–6967 [DOI] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE 1998 Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1:611–617 [DOI] [PubMed] [Google Scholar]
- Chen SL, Chang YJ, Wu YH, Lin KH 2003 Mitogen-activated protein kinases potentiate thyroid hormone receptor transcriptional activity by stabilizing its protein. Endocrinology 144:1407–1419 [DOI] [PubMed] [Google Scholar]
- Bhat MK, Ashizawa K, Cheng SY 1994 Phosphorylation enhances the target gene sequence-dependent dimerization of thyroid hormone receptor with retinoid X receptor. Proc Natl Acad Sci USA 91:7927–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden T, Wondisford FE, Hollenberg AN 1997 Isolation and characterization of a novel ligand-dependent thyroid hormone receptor-coactivating protein. J Biol Chem 272:29834–29841 [DOI] [PubMed] [Google Scholar]
- Meier CA, Parkison C, Chen A, Ashizawa K, Meier-Heusler SC, Muchmore P, Cheng SY, Weintraub BD 1993 Interaction of human β1 thyroid hormone receptor and its mutants with DNA and retinoid X receptor β. T3 response element-dependent dominant negative potency. J Clin Invest 92:1986–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z 2001 How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Hirakawa K, Haruyama T, Akaike T 2001 Direct production of an activated matrix metalloproteinase-9 (gelatinase B) from mammalian cells. FEBS Lett 502:63–67 [DOI] [PubMed] [Google Scholar]
- Stawowy P, Meyborg H, Stibenz D, Borges Pereira Stawowy N, Roser M, Thanabalasingam U, Veinot JP, Chretien M, Seidah NG, Fleck E, Graf K 2005 Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation 111:2820–2827 [DOI] [PubMed] [Google Scholar]
- Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ 2001 Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci USA 98:10326–10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua G, Sobel ME, Liotta LA, Steeg PS 1989 Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res 49:5185–5190 [PubMed] [Google Scholar]
- Tzimas GN, Chevet E, Jenna S, Nguyen DT, Khatib AM, Marcus V, Zhang Y, Chretien M, Seidah N, Metrakos P 2005 Abnormal expression and processing of the proprotein convertases PC1 and PC2 in human colorectal liver metastases. BMC Cancer 5:149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ 2005 Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog 44:151–161 [DOI] [PubMed] [Google Scholar]
- Yen CC, Huang YH, Liao CY, Liao CJ, Cheng WL, Chen WJ, Lin KH 2006 Mediation of the inhibitory effect of thyroid hormone on proliferation of hepatoma cells by transforming growth factor-β. J Mol Endocrinol 36:9–21 [DOI] [PubMed] [Google Scholar]
- Miller LD, Park KS, Guo QM, Alkharouf NW, Malek RL, Lee NH, Liu ET, Cheng SY 2001 Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Mol Cell Biol 21:6626–6639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH 1999 TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Marshall CJ 1996 Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv 27:101–125 [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J 1996 Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18:567–577 [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Kyriakis JM, Avruch J, Zon LI, Zanke B, Templeton DJ 1996 Reconstitution of novel signalling cascades responding to cellular stresses. Philos Trans R Soc Lond B Biol Sci 351:135–141 [DOI] [PubMed] [Google Scholar]
- Kamimura H, Konda Y, Yokota H, Takenoshita S, Nagamachi Y, Kuwano H, Takeuchi T 1999 Kex2 family endoprotease furin is expressed specifically in pit-region parietal cells of the rat gastric mucosa. Am J Physiol 277(1 Pt 1):G183–G190 [DOI] [PubMed] [Google Scholar]
- Laprise MH, Grondin F, Cayer P, McDonald PP, Dubois CM 2002 Furin gene (fur) regulation in differentiating human megakaryoblastic Dami cells: involvement of the proximal GATA recognition motif in the P1 promoter and impact on the maturation of furin substrates. Blood 100:3578–3587 [DOI] [PubMed] [Google Scholar]
- Blanchette F, Rudd P, Grondin F, Attisano L, Dubois CM 2001 Involvement of Smads in TGFβ1-induced furin (fur) transcription. J Cell Physiol 188:264–273 [DOI] [PubMed] [Google Scholar]