Abstract
The adipocyte enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) amplifies local glucocorticoid action by generating active glucocorticoids from inactive metabolites and has emerged as a key player in the pathogenesis of central obesity and metabolic syndrome. However, the regulation of adipocyte 11β-HSD1 is incompletely understood. Therefore, the present study was designed to investigate the effects of insulin and glucocorticoid as well as their underlying molecular mechanisms on 11β-HSD1 activity and expression in 3T3-L1 adipocytes and determine whether the in vitro findings could be confirmed in vivo. Our main in vitro findings are 1) insulin stimulated whereas dexamethasone inhibited 11β-HSD1 activity and expression in a time- and concentration-dependent manner; 2) the effect of dexamethasone was mimicked by both cortisol and corticosterone but blocked by the glucocorticoid receptor antagonist RU486; 3) the p38 MAPK inhibitor SB220025, but not the ERK inhibitor U0126 or the phosphatidylinositol 3-kinase inhibitor LY294002, prevented insulin stimulation of 11β-HSD1 activity; and 4) although dexamethasone did not alter the half-life of 11β-HSD1 mRNA, insulin doubled it. Taken together, these in vitro results demonstrate that insulin stimulates adipocyte 11β-HSD1 through a posttranscriptional mechanism that involves activation of the p38 MAPK signaling pathway, whereas dexamethasone exerts an opposite effect by a glucocorticoid receptor-mediated transcriptional mechanism. In contrast, both insulin and dexamethasone augmented 11β-HSD1 activity and expression in rat white adipose tissue in vivo, thus confirming the role of insulin but revealing a fundamental difference regarding the role of dexamethasone in regulating adipocyte 11β-HSD1 between the two model systems.
THE PATHOPHYSIOLOGICAL significance of glucocorticoid is exemplified in Cushing’s syndrome, wherein patients with glucocorticoid excess develop central obesity and its associated metabolic disorders (1,2). The adipocyte reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent endoplasmic reticulum (ER) enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1; encoded by HSD11B1 gene), which amplifies local glucocorticoid action by converting the inactive glucocorticoid cortisone to active cortisol (11-dehydrocorticosterone to corticosterone in rodents), has emerged as a key player in the pathogenesis of central obesity and metabolic syndrome, because transgenic mice overexpressing 11β-HSD1 specifically in adipose tissue recapitulate Cushing’s syndrome (3). Conversely, 11β-HSD1-deficient mice exhibit reduced visceral adiposity upon high-fat feeding (4), an improved lipid and lipoprotein profile, enhanced glucose tolerance, and increased insulin sensitivity (5,6). Moreover, adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity (7), whereas 11β-HSD1 activity and expression in human adipose tissue are increased in obesity (8,9,10,11,12,13,14,15,16). Thus, these findings underscore the critical importance of gaining a thorough understanding of regulation of adipocyte 11β-HSD1.
Although a number of factors are known to regulate adipocyte 11β-HSD1 activity and expression, including peroxisome proliferator-activated receptor-γ (17), liver X receptor (18), dehydroepiandrosterone (19), CCAAT/enhancer-binding protein (C/EBP)α and C/EBPβ (20), bezafibrate (21), and ceramide and AMP-activated protein kinase (22), two principal candidates have emerged in the past few years. Several in vivo studies in the human have focused on the effect of insulin with inconsistent results. Some studies have shown a stimulatory effect of insulin on 11β-HSD1 expression and/or activity (23,24), whereas others have reported an inhibitory (25) or no effect (26) on these parameters in human adipose tissue. One in vitro study has revealed an inhibitory effect of insulin on adipocyte 11β-HSD1 mRNA, but no data on 11β-HSD1 activity were reported (27). Furthermore, the molecular mechanisms by which insulin regulates adipocyte 11β-HSD1 remain to be determined. Accumulating evidence has also implicated a key role for hexose-6-phosphate dehydrogenase (H6PD), an ER-resident enzyme that is functionally coupled to 11β-HSD1 by generating the obligate cofactor NADPH, in modulating 11β-HSD1 activity (28,29,30,31).
11β-HSD1 is anchored in the ER membrane with the bulk of the peptide including its catalytic site inside the ER lumen (32,33,34), and its reductase activity requires NADPH as the cofactor (29). Most cytosolic NADPH is produced by the cytosolic enzyme glucose-6-phosphate dehydrogenase. Because NADPH dose not freely cross the ER membrane, the ER-resident enzyme H6PD is responsible for generating this required cofactor for 11β-HSD1 (29,35). Indeed, H6PD is colocalized with 11β-HSD1 in many mammalian tissues (36), and H6PD-deficient mice lack 11β-HSD1 reductase activity (i.e. unable to convert 11-dehydrocorticosterone to corticosterone) (37). Furthermore, forced expression of H6PD increases 11β-HSD1 reductase activity in HEK-293 cells (38). Conversely, small interfering RNA-mediated H6PD knockdown causes a significant decrease in 11β-HSD1 reductase activity in vitro (39). Taken together, these studies suggest that H6PD may be a major determinant of 11β-HSD1 activity. However, the relative contribution of variations in H6PD expression (in relation to those in 11β-HSD1 expression) to changes in 11β-HSD1 activity in response to hormonal regulation in vitro and in vivo is uncertain, because previous studies were carried out in an environment where 11β-HSD1 expression was unaltered. Indeed, one recent study has cast some doubt on the role of H6PD in modifying 11β-HSD1 activity in vivo in humans (23).
There is robust evidence that glucocorticoid stimulates HSD11B1 gene expression and 11β-HSD1 activity in vivo and in vitro in nonadipose cells/tissues (40). Although one recent study provided evidence that glucocorticoid receptor (GR) and C/EBPα were involved in cortisol-induced 11β-HSD1 mRNA expression via binding to HSD11B1 promoter in human amnion fibroblasts (41), the molecular mechanisms underlying glucocorticoid induction of HSD11B1 remain largely unknown. Moreover, there is conflicting information regarding the role of glucocorticoid in regulating adipocyte 11β-HSD1. A previous in vitro study showed that dexamethasone decreased 11β-HSD1 mRNA by 80% in 3T3-F442A adipocytes (27). However, this is in marked contrast to an in vivo study by Livingstone and colleagues (42), who implicated a role for glucocorticoid in up-regulating adipocyte 11β-HSD1, because adrenalectomy reduced 11β-HSD1 activity in omental fat of obese Zucker rats.
In summary, although there is evidence that insulin, glucocorticoid, and H6PD regulate adipocyte 11β-HSD1 activity and/or expression, the literature is both inconsistent and incomplete. Therefore, the present study was undertaken to better characterize the regulation of adipocyte 11β-HSD1 activity and expression by insulin and dexamethasone, and to define the underlying molecular mechanisms and signal transduction pathways involved, using 3T3-L1 adipocytes as an in vitro model system. Furthermore, experiments were performed to gain insight into the relative contribution of variations in H6PD and HSD11B1 gene expression to changes in 11β-HSD1 activity in vitro by examining the concurrent effect of insulin and dexamethasone on H6PD expression. In addition, parallel in vivo studies in the rat were conducted to determine whether our in vitro findings could be confirmed.
Materials and Methods
Overview
In this study, we used the well-established cultured 3T3-L1 adipocytes as an in vitro model system in which to characterize the effects, and define the molecular mechanisms, of insulin and dexamethasone on 11β-HSD1 activity and expression. The primary focus of our study was on 11β-HSD1 reductase activity, because it represents the main biological function of this enzyme in intact cells and in vivo (43,44). Changes in 11β-HSD1 mRNA were determined by real-time quantitative RT-PCR (qRT-PCR), and found to be closely associated with alterations in 11β-HSD1 enzyme activity. Consequently, 11β-HSD1 mRNA levels were taken as indicators of 11β-HSD1 expression. Finally, we studied the effects of insulin and dexamethasone on 11β-HSD1 activity and expression as well as H6PD expression in rat white adipose tissue in vivo to determine whether our in vitro findings could be confirmed.
Reagents and supplies
[1,2,3-3H]Cortisone (50 Ci/mmol) and [1,2,6,7-3H]corticosterone (70 Ci/mmol) were purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO) and PerkinElmer Life & Analytical Sciences (Woodbridge, Ontario, Canada), respectively. Nonradioactive cortisone, cortisol, corticosterone, and 11-dehydrocorticosterone were obtained from Steraloids Inc. (Wilton, NH). RU-486, 5,6-dichlorobenzimidazole 1-β-d-ribfuranoside (DRB), U0126, LY294002, and SB220025 were purchased from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Polyester-backed thin-layer chromatography plates were obtained from Fisher Scientific Ltd. (Nepean, Ontario, Canada). All solvents used were from VWR Canlab (Mississauga, Ontario, Canada). Cell culture supplies were obtained from either Invitrogen Life Technologies (Burlington, Ontario, Canada) or Fisher Scientific. General molecular biology reagents were from Invitrogen or Pharmacia Canada Inc. (Baie d'Urfe, Quebec, Canada). Oligonucleotides were purchased from Sigma Genosys (Oakville, Ontario, Canada).
3T3-L1 cultures and differentiation
The murine preadipocyte 3T3-L1 cell line was obtained from the American Type Culture Collection (Manassas, VA). 3T3-L1 cells were cultured and differentiated, as previously described (45). Briefly, they were cultured in growth medium, consisting of DMEM (Sigma) supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin (Invitrogen), and 10% fetal bovine serum (Sigma). Cultures were maintained in a humidified incubator at 5% CO2 and 37 C. Medium was replaced every 2–3 d.
At 2 d after confluence (d 0), cells were induced to differentiate by the addition of a standard differentiation cocktail containing 500 μm 3-isobutyl-1-methylxanthine (Sigma), 0.25 μm dexamethasone (Alpharma, Boucherville, Quebec, Canada), and 1 μg/ml insulin (Eli Lilly Canada Inc., Toronto, Ontario, Canada). At d 2, the medium was replaced with growth medium supplemented with 1 μg/ml insulin and then replaced at d 4 and 6 with growth medium. At d 8, differentiation was 95% or higher, as determined by Oil red O staining. All treatments were carried out on differentiated 3T3-L1 adipocytes and preceded by a 2-h starving period during which cells were washed and incubated in serum-free medium. Each treatment was performed in triplicate under serum-free conditions, and a total of four to six independent experiments were carried out. For each treatment condition, controls were included and treated for the same time with an identical volume of the vehicle, as described in detail in figure legends.
In vivo studies
The use of animals in this study was approved by the Council on Animal Care at the University of Western Ontario, following the guidelines of the Canadian Council on Animal Care. Male Wistar rats at 7 wk of age were purchased from Charles River Laboratories (Wilmington, MA). They were housed at 22 C on a 12-h light, 2-h dark (0700–1900 h) cycle, and allowed free access to standard rat chow and drinking water for 5–7 d before treatment. They were then randomly assigned to one of the three treatment groups (control and glargine- and dexamethasone-treated), respectively. All animals were weighed daily before treatment, and the dosage of drugs was adjusted daily based on their body weight. The long-acting insulin analog glargine (0.5 IU/kg body weight) and the synthetic glucocorticoid dexamethasone (120 μg/kg body weight) were injected sc daily at 1000 h, as previously described (45). The reason for using glargine instead of insulin is that administration of this analog in humans results in a constant level of insulin in circulation thereby eliminating the need to adjust blood glucose levels by glucose clamp (46). The dosage of glargine (0.5 IU/kg body weight) and dexamethasone (120 μg/kg body weight) represented the therapeutic dosage given to type 2 diabetic patients (46) and stress levels of glucocorticoid (47), respectively. Drugs were diluted in 0.9% saline solution for injection, and a matched volume of the saline solution was injected daily into the control group. On the eighth day of treatment, animals were killed, and epididymal white adipose tissues were collected, flash-frozen in liquid nitrogen, and stored at −80 C.
Assay of 11β-HSD1 activity: radiometric conversion assay
Intact cell assay.
The level of 11β-HSD1 reductase activity in intact 3T3-L1 adipocytes was determined by measuring the rate of conversion of cortisone to cortisol, as described previously (48). Briefly, 3T3-L1 adipocytes were incubated for 30 min at 37 C in serum-free medium containing approximately 100,000 dpm [3H]cortisone and 12 μm unlabeled cortisone. At the end of incubation, the medium was collected and steroids extracted. The extracts were dried and the residues resuspended. A fraction of the resuspension was spotted on a thin-layer chromatography plate that was developed in chloroform/methanol (9:1, vol/vol). The bands containing the labeled cortisone and cortisol were identified by UV light of the cold carriers, cut out into scintillation vials, and counted in Scintisafe Econo 1 (Fisher Scientific). The rate of cortisone to cortisol conversion was calculated, with the blank values (defined as the amount of conversion in the absence of cells) subtracted, and expressed as percentage of control. Results are shown as mean ± sem.
Tissue homogenate assay.
The level of 11β-HSD1 dehydrogenase activity in adipose tissue homogenates was determined by measuring the rate of conversion of corticosterone to 11-dehydrocorticosterone, as described (49). Briefly, white adipose tissues (0.1–0.2 g) were homogenized in 10 vol ice-cold 10 mm sodium phosphate buffer (pH 7.0) containing 0.25 m sucrose. Protein concentration was determined by the Bradford method using a Bio-Rad (Mississauga, Ontario, Canada) protein assay kit with BSA as standard. The conversion assay tubes contained adipose tissue homogenate (100–200 μg protein), 100,000 dpm [3H]corticosterone, and final concentrations of nonradioactive corticosterone and cofactor NADP at 0.1 and 250 μm, respectively. After incubation in a shaking water bath at 37 C for 30 min (preliminary studies indicated that the rate of reaction was linear with incubation time from 10–60 min, and the amount of tissue homogenates from 50–500 μg protein), the reaction was arrested, and the steroids were extracted and processed similarly as described above. The rate of corticosterone to 11-dehydrocorticosterone conversion was calculated from the specific activity of the labeled corticosterone and the radioactivity of 11-dehydrocorticosterone, and results are expressed as the amount of 11-dehydrocorticosterone (picomoles) formed per minute per milligram protein.
Assessment of 11β-HSD1 mRNA and H6PD mRNA: real-time quantitative RT-PCR
To determine whether changes in 11β-HSD1 activity after the different treatment regimens were associated with alterations in 11β-HSD1 mRNA levels, and to ascertain whether these treatments also affected H6PD expression, the relative abundance of 11β-HSD1 mRNA and H6PD mRNA in 3T3-L1 adipocytes and in rat white adipose tissues was assessed by a two-step qRT-PCR, as described previously (45,50).
Briefly, total RNA was extracted from 3T3-L1 adipocytes using RNeasy Mini Kit (QIAGEN Inc., Mississauga, Ontario, Canada) coupled with on-column deoxyribonuclease digestion with the ribonuclease-free deoxyribonuclease set (QIAGEN), whereas total RNA from homogenized adipose tissues was extracted using Trizol (Invitrogen) and subsequently purified with the RNeasy Mini Kit, following the manufacturers’ instructions. One microgram of total RNA was reverse-transcribed in a total volume of 20 μl using the High Capacity cDNA Archive Kit (Applied Biosystems, Forest City, CA) according to the manufacturer’s instructions. For every RT reaction set, one RNA sample was set up without reverse transcriptase enzyme to provide a negative control. Gene transcript levels of 28S rRNA (housekeeping gene), 11β-HSD1, and H6PD in both 3T3-L1 adipocytes and rat adipose tissues were quantified separately by custom-designed SYBR Green I chemistry-based assays. Gene-and species-specific primers (Table 1) were designed using Primer Express Software (Applied Biosystems, Forest City, CA), and the optimal concentrations for each gene were determined empirically. All primers were purchased from Sigma Genosys. The SYBR Green I assay was performed with the SYBR Green PCR Master Mix (Applied Biosystems) and a modified universal thermal cycling condition (2 min at 50 C and 10 min at 95 C, followed by 40 cycles of 10 sec each at 95 C, 60 C, and 72 C) with the standard disassociation/melting parameters (15 sec each at 95 C, 60 C, and 95 C) on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The specificity of the SYBR Green I assay was verified by performing a melting curve analysis and by subsequent sequencing of the PCR products.
Table 1.
Primers for qRT-PCR
| Gene | Primer sequence |
|---|---|
| Mouse 11β-HSD1 | |
| Forward | 5′-GTGTCTCGCTGCCTTGAACTC-3′ |
| Reverse | 5′-TTTCCCGCCTTGACAATAAATT-3′ |
| Mouse H6PD | |
| Forward | 5′-GGGCCAACAACATAAACATTTCA-3′ |
| Reverse | 5′-GGACGGGCCATCCTAACC-3′ |
| Mouse 28S rRNA | |
| Forward | 5′-TTGAAAATCCGGGGGAGAG-3′ |
| Reverse | 5′-ACATTGTTCCAACATGCCAG-3′ |
| Rat 11β-HSD1 | |
| Forward | 5′-TGGAAGACATGGCTTTTGCA-3′ |
| Reverse | 5′-TCCAGTCCACCCAAGAGCTT-3′ |
| Rat H6PD | |
| Forward | 5′-GCCGCAAGGAGTCCTTCAT-3′ |
| Reverse | 5′-GTACAGGCGTGGGACTTCAAA-3′ |
| Rat 28S rRNA | |
| Forward | 5′-GAATCCGCTAGGGTGTGTAACAA-3′ |
| Reverse | 5′-GCTCCAGCGCCATCCAT-3′ |
Levels of 28S rRNA, 11β-HSD1 mRNA, and H6PD mRNA in each RNA sample were quantified by the relative standard curve method (Applied Biosystems). Briefly, standard curves for 28S rRNA, 11β-HSD1, and H6PD were generated by performing a dilution series of the untreated control cDNA. For each RNA sample, the relative amount of 28S rRNA, 11β-HSD1 mRNA, and H6PD mRNA was obtained, and the ratio of 11β-HSD1 mRNA to 28S rRNA as well as the ratio of H6PD mRNA to 28S rRNA were calculated. For each experiment, the amount of 11β-HSD1 mRNA or H6PD mRNA at any given time point under various treatment conditions is expressed relative to the amount of transcript present in the untreated control.
Assessment of 11β-HSD1 mRNA stability
The effects of insulin and dexamethasone on the rate of 11β-HSD1 mRNA decay were determined as previously described (51). Briefly, 3T3-L1 adipocytes were cultured as outlined above and treated with insulin (100 nm) or dexamethasone (100 nm) for 24 h. Transcription was then stopped with DRB (100 μm), and cells were harvested at discrete times (0–48 h) thereafter for RNA isolation and qRT-PCR analysis.
Assessment of signal transduction pathway and receptor involvement
3T3-L1 adipocytes were cultured as outlined above and pretreated with RU-486 (1 μm) or various kinase inhibitors (10 μm U0126, 30 μm LY294002, or 10 μm SB220025) for 1 h before the addition of dexamethasone (100 nm) or insulin (100 nm). At the end of 48 h treatment, the level of 11β-HSD1 activity in intact cells was determined as described above.
Statistical analyses
Results are presented as mean ± sem of four to six independent experiments, as indicated. Statistical analyses of 11β-HSD1 activity and mRNA as well as H6PD mRNA data were performed using one-way ANOVA followed by Tukey’s post hoc test, except that the data from combined treatment of insulin and dexamethasone were analyzed by two-way ANOVA. Significance was set at P < 0.05. Calculations were performed using Prism 3.0 GraphPad software (San Diego, CA).
Results
Effects of insulin and dexamethasone on 11β-HSD1 activity and mRNA: time course
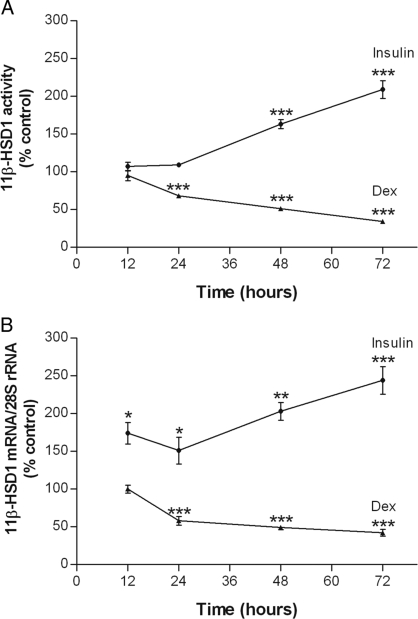
To gain some fundamental insight into hormonal regulation of adipocyte 11β-HSD1, the time-dependent effects of insulin and dexamethasone on 11β-HSD1 activity in 3T3-L1 adipocytes were studied first by a standard radiometric conversion assay. As shown in Fig. 1A, insulin increased 11β-HSD1 activity significantly by 48 h (P < 0.001), raising its levels to 209 ± 12% of control by 72 h (P < 0.001). In contrast, dexamethasone decreased 11β-HSD1 activity by 24 h (P < 0.001) and did so progressively thereafter such that by 72 h, it reduced the enzyme activity to 34 ± 2% of control (P < 0.001).
Figure 1.
Time-dependent effects of insulin and dexamethasone on 11β-HSD1 activity and 11β-HSD1 mRNA. Differentiated 3T3-L1 adipocytes were treated with insulin (100 nm) or dexamethasone (Dex; 100 nm) for various times. For each time point, controls were included and treated with an appropriate volume of vehicle. Levels of 11β-HSD1 activity in intact cells (A) were determined by a standard radiometric conversion assay. Total cellular RNA was extracted, and the steady-state level of 11β-HSD1 mRNA (B) was measured by qRT-PCR. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control.
To determine whether the effects of insulin and dexamethasone on 11β-HSD1 activity were mediated at the level of 11β-HSD1 expression, we assessed the steady-state level of 11β-HSD1 mRNA with qRT-PCR. As shown in Fig. 1B, there were corresponding changes in 11β-HSD1 mRNA after dexamethasone treatment. In the case of insulin, although consistent with its effects on enzyme activity, it increased 11β-HSD1 mRNA as early as 12 h (P < 0.05).
Effects of insulin and dexamethasone on 11β-HSD1 activity and mRNA: dose-response curve
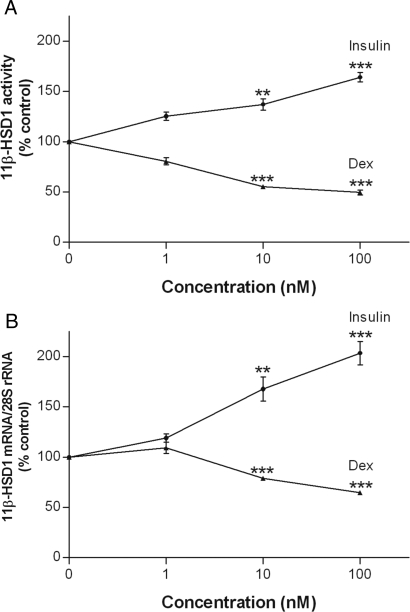
To gain further insight into the regulation of 11β-HSD1 by insulin and dexamethasone, 3T3-L1 adipocytes were treated with increasing concentrations of both hormones (1, 10, and 100 nm), a range that represents physiological to pathological and therapeutic levels in vivo. Although they were ineffective at 1 nm, insulin increased, whereas dexamethasone decreased levels of both 11β-HSD1 activity (Fig. 2A) and mRNA (Fig. 2B) at 10 and 100 nm concentrations (P < 0.01).
Figure 2.
Concentration-dependent effects of insulin and dexamethasone on 11β-HSD1 activity and 11β-HSD1 mRNA. Differentiated 3T3-L1 adipocytes were treated with various concentrations of insulin or dexamethasone (Dex) for 48 h. Controls were treated at the same time with an appropriate volume of vehicle. Levels of 11β-HSD1 activity in intact cells (A) were determined by a standard radiometric conversion assay. Total cellular RNA was extracted, and the steady-state level of 11β-HSD1 mRNA (B) was measured by qRT-PCR. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. **, P < 0.01; ***, P < 0.001 vs. control.
Combined effects of insulin and dexamethasone on 11β-HSD1 activity and mRNA
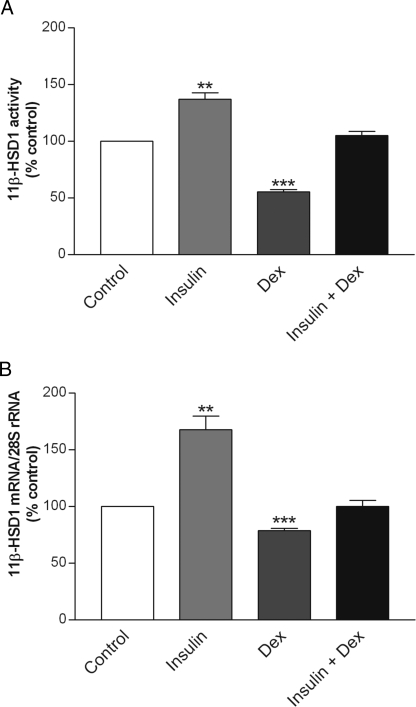
To determine whether concurrent treatment with both hormones would lead to increases, decreases, or no changes in 11β-HSD1 activity and expression, we examined the effects of insulin and dexamethasone combined on 11β-HSD1 activity and mRNA. The 3T3-L1 adipocytes were incubated for 48 h in the presence of both hormones at 10 nm each. As shown in Fig. 3A, despite their robust individual effects, the combined treatment of insulin and dexamethasone produced no net changes in 11β-HSD1 activity. Similarly, 11β-HSD1 mRNA remained unchanged after the combined treatment (Fig. 3B).
Figure 3.
Combined effects of insulin and dexamethasone on 11β-HSD1 activity and 11β-HSD1 mRNA. Differentiated 3T3-L1 adipocytes were treated with insulin (10 nm), dexamethasone (Dex; 10 nm), or in combination (10 nm each) for 48 h. Controls were treated at the same time with an appropriate volume of vehicle. Levels of 11β-HSD1 activity in intact cells (A) were determined by a standard radiometric conversion assay. Total cellular RNA was extracted, and the steady-state level of 11β-HSD1 mRNA (B) was measured by qRT-PCR. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. **, P < 0.01; ***, P < 0.001 vs. control.
Effects of insulin and dexamethasone on the rate of 11β-HSD1 mRNA decay
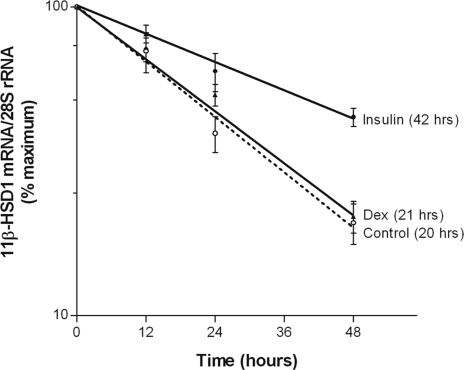
Insulin- and dexamethasone-induced changes in steady-state levels of 11β-HSD1 mRNA can be attributed to alterations in HSD11B1 gene transcription and/or mRNA stability. To ascertain which of the two mechanisms was involved, we assessed the rate of 11β-HSD1 mRNA decay with the use of a transcriptional inhibitor, DRB. The half-life of 11β-HSD1 mRNA in 3T3-L1 adipocytes was determined to be 20 h under basal conditions, and it was extended to 42 h by insulin. In contrast, dexamethasone did not alter the mRNA half-life (Fig. 4).
Figure 4.
Effects of insulin and dexamethasone on 11β-HSD1 mRNA degradation. Differentiated 3T3-L1 adipocytes were treated with vehicle (controls), insulin (100 nm), or dexamethasone (Dex; 100 nm) for 24 h and then with DRB (100 μm) for various times. At the indicated time points, total cellular RNA was extracted and the steady-state level of 11β-HSD1 mRNA was measured by qRT-PCR. Data are presented as mean ± sem of four independent experiments, each performed in triplicate.
Effects of various kinase inhibitors on the insulin-induced increase in 11β-HSD1 activity
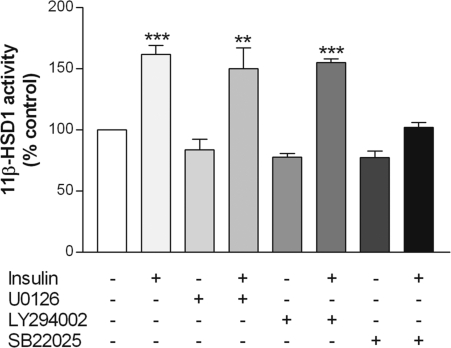
The effects of insulin are known to be mediated by ERK, phosphatidylinositol 3-kinase (PI3K), and/or p38 MAPK in various cell types, including adipocytes (52). To define the signal transduction pathway by which insulin stimulates 11β-HSD1, we used pharmacological inhibitors that specifically target the three potential pathways. Treatment of 3T3-L1 adipocytes with either the ERK inhibitor U0126 or the PI3K inhibitor LY294002 did not alter 11β-HSD1 activity in the absence or presence of insulin. In contrast, although the p38 MAPK inhibitor SB220025 alone had no effect, it blocked the insulin-induced increase in 11β-HSD1 activity (Fig. 5).
Figure 5.
Effects of various kinase inhibitors on 11β-HSD1 activity. Differentiated 3T3-L1 adipocytes were preincubated for 1 h with U0126 (ERK 1/2 inhibitor; 10 μm), LY294002 (PI3K inhibitor; 30 μm), or SB220025 (p38 inhibitor; 10 μm), followed by the addition of insulin (100 nm) for 48 h. Controls were treated at the same time with an appropriate volume of vehicle. Levels of 11β-HSD1 activity in intact cells were determined by a standard radiometric conversion assay. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. **, P < 0.01; ***, P < 0.001 vs. control.
Effects of RU486 on the dexamethasone-induced decrease in 11β-HSD1 activity
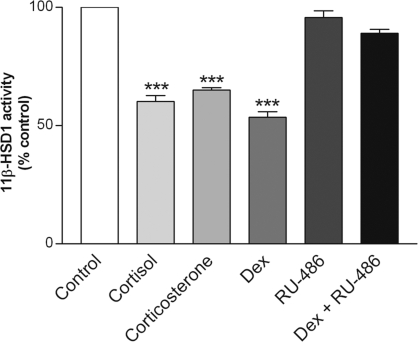
To determine whether the inhibitory effect of dexamethasone on 11β-HSD1 was mediated by the GR, 3T3-L1 adipocytes were treated with the GR antagonist RU486. Although RU486 alone had no effect, it prevented dexamethasone-induced decreases in 11β-HSD1 activity (Fig. 6). To gain further insight into the role of glucocorticoid in regulating 11β-HSD1, 3T3-L1 adipocytes were treated with the two endogenous/natural glucocorticoids, cortisol and corticosterone, each at 100 nm. As shown in Fig. 6, both steroids caused a similar reduction in 11β-HSD1 activity as seen with the synthetic glucocorticoid dexamethasone.
Figure 6.
Effects of RU-486 on dexamethasone-induced decreases in 11β-HSD1 activity. Differentiated 3T3-L1 adipocytes were pretreated for 1 h with the GR antagonist RU-486 (1 μm), followed by the addition of dexamethasone (Dex; 100 nm) for 48 h. Controls were treated at the same time with an appropriate volume of vehicle. Levels of 11β-HSD1 activity in intact cells were determined by a standard radiometric conversion assay. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. ***, P < 0.001 vs. control.
Effects of insulin and dexamethasone on H6PD mRNA
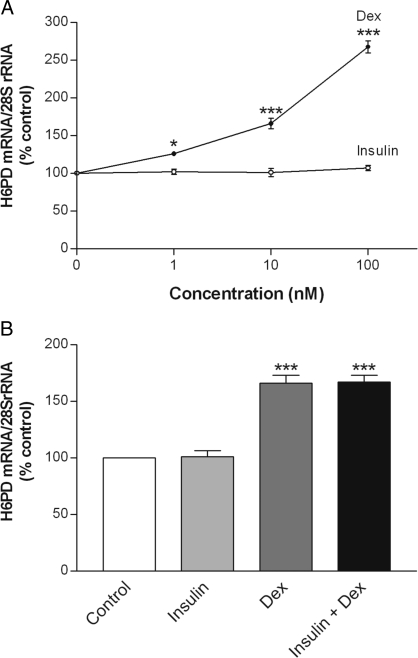
To determine whether insulin and dexamethasone had similar effects on the expression of H6PD, an ER enzyme that is responsible for generating the obligate cofactor NADPH for 11β-HSD1, the steady-state level of H6PD mRNA was assessed by qRT-PCR. In marked contrast to its inhibitory effects on 11β-HSD1, dexamethasone increased H6PD mRNA levels in a concentration-dependent manner, being effective at 1 nm (P < 0.05; Fig. 7A). Although insulin stimulated 11β-HSD1 activity and expression, it did not alter H6PD mRNA abundance. We also examined the effect of concurrent treatment with both hormones on H6PD expression. As shown in Fig. 7B, the combined treatment of insulin and dexamethasone resulted in a similar increase in the level of H6PD mRNA as seen with dexamethasone alone.
Figure 7.
Effects of insulin and dexamethasone on H6PD mRNA. Differentiated 3T3-L1 adipocytes were treated for 48 h with various concentrations of insulin or dexamethasone (A) or for 48 h in combination with each at 10 nm concentration (B). Controls were treated at the same time with an appropriate volume of vehicle. Total cellular RNA was extracted, and the steady-state level of H6PD mRNA was measured by qRT-PCR. Data are expressed as a percentage of control and presented as mean ± sem of four independent experiments, each performed in triplicate. *, P < 0.05; ***, P < 0.001 vs. control.
Effects of insulin and dexamethasone in vivo
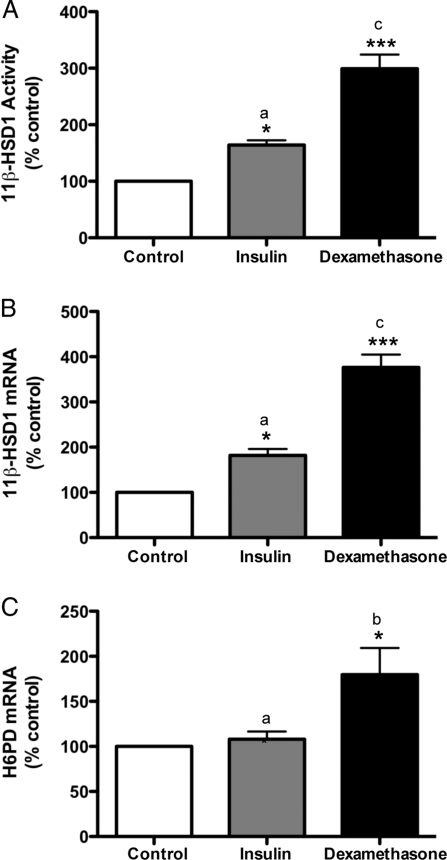
Having established a dynamic role for insulin and dexamethasone in regulating 11β-HSD1 in 3T3-L1 adipocytes, we then determined whether our in vitro findings could be confirmed in vivo. To do so, we treated male Wistar rats for 7 d with the long-acting insulin analog glargine or dexamethasone at clinically or physiologically relevant doses (45). Consistent with its effects on 3T3-L1 adipocytes, insulin augmented adipose tissue levels of both 11β-HSD1 activity (164 ± 8% of control; Fig. 8A) and mRNA (182 ± 14% of control; Fig. 8B). In contrast to its inhibitory effects in vitro, however, dexamethasone stimulated 11β-HSD1 activity (299 ± 25% of control; Fig. 8A) and mRNA (376 ± 28% of control; Fig. 8B) expression in rat adipose tissue in vivo. Furthermore, the magnitude of the increase in adipose tissue levels of both 11β-HSD1 activity (Fig. 8A) and mRNA (Fig. 8B) was greater (P < 0.001 in both cases) after dexamethasone than that after insulin treatment.
Figure 8.
Effects of insulin and dexamethasone in vivo. Male Wistar rats at 7 wk of age were injected sc with the long-acting insulin analog glargine (0.5 IU/kg·d) or the synthetic glucocorticoid dexamethasone (120 μg/kg·d) for 7 d. At the end of treatment, epididymal adipose tissues were collected. Levels of 11β-HSD1 activity (A) and mRNA (B) as well as H6PD mRNA (C) in adipose tissues were determined by a standard radiometric conversion assay and qRT-PCR, respectively. Data are expressed as a percentage of control and presented as mean ± sem (n = 4 rats). *, P < 0.05; ***, P < 0.001 vs. control; a vs. b, P < 0.05; a vs. c, P < 0.001.
Despite our observed contrasting effect of dexamethasone on adipocyte 11β-HSD1 in vitro and in vivo, dexamethasone increased (180 ± 30% of control), whereas insulin did not alter H6PD mRNA abundance in rat adipose tissue (Fig. 8C), a finding closely resembling that in 3T3-L1 adipocytes.
Discussion
In the present study, we demonstrate that insulin stimulates whereas dexamethasone inhibits 11β-HSD1 activity and expression via posttranscriptional and transcriptional mechanisms, respectively, in 3T3-L1 adipocytes. Our in vitro results also implicate a key role for the p38 MAPK signaling pathway in mediating insulin-stimulated 11β-HSD1 activity. Importantly, we show that both insulin and dexamethasone augment 11β-HSD1 activity and expression in rat white adipose tissue in vivo, thus confirming the role of insulin but revealing a fundamental difference regarding the role of dexamethasone in regulating adipocyte 11β-HSD1 between the two model systems. Furthermore, we provide evidence that dexamethasone stimulates, whereas insulin does not affect, H6PD expression in both 3T3-L1 adipocytes in vitro and rat adipose tissue in vivo, indicating that changes in 11β-HSD1 activity are associated with alterations in 11β-HSD1, but not H6PD, expression. Taken together, our present study has not only defined molecular mechanisms as well as signaling pathways underlying hormonal regulation of adipocyte 11β-HSD1 in vitro but also underscored the importance of conducting parallel in vivo studies when 3T3-L1 adipocytes are used.
Although a number of in vivo and in vitro studies have investigated the regulation of 11β-HSD1 by insulin and glucocorticoid in preadipocytes (53,54), skin fibroblasts (55), testis (56), hippocampus (57,58), liver (57,59), and hepatocytes (60,61), there is relatively little information regarding hormonal regulation of adipocyte 11β-HSD1. The general consensus is that glucocorticoid stimulates whereas insulin inhibits 11β-HSD1 activity and/or expression in nonadipocytes and nonadipose tissues (40). There are several in vivo studies in the human, which examined the effect of insulin on 11β-HSD1 in adipose tissue. These studies have produced inconsistent results with an increase (23,24), a decrease (25), or no change (26) in 11β-HSD1 expression and/or activity in human adipose tissue after acute insulin administration. The regulation of adipocyte 11β-HSD1 by glucocorticoid is largely unknown.
Livingstone and colleagues (42) showed that adrenalectomy reduced 11β-HSD1 activity in omental fat of obese Zucker rats, implicating a role for glucocorticoid in up-regulating adipose 11β-HSD1. In contrast, an in vitro study by Napolitano et al. (27) showed that treatment of 3T3-F442A adipocytes for 48 h with 5 μg/ml insulin (∼875 nm) and 100 nm dexamethasone resulted in 50 and 80% decreases in 11β-HSD1 mRNA, respectively. The effects of these hormones on 11β-HSD1 activity were not reported. Thus, our present results extended the previous observations by revealing parallel changes in 11β-HSD1 activity and mRNA. Furthermore, we defined the time- and concentration-dependent effects of these two hormones as well as their underlying molecular mechanisms. Importantly, we determined the effects of both insulin and dexamethasone on adipocyte 11β-HSD1 in vivo.
One major gap in the understanding of hormonal regulation of adipocyte 11β-HSD1 pertains to the underlying molecular mechanisms. Therefore, in the present study, we determined whether changes in steady-state levels of 11β-HSD1 mRNA after treatment with insulin and dexamethasone were due to alterations in HSD11B1 gene transcription or mRNA stability. Using a standard mRNA decay assay, we found that whereas dexamethasone did not alter the half-life of 11β-HSD1 mRNA, insulin doubled it. Importantly, the magnitude of insulin-stimulated increases in 11β-HSD1 mRNA and its half-life was similar, suggesting that the effects of dexamethasone and insulin on HSD11B1 gene expression were mediated by a transcriptional and a posttranscriptional mechanism, respectively. Obviously, future studies will be required to confirm the effect of dexamethasone on HSD11B1 gene transcription, and determine the precise molecular mechanisms by which insulin stabilizes 11β-HSD1 mRNA.
To gain insight into the signal transduction pathway mediating insulin-stimulated 11β-HSD1 activity, we investigated the effects of pharmacological inhibitors targeting various signaling pathways in 3T3-L1 adipocytes. Treatment with the PI3K inhibitor LY294002, the ERK1/2 inhibitor U0126, and the p38 MAPK inhibitor SB22025 did not affect basal levels of 11β-HSD1 activity. However, SB22025 blocked insulin-induced increases in 11β-HSD1 activity, whereas both LY294002 and U0126 were ineffective. This suggested that the stimulatory effects of insulin on 11β-HSD1 were mediated by the p38 MAPK signaling pathway. It is interesting to note that p38 MAPK is essential for 3T3-L1 adipocyte differentiation (62), during which 11β-HSD1 is induced (53,63,64). Moreover, C/EBPβ, a transcriptional factor that is critically involved in basal HSD11B1 gene transcription in 3T3-L1 preadipocytes (20), is also a target of p38 MAPK during 3T3-L1 adipogenesis (62). Collectively, these studies implicate a key role for the p38 MAPK signaling pathway in mediating both basal and insulin-stimulated 11β-HSD1 expression in 3T3-L1 adipocytes.
Considering that dexamethasone is a synthetic glucocorticoid, we determined whether the two major endogenous/natural glucocorticoids, cortisol and corticosterone, had similar effects on adipocyte 11β-HSD1 in vitro. We showed that both steroids were equally effective in reducing 11β-HSD1 activity as dexamethasone, demonstrating that the HSD11B1 gene is subject to repression by glucocorticoid in 3T3-L1 adipocytes. This contrasts with the stimulatory effects of glucocorticoid not only in rat adipose tissue in vivo, as shown in the present study and also implicated in a previous study (42), but also in nonadipocytes in vitro and nonadipose tissues in vivo as reported in the literature (40). Given the popularity of 3T3-L1 adipocytes as a bona fide in vitro model system (17,18,19,21,22,53,65,66), the discrepancy between our in vitro and in vivo findings regarding the role of dexamethasone in regulating adipocyte 11β-HSD1 is intriguing. Although the reasons for this discrepancy are not apparent, it is tempting to speculate that some components of glucocorticoid signaling may be radically altered in 3T3-L1 adipocytes. They may include an altered ratio of GRα/GRβ, a distinct glucocorticoid response element (within the HSD11B1 promoter), and/or an aberrant pattern of expression/activity of one or more of transcriptional coregulators. Irrespective of which of these possibilities is responsible for glucocorticoid repression of HSD11B1 gene expression in 3T3-L1 adipocytes, however, our present findings underscore the critical importance of conducting parallel in vivo studies when this cell line is used.
In contrast, both our in vitro and in vivo results demonstrate that insulin stimulates adipocyte 11β-HSD1. Thus, our present findings are consistent with some but not all the human studies (regarding the regulation of adipocyte 11β-HSD1) in vivo (8,9,10,11,12,13,14,15,16) and differ with insulin suppression of 11β-HSD1 in nonadipocytes and nonadipose tissues (40). Although our data also appear to contradict a previous in vitro study in which insulin (at ∼875 nm) reduced adipocyte 11β-HSD1 mRNA, they agree with each other because this inhibitory effect of insulin was shown to be mediated through the IGF-I, not the insulin, receptor (27). Importantly, at concentrations that are comparable to those used in the present study and not expected to activate the IGF-I receptor, insulin increased 11β-HSD1 expression in 3T3-F442A adipocytes (27). Taken together, these studies suggest that insulin dynamically regulates 11β-HSD1 in a cell-specific and concentration-dependent manner.
Although 11β-HSD1 possesses both dehydrogenase and reductase activities in its purified form and in tissue homogenates, it functions predominantly as a reductase in intact cells and in vivo (43,44). Recent studies have implicated a key role for H6PD, an ER resident enzyme, in controlling 11β-HSD1 reductase activity by generating the obligate cofactor NADPH (29,37). Furthermore, a functional H6PD-11β-HSD1 system exists in rat adipose tissue microsomes (67). However, the relative contribution of variations in H6PD expression/activity to changes in 11β-HSD1 reductase activity in intact cells and in vivo is uncertain. Indeed, one study by Wake and colleagues (23) questioned the role of H6PD in modifying 11β-HSD1 reductase activity in human adipose tissue in vivo, because they did not observe a change in the direction of 11β-HSD1 activity in subjects who were expected to have an altered H6PD activity. Another recent study also provided evidence that is incompatible with the notion that H6PD is a major determinant of 11β-HSD1 reductase activity (68). In mesenteric fat of rats exposed to sucrose, 11β-HSD1 mRNA increased 23-fold, whereas H6PD mRNA increased only 3.5-fold. Surprisingly, hepatic 11β-HSD1 mRNA was suppressed by 46%, whereas hepatic H6PD mRNA was doubled.
Against this background, in the present study, we first determined the concurrent effect of insulin and dexamethasone on H6PD mRNA expression in 3T3-L1 adipocytes in vitro, to gain insight into the role of H6PD in modulating 11β-HSD1 reductase activity. In marked contrast to its inhibitory effects on 11β-HSD1 activity and expression, dexamethasone increased H6PD mRNA abundance. Likewise, although insulin stimulated 11β-HSD1 activity and expression, it had no effect on H6PD expression. Importantly, our in vivo results confirmed these in vitro findings, because dexamethasone stimulated whereas insulin did not alter H6PD expression in rat adipose tissue. Furthermore, although dexamethasone increased adipose tissue levels of both 11β-HSD1 mRNA and H6PD mRNA, the magnitude of the increase in 11β-HSD1 mRNA was far greater than that in H6PD mRNA. Thus, both our in vitro and in vivo data indicated a discordant regulation of expression of these two functionally linked ER enzymes, but a close association between 11β-HSD1 activity and expression. Collectively, our present findings would argue against a key role for H6PD in determining adipocyte 11β-HSD1 activity. However, one caveat of our present study is that we did not measure H6PD activity. Consequently, it remains possible that H6PD activity might be altered differently from H6PD mRNA.
In addition, it is plausible that the observed increase in 11β-HSD1 activity and mRNA in adipose tissue in vivo after 7 d of treatment with dexamethasone could be attributed to elevated plasma levels of insulin, which is a known consequence of glucocorticoid administration in both rats (69,70) and humans (71). It is also likely that plasma levels of corticosterone are reduced in dexamethasone-treated animals because of the well-established negative feedback effect of glucocorticoid. Regrettably, both scenarios could not be verified in the present study due to a human error in processing the blood samples. It is noteworthy that 11β-HSD1 activity and expression as well as H6PD expression were determined in whole adipose tissue homogenates rather than mature adipocytes. Consequently, it is probable that the presence of nonadipocytes and preadipocytes may have an impact on our in vivo results. Obviously, future studies will be required to address this issue.
In conclusion, our present results demonstrate that insulin and dexamethasone exert opposite effects on 11β-HSD1 activity and expression in 3T3-L1 adipocytes by distinct mechanisms. In contrast, both insulin and dexamethasone stimulate adipocyte 11β-HSD1 in vivo. Given that circulating levels of insulin and/or glucocorticoid are elevated in stress, prediabetic, and other common conditions in the human, our present findings would suggest that the dysregulated insulin and/or glucocorticoid might contribute to 11β-HSD1-mediated central obesity and metabolic syndrome. Moreover, our data also suggest that 11β-HSD1, not H6PD, expression is the primary determinant of 11β-HSD1 activity, thus supporting the current strategy of developing selective inhibitors of 11β-HSD1 to combat metabolic disorders (72,73).
Acknowledgments
We thank Ms. Dong Yang for her technical assistance.
Footnotes
This work was supported by the Canadian Institutes of Health Research (Operating Grant MOP-79484.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: C/EBP, CAAT/enhancer-binding protein; DRB, 5,6-dichlorobenzimidazole 1-β-d-ribfuranoside; ER, endoplasmic reticulum; GR, glucocorticoid receptor; H6PD, hexose-6-phosphate dehydrogenase; 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PI3K, phosphatidylinositol 3-kinase; qRT-PCR, quantitative RT-PCR.
References
- Newell-Price J, Bertagna X, Grossman AB, Nieman LK 2006 Cushing’s syndrome. Lancet 367:1605–1617 [DOI] [PubMed] [Google Scholar]
- Raff H, Findling JW 2003 A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med 138:980–991 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS 2001 A transgenic model of visceral obesity and the metabolic syndrome. Science 294:2166–2170 [DOI] [PubMed] [Google Scholar]
- Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR 2004 Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 53:931–938 [DOI] [PubMed] [Google Scholar]
- Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ 1997 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 94:14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NM, Holmes MC, Fievet C, Staels B, Tailleux A, Mullins JJ, Seckl JR 2001 Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11β-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem 276:41293–41300 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS 2005 Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 54:1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR 2001 Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 86:1418–1421 [DOI] [PubMed] [Google Scholar]
- Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi MC, Grino M 2002 Expression of the mRNA coding for 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab 87:2701–2705 [DOI] [PubMed] [Google Scholar]
- Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O, Andrew R, Olsson T 2002 Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab 87:3330–3336 [DOI] [PubMed] [Google Scholar]
- Wake DJ, Rask E, Livingstone DE, Soderberg S, Olsson T, Walker BR 2003 Local and systemic impact of transcriptional up-regulation of 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. J Clin Endocrinol Metab 88:3983–3988 [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Wake DJ, Nair S, Bunt J, Livingstone DE, Permana PA, Tataranni PA, Walker BR 2003 Subcutaneous adipose 11β-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab 88:2738–2744 [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, Luft FC, Sharma AM 2004 Regulation of 11β-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res 12:9–17 [DOI] [PubMed] [Google Scholar]
- Kannisto K, Pietilainen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Jarvinen H 2004 Overexpression of 11β-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab 89:4414–4421 [DOI] [PubMed] [Google Scholar]
- Mariniello B, Ronconi V, Rilli S, Bernante P, Boscaro M, Mantero F, Giacchetti G 2006 Adipose tissue 11β-hydroxysteroid dehydrogenase type 1 expression in obesity and Cushing’s syndrome. Eur J Endocrinol 155:435–441 [DOI] [PubMed] [Google Scholar]
- Paulsen SK, Pedersen SB, Fisker S, Richelsen B 2007 11β-HSD type 1 expression in human adipose tissue: impact of gender, obesity, and fat localization. Obesity (Silver Spring) 15:1954–1960 [DOI] [PubMed] [Google Scholar]
- Berger J, Tanen M, Elbrecht A, Hermanowski-Vosatka A, Moller DE, Wright SD, Thieringer R 2001 Peroxisome proliferator-activated receptor-γ ligands inhibit adipocyte 11β-hydroxysteroid dehydrogenase type 1 expression and activity. J Biol Chem 276:12629–12635 [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Oppermann U, Steffensen KR, Schuster GU, Gustafsson JA 2002 Liver X receptors downregulate 11β-hydroxysteroid dehydrogenase type 1 expression and activity. Diabetes 51:2426–2433 [DOI] [PubMed] [Google Scholar]
- Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A 2005 Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab 288:E957–E964 [DOI] [PubMed] [Google Scholar]
- Gout J, Tirard J, Thevenon C, Riou JP, Begeot M, Naville D 2006 CCAAT/enhancer-binding proteins (C/EBPs) regulate the basal and cAMP-induced transcription of the human 11β-hydroxysteroid dehydrogenase encoding gene in adipose cells. Biochimie (Paris) 88:1115–1124 [DOI] [PubMed] [Google Scholar]
- Nakano S, Inada Y, Masuzaki H, Tanaka T, Yasue S, Ishii T, Arai N, Ebihara K, Hosoda K, Maruyama K, Yamazaki Y, Shibata N, Nakao K 2007 Bezafibrate regulates the expression and enzyme activity of 11β-hydroxysteroid dehydrogenase type 1 in murine adipose tissue and 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 292:E1213–E1222 [DOI] [PubMed] [Google Scholar]
- Arai N, Masuzaki H, Tanaka T, Ishii T, Yasue S, Kobayashi N, Tomita T, Noguchi M, Kusakabe T, Fujikura J, Ebihara K, Hirata M, Hosoda K, Hayashi T, Sawai H, Minokoshi Y, Nakao K 2007 Ceramide and adenosine 5′-monophosphate-activated protein kinase are two novel regulators of 11β-hydroxysteroid dehydrogenase type 1 expression and activity in cultured preadipocytes. Endocrinology 148:5268–5277 [DOI] [PubMed] [Google Scholar]
- Wake DJ, Homer NZ, Andrew R, Walker BR 2006 Acute in vivo regulation of 11β-hydroxysteroid dehydrogenase type 1 activity by insulin and intralipid infusions in humans. J Clin Endocrinol Metab 91:4682–4688 [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Corner A, Kannisto K, Kolak M, Makkonen J, Korsheninnikova E, Nyman T, Hamsten A, Fisher RM, Yki-Jarvinen H 2006 Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia 49:132–140 [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Andrew R, Homer NZ, Andrews RC, Smith K, Walker BR 2005 Increased in vivo regeneration of cortisol in adipose tissue in human obesity and effects of the 11β-hydroxysteroid dehydrogenase type 1 inhibitor carbenoxolone. Diabetes 54:872–879 [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Forsgren M, Wallberg-Henriksson H, Zierath JR 2004 Insulin action on expression of novel adipose genes in healthy and type 2 diabetic subjects. Obes Res 12:25–31 [DOI] [PubMed] [Google Scholar]
- Napolitano A, Voice MW, Edwards CR, Seckl JR, Chapman KE 1998 11β-Hydroxysteroid dehydrogenase 1 in adipocytes: expression is differentiation-dependent and hormonally regulated. J Steroid Biochem Mol Biol 64:251–260 [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Benedetti A, Fulceri R, Senesi S 2004 Cooperativity between 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum. J Biol Chem 279:27017–27021 [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Walker EA, Stewart PM 2005 Hexose-6-phosphate dehydrogenase and redox control of 11β-hydroxysteroid dehydrogenase type 1 activity. Endocrinology 146:2539–2543 [DOI] [PubMed] [Google Scholar]
- Czegle I, Piccirella S, Senesi S, Csala M, Mandl J, Banhegyi G, Fulceri R, Benedetti A 2006 Cooperativity between 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase is based on a common pyridine nucleotide pool in the lumen of the endoplasmic reticulum. Mol Cell Endocrinol 248:24–25 [DOI] [PubMed] [Google Scholar]
- Nashev LG, Chandsawangbhuwana C, Balazs Z, Atanasov AG, Dick B, Frey FJ, Baker ME, Odermatt A 2007 Hexose-6-phosphate dehydrogenase modulates 11β-hydroxysteroid dehydrogenase type 1-dependent metabolism of 7-keto- and 7β-hydroxy-neurosteroids. PLoS ONE 2:e561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J 1995 Lumenal orientation and post-translational modifications of the liver microsomal 11β-hydroxysteroid dehydrogenase. J Biol Chem 270:2305–2312 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Arnold P, Stauffer A, Frey BM, Frey FJ 1999 The N-terminal anchor sequences of 11β-hydroxysteroid dehydrogenases determine their orientation in the endoplasmic reticulum membrane. J Biol Chem 274:28762–28770 [DOI] [PubMed] [Google Scholar]
- Mziaut H, Korza G, Hand AR, Gerard C, Ozols J 1999 Targeting proteins to the lumen of endoplasmic reticulum using N-terminal domains of 11β-hydroxysteroid dehydrogenase and the 50-kDa esterase. J Biol Chem 274:14122–14129 [DOI] [PubMed] [Google Scholar]
- Ozols J 1993 Isolation and the complete amino acid sequence of lumenal endoplasmic reticulum glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci USA 90:5302–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Romero DG, de Rodriguez AF, Warden MP, Krozowski Z, Gomez-Sanchez CE 2008 Hexose-6-phosphate dehydrogenase and 11β-hydroxysteroid dehydrogenase-1 tissue distribution in the rat. Endocrinology 149:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery GG, Walker EA, Draper N, Jeyasuria P, Marcos J, Shackleton CH, Parker KL, White PC, Stewart PM 2006 Hexose-6-phosphate dehydrogenase knock-out mice lack 11β-hydroxysteroid dehydrogenase type 1-mediated glucocorticoid generation. J Biol Chem 281:6546–6551 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A 2004 Hexose-6-phosphate dehydrogenase determines the reaction direction of 11β-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett 571:129–133 [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA, Stewart PM 2005 Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11β-hydroxysteroid dehydrogenase type 1. J Mol Endocrinol 34:675–684 [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM 2004 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25:831–866 [DOI] [PubMed] [Google Scholar]
- Yang Z, Guo C, Zhu P, Li W, Myatt L, Sun K 2007 Role of glucocorticoid receptor and CCAAT/enhancer-binding protein α in the feed-forward induction of 11β-hydroxysteroid dehydrogenase type 1 expression by cortisol in human amnion fibroblasts. J Endocrinol 195:241–253 [DOI] [PubMed] [Google Scholar]
- Livingstone DE, Kenyon CJ, Walker BR 2000 Mechanisms of dysregulation of 11β-hydroxysteroid dehydrogenase type 1 in obese Zucker rats. J Endocrinol 167:533–539 [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Chapman KE, Edwards CR, Seckl JR 1995 11β-Hydroxysteroid dehydrogenase is an exclusive 11β-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology 136:4754–4761 [DOI] [PubMed] [Google Scholar]
- Low SC, Chapman KE, Edwards CR, Seckl JR 1994 ‘Liver-type’ 11β-hydroxysteroid dehydrogenase cDNA encodes reductase but not dehydrogenase activity in intact mammalian COS-7 cells. J Mol Endocrinol 13:167–174 [DOI] [PubMed] [Google Scholar]
- Yang K, Guan H, Arany E, Hill DJ, Cao X 7 March 2008 Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J 10.1096/fj.07-100735 [DOI] [PubMed] [Google Scholar]
- Heise T, Pieber TR 2007 Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab 9:648–659 [DOI] [PubMed] [Google Scholar]
- Kalinyak JE, Griffin CA, Hamilton RW, Bradshaw JG, Perlman AJ, Hoffman AR 1989 Developmental and hormonal regulation of glucocorticoid receptor messenger RNA in the rat. J Clin Invest 84:1843–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Khalil MW, Strutt BJ, Killinger DW 1997 11β-Hydroxysteroid dehydrogenase 1 activity and gene expression in human adipose stromal cells: effect on aromatase activity. J Steroid Biochem Mol Biol 60:247–253 [DOI] [PubMed] [Google Scholar]
- Langlois DA, Matthews SG, Yu M, Yang K 1995 Differential expression of 11β-hydroxysteroid dehydrogenase 1 and 2 in the developing ovine fetal liver and kidney. J Endocrinol 147:405–411 [DOI] [PubMed] [Google Scholar]
- Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, Yang K 2005 Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab 288:E663–E673 [DOI] [PubMed] [Google Scholar]
- Julan L, Guan H, van Beek JP, Yang K 2005 Peroxisome proliferator-activated receptor δ suppresses 11β-hydroxysteroid dehydrogenase type 2 gene expression in human placental trophoblast cells. Endocrinology 146:1482–1490 [DOI] [PubMed] [Google Scholar]
- Keeton AB, Amsler MO, Venable DY, Messina JL 2002 Insulin signal transduction pathways and insulin-induced gene expression. J Biol Chem 277:48565–48573 [DOI] [PubMed] [Google Scholar]
- Kim J, Temple KA, Jones SA, Meredith KN, Basko JL, Brady MJ 2007 Differential modulation of 3T3-L1 adipogenesis mediated by 11β-hydroxysteroid dehydrogenase-1 levels. J Biol Chem 282:11038–11046 [DOI] [PubMed] [Google Scholar]
- Handoko K, Yang K, Strutt B, Khalil W, Killinger D 2000 Insulin attenuates the stimulatory effects of tumor necrosis factor α on 11β-hydroxysteroid dehydrogenase 1 in human adipose stromal cells. J Steroid Biochem Mol Biol 72:163–168 [DOI] [PubMed] [Google Scholar]
- Hammami MM, Siiteri PK 1991 Regulation of 11β-hydroxysteroid dehydrogenase activity in human skin fibroblasts: enzymatic modulation of glucocorticoid action. J Clin Endocrinol Metab 73:326–334 [DOI] [PubMed] [Google Scholar]
- Nwe KH, Hamid A, Morat PB, Khalid BA 2000 Differential regulation of the oxidative 11β-hydroxysteroid dehydrogenase activity in testis and liver. Steroids 65:40–45 [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Chapman KE, Seckl JR 1999 Tissue- and temporal-specific regulation of 11β-hydroxysteroid dehydrogenase type 1 by glucocorticoids in vivo. J Steroid Biochem Mol Biol 68:245–250 [DOI] [PubMed] [Google Scholar]
- Moisan MP, Seckl JR, Edwards CR 1990 11β-Hydroxysteroid dehydrogenase bioactivity and messenger RNA expression in rat forebrain: localization in hypothalamus, hippocampus, and cortex. Endocrinology 127:1450–1455 [DOI] [PubMed] [Google Scholar]
- Yang K, Berdusco ET, Challis JR 1994 Opposite effects of glucocorticoid on hepatic 11β-hydroxysteroid dehydrogenase mRNA and activity in fetal and adult sheep. J Endocrinol 143:121–126 [DOI] [PubMed] [Google Scholar]
- Voice MW, Seckl JR, Edwards CR, Chapman KE 1996 11β-Hydroxysteroid dehydrogenase type 1 expression in 2S FAZA hepatoma cells is hormonally regulated: a model system for the study of hepatic glucocorticoid metabolism. Biochem J 317(Pt 2):621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Nakagawa Y, Nasuda K, Saegusa H, Igarashi Y 1996 Effect of growth hormone, insulin and dexamethasone on 11β-hydroxysteroid dehydrogenase activity on a primary culture of rat hepatocytes. Life Sci 59:227–234 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lisanti MP, Scherer PE 1998 Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem 273:32111–32120 [DOI] [PubMed] [Google Scholar]
- Jessen BA, Stevens GJ 2002 Expression profiling during adipocyte differentiation of 3T3-L1 fibroblasts. Gene 299:95–100 [DOI] [PubMed] [Google Scholar]
- Bujalska IJ, Walker EA, Hewison M, Stewart PM 2002 A switch in dehydrogenase to reductase activity of 11β-hydroxysteroid dehydrogenase type 1 upon differentiation of human omental adipose stromal cells. J Clin Endocrinol Metab 87:1205–1210 [DOI] [PubMed] [Google Scholar]
- Schuster D, Maurer EM, Laggner C, Nashev LG, Wilckens T, Langer T, Odermatt A 2006 The discovery of new 11β-hydroxysteroid dehydrogenase type 1 inhibitors by common feature pharmacophore modeling and virtual screening. J Med Chem 49:3454–3466 [DOI] [PubMed] [Google Scholar]
- Liu Y, Park F, Pietrusz JL, Jia G, Singh RJ, Netzel BC, Liang M 2008 Suppression of 11β-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3-L1 adipogenesis. Physiol Genomics 32:343–351 [DOI] [PubMed] [Google Scholar]
- Marcolongo P, Piccirella S, Senesi S, Wunderlich L, Gerin I, Mandl J, Fulceri R, Banhegyi G, Benedetti A 2007 The glucose-6-phosphate transporter-hexose-6-phosphate dehydrogenase-11β-hydroxysteroid dehydrogenase type 1 system of the adipose tissue. Endocrinology 148:2487–2495 [DOI] [PubMed] [Google Scholar]
- London E, Lala G, Berger R, Panzenbeck A, Kohli AA, Renner M, Jackson A, Raynor T, Loya K, Castonguay TW 2007 Sucrose access differentially modifies 11β-hydroxysteroid dehydrogenase-1 and hexose-6-phosphate dehydrogenase message in liver and adipose tissue in rats. J Nutr 137:2616–2621 [DOI] [PubMed] [Google Scholar]
- Guillaume-Gentil C, Assimacopoulos-Jeannet F, Jeanrenaud B 1993 Involvement of non-esterified fatty acid oxidation in glucocorticoid-induced peripheral insulin resistance in vivo in rats. Diabetologia 36:899–906 [DOI] [PubMed] [Google Scholar]
- Dumas JF, Bielicki G, Renou JP, Roussel D, Ducluzeau PH, Malthiery Y, Simard G, Ritz P 2005 Dexamethasone impairs muscle energetics, studied by 31P NMR, in rats. Diabetologia 48:328–335 [DOI] [PubMed] [Google Scholar]
- Willi SM, Kennedy A, Wallace P, Ganaway E, Rogers NL, Garvey WT 2002 Troglitazone antagonizes metabolic effects of glucocorticoids in humans: effects on glucose tolerance, insulin sensitivity, suppression of free fatty acids, and leptin. Diabetes 51:2895–2902 [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Stewart PM 2005 Mechanisms of disease: selective inhibition of 11β-hydroxysteroid dehydrogenase type 1 as a novel treatment for the metabolic syndrome. Nat Clin Pract Endocrinol Metab 1:92–99 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Odermatt A 2007 Readjusting the glucocorticoid balance: an opportunity for modulators of 11β-hydroxysteroid dehydrogenase type 1 activity? Endocr Metab Immune Disord Drug Targets 7:125–140 [DOI] [PubMed] [Google Scholar]