Abstract
Orexins mediate a variety of physiological processes, including feeding behavior, the circadian pathway, and cortisol secretion. Steroidogenesis is regulated by a variety of neuropeptides, and one of the key rate-limiting steps is cholesterol transport across the mitochondrial membrane by the steroidogenic acute regulatory protein (StAR). StAR expression can be regulated through several different signaling pathways. Despite the clear link between orexins and steroid production, the actions of the orexin family of hormones on steroid biosynthesis are not fully understood. We present data showing that 100 nm of both orexins A and B for 4 or 24 h significantly up-regulates StAR, in H295R pluripotent adrenocortical cells. We present the dose-dependent and time-dependent characteristics of StAR up-regulation at the protein level, showing significant increases after 4 h at a relatively low agonist concentration (1 nm). We have provided a key analysis of the precise G protein-coupled signaling pathways required for the up-regulation of StAR in response to orexins A and B. This has involved dominant-negative G protein analysis, and the direct inhibition of the protein kinase A, protein kinase C, ERK1/2, and p38 pathways. This shows a fundamental role for multiple G protein-coupled and MAPK-mediated signaling pathways leading to StAR expression. Antagonist analysis also showed that orexin effects on StAR were primarily, but not exclusively, acting through the orexin receptor type 1. This is the first study linking orexin action on StAR expression and comprehensively describes the signaling pathways involved in regulating the complexity of hormone biosynthesis.
OREXIN A (ORA) AND B (ORB) are two hypothalamic peptides that originate from the posttranslational proteolytic cleavage of a common precursor, the prepro-orexin gene (1). Their effects are articulated through signaling cascades via two G protein-coupled receptors (GPCRs), orexin receptor types 1 (OX1R) and 2 (OX2R). These receptors are members of the rhodopsin-like family A GPCRs. They share approximately 64% amino acid identity and couple to multiple G proteins, activating through several intracellular signaling pathways (2,3). ORA binds with equal affinity for both receptors, whereas ORB has an approximate 10-fold higher affinity for OX2R (4).
We and others have previously published data showing expression of orexin receptors in human fetal and adult adrenal membranes, and the implications in energy balance (5,6,7), including the demonstration that orexin receptors couple to multiple G proteins within the adrenal gland. Studies involving adrenalectomy and glucocorticoid antagonists in obese mice implicated glucocorticoids in the development of their phenotype (8). Interestingly, in dispersed adrenocortical cells, orexins induced corticosterone production in rats and cortisol secretion in humans acting through OX1R (9), and the expression of both orexin-receptor subtypes was up-regulated in adenomas (10). These receptors are widely expressed in the central nervous system and in the periphery, including in adipose tissue, the endocrine cells of the gut and the adrenal gland, all of which play a role in the integration of metabolic activity and energy balance.
It has been shown that there exists a close interrelationship between body weight homeostasis and adrenal secretory activity, notably of steroid hormones (11,12). The biosynthesis of these steroid hormones (steroidogenesis) occurs predominantly in the mitochondria via the successive enzymatic breakdown of cholesterol (13). There are numerous enzymes involved, regulated via transcription and activation by a host of protein molecules (14).
The first crucial protein involved in steroidogenesis is the 30-kDa steroidogenic acute regulatory protein (StAR), first identified in 1994 (15). StAR is expressed predominantly in the steroid-producing cells of the body, required for the obligatory first step of acute steroidogenesis, the transport of cholesterol from the outer to the inner mitochondrial membrane (16). Reduced StAR expression is currently the only known cause of the steroid-deficiency disease, familial lipoid adrenal hyperplasia (17).
Several proteins are known to lead to StAR expression or inhibition, including ACTH, epidermal growth factor, IGF-I, TGF-β, and angiotensin (18,19). In addition, the expression of StAR can be initiated through multiple signaling pathways, including protein kinase A (PKA) and protein kinase C (PKC)-dependent mechanisms (20). This complexity makes the study of StAR expression and regulation very difficult, and likely reflects the constantly changing steroid requirements.
The effects of orexins on steroid production and the underlying signaling mechanisms are not yet fully understood. Given the effects of orexins on cortisol and the implications of these actions to energy balance, we aimed to investigate further the effects of these peptides on the StAR gene, as the rate-determining step in the steroid biosynthesis pathway, in a human adrenocortical cell (H295R) model. H295R cells act as pluripotent adrenocortical cells capable of producing all major zone-specific adrenal steroids (21).
This report describes the up-regulation of StAR gene expression in response to both ORA and ORB in H295R adrenal cells. The effect is analyzed in detail at the protein level, using dominant-negative G proteins, signaling pathway inhibitors, and receptor-specific antagonists to identify the exact G protein-coupling systems involved. We present evidence that ORA mediates its actions on StAR expression predominantly through the OX1R, but mediated by Gq/11, Gs, and Gi coupling. ORB acts through both OX1R and OX2R but is primarily affecting StAR expression through Gq coupling. Furthermore ERK1/2 and p38, mediators from two of the four major MAPK kinase (MEK) kinase/MEK/MAPK signaling cascades, are also required for full StAR expression in response to both ORA and ORB. Our findings are crucial toward understanding how orexins signal through multiple pathways during the regulation of steroid biosynthesis.
Materials and Methods
Biochemical reagents
Human ORA and ORB were obtained from Phoenix Pharmaceuticals (Belmont, CA). Insulin, transferring, selenium and Ultraserum-G (PALL Life Sciences, Cergy, France), growth supplement for H295R cells, were obtained from Sigma-Aldrich Co. Ltd. (Gillingham, Dorset, UK). Accutase required for splitting cells was obtained from TCS Cellworks (Buckinghamshire, UK), and ECL plus Western blotting detection reagents was from Amersham Biosciences (Buckinghamshire, UK). Agarose was obtained from MBI Fermentas (York, UK) and Helena BioSciences Europe (Tyne & Wear, UK).
Inhibitors
The following inhibitors were obtained from Calbiochem (Darmstadt, Germany): 2′,5′-dideoxyadenosine is a potent and specific inhibitor of adenylate cyclase, the enzyme catalyzing cAMP production; myristoylated PKA inhibitor (PKAi) amide 14–22, a selective inhibitor of PKA; 2-[1-[2-(1-methylpyrrolidino)ethyl]-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide (Bis), a selective inhibitor of PKC (PKCi); 1,4-diamino-2,3-dicyano-1,4-bis (2-aminophenylthio) butadiene (U0126), a potent and specific inhibitor of MEK1 and MEK2; and pertussis toxin is an enzyme derived from the bacteria Bordetella pertussis, which catalyzes ADP-ribosylation of the α-subunit of inhibitory G proteins (Gi) and Gi-like G proteins, resulting in dissociation of the G protein from the receptor, allowing effector enzymes to remain activated.
Antibodies
Goat polyclonal anti-OXR1 antibody (Santa Cruz, Biotechnology, Santa Cruz, CA), mouse monoclonal anti-OX2R antibody, anti-StAR, and anti-β-actin antibody were purchased from Abcam (Cambridge, UK). Polyclonal horseradish peroxidase-conjugated goat antirabbit, antimouse immunoglobulin/HRP was from DakoCytomation (Glostrup, Denmark).
Other materials
Precision Plus Protein Standard was from Bio-Rad Laboratories Ltd. (Hertfordshire, UK). The mammalian expression vector pcDNA3.1(+) was from Invitrogen (Paisley, UK). Human G protein α q [dominant-negative (Q209L/D227N) Gq], human G protein α S long [dominant-negative (Q227L/D295N) Gs], and human G protein α i [dominant-negative (Q205L/D273N) Gi] were obtained from University of Missouri-Rolla cDNA Resource Centre, University of Missouri-Rolla (Rolla, MO). Polyvinylidene difluoride (PVDF) membrane was purchased from Amersham Biosciences; all of the primers were obtained from TAGN (Newcastle, UK).
Cell culture
H295R human adrenocortical cells were cultured in H295R complete media containing DMEM/F12 (1:1) supplemented with 2% Ultroser G (Biosepra, Villeneuve-la-Garenne, France) and insulin, transferring, selenium (Discovery Labware, Bedford, MA), in six-well plates for 24 h after reaching confluence. Media were replaced with 3 ml fresh media containing different agents and cultured for 4 h for protein experiments, and both 4 and 24 h for the RT-PCR experiments. At the end of the incubation period, cells were washed with ice-cold PBS and subjected to RNA or protein extraction and analysis as described below for RT-PCR or Western blotting.
RT-PCR
Total RNA was extracted using the QIAGEN RNeasy Mini Kit (West Sussex, UK) and reverse-transcribed into cDNA as previously described (6). Steroidogenic gene expression was measured by RT-PCR, using 3 μg RNA and random primers as RT primers. A control reaction that omitted reverse transcriptase was included to check for the presence of genomic DNA. Steroidogenic gene expressions were amplified using a Hybaid Thermal Cycler in 50 ml reaction medium containing 1 U Taq polymerase (Fermantes, Lithuania, UK), 20 pmol of each sense and antisense primer, and deoxynucleotide triphosphate (10 mmol/liter each), using the following cycling conditions: 94 C for 1 min, then 38 cycles of 94 C for 60 sec, 60 C for 45 s, and 72 C for 30 s, followed by a 10-min extension at 72 C. The sequences for the sense and antisense primers (respectively) were: StAR, 5′-GGCTACTCAGCATCGACCTC-3′ and 5′-CATCCCACTGTCACCAGATG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TGAACGGGAAGCTCACTGG-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′; OX1R, 5′-CCTTCCTGGCTGAAGTGAAG-3′ and 5′-AGTGGGAGAAGGTGAAGCAG-3′; and OX2R, 5′-GTCGCAACTGGTCATCTGCT-3′ and 5′-CGTCCTCATGTGGTGGTTCT-3′. PCR products were stained with ethidium bromide and visualized by electrophoresis through 2% agarose gels. Sequencing of the PCR products confirmed the sequence identities. OX1R and OX2R gene expression was also measured in a similar way.
Quantification of StAR, OX1R, and OX2R mRNA
The concentration of target mRNA was measured by RT, followed by real-time PCR performed on a Roche Light Cycler system (Roche Molecular Biochemicals, Mannheim, Germany) using the relevant primers from the aforementioned list. Quantitative PCRs were performed using 2.5 ml cDNA in 5.5 ml PCR SYBR Green-1 Light Cycler Master Mix (Biogene, Cambridgeshire, UK), and 1 ml sense and antisense primers. A series of three dilutions for each cDNA was used to ensure linear amplification. Protocol conditions consisted of denaturation of 95 C for 60 sec, followed by 40 cycles of 94 C for 1 sec, 60 C for 8 sec, and 72 C for 15 sec, followed by melting-curve analysis. For analysis, quantitative amounts of the gene of interest were standardized against the housekeeping gene GAPDH. Negative controls for all the reactions included preparations lacking cDNA or RNA-lacking reverse transcriptase in place of the cDNA. The relative mRNA levels were expressed as a ratio using the “δ–δ method” for comparing relative expression results between treatments in real-time PCR (22).
Transfection and subsequent overexpression of dominant G protein subunits for inhibiting the corresponding pathway
H295R cells were transfected using Nucleofector Technology (Amaxa Biosystems, Cologne, Germany). Three million log-phase cells were resuspended in 100 μl Nucleofector Solution R, mixed with 3 μg plasmid DNA, and electroporated using the proprietary program P-20. Cells were allowed to recover for 15 min in DMEM/F12 media at room temperature and then plated in 12-well plates with 2 ml H295R complete media per well. Cells were cultured for 18 h. The media were then removed and the cells incubated with prewarmed serum-free media overnight before 4-h peptide treatment. Cells were lysed with Laemmli buffer (Sigma, Rockford, UK) for protein studies.
Western blotting
Protein lysates were prepared by adding equal amounts of Laemmli buffer to each well, and samples were denatured by sonication and boiling. Samples were separated by SDS-PAGE (10% resolving gel) and transferred to PVDF membranes at 100 V for 1 h in a transfer buffer containing 20 mm Tris, 150 mm glycine, and 20% methanol. The PVDF membranes were incubated with primary antibody for StAR (Abcam) at a 1:7,000 dilution and β-actin (Abcam) at 1:25,000 or OX1R and OX2R (Santa Cruz) at 1:1,500 dilution in Tris-buffered saline-0.1% Tween, and 5% BSA overnight at 4 C. The membranes were washed, incubated with a secondary antirabbit (StAR), antimouse (β-actin) horseradish peroxidase-conjugated antibody (1:2000) for 1 h at room temperature, and washed for 60 min with Tris-buffered saline-0.1% Tween. Antibody complexes were visualized using the ECL Plus, chemiluminescence detection kit. The densities were measured using a scanning densitometer coupled to Scion Image scanning software (Scion Corp., Frederick, MD).
Using pathway inhibitors/receptor antagonists to compare StAR expression
PKAI, PKCi, U0126 (MEK/ERK1/2 inhibition), and SB203580 [p38 inhibition (p38i)] as well as the OX1R antagonist SB334867 were added to 3 ml fresh media and added to confluent, cultured cells for 4 h. Inhibitors were added at least 30 min before the other reagents and remained during the incubation period. Cells were then processed as described previously.
alamarBlue (BioSource International Inc., Camarillo, CA) cell proliferation assay
Cells were seeded on a 96-well plate at 1 × 104 cells per well in quadruplet. before treatment with different concentrations of ORA and ORB for 24 h at 37 C/5% CO2. The alamarBlue assay was performed according to the manufacturer’s instructions. Ten percent alamarBlue reagent was added to 96-well plates, incubated for the specified time, and quantified spectrophotometrically for absorbance with a microplate reader (TECAN Group Ltd., Männedorf, Switzerland) at wavelengths of 570 and 600 nm. Nonseeded wells containing media alone were included as a control.
Statistical analysis
Nonparametric tests were used. Data are presented as means ± sem unless indicated otherwise. Differences between two groups were assessed using the Mann-Whitney U test. Data involving more than two groups were assessed by Friedman’s ANOVA with Dunn’s test for post hoc analysis. For Western immunoblotting experiments, the densities were measured using a scanning densitometer coupled to scanning software Scion Image. Spearman rank correlation was used for calculation of associations between variables; P < 0.05 was considered significant.
Results
RT-PCR to assess the effect of orexin stimulation on gene expression of StAR in H295R adrenal cell
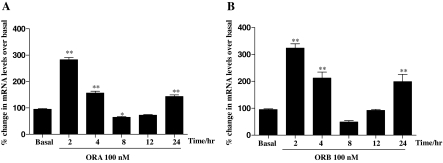
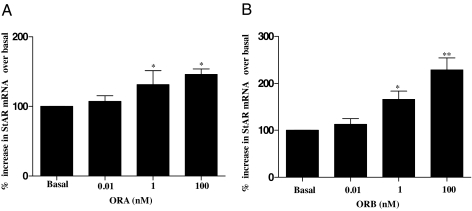
Time-dependent RT-PCR of StAR, in response to 100 nm stimulation by both orexin peptides ORA or ORB, showed for the first time significant overexpression (Fig. 1). A biphasic response was observed as StAR increased (∼300% for both ORA and ORB) when H295R cells were treated for 2 h. The response gradually reduced, increasing again after 24 h. StAR expression is recognized as one of the key rate-limiting steps in steroid biosynthesis, and StAR deficiency is currently the only known cause in the pathophysiology of lipoid congenital adrenal hyperplasia (23). To assess the relative expression of StAR more thoroughly, we compared the dose-dependent RT-PCR analysis using three different concentrations of ORA and ORB. Figure 2 clearly shows that significant StAR mRNA was seen after 4 h after stimulation by just 1 nm, increasing slightly with 100 nm treatment.
Figure 1.
Time-course effects of orexins on StAR mRNA levels in H295R cells. Cells were cultured for the indicated periods in the presence or absence (basal) of ORA (100 nm) (A) or ORB (100 nm) (B). Total RNA was isolated, reverse transcribed, and used as a template for quantitative PCR using StAR, and GAPDH specific primers. Results were normalized against GAPDH expression and expressed as percentage of basal. Data points are means ± se from six independent experiments. Significance was determined by Friedman’s ANOVA with Dunn’s test for post hoc analysis (*, P < 0.05; **, P < 0.01).
Figure 2.
Concentration-dependent effects of orexins on StAR mRNA levels in H295R cells. Cells were cultured for the indicated periods in the presence or absence (basal) of 0.01, 1, and 100 nm ORA (A) or ORB (B). Total RNA was isolated, reverse transcribed, and used as a template for quantitative PCR using StAR, and GAPDH specific primers. Result were normalized against GAPDH expression and expressed as percentage of basal. Data points are means ± se from six independent experiments. Significance was determined by Friedman’s ANOVA with Dunn’s test for post hoc analysis (*, P < 0.05; **, P < 0.01).
Western blot analysis of the time and dose dependent expression of StAR in response to ORA and ORB
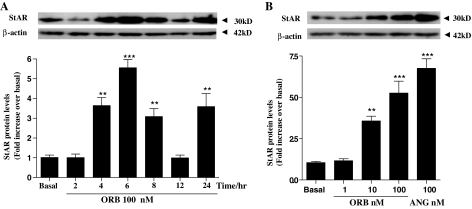
Although mRNA is a good indicator of expression, this does not necessarily reflect the actual cellular protein levels. To address this, we used a series of Western blots with a StAR antibody comparing protein expression after 2, 4, 6, 8, 12, and 24 h after treatment with 100 nm ORA (Fig. 3A) or 100 nm ORB (Fig. 4A). Both showed an interesting and similar temporal pattern of StAR expression with an initial maximum after approximately 4 h, expression declining, and then increasing again after 24 h. In addition, we compared the levels of dose-dependent effects on StAR expression in response to 1, 10, and 100 nm ORA (Fig. 3B) and ORB (Fig. 4B). This shows a clear dose-response effect of both orexin forms on StAR expression reaching significance at 10 nm ligand concentration, increasing further with 100 nm treatment.
Figure 3.
Time-course and concentration-dependent effects of ORA on StAR expression in H295R cells. Cells were cultured for the indicated periods in response to 100 nm ORA for 2, 4, 6, 8, 12, and 24 h after treatment (A), and 4-h treatment of 0, 1, 10, and 100 nm ORA (B). Cell lysates were extracted in radioimmunoprecipitation assay buffer, and total protein was quantified using the bicinchoninic acid assay. Equal protein was loaded into each well. StAR and β-actin protein levels were determined by Western blot and quantified using densitometric analysis. StAR levels were normalized against β-actin. Data points are means ± se from six independent experiments. B, The effect of treatment with 100 nm angiotensin (ANG) was included to show an approximate maximum StAR expression. Significant difference from basal (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Figure 4.
Time-course and concentration-dependent effects of ORB on StAR expression in H295R cells. Cells were cultured for the indicated periods in response to 100 nm ORB for 2, 4, 6, 8, 12, and 24 h after treatment (A), and 4-h treatment of 0, 1, 10, and 100 nm ORB (B). StAR levels were determined and quantified by Western blot exactly as described for Fig. 3. Data points are means ± se from six independent experiments. B, The effect of treatment with 100 nm angiotensin (ANG) was included to show an approximate maximum StAR expression. *, Significant difference from basal using Friedman’s ANOVA with Dunn’s test for post hoc analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The effects of dominant-negative G proteins on orexin-mediated StAR expression
It is known that ORA and ORB work through two related GPCRs (OX1R and OX2R) (1) and that ORA is equipotent for both (∼30 nm), whereas ORB has a higher affinity for OX2R than for OX1R (∼30 and 300 nm, respectively) (4). In addition, the effects of orexin receptors are known to be mediated through several different pathways, with promiscuity for Gq, Gs, and Gi heterotrimeric G proteins (5).
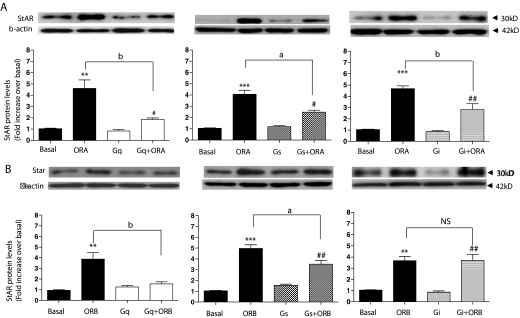
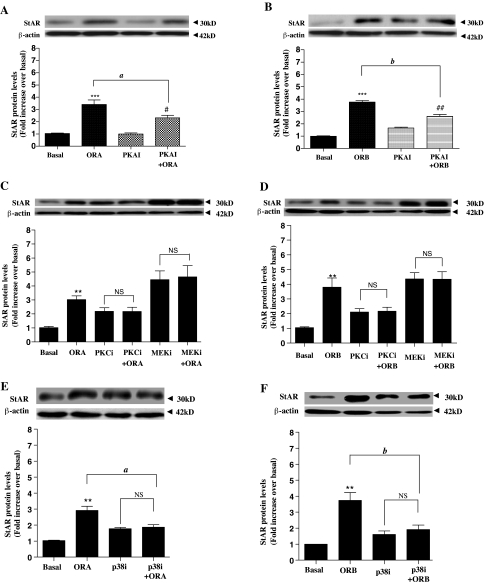
To decipher the precise G protein-signaling pathways required for the significant effects of orexin-mediated StAR activation, dominant-negative Gq, Gs, and Gi constructs were transfected into the H295R adrenal cell line and stimulated with 100 nm of either ORA or ORB for 4 h. Western blot analyses of the subsequent StAR expression levels are shown in Fig. 5. Interestingly, none of the three G proteins could be down-regulated without a significant reduction in StAR expression in response to ORA (Fig. 5A), with approximately half of the expression reduced by inhibition of Gq, Gs, and Gi. Although Gq inhibition had the greatest deleterious effect, none of these with either ORA or ORB at 100 nm for 4 h led to a complete inhibition of StAR expression down to basal levels. Surprisingly, the same inhibition profile was not seen for the ORB-mediated StAR expression (Fig. 5B). Transfection of the dominant-negative Gq led to an almost complete reduction of StAR expression, from 3.7 ± 0.61-fold over basal for the wild type down to levels comparable with basal (1.3 ± 0.19-fold), and Gs inhibition showed a small but significant (0.01 < P < 0.05) reduction. In contrast to the ORA data, the reduction in StAR expression using the dominant-negative Gi transfection was not significantly different from that seen in wild-type H295R cells (from 3.7 ± 0.36-fold over basal to 3.6 ± 0.54-fold).
Figure 5.
The effect of inducing dominant-negative Gq, Gs, and Gi G protein expression in H295R cells on StAR expression. Dominant negative vectors were transfected into H295R cells (∼70% confluence) in 12-well plates. After a further 24 h, cells were cultured for the indicated periods in response to 100 nm ORA (A) and ORB (B). Basal levels were obtained from ORA and ORB-treated cells transfected with pcDNA3.1 alone. StAR levels were determined and quantified by Western blot exactly as described for Fig. 3. Data points are means ± se from six independent experiments. *, Significant difference from basal for orexin-stimulated expression (*, P < 0.05; **, P < 0.01; ***, P < 0.001). #, Significant difference from basal for orexin-stimulated expression in the presence of the dominant-negative G protein using Friedman’s ANOVA with Dunn’s test for post hoc analysis (#, P < 0.05; ##, P < 0.01). Significance between stimulated expression ± dominant-negative G protein is indicated (a, P < 0.01; and b, P < 0.05). NS, Not significant.
Inhibition of multiple kinase signaling pathway components on orexin-mediated StAR expression
The precise expression levels and down-regulation effect of the dominant-negative G proteins themselves cannot be easily measured. To confirm the different relative effects of ORA/ORB-mediated G protein-signaling pathways on StAR expression levels, we compared the relative StAR expression (in H295R cells) in response to both ORA and ORB in the presence of PKAi and PKCi. PKA and PKC are key mediators of the Gs and Gq pathways. In addition, orexins have been proposed to play a role in adrenal cells in ERK1/2 and p38 activation (unpublished data). ERK1/2 + p38 are representative proteins from two of the four mammalian MEK kinase/MEK/MEK cascades (24). Therefore, we included the inhibitors U0126 (MEK1/2/ERK1/2 inhibition) and p38i in our experimental design. The data in Fig. 6 showed that PKAi led to a partial reduction in response to both ORA (from 3.4 ± 0.36-fold to 2.3 ± 0.20-fold over basal) and ORB (from 3.8 ± 0.13-fold to 1.2 ± 0.27-fold over basal). PKCi led to an almost complete reduction of StAR expression in response to both ORA (from 3.8 ± 0.16-fold to 1.2 ± 0.29-fold) and ORB (from 3.8 ± 0.13-fold to 1.2 ± 0.27-fold). Furthermore, MEK1/2 (ERK1/2) inhibition (MEKi), as well as p38i (through SB203580), abolished the relative increase in StAR expression in response to both ORA and ORB (Fig 6, C–F). RT-PCR analysis showed that MEK inhibition increased StAR mRNA levels, consistent with that observed for protein (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Figure 6.
Comparing the StAR expression in H295R cells in the presence of several pathway inhibitors. The indicated inhibitors were added to H295R cells (∼70% confluence) in 12-well plates and incubated for at least 30 min. Cells were then cultured for the indicated periods in response to: PKAI stimulated by 100 nm ORA (A); PKAI stimulated by 100 nm ORB (B); PKCi through Bis and MEK1/2/ERK1/2-inhibition stimulated by 100 nm ORA (C); PKCi through Bis and MEKi stimulated by 100 nm ORB (D); p38i through SB203580 stimulated by 100 nm ORA (E); and p38i through SB203580 stimulated by 100 nm ORB (F). D, Angiotensin-stimulated StAR expression was included as a control for approximate maximum expression. StAR levels were determined and quantified by Western blot exactly as described for Fig. 3. Data points are means ± se from six independent experiments. *, Significant difference from basal for orexin-stimulated expression using Friedman’s ANOVA with Dunn’s test for post hoc analysis (**, P < 0.01; ***, P < 0.001). #, Significant difference from basal for orexin-stimulated expression in the presence of the inhibitors (#, P < 0.05; ##, P < 0.01). Significance between stimulated expression ± inhibitors is indicated (a, P < 0.05; and b, P < 0.01). NS, Not significant.
These data are consistent with the dominant-negative G protein data suggesting that ORA-mediated StAR expression is mediated by multiple G protein-signaling pathways, whereas the ORB effects are predominantly, but not exclusively, through the Gq-coupled system. In addition, these multiple signaling pathways leading to StAR expression are mediated through both ERK1/2-dependent and p38-dependent MAPK cascades.
Using the OX1R-specific nonpeptide antagonist SB334867 to discriminate orexin receptor-specific effects on StAR expression
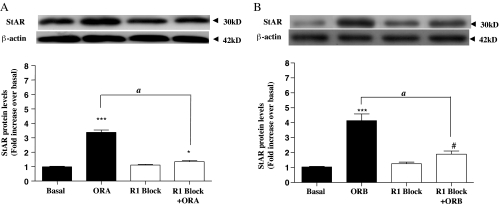
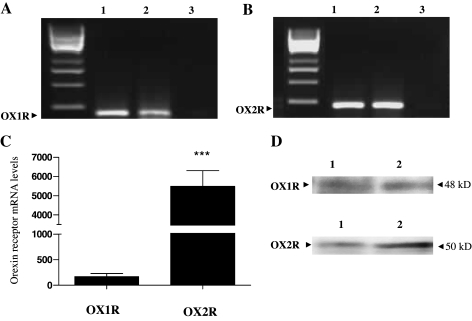
As described previously, ORA is equipotent for OX1R and OX2R, whereas ORB favorably binds OX2R. SB334867 is a nonpeptide antagonist that is highly potent and discriminative for the OX1R (25). To determine whether the difference between the ORA-mediated and ORB-mediated signaling pathways leading to increased StAR expression is reflected, in part, by differential interaction with the two GPCRs, StAR expression was compared for both orexin agonists in the presence and absence of 10 μm SB334867. The results were intriguing. ORA stimulated StAR expression was completely attenuated in the presence of the OX1R-specific antagonist (Fig. 7A), despite the equipotent affinity for the two receptors. Even more surprisingly, the significant majority of StAR expression in response to ORB was also blocked by the OX1R-specific antagonist (Fig. 7B). This suggests that despite a relative affinity for ORB 5- to 10-fold lower for OX1R than for the OX2R, most, but not all, of the StAR expression after ORB activation was mediated through OX1R, despite the fact that mRNA for both receptors was detected in these cells using RT-PCR techniques and direct Western blotting (Fig. 8), although OX2R levels were higher than those of OX1R (Fig. 8, C and D).
Figure 7.
The effects of 10 μm OX1R-specific nonpeptide antagonist SB334867 on StAR expression. The antagonist was added to H295R cells (∼70% confluence) in 12-well plates and incubated for 1 h. Cells were then cultured for the indicated periods in response to 100 nm ORA (A) and ORB (B). StAR levels were determined and quantified by Western blot exactly as described for Fig. 3. Data points are means ± se from six independent experiments. *, Significant difference from basal for orexin-stimulated expression using Friedman’s ANOVA with Dunn’s test for post hoc analysis (*, P < 0.05; ***, P < 0.001). #, Significant difference (P < 0.05) from basal for orexin-stimulated expression in the presence of the antagonist. Significance between stimulated expression ± antagonist is indicated (A, P < 0.01).
Figure 8.
RT-PCR and Western blot analyses showing the presence of mRNA and protein of both orexin receptor subtypes in H295R cells. Cells were grown for 48 h. RNA was isolated, reverse transcribed, and used as a template for RT-PCR and quantitative PCR using OX1R, OX2R, and GAPDH specific primers. Protein was extracted in sample buffer. RT-PCR for OX1R (A) and OX2R (B). PCR products were run on a 1% agarose gel. Lanes correspond to: 1) the cloned receptor positive control, 2) endogenous human receptor, and 3) water. C, Quantitative PCR showing endogenous expression of OX1R and OX2R. mRNA was normalized against GAPDH expression and expressed as percentage of basal. D, OX1R and OX2R protein levels were determined by Western blot exactly as described for Fig. 3. In each case, similar results were obtained from mRNA isolated from four independent experiments of H295R cells (***, P < 0.001).
alamarBlue cell proliferation assay
Neither orexin (ORA or ORB) increased H295R cell proliferation after 4 or 24 h stimulation with 100 nm agonist (supplemental Fig. 2).
Discussion
Orexins are known to be involved in a host of physiological processes, including obesity, sleep regulation, and steroid secretion (26,27). We have previously reported the presence of orexin receptors in the human adrenal gland that couple to multiple G proteins, activating both cAMP and inositol 1,4,5-trisphosphate (6), and others have reported Gq/11-independent calcium influx through the OX1R (28). Steroidogenesis is a crucial factor in adrenal physiology leading to the production and secretion of several key hormones. Because steroidogenic cells store very little steroid, a rapid steroidogenic response requires rapid synthesis of new steroid (20). The chronic regulation of steroidogenic capacity is at the transcriptional level, primarily of genes coding for the steroidogenic enzymes (29). These include a number of enzymes with proposed roles in the pathophysiology of a number of diseases (14,30).
The stimulation of adrenocortical steroidogenesis involves up-regulation of StAR, a critical molecule in steroidogenesis, mediating the transfer of cholesterol from the outer to the inner mitochondrial membrane for steroid biosynthesis to take place, acting as a first-rate limiting step (31,32). Acute regulation of steroidogenesis is at the level of substrate access to p450scc, which is regulated through cholesterol transport into the mitochondria and can take place only in the presence of StAR to mediate the cholesterol transfer in adrenal cells.
Our initial treatment of the pluripotent, adrenal H295R cells with one of ORA or ORB resulted in a significant increase in StAR mRNA levels, peaking at 2 h, followed by a gradual decline and then a second significant increase after 24 h. This novel study also demonstrated a significant up-regulation of StAR at the protein level by ORA and ORB in human adrenocortical cells. The importance of the StAR and the novelty of this finding led us to concentrate our study on the cell signaling and mechanisms required for orexin-mediated StAR expression. We observed a dose-dependent increase of StAR mRNA with a relatively potent effect, reaching significance at an orexin concentration of 1 nm (for both ORA and ORB). The reported affinity of orexins for their receptors is around 30 nm, suggesting a receptor occupancy at this concentration of less than 10%; although ORA is equipotent for OX1R and OX2R, ORB only binds OX2R with such high affinity.
The subsequent Western blot analysis of dose-dependent and time-dependent StAR expression was consistent with the mRNA data and reflects effects at a relatively low receptor occupancy, assuming, of course, that the reported receptor affinities are the same here as previously reported. This is not considering the possible affinity differences with different cells and the possibility of hypersensitization through other orexin receptors, or indeed through an unknown third-party molecule (33). It was interesting that the temporal analysis of StAR mRNA and protein expression seen after 2 and 4 h, respectively, declined and then spiked again at 24 h. It is not clear if this reflects a cycling effect or indirect gene expression in response to a second component. This apparent cycling phenomenon has previously been seen for StAR mRNA expression in response to angiotensin II treated bovine adrenocortical cells (18). It is also important to note that the activation of StAR through phosphorylation and other signaling molecules are important factors in the transport of cholesterol, and there may be a role for hormones, including orexin, in these processes (34). It has been observed previously that StAR expression and phosphorylation were both up-regulated in response to angiotensin II (35,36).
Orexins mediate their actions through two GPCRs (OX1R and OX2R), both of which can signal through multiple G proteins, and are expressed widely in the hypothalamus and adrenal gland (6,37). To assess the precise signaling pathways leading to StAR expression, we compared the effect of expressing different dominant-negative G proteins in H295R adrenal cells on orexin-induced StAR production. Our data strongly suggest that StAR expression in response to ORB acts predominantly through Gq-mediated signaling (with a small Gs effect), whereas all three pathways (Gq, Gs, and Gi) were significantly involved in the up-regulation of StAR in adrenal cells in response to ORA. The inhibition studies provide further evidence for the concomitant activation of more than one G protein-coupling pathway leading to StAR expression. Although PKCi completely disrupted StAR expression in response to either orexin, PKAI after ORA or ORB stimulation was also significantly reduced. The downstream MAPK signaling cascades involving both ERK1/2 and p38 were also required for the increase in StAR levels. This is consistent with previous studies showing that cAMP/PKA (23) and inositol 1,4,5-trisphosphate/PKC (38) are the major signaling cascades regulating steroidogenesis as well as StAR by GPCRs. In addition, PKA is considered to be an important regulator of StAR activity by posttranslational phosphorylation (39). Furthermore, direct involvement of ERK1/2 in acute StAR regulation has been shown in rat Leydig cells (40). Several studies have reported the involvement of various signaling cascades, including the cAMP/PKA/PKC/cAMP response element-binding protein pathways, in steroidogenesis and in the up-regulation of steroidogenic enzymes and StAR, including the ERK1/2 (34,41) and p38 MAPK cascades (42,43,44). Although basal levels in the presence of inhibitors were occasionally higher than in their absence, particularly for the MEK inhibitor U0126, this phenomenon has also been seen in both primary cells and mouse Leydig cells, and was thought to represent an issue of stimulus specificity inducing StAR transcriptional activity, but not steroidogenesis, as a direct response to inhibitor treatment (45,46). We also observed a similar increase in StAR mRNA with MEKi (data not shown). Intriguingly, the OX1R-specific antagonist SB334867 showed that StAR expression through ORA was acting, almost exclusively, through OX1R, and even ORB-mediated StAR expression was predominantly a response to the OX1R. This occurs even though the affinity for ORB at OX1R is only 300 nm, around 10-fold lower than the affinity for OX2R. It was previously suggested that cortisol synthesis occurred through the OX1R (9), and this appears to be generally true in terms of StAR expression, although the ORB effect seems able to use an OX2R component. It is distinctly possible that these effects are mediated through the OX2R or, indeed, through a heterodimer, either of OX1R/OX2R, or through one orexin receptor and an undetermined third party. Indeed, OX1R/cannabinoid CB1 receptor heterodimers have been hypersensitive to orexin stimulation (33). The RT-PCR showed expression of both OX2R and OX1R mRNA, and it may be interesting to analyze the precise protein expression levels of these receptors in H295R cells.
These studies show that StAR expression is significantly higher even at concentrations similar to that in circulation. It was previously observed that in dispersed rat adrenocortical cells, both orexins stimulated corticosterone but not aldosterone production in a dose-dependent manner (26). In human adrenocortical cells, ORA but not ORB enhanced cortisol secretion (27), and both orexins (with ORB being more potent) enhanced proliferative activity of zona glomerulosa cells in immature rats (26). These studies combined with our observations showing that increases in StAR expression in response to orexins suggest a direct link between StAR up-regulation and steroidogenesis. However, it would be interesting to assess directly the effect of transcriptional or translational inhibition of StAR expression on steroidogenesis. A recent review (20) discussed the independent as well as synergistic effects of multiple signaling pathways leading to the regulation of StAR, like this study, emphasizing the fundamental complexity of steroid biosynthesis. In addition, although our orexin-mediated effects on StAR expression are broadly comparable with those of angiotensin, the angiotensin was only used as a positive control reflecting cellular function. Therefore, it would be an interesting future study to assess the potential physiological importance of orexins through comparison with other known stimulatory molecules, such as (Bu)2-cAMP and potassium (36).
To conclude, this is the first time that StAR expression has been shown to be up-regulated by orexin. We present evidence that ORA-mediated StAR expression is exclusively channeled through the OX1R, coupling through all three major G protein-signaling pathways (Gq, Gs, and Gi). StAR expression in response to ORB can be mediated by the OX1R or OX2R, although this is predominantly through the Gq subunit and, to a lesser extent, the Gs-signaling pathway. Furthermore, for both ORA and ORB-stimulated StAR expression, the major MEK signaling cascade mediators ERK1/2 and p38 are crucial components.
Supplementary Material
Footnotes
This work was supported by the Coventry General Charities. J.C. is funded by The British Heart Foundation (PG/03/131/16192).
First Published Online May 1, 2008
Abbreviations: Bis, 2-[1-[2-(1-Methylpyrrolidino)ethyl]-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPCR, G protein-coupled receptor; MEK, MAPK kinase; MEKi, MAPK kinase 1/2 (ERK1/2) inhibition; ORA, orexin A; ORB, orexin B; OX1R, orexin receptor type 1; OX2R, orexin receptor type 2; p38i, p38 inhibition; PKA, protein kinase A; PKAi, protein kinase A inhibitor; PKC, protein kinase C; PKCi, protein kinase C inhibitor; PVDF, polyvinylidene difluoride; StAR, steroidogenic acute regulatory protein; U0126, 1,4-diamino-2,3-dicyano-1,4-bis (2-aminophenylthio) butadiene.
References
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M 1998 Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:697 [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Ostman M, Ammoun S, Akerman KE, Kukkonen JP 2005 OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem 280:6570–6579 [DOI] [PubMed] [Google Scholar]
- Karteris E, Randeva HS 2003 Orexin receptors and G-protein coupling: evidence for another “promiscuous” seven transmembrane domain receptor. J Pharmacol Sci 93:126–128 [DOI] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP 2003 Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther 305:507–514 [DOI] [PubMed] [Google Scholar]
- Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW 2001 Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab 86:4512–4519 [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW 2001 Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab 86:4808–4813 [DOI] [PubMed] [Google Scholar]
- Johren O, Bruggemann N, Dominiak P 2004 Orexins (hypocretins) and adrenal function. Horm Metab Res 36:370–375 [DOI] [PubMed] [Google Scholar]
- Perello M, Moreno G, Gaillard RC, Spinedi E 2004 Glucocorticoid-dependency of increased adiposity in a model of hypothalamic obesity. Neuro Endocrinol Lett 25:119–126 [PubMed] [Google Scholar]
- Ziolkowska A, Spinazzi R, Albertin G, Nowak M, Malendowicz LK, Tortorella C, Nussdorfer GG 2005 Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the OX1 receptor. J Steroid Biochem Mol Biol 96:423–429 [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Rucinski M, Neri G, Malendowicz LK, Nussdorfer GG 2005 Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab 90:3544–3549 [DOI] [PubMed] [Google Scholar]
- Goodfriend TL, Egan BM, Kelley DE 1998 Aldosterone in obesity. Endocr Res 24:789–796 [DOI] [PubMed] [Google Scholar]
- Lamounier-Zepter V, Bornstein SR, Ehrhart-Bornstein M 2004 Mechanisms of obesity-related hypertension. Horm Metab Res 36:376–380 [DOI] [PubMed] [Google Scholar]
- Miller WL 2007 Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676 [DOI] [PubMed] [Google Scholar]
- New MI 2003 Inborn errors of adrenal steroidogenesis. Mol Cell Endocrinol 211:75–83 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- Stocco DM 2001 Tracking the role of a star in the sky of the new millennium. Mol Endocrinol 15:1245–1254 [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss 3rd JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL 1995 Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM 2000 Regulation by adrenocorticotropin (ACTH), angiotensin II, transforming growth factor-β, and insulin-like growth factor I of bovine adrenal cell steroidogenic capacity and expression of ACTH receptor, steroidogenic acute regulatory protein, cytochrome P450c17, and 3β-hydroxysteroid dehydrogenase. Endocrinology 141:1599–1607 [DOI] [PubMed] [Google Scholar]
- Reinhart AJ, Williams SC, Stocco DM 1999 Transcriptional regulation of the StAR gene. Mol Cell Endocrinol 151:161–169 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR 2005 Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI 1994 The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100:45–50 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Lange IG, Daxenberger A, Meyer HH 2001 Tissue-specific expression pattern of estrogen receptors (ER): quantification of ERα and ERβ mRNA with real-time RT-PCR. APMIS 109:345–355 [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM 2005 Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord 5:93–108 [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ 2007 Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291–3310 [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC 2001 SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol 132:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz LK, Tortorella C, Nussdorfer GG 1999 Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol 70:185–188 [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG 2001 Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab 86:778–782 [DOI] [PubMed] [Google Scholar]
- Magga J, Bart G, Oker-Blom C, Kukkonen JP, Akerman KE, Nasman J 2006 Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol 71:827–836 [DOI] [PubMed] [Google Scholar]
- Simpson ER, Waterman MR 1988 Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol 50:427–440 [DOI] [PubMed] [Google Scholar]
- Hammer F, Stewart PM 2006 Cortisol metabolism in hypertension. Best Pract Res Clin Endocrinol Metab 20:337–353 [DOI] [PubMed] [Google Scholar]
- Miller WL 1998 Early steps in androgen biosynthesis: from cholesterol to DHEA. Baillieres Clin Endocrinol Metab 12:67–81 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 51:197–205 [DOI] [PubMed] [Google Scholar]
- Hilairet S, Bouaboula M, Carriere D, Le Fur G, Casellas P 2003 Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem 278:23731–23737 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, Jo Y, Stocco DM 2006 cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37:81–95 [DOI] [PubMed] [Google Scholar]
- Betancourt-Calle S, Calle RA, Isales CM, White S, Rasmussen H, Bollag WB 2001 Differential effects of agonists of aldosterone secretion on steroidogenic acute regulatory phosphorylation. Mol Cell Endocrinol 173:87–94 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Pezzi V, Stocco DM, Rainey WE 1995 The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol 115:215–219 [DOI] [PubMed] [Google Scholar]
- Lopez M, Senaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, Casanueva F, Dieguez C 1999 Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology 140:5991–5994 [DOI] [PubMed] [Google Scholar]
- Catt KJ, Carson MC, Hausdorff WP, Leach-Harper CM, Baukal AJ, Guillemette G, Balla T, Aguilera G 1987 Angiotensin II receptors and mechanisms of action in adrenal glomerulosa cells. J Steroid Biochem 27:915–927 [DOI] [PubMed] [Google Scholar]
- Fleury A, Mathieu AP, Ducharme L, Hales DB, LeHoux JG 2004 Phosphorylation and function of the hamster adrenal steroidogenic acute regulatory protein (StAR). J Steroid Biochem Mol Biol 91:259–271 [DOI] [PubMed] [Google Scholar]
- Martinelle N, Holst M, Soder O, Svechnikov K 2004 Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology 145:4629–4634 [DOI] [PubMed] [Google Scholar]
- Krug AW, Vleugels K, Schinner S, Lamounier-Zepter V, Ziegler CG, Bornstein SR, Ehrhart-Bornstein M 2007 Human adipocytes induce an ERK1/2 MAP kinases-mediated upregulation of steroidogenic acute regulatory protein (StAR) and an angiotensin II-sensitization in human adrenocortical cells. Int J Obes (Lond) 31:1605–1616 [DOI] [PubMed] [Google Scholar]
- Foster RH 2004 Reciprocal influences between the signalling pathways regulating proliferation and steroidogenesis in adrenal glomerulosa cells. J Mol Endocrinol 32:893–902 [DOI] [PubMed] [Google Scholar]
- Otis M, Gallo-Payet N 2007 Role of MAPKs in angiotensin II-induced steroidogenesis in rat glomerulosa cells. Mol Cell Endocrinol 265- 266:126–130 [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Stocco DM, Soder O 2003 Interleukin-1α stimulates steroidogenic acute regulatory protein expression via p38 MAP kinase in immature rat Leydig cells. J Mol Endocrinol 30:59–67 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM 2006 Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse Leydig cells. Mol Endocrinol 20:362–378 [DOI] [PubMed] [Google Scholar]
- Martinat N, Crepieux P, Reiter E, Guillou F 2005 Extracellular signal-regulated kinases (ERK) 1, 2 are required for luteinizing hormone (LH)-induced steroidogenesis in primary Leydig cells and control steroidogenic acute regulatory (StAR) expression. Reprod Nutr Dev 45:101–108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.