Abstract
Metabolic syndrome accelerates the atherosclerotic process, and the earliest event of which is endothelial dysfunction. Ghrelin, a newly discovered gastric peptide, improves endothelial function and inhibits proatherogenic changes. In particular, low ghrelin concentration has been associated with several features of metabolic syndrome, including obesity, insulin resistance, and high blood pressure. However, the molecular mechanisms underlying ghrelin vascular actions remain largely unclear. Here, we showed that ghrelin activated endothelial nitric oxide (NO) synthase (eNOS) in cultured endothelial cells (ECs) and in intact vessels. Specifically, ghrelin rapidly induced phosphorylation of eNOS on an activation site and production of NO in human umbilical vein ECs and bovine aortic ECs. The eNOS phosphorylation was also observed in mouse aortas ex vivo perfused with ghrelin and in aortic tissues isolated from mice injected with ghrelin. Mechanistically, ghrelin stimulated AMP-activated protein kinase (AMPK) and Akt activation in cultured ECs and intact vessels. Inhibiting AMPK and Akt with their pharmacological inhibitors, small interference RNA and adenoviruses carried dominant-negative mutants, markedly attenuated ghrelin-induced eNOS activation, and NO production. Furthermore, ghrelin receptor/Gq protein/calcium-dependent pathway mediates activation of AMPK, Akt, and eNOS, and calmodulin-dependent kinase kinase is a potential convergent point to regulate Akt and AMPK activation in ghrelin signaling. Importantly, eNOS activation is critical for ghrelin inhibition of vascular inflammation. Together, both in vitro and in vivo data demonstrate a new role of ghrelin signaling for eNOS activation, and highlight the therapeutic potential for ghrelin to correct endothelial dysfunction associated with atherosclerotic vascular diseases and metabolic syndrome.
METABOLIC SYNDROME, the clustering of hyperglycemia, hyperlipidemia, hypertension, and obesity, is a risk factor for the development of atherosclerosis (1). Both obesity and insulin resistance have recently been associated with endothelial dysfunction, the earliest event of atherosclerotic process (2,3). Endothelial dysfunction is characterized by decreased bioavailability of endothelial nitric oxide (NO) (4,5). Endothelial-derived NO is produced by the endothelial isoform of NO synthase (eNOS) upon the conversion of the substrate l-arginine to l-citrulline, and eNOS activation is tightly regulated by several molecular mechanisms, including Ca2+/calmodulin binding, phosphorylation by protein kinases, and dephosphorylation by protein phosphatases (6,7,8). In particular, Akt (also called protein kinase B) has been an important kinase phosphorylating human eNOS at Ser-1177 (Ser-1179 in bovine eNOS), with ensuing increased activity of eNOS in response to various stimuli such as fluid shear stress and insulin (9,10,11,12). Recently, AMP-activated protein kinase (AMPK), a trimeric enzyme comprising a catalytic α-subunit and regulatory β,γ-subunits (13,14), has also been suggested to phosphorylate eNOS at Ser-1177, resulting in eNOS activation (15,16,17). Interestingly, it has been reported that both adipocytokine adiponectin and the antidiabetic drug metformin stimulate eNOS AMPK-dependent eNOS activation (18,19,20), which may contribute to their beneficial effects on improving vascular endothelial functions and reducing cardiovascular events in the patients with type 2 diabetes. Understanding the molecular mechanisms of eNOS activation in endothelium is important for the development of new strategies to prevent endothelial dysfunction and cardiovascular disease associated with metabolic syndrome.
Ghrelin is a newly discovered hormone from the stomach, which induces the release of GH and stimulates food intake, energy balance, and adiposity (21,22,23). Emerging evidence from us and others indicates that ghrelin has a variety of GH releasing-independent cardiovascular activities, including an increase of myocardial contractility and remodeling (24,25,26,27,28), and an enhancement of vasodilation that regulates blood pressure in animal models and humans (29,30). Of note, low ghrelin concentration has been associated with several features of metabolic syndrome, including obesity (31,32), insulin resistance (33), and high pressure (34). Most importantly, local administration of human ghrelin ameliorates endothelial dysfunction in patients with metabolic syndrome (35). However, the molecular mechanisms responsible for these cardiovascular effects of ghrelin remain poorly understood.
We here examined the effects of ghrelin on eNOS activation in vitro, ex vivo, and in vivo, and signal pathways involved in the process. Our studies showed that ghrelin rapidly stimulated eNOS phosphorylation on Ser-1177 in cultured endothelial cells (ECs) and intact aortic vessels, resulting in an acute increase in NO production that is involved in ghrelin antiinflammatory effects. We further demonstrated that Akt and AMPK are the major mediators for ghrelin activation of eNOS both in vitro and in vivo.
Materials and Methods
Materials
Synthesized and HPLC purified human ghrelin was purchased from Sigma-Aldrich (St. Louis, MO). The phosphatidylinositol 3′OH-kinase (PI3K) wortmannin, AMPK inhibitor compound C, calmodulin-dependent kinase kinases (CaMKKs) inhibitor STO-609, eNOS inhibitor N (G)-nitro-l-arginine methyl este (L-NAME), and protein Gq inhibitor GP antagonist 2A were from Calbiochem (San Diego, CA). Calcium chelator BAPTA/AM and NO reactive dye 4,5-diaminofluorescein diacetate (DAF2-DA) were also from Calbiochem. Antibodies specific for phosphorylated eNOS (Ser-1177), Akt (Ser-473), and AMPK (Thr-172) were obtained from Cell Signaling Technologies (Beverly, MA). eNOS antibody was from BD Transduction Laboratories (BD, Franklin Lakes, NJ). β-Actin antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). GH secretagogue receptor (GHSR) antibody was obtained from Alpha Diagnostics International Inc. (San Antonio, TX).
EC culture
Cultured human umbilical vein ECs (HUVECs) were prepared by collagenase treatment of human umbilical veins with the approval from the University of Rochester Research Subjects Review Board. Cells were cultured and characterized as previously described (36,37), and used in experiments at passage 2–4. Bovine aortic ECs were purchased from Clonetics (San Diego, CA) and cultured as described previously (36,37), and used in experiments at passage 4–8. Confluent cells were serum starved for 24 h and then exposed to ghrelin. For the inhibitor studies, cells were pretreated with various inhibitors for 30 min in serum-depleted medium before ghrelin treatment.
Adenoviruses and infection
We used a recombinant adenovirus expressing a dominant-negative mutant of Akt (Ad-Akt-DN) (38) or Myc-tagged AMPK2α (Ad-AMPK-DN) (39) as described previously. The adenovirus encoding β-galactosidase gene (LacZ) was used as a control. Confluent ECs were infected with recombinant adenoviruses at the indicated multiplicity of infection and incubated for 24 h before experiments.
Transfection of ECs with small interference RNA (siRNA)
siRNA duplex against human GHSR and human AMPK, and scrambled control siRNA were obtained from Dharmacon (Lafayette, CO). The sequences of specific siRNA targeting human GHSR were sense 5′-GGGUGAAGCUGGUCAUCUUUU-3′ and antisense 5′-AAGAUGACCAGCUUCACCCUU-3′. Human AMPKα1 siRNA was sense 5′-GUAGAGCAAUCAAACAAUUUU-3′ and antisense 5′-AAUUGUUUGAUUGCUCUACUU-3′. siRNA duplex against human CaMKKβ was obtained from Ambion, Inc. (Austin, TX), and the sequences were sense 5′-AGCUGAUUGUUGUGGUCAAtt-3′ and antisense 5′-UUGACCACAACAAUCAGCUtt-3′. For siRNA transfection, proliferating ECs after seeded into 60-mm dishes for 24 h were transfected with control siRNA (100 nm) and GHSR siRNA (100 nm) using Lipofectamine 2000 (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s protocol as described previously (36,37). Forty-eight hours later, cells were exposed to ghrelin and harvested.
Western blot analysis
ECs were harvested with a lysis buffer as described previously (36,37,40). Frozen mouse aortas were thawed and homogenized in the same buffer as described previously. EC lysates and mouse aortic extracts were resolved on SDS-PAGE according to standard protocols. After being transferred to membranes, the samples were immunoblotted with primary antibodies, followed by IRDye infrared secondary antibodies (LI-COR Biosciences, Lincoln, NE). Bands for immunoreactive proteins were visualized by an Odyssey infrared imaging system, and density was quantified using Odyssey software (LI-COR Biosciences).
NO production assay
NO released from ECs was measured as its nitrogen oxide metabolites (nitrite/nitrate) accumulated in the medium, using a chemiluminescence detector as previously described (40). The production of NO was also examined by a cell-permeable form of the NO reactive dye, DAF2-DA (5 μm), as described previously (16,41). The signal generated by the reaction of DAF2-DA and NO was monitored by fluorescence microscopy (Olympus BX51; Olympus, Hamburg, Germany), and the images were captured and analyzed by Spot software system (RT Color Diagnostic Instruments, Sterling Heights, MI).
Measurement of cellular cyclic GMP (cGMP) concentration in ECs
HUVECs were seeded in the 96-well plates. After treating the cells with ghrelin, cells were washed with PBS, and the cellular cGMP concentrations were analyzed using a commercial enzyme immunoassay kit (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer’s instructions (42).
Animal experiments
The protocols for animal experiments were approved by the University Committee on Animal Resources. All experiments were performed in 8-wk-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Mice were injected with ghrelin at 25 μg/kg body weight into the tail vein. Saline was injected to control animals as a vehicle control. After 30 min, mice were killed, and the aortas were then removed and stored at −80 C. For ex vivo perfusion, the aortas were isolated from mice and perfused with 100 nm ghrelin or saline (control group) for 15 min, as described previously (43), and then stored at −80 C.
Adhesion of U937 mononuclear cells to HUVEC monolayers
To evaluate monocyte adhesion, HUVEC monolayers in six-well plates were incubated in M199 containing 0.25% fetal bovine serum with human TNF-α (5 ng/ml) for 6 h in the absence or presence of ghrelin (100 nm). The media were then removed, and U937 cells were added to wells (6 × 104 per well) and incubated for 10 min at 37 C. Unbound cells in the wells were then washed out for three times with serum-free medium. The adherent cells were counted in five randomly selected optical fields in each well. Phase-contrast microphotographs of the cells in plates were taken under a microscope (IMT-2; Olympus) with a digital camera.
Statistical analysis
Data are the means ± sem. Differences were analyzed using ANOVA, followed by appropriate multiple comparison tests. A P < 0.05 value was considered statistically significant.
Results
Ghrelin stimulates eNOS phosphorylation and NO production in cultured ECs
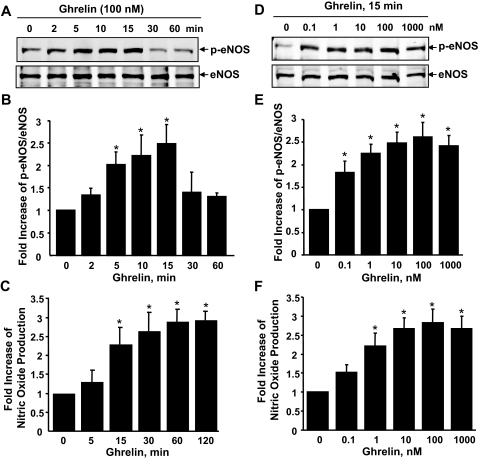
To test whether ghrelin phosphorylates eNOS at activation site Ser-1177 in ECs, HUVECs were treated with ghrelin for up to 60 min. As shown in Fig. 1, A and B, the phosphorylation of Ser-1177 of eNOS in HUVECs increased transiently, with a peak level at 15 min. Treatment with ghrelin did not change the levels of eNOS in ECs. To determine whether ghrelin can stimulate NO production in cultured ECs, we used three methods, chemiluminescence assay for measuring nitrite/nitrate accumulated in cultured medium (40,43), the NO-specific fluorescent dye DAF-2 DA (16,41), and enzyme immunoassay for cGMP level in cell lysates (42), to assess NO production in ECs. Consistent with eNOS phosphorylation, NO accumulation in the cultured medium from ECs was significantly increased after ghrelin treatment (Fig. 1C). Quantitative assays for cGMP level in cell lysates showed an enhancement of cGMP by ghrelin in a time-dependent manner (supplemental data S1-A and S1-B, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). The NO-specific fluorescent dye also detected NO production in response to ghrelin (supplemental data S1-D). Moreover, when ECs were pretreated with eNOS inhibitor L-NAME (200 μm), the production of NO in response to ghrelin was totally blocked (supplemental data S1-C), indicating ghrelin-induced NO production is eNOS dependent. In addition, as shown in Fig. 1, D–F, eNOS phosphorylation and NO production significantly increased in a ghrelin dose-dependent manner as well.
Figure 1.
Ghrelin stimulates eNOS phosphorylation and NO production in ECs. HUVECs were incubated with ghrelin (100 nm) at the indicated times (A–C) or with various doses for 15 min (D and E). The phosphorylation of eNOS at Ser-1179 (p-eNOS) and the level of eNOS expression in cell lysates were analyzed by Western blots. The medium was collected for chemiluminescence analysis of NO production. All quantitative data are the levels of phosphorylated eNOS normalized to those of total eNOS, and presented as mean ± sem (n = 4). *, P < 0.05 vs. control without ghrelin stimulation.
Ghrelin-activated Akt is involved in eNOS phosphorylation and NO production
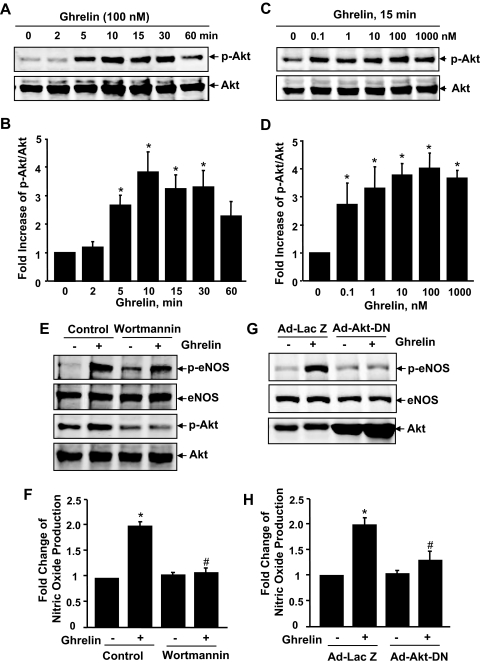
To understand the molecular mechanisms underlying ghrelin activation of eNOS, we first focused on PI3K-dependent Akt, a known eNOS kinase (9,10,11). As shown in Fig. 2, A–D, ghrelin induced Akt phosphorylation on activation site Ser-473 in a time- and dose-dependent manner. The PI3K inhibitor wortmannin abolished ghrelin-induced Akt activation, and significantly inhibited eNOS activation and the consequent NO production (Fig. 2, E and F). Overexpression of adenovirus encoding Akt dominant negative mutant (Ad-Akt-DN) in ECs dramatically attenuated eNOS activation and NO production by ghrelin (Fig. 2, G and H), indicating an important role of Akt in ghrelin-induced eNOS activation.
Figure 2.
Akt is involved in ghrelin-induced eNOS activation. A–D, HUVECs were incubated with ghrelin (100 nm) at the indicated times (A and B, n = 4) or with various doses for 15 min (C and D, n = 4). E, HUVECs were pretreated with vehicle (dimethylsulfoxide), or 100 nm wortmannin for 30 min before exposure to 100 nm ghrelin for 15 min (n = 3). G, HUVECs were infected with 50 MOI (multiplicity of infection) Ad-LacZ or Ad-Akt-DN and then exposed to ghrelin (n = 3). The phosphorylation of Akt at Ser-473 (p-Akt) and eNOS at Ser-1177 (p-eNOS) and total levels of Akt and eNOS expression were analyzed. F and H, HUVECs were treated as in E and G except for a 30-min treatment of ghrelin (n = 3). NO production was detected by chemiluminescence analysis. *, P < 0.05 vs. control without ghrelin stimulation. #, P < 0.05 vs. the group treated with ghrelin.
Ghrelin activates AMPK that plays a critical role in ghrelin activation of eNOS
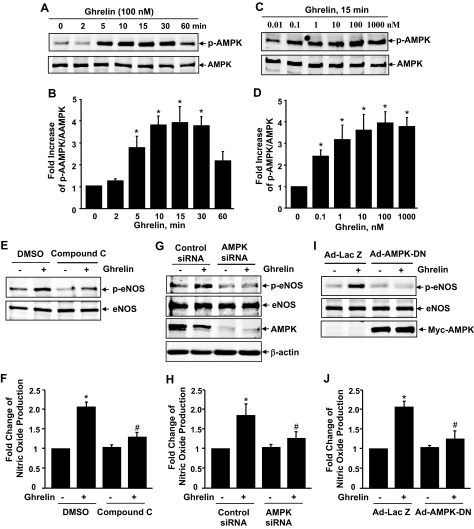
Because wortmannin only partially inhibited eNOS phosphorylation and NO production, whereas Akt phosphorylation was completely inhibited, there must be other kinase(s) involved in eNOS phosphorylation by ghrelin. Recently, AMPK, an energy sensing enzyme involved in cellular energy homeostasis, has been identified to phosphorylate directly eNOS at Ser-1177 (15,16,17,44). Thus, we investigated the potential role of AMPK in ghrelin activation of eNOS with multiple approaches, including the pharmacological inhibitor, siRNA, and dominant negative mutant of AMPK. In HUVECs, ghrelin stimulated AMPK phosphorylation on activation site Thr-172 in a time- and dose-dependent manner (Fig. 3, A–D). The AMPK antagonist compound C significantly inhibited ghrelin-induced phosphorylation of eNOS and NO production (Fig. 3, E and F). To examine further the involvement of AMPK, we knocked down AMPK expression with siRNA (Fig. 3G). Silencing AMPK by siRNA also significantly attenuated ghrelin-induced phosphorylation at Ser-1177 and NO production (Fig. 3, F and G). To further determine the requirement of AMPK activity for ghrelin activation of eNOS, we used a recombinant adenovirus expressing a dominant negative form of Myc-tagged AMPK (Ad-AMPK-DN) (39), HUVECs infected with Ad-LacZ (control) or Ad-AMPK-DN, and then treated with 100 nm ghrelin for 15 min. As shown in Fig. 3I, cells infected with the Ad-LacZ control showed an increased phosphorylation of AMPK at Thr-172 and eNOS at Ser-1177. However, Ad-AMPK-DN overexpression, indicated by positive anti-Myc immunoblotting, markedly attenuated ghrelin-induced phosphorylation of eNOS at Ser-1177. Consequently, ghrelin-enhanced NO production was substantially inhibited in cells treated with Ad-AMPK-DN compared with that in control cells (Fig. 3J). Together, these results clearly demonstrate a key role for AMPK in mediating ghrelin-induced eNOS activation.
Figure 3.
Ghrelin activates AMPK that is also involved in eNOS activation. A–D, HUVECs were incubated with ghrelin (100 nm) at indicated times (A and B, n = 4) or with various doses for 15 min (C and D, n = 4). E, HUVECs were pretreated with vehicle [dimethylsulfoxide (DMSO)], or 10 μm compound C for 30 min before exposure to 100 nm ghrelin for 15 min (n = 3). G, HUVECs were pretreated with 100 nm control siRNA, or 100 nm AMPK siRNA for 48 h before exposure to 100 nm ghrelin for 15 min (n = 3). I, HUVECs were infected with 100 MOI (multiplicity of infection) Ad-LacZ or Ad-AMPK-DN and then exposed to ghrelin (n = 3). The phosphorylation of AMPK at Thr-172 (p-AMPK) and eNOS at Ser-1177 (p-eNOS) and total levels of AMPK and eNOS expression were analyzed. F, H, and J, HUVECs were treated the same as in E, G, and I, respectively, except for a 30-min treatment of ghrelin. NO production was detected by chemiluminescence analysis. *, P < 0.05 vs. control without ghrelin stimulation. #, P < 0.05 vs. the group treated with ghrelin.
To understand further the role of Akt and AMPK in ghrelin-induced eNOS activation, we asked whether there is a cross talk between Akt and AMPK. Although wortmannin and Ad-Akt-DN totally blocked ghrelin-induced Akt activation (Fig. 3), it has no effect on ghrelin-induced AMPK activation, and the phosphorylation at Ser-79 of acetyl-CoA carboxylase (supplemental data S3-A and S3-B), a direct target of AMPK (13). Conversely, inhibiting AMPK by compound C, AMPK siRNA, and Ad-AMPK-DN did not affect phosphorylation of Akt and its substrate GSK-3α (supplemental data S4). These results suggest that Akt and AMPK are two mutually independent kinases involved in eNOS activation in ghrelin signaling.
Ghrelin receptor GHSR mediates Akt, AMPK, and eNOS activation in ECs
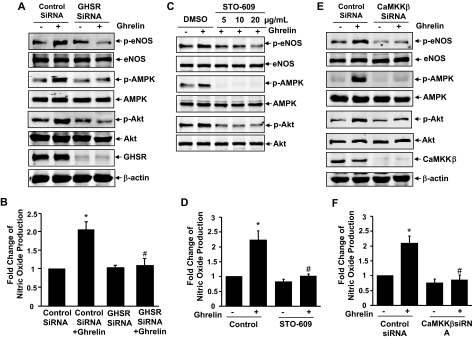
Many biological actions of ghrelin are initiated by binding of ghrelin to its cognate cell surface receptor GHSR-1a (21,22,23). Thus, we studied the role of GHSR-1a in ghrelin-stimulated eNOS activation using siRNA. Transfection of HUVECs with human GHSR-1a siRNA (100 nm) significantly reduced GHSR expression compared with that with scrambled control siRNA (Fig. 4A). The effect of siRNA on GHSR was specific because it did not affect the levels of β-actin, Akt, AMPK, and eNOS expression. As expected, knockdown of GHSR expression by siRNA markedly inhibited ghrelin-stimulated phosphorylation of Akt, AMPK, and eNOS (Fig. 4A). The production of NO in response to ghrelin was also substantially inhibited in cells transfected with GHSR-1a siRNA (Fig. 4B).
Figure 4.
GHSR mediates ghrelin activation of AMPK, Akt, and eNOS in a CaMKKβ-dependent manner. A, HUVECs were transfected with scrambled control siRNA (100 nm) or human GHSR siRNA (100 nm) for 48 h, and then exposed to ghrelin for 15 min (A) or 30 min (B). Cell lysates were analyzed for Western blots (n = 3). NO production in the medium was detected by chemiluminescence analysis (n = 3). C and D, HUVECs were pretreated with vehicle [dimethylsulfoxide (DMSO)], STO-609 at the indicated does for 30 min before exposure to 100 nm ghrelin for 15 min (C) or 30 min (D) (n = 3). E and F, HUVECs were pretreated with control siRNA or CaMKKβ siRNA and then exposed to 100 nm ghrelin for 15 min (E) or 30 min (F) (n = 3). The assays were performed the same as in A and B. *, P < 0.05 vs. control without ghrelin stimulation. #, P < 0.05 vs. the group treated with ghrelin. p-AMPK, Phosphorylation of AMPK at Thr-172; p-eNOS, phosphorylation of eNOS at Ser-1179.
CaMKKβ is involved in mediating Akt, AMPK, and eNOS activation in response to ghrelin
G protein activation and calcium mobilization are the major downstream signal events of GHSR-1a by ghrelin stimulation (21,22,23). Using Gq protein antagonist GP-2A and intracellular calcium chelator BAPTA/AM, we found that Gq protein and intracellular calcium are involved in eNOS phosphorylation and NO production (supplemental data S5 and S6). Interestingly, it has recently been reported that CaMKKs, in particular its β-isoform (CaMKKβ), are involved in AMPK activation (14), and CaMKK has also activated Akt (45). Therefore, we investigated the potential role of CaMKK for mediating Akt and AMPK activation in response to ghrelin. As shown in Fig. 4C, the treatment of HUVECs with the potent CaMKK inhibitor STO-609 dose dependently inhibited ghrelin-induced phosphorylation of both Akt and AMPK. Consequently, eNOS activation and NO production in response to ghrelin were significantly blocked by STO-609 (Fig. 4D). To examine further the role of CaMKKβ in ghrelin signaling, we treated ECs with specific CaMKKβ siRNA. Consistent with results of the pharmacological inhibitor, knockdown CaMKKβ by siRNA significantly inhibited Akt, AMPK, and eNOS activation and NO production (Fig. 4, E and F). Collectively, our results suggest that CaMKKβ is a potential convergent point to mediate Akt and AMPK activation in ghrelin signaling.
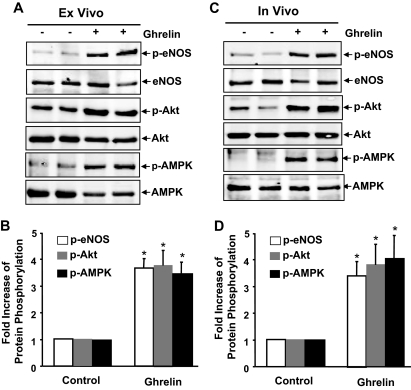
Ghrelin activates AMPK, Akt, and eNOS in intact vessels ex vivo and in vivo
To examine whether ghrelin stimulates eNOS, Akt, and AMPK activation in isolated vessels, the fresh aortas isolated from mice were perfused ex vivo with ghrelin (100 nm) for 15 min and then subjected to Western blot assay. As shown in Fig. 5A, the levels of phosphorylated AMPK, Akt, and eNOS were also markedly increased in ghrelin perfused aortas compared with those perfused with saline. Although eNOS was only expressed in ECs in the aortas, AMPK and Akt were from the mixture of cell types from the aortas. To show the contribution of Akt and AMPK in eNOS activation in intact vessels, we pretreated with isolated mouse aortas with wortmannin or compound C and then exposed to ghrelin. We observed that both wortmannin and compound C inhibited eNOS phosphorylation in intact vessels (supplemental data S7).
Figure 5.
GHSR activates eNOS, Akt, and AMPK in intact vessels ex vivo and in vivo. A and B, Fresh aortas were isolated from C57BL/6 mice, and then ex vivo perfused with 100 nm ghrelin or saline (control group) for 15 min. C and D, C57BL/6 mice were subjected to tail vein injection with ghrelin (25 ng/kg) or the same amount of saline (control group) for 30 min, and then aortas were isolated. Tissue extracts from aortas were analyzed by Western blotting with various antibodies as indicated. The data represent results of three independent sets of experiments from six animals. Representative immunoblots (A and C) and quantitative data of protein phosphorylation (B and D) were shown. *, p < 0.05 vs. control without ghrelin stimulation. p-AMPK, Phosphorylation of AMPK at Thr-172; p-eNOS, phosphorylation of eNOS at Ser-179.
To explore further whether eNOS can be activated by ghrelin in vivo, C57BL/6J mice were injected with ghrelin at 25 μg/kg body weight into the tail vein, then aortas were removed after 30 min to detect the level of eNOS, Akt, and AMPK phosphorylation. As shown in Fig. 5B, the levels of phosphorylated AMPK Thr-172 and Akt Ser-473 in the aorta were significantly increased after ghrelin administration. The phosphorylation of eNOS at Ser-1177 was also elevated, which is correlated with that of phosphorylated AMPK and Akt.
Ghrelin-activated eNOS is involved in the inhibition of monocyte adhesion on ECs
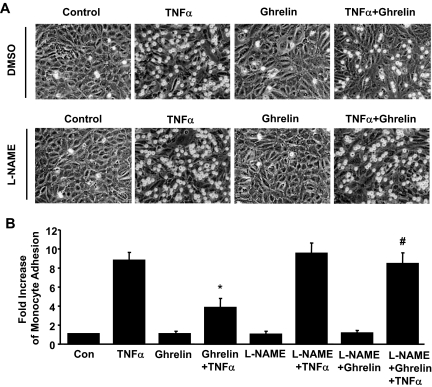
To demonstrate the physiological relevance of our findings, we examined the effect of ghrelin on proinflammatory cytokine-induced monocyte adhesion on ECs. HUVECs were pretreated with 100 nm ghrelin for 30 min, followed with addition of 5 ng/ml TNFα for 6 h. Monocytes U937 were then added, and adhered monocytes on ECs were counted. Consistent with a previous report (46), treatment with ghrelin significantly inhibited TNFα-stimulated U937 binding to ECs (Fig. 6). The pretreatment of L-NAME (200 nm) for 30 min before the application of ghrelin markedly reduced the inhibitory effect of ghrelin on TNFα-induced monocyte adhesion on ECs.
Figure 6.
Ghrelin activation of eNOS mediates its inhibition of monocyte adhesion on ECs. A, HUVECs were pretreated with vehicle [dimethylsulfoxide (DMSO)], or 200 μm L-NAME for 30 min before exposure to 100 nm ghrelin for 30 min, and then exposed to TNFα (5 ng/ml) or vehicle for 6 h. B, U937 monocyte adhesion on TNFα- or vehicle-stimulated HUVECs was analyzed. Images of U937 monocyte adhesion on HUVECs are shown. Data shown are pooled from four independent experiments. *, P < 0.05 vs. control (Con) without TNFα stimulation alone. #, P < 0.05 vs. the group treated with TNFα plus ghrelin.
Discussion
Emerging evidence suggests that ghrelin has important, beneficial effects on the vascular system through promoting vasodilation and ameliorating endothelial dysfunction (30,35). However, the molecular mechanisms of ghrelin cardiovascular effects are still not well defined. In the present study, one of the principal findings is that ghrelin activates eNOS in cultured ECs and intact vessels. Furthermore, we have demonstrated that ghrelin stimulates AMPK and Akt activation through GHSR- and CaMKK-dependent pathway, which plays an essential role in mediating ghrelin-stimulated eNOS activation. These cumulative observations indicate that ghrelin is a novel regulator of eNOS activation via GHSR-mediated signal pathways, which may represent an important mechanism for ghrelin cardiovascular protection observed in animal models and humans.
We first demonstrate the positive effect of ghrelin on the phosphorylation of eNOS Ser-1177 in cultured ECs. Such phosphorylation is associated with an increase of eNOS activation, which is also revealed by increased NO production. Our data are consistent with the recent report showing that ghrelin stimulated production of NO from endothelial cells (49). These effects were seen at concentrations of ghrelin that are within the physiological range (47). The positive effects of ghrelin on eNOS activation should not be limited to HUVECs because ghrelin also induces eNOS phosphorylation and NO production in bovine aortic ECs. Although the chronic effects of ghrelin on eNOS expression and activation have not been characterized yet, the present data indicate that eNOS phosphorylation and activation in ECs were increased by ghrelin in a rapid and transient manner. This temporal response is similar to that stimulated by other vascular protective factors, such as fluid shear stress, insulin, estradiol, statins, and high-density lipoprotein (6). Importantly, the acute phosphorylation and activation of eNOS in mouse aorta were also observed after ghrelin administration in vivo and ex vivo (Fig. 6). Notably, ghrelin was discovered as a GH-releasing peptide acting on neurons in the hypothalamus and pituitary, which mediates ghrelin action in regulation of food intake and energy homeostasis (21,22,23). However, the detected eNOS phosphorylation in cultured ECs and perfused mouse aortas ex vivo suggests that ghrelin activation of eNOS is GH releasing independent, which is consistent with the results showing that ghrelin improves endothelial dysfunction in GH-deficient rats (30). In contrast to eNOS phosphorylation at Ser-1177, ghrelin did not change eNOS phosphorylation at Thr-495, the site that inactivates eNOS (6,7). Furthermore, we found that eNOS activation, at least in part, mediated the ghrelin inhibitory effect on endothelial proinflammatory responses in cultured ECs (46) because eNOS inhibitor L-NAME significantly attenuated such an effect of ghrelin. Together, our data reveal a novel role of ghrelin in regulation of eNOS activity, which may represent an important mechanism for ghrelin vascular protection.
Several protein kinases are implicated in eNOS phosphorylation and activation, including Akt and AMPK (9,10,15). In this study we showed that ghrelin time dependently stimulated Akt and AMPK phosphorylation in both cultured ECs and intact vessels. Using specific pharmacological inhibitors for PI3K and a dominant negative mutant of Akt, a critical role of the PI3K-Akt pathway in ghrelin stimulation of eNOS activity is established. In particular, we found that the PI3K inhibitor wortmannin abolished ghrelin-induced Akt activation, and both wortmannin and Ad-Akt-DN significantly inhibited eNOS phosphorylation and NO production by ghrelin stimulation. Furthermore, it appears that AMPK also plays an important role in ghrelin-stimulated eNOS phosphorylation and NO production. Ample experimental evidence supports such a concept, including the inhibition of eNOS Ser-1177 phosphorylation and NO production by compound C, AMPK siRNA, and Ad-AMPK-DN. Specifically, we showed that ghrelin rapidly stimulated phosphorylation of AMPK Thr-172 in ECs. Moreover, eNOS Ser-1177 phosphorylation was largely inhibited in ECs treated with compound C, AMPK siRNA, or Ad-AMPK-DN, whereas Akt Ser-473 phosphorylation was not significantly affected in these cells (data not shown). Together, these results suggest a pivotal role of Akt and AMPK for ghrelin activation of eNOS, which agrees with recent reports showing the involvement of both Akt and AMPK in eNOS phosphorylation and NO production in ECs in response to high-density lipoprotein and fluid shear stress (16,17). However, others have shown that in response to adiponectin, eNOS phosphorylation and NO production in ECs are only dependent on AMPK, but not Akt (48). Thus, the contribution of individual eNOS kinase to eNOS phosphorylation at Ser-1177 might differ dependent on the stimulus. Collectively, data presented in this study demonstrate that Akt and AMPK are the major mediators for ghrelin activation of eNOS.
GHSR is a seven-transmembrane G protein-coupled receptor for ghrelin, and stimulation of GHSR with ghrelin leads to activation of G protein, calcium mobilization, and multiple downstream signalings (21,22,23). In the present study, we used siRNA technology to examine the role of GHSR in ghrelin-mediated eNOS activation. Specific knockdown of GHSR expression by siRNA significantly attenuated the positive effects of ghrelin on eNOS, Akt, and AMPK phosphorylation in ECs. Inhibiting Gq protein and intracellular calcium also blocked such effects of ghrelin. We further showed that inhibiting CaMKKβ by the pharmacological inhibitor and siRNA abolished ghrelin-induced phosphorylation of Akt, AMPK, and eNOS activation as well as NO production, suggesting that CaMKKβ may be a convergent point to mediate AMPK and Akt activation in ghrelin signaling.
In summary, we demonstrate that ghrelin activates eNOS in ECs through GHSR-mediated Akt and AMPK signal pathways. Given the importance of eNOS activity in regulation of endothelial function, ghrelin activation of eNOS should be beneficial through preventing endothelial dysfunction associated with cardiovascular disease. Consistent with the notion, it has recently been reported that ghrelin infusion markedly improved endothelial function in patients with metabolic syndrome (35), and the supplement of ghrelin inhibits proatherogenic changes in experimental models (30). However, whether ghrelin-mediated eNOS activation contributes to its vascular protection in vivo needs further investigations. Therefore, the identification of ghrelin regulatory pathways on eNOS activation may provide new strategies for preventing endothelial dysfunction in patients with cardiovascular disease and diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Morris J. Birnbaum for the gift of AMP-activated protein kinase adenovirus constructs. We also thank Ms. Chelsea Wong for excellent technical assistance.
Footnotes
This work was partially supported by Thomas R. Lee Career Development Award 1-06-CD-13 from American Diabetes Association Grant-In-Aid Award 0755916T from American Heart Association and RO1 HL-080611 grant from the National Institutes of Health (to Z.-G.J.).
Disclosure Statement: The authors have noting to declare.
First Published Online May 1, 2008
Abbreviations: AMPK, AMP-activated protein kinase; CaMKK, calmodulin-dependent kinase kinase; cGMP, cyclic GMP; DAF2-DA, dye 4,5-diaminofluorescein diacetate; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; GHSR, GH secretagogue receptor; HUVEC, human umbilical vein endothelial cell; L-NAME, N (G)-nitro-l-arginine methyl este; NO, nitric oxide; PI3K, phosphatidylinositol 3′OH-kinase; siRNA, small interference RNA.
References
- Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H 2002 Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation 105:e138–e143 [DOI] [PubMed] [Google Scholar]
- Landmesser U, Hornig B, Drexler H 2004 Endothelial function: a critical determinant in atherosclerosis? Circulation 109(Suppl 1):II27-II33 [DOI] [PubMed] [Google Scholar]
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C 2005 Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493 [DOI] [PubMed] [Google Scholar]
- Zeiher AM 1996 Endothelial vasodilator dysfunction: pathogenetic link to myocardial ischaemia or epiphenomenon? Lancet 348(Suppl 1):s10–s12 [DOI] [PubMed] [Google Scholar]
- Harrison DG 1997 Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100:2153–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC 2005 Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz 100(Suppl 1):15–18 [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Igarashi J, Greif D, Michel T 2006 The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46:235–276 [DOI] [PubMed] [Google Scholar]
- Jin ZG 2006 Where is endothelial nitric oxide synthase more critical: plasma membrane or Golgi? Arterioscler Thromb Vasc Biol 26:959–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM 1999 Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC 1999 Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljada A, Dandona P 2000 Effect of insulin on human aortic endothelial nitric oxide synthase. Metabolism 49:147–150 [DOI] [PubMed] [Google Scholar]
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL 2007 The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest 117:1961–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA 2003 AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31:162–168 [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ 2005 Activating AMP-activated protein kinase without AMP. Mol Cell 19:289–290 [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE 1999 AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289 [DOI] [PubMed] [Google Scholar]
- Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA 2004 High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA 101:6999–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland Jr T, Shyy JY 2006 AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 26:1281–1287 [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ 2003 Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278:45021–45026 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH 2006 Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505 [DOI] [PubMed] [Google Scholar]
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ 2008 Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57:696–705 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Locatelli V, Rossoni G, Schweiger F, Torsello A, De Gennaro Colonna V, Bernareggi M, Deghenghi R, Muller EE, Berti F 1999 Growth hormone-independent cardioprotective effects of hexarelin in the rat. Endocrinology 140:4024–4031 [DOI] [PubMed] [Google Scholar]
- Xu XB, Cao JM, Pang JJ, Xu RK, Ni C, Zhu WL, Asotra K, Chen MC, Chen C 2003 The positive inotropic and calcium-mobilizing effects of growth hormone-releasing peptides on rat heart. Endocrinology 144:5050–5057 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K 2004 Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110:3674–3679 [DOI] [PubMed] [Google Scholar]
- Pang JJ, Xu RK, Xu XB, Cao JM, Ni C, Zhu WL, Asotra K, Chen MC, Chen C 2004 Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro. Am J Physiol Heart Circ Physiol 286:H1063–H1069 [DOI] [PubMed] [Google Scholar]
- Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C 2005 GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol 289:H1643–H1651 [DOI] [PubMed] [Google Scholar]
- Shinde UA, Desai KM, Yu C, Gopalakrishnan V 2005 Nitric oxide synthase inhibition exaggerates the hypotensive response to ghrelin: role of calcium-activated potassium channels. J Hypertens 23:779–784 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nagaya N, Teranishi Y, Imazu M, Yamamoto H, Shokawa T, Kangawa K, Kohno N, Yoshizumi M 2003 Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochem Biophys Res Commun 310:830–835 [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML 2001 Circulating ghrelin levels are decreased in human obesity. Diabetes 50:707–709 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J 2004 Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101:10434–10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O 2003 Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52:2546–2553 [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Hulten LM, Hulthe J 2003 Plasma ghrelin, body fat, insulin resistance, and smoking in clinically healthy men: the atherosclerosis and insulin resistance study. Metabolism 52:1460–1463 [DOI] [PubMed] [Google Scholar]
- Tesauro M, Schinzari F, Iantorno M, Rizza S, Melina D, Lauro D, Cardillo C 2005 Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112:2986–2992 [DOI] [PubMed] [Google Scholar]
- Jin ZG, Wong C, Wu J, Berk BC 2005 Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate Akt and eNOS activation in endothelial cells. J Biol Chem 280:12305–12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Jin ZG 2005 Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem 280:33262–33269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K 2000 Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest 106:493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ 2003 Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem 278:43074–43080 [DOI] [PubMed] [Google Scholar]
- Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC 2003 Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 93:354–363 [DOI] [PubMed] [Google Scholar]
- Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B 2004 HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 113:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Gerstenblith G, Lakatta EG, Rosas GO, Vandegaer K, Ricker KM, Hare JM 2001 Upregulation of the nitric oxide-cGMP pathway in aged myocardium: physiological response to l-arginine. Circ Res 88:97–102 [DOI] [PubMed] [Google Scholar]
- Lungu AO, Jin ZG, Yamawaki H, Tanimoto T, Wong C, Berk BC 2004 Cyclosporin A inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J Biol Chem 279:48794–48800 [DOI] [PubMed] [Google Scholar]
- Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA 2002 Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277:32552–32557 [DOI] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR 1998 Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396:584–587 [DOI] [PubMed] [Google Scholar]
- Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL 2004 Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation 109:2221–2226 [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S 2002 Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87:240–244 [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K 2004 Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iantorno M, Chen H, Kim JA, Tesauro M, Lauro D, Cardillo C, Quon MJ2007 Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. Am J Physiol Endocrinol Metab 292:E756–E764 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.