Abstract
Estrogen and its receptors influence growth and differentiation by stimulating the production and secretion of growth factors. Our previous studies indicate an increased expression of estrogen receptor (ER)-α and decreased growth factor synthesis in the olfactory bulb of reproductive senescent female rats as compared with young animals. The present study tests the hypothesis that abnormal overexpression of ERα contributes to decreased growth factor synthesis. We developed the HeLa-Tet-On cell line stably transfected with ERα (HTERα) that expresses increasing amounts of ERα with increasing doses of doxycycline (Dox). Increasing doses of Dox had no effect on vascular endothelial growth factor (VEGF) secretion in HTERα cells. However, in the presence of 40 nm 17β-estradiol, VEGF secretion increased in low-dose Dox-exposed HTERα cultures, which was attenuated by the ERα antagonist, 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]1H-pyrazole dihydrochloride. However, at high-dose Dox and, consequently, high ERα levels, estradiol failed to increase VEGF. In the HeLa X6 cell line in which the Tet-On construct is upstream of an unrelated gene (Pitx2A), estradiol failed to induce VEGF at any Dox dose. Furthermore, in the HTERα cell line, estradiol selectively down-regulates phospho-ERK2 and phospho-Akt at high ERα expression. This study clearly demonstrates that the dose of receptor critically mediates estradiol’s ability to regulate growth factors and survival kinases. The present data also support the hypothesis that 17β-estradiol treatment to an ERα overexpressing system, such as the senescent brain, could reverse the normally observed beneficial effect of estrogen.
THE OVARIAN STEROID, estrogen plays a critical role in the development of the central nervous system, cardiovascular system, and skeletal system in addition to its role in reproduction and may play a protective role in neurodegenerative (1,2), cardiovascular (3), and bone diseases (2,4,5). Estrogens effects are mediated by a special class of transcription factors, estrogen receptors (ERs), which belong to a steroid receptor superfamily. The estrogen receptor is a ligand-dependent transcription factor that regulates the expression of estrogen-responsive genes in target tissues. The three major forms of ER are ERα (6), ERβ (7), and truncated ER product as reported in the rat pituitary (8). Although ERα and ERβ share 95% homology in DNA-binding domains, they differ from each other by 45% in their ligand-binding domain, and both are encoded by different genes. The transcriptional efficiency of ERα and ERβ varies with the type of promoter of the target gene (9).
The brain is an important nonreproductive target organ for estrogen, and estrogen regulates the expression of key enzymes, cytoskeletal proteins, and growth factors (summarized in Ref. 10). Many of these processes critically affect neural development (11,12,13,14,15), and several of them, such as its actions on growth factors (10,16,17) and the cholinergic system (4,18,19,20), continue to occur in the adult brain as well. Direct neuroprotective actions of estradiol have been demonstrated by several in vivo and in vitro studies. Estrogen improves learning and memory in rodents (21,22) and cognitive function in aged monkeys (23). 17β-Estradiol decreases the infarct size after occlusion of the middle cerebral artery (2,24) and attenuates the loss of cholinergic function and growth factors after excitotoxic injury (25,26). In many tissues, both in the brain and the periphery, estrogen mediates its cytotrophic effects via regulation of local growth factors and their receptors (27,28).
However, studies from our laboratory show that 17β- estradiol’s neurotrophic effects are not universal. Thus, although acute and chronic 17β-estradiol treatment increased brain-derived neurotrophic factor (BDNF) mRNA and protein expression in the young adult rat brain (16,17,29,30,31,32), 17β-estradiol fails to increase BDNF in the forebrain of older, acyclic (reproductive senescent) female rats (33). Moreover, the immunosuppressive actions of 17β-estradiol demonstrated in inflammation models in young adult females (34,35) was actually reversed in senescent females (35).
Although the mechanism underlying estrogen’s age-dependent actions are not fully understood, one possibility may be the age-dependent alterations in estrogen receptors. For example, the diametrically opposite actions of 17β-estradiol on BDNF expression in the olfactory bulbs of young and senescent females was paralleled by increased expression of ERα in this region in senescent animals compared with young adults (33). Such increases in ERα immunoreactivity have also been observed in other regions of aged female rats, such as the hypothalamus (36).
In the present study, we tested the hypothesis that overexpression of ERα will inhibit the growth-promoting actions of 17β-estradiol. For these studies, a Hela-Tet-On ERα cell line was engineered, in which ER expression was controlled by graded exposure to doxycycline (Dox). Our results indicate that estradiol elevates vascular endothelial growth factor (VEGF) synthesis at low and moderate levels of ERα but fails to increase VEGF at high ERα levels. Furthermore, at high levels of ERα expression, 17β-estradiol treatment suppresses activation of the survival kinases ERK and Akt, suggesting that the dose of ERα is critical in determining growth-promoting actions of estradiol.
Materials and Methods
Construction and analysis of HeLa-ERα cell line
Construction of plasmid.
Human ERα cDNA (kindly provided by Dr. Steve Safe, Texas A&M University) was propagated by cloning into the EcoRI restriction site of the pBluescript SK vector (gift of Dr. Patrick Dunn, Texas A&M University). ERα cDNA was ligated within BamHI and SalI restriction sites, which allows reconstruction of the cDNA into the pTRE2hyg plasmid. The pTRE2hyg plasmid (CLONTECH, Palo Alto, CA) contains the hygromycin-resistance gene localized downstream of tetracycline-response element (TRE) and the cytomegalovirus minimal promoters, enabling activation of ERα expression in the presence of tetracycline or the analogs such as Dox. The pTRE2hygERα construct was verified by restriction digestion and sequencing. The forward and reverse primers for sequencing were: 5′-CGCCTGGAGACGCCATC-3′ and 5′-CCATTCTAAACAACACCCTG-3′, respectively.
Cell culture, gene transfection, and cloning of plasmids
The Hela Tet-On cell line (Invitrogen, Carlsbad, CA) transfected with the plasmid pTet-On and stably expressing reverse tetracycline-controlled transactivator was purchased from CLONTECH. Hela Tet-On cells were maintained at 37 C and 5% CO2 with culture medium including 90% DMEM, 10% Tet-free fetal bovine serum, 4 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml G418. At 90% confluency, the pTRE2hygERα was transfected into Tet-On HeLa cells using the Lipofectamine 2000 reagent (Invitrogen). The cultures were subjected to selection in presence of 200 μg/ml hygromycin (CLONTECH) and 500 μg/ml G418 for more than 2 wk. Clones were then exposed to Dox to assess transfection efficiency. Clones confirmed for stable transfection were passaged and maintained for experimental purposes. Cells were grown in media containing 80% phenol red-free DMEM, 20% gelded horse serum, 4 mm l-glutamine, 500 μg/ml geneticin, and 200 μg/ml hygromycin and were treated with Dox/17β-estradiol (Sigma-Aldrich, St. Louis, MO). HeLa Tet-On cells transfected with an unrelated gene, pTRE-GFP Pitx2A (X6 cell line) used as control cell line, was a kind gift from Dr. Q. Wei (Kansas State University, Manhattan, KS).
RNA extraction and RT-PCR
Total RNA was extracted from the transfected cells using Trizol reagent (Invitrogen) using our previously established methods (37). The concentration of RNA was determined by using Ribogreen RNA quantification kit (Molecular Probes/Invitrogen, Carlsbad, CA). First-strand cDNA synthesis was performed with an Invitrogen first-strand synthesis kit using 2 μg total RNA and oligo deoxythymidine as the primer. The reaction was performed at 50 C for 5 min, 42 C for 60 min, and then heated at 97 C for 5 min. A 2-μl aliquot from each reverse transcription reaction mixture was used for PCR amplification using previously established methods (37). PCRs for VEGF were performed using previously published primers (38). The primer sequences were: forward, 5′-AGGAGGGCAGAATCATCACG-3′, reverse, 5′-CAAGGCCCACAGGGATTTTCT-3′, and the reaction was amplified for 30 cycles, beginning with 95 C for 5 min, and each subsequent cycle at 94 C for 1 min; 55 C for 1 min, and 72 C for 1 min. As an internal control, reverse transcribed product from each treatment condition were also subjected to PCR amplification for a housekeeping gene (cyclophilin) as described elsewhere (37). Primer sequences were: forward, TGG TCA ACC CCA CCG TGT TCT TCG; reverse, TGC CAT CCA GCC ACT CAG TCT TGG. The PCR cycles for cyclophilin are as follows: 95 C for 2 min, 20 cycles of 95 C for 30 sec, 62 C for 1 min, and 72 C for 2 min. PCR products were separated on a 1.5% ethidium bromide-agarose gel and photographed using Molecular Analyst (Bio-Rad, Hercules, CA).
Protein extraction and Western blot analysis
Cultures were rinsed with DMEM, and cell proteins were harvested in lysis buffer [50 mm Tris (pH 7.4), 150 mm NaCl, 10% glycerol, 1 mm EGTA, 1 mm Na-orthovanadate (pH 10), 5 μm ZnCl2, 100 mm NaF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride in dimethylsulfoxide, 1% Triton X-100] and centrifuged at 18,000 rpm for 15 min. Supernatant was collected and stored at −20 C until further analysis. Protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL). Samples (25 μg) were size fractionated on 10% PAGE and transferred to nylon membranes (Millipore, Bedford, MA). Blots were blocked with 1× Tris-buffered saline containing 0.05% Tween 20 and 5% nonfat dry milk. Subsequently blots were incubated with primary (1:200, ERα AB16; Labvision, Fremont, CA) and secondary antibodies (1:5000, goat antirabbit; Upstate, Billerica, MA), subjected to three washes in 1× Tween Tris-buffered saline buffer between the two incubations. The immunosignal was detected to x-ray film (Bio-Rad) using chemiluminescence reagents (Renaissance; NEN Life Science Products, Boston, MA). In the case of ERα-positive bands for Dox 10- and Dox 50-treated cells, the bands were quantified using computer-assisted densitometric analysis (Quantity One; Bio-Rad). Group differences between the band intensity for Dox 10 and Dox 50 groups were analyzed by a t test.
Immunocytochemistry
Cells were rinsed with PBS three times and fixed in 4% paraformaldehyde for 30 min followed by washes in PBS (three times). Cells were blocked for an hour with buffer containing 5% goat serum and sequentially incubated with primary antibodies raised against different epitopes of the C terminus of ERα (1:200; MC20; Santa Cruz Laboratories, Santa Cruz, CA) followed by the appropriate secondary antibodies 1:2000 dilution; Alexafluor 594; Molecular Probes). Labeled cells were coverslipped with Prolong antifade mounting media (Molecular Probes) and images were digitized using the QCapture software (Q-Imaging, Surrey, Canada).
VEGF ELISA
VEGF levels in cell culture media and cell lysates were measured using a quantitative sandwich enzyme immunoassay kit (R&D Systems, Minneapolis, MN) and the manufacturer’s instructions. Briefly, samples and standards were loaded in duplicate onto a 96-well microplate precoated with affinity-purified polyclonal antibody specific for human VEGF. An equal volume of assay diluent was pipetted into all wells containing samples and standards. After 2 h of incubation at room temperature, the unbound antigens were washed five times with wash buffer. Thereafter 100 μl of horseradish peroxidase-conjugated polyclonal antibody was added to each well, and the plate was incubated again for 2 h. The unbound antibody-enzyme was removed by washes (five times). The presence of VEGF was detected by adding 100 μl of substrate for 30 min. The enzyme reaction was stopped by adding 2 n sulfuric acid. The colored reaction product was read at 450 nm in an ELISA plate reader with a correction at 590 nm. The concentration of VEGF present in the samples was interpolated from a linear standard curve using the KC4 software application (BioTek, Winooski, VT).
Phospho-ERK assay
Phosphorylated (p) and total ERK expression was analyzed by Western blots, performed as described above. Briefly, 50 μg of total proteins from each sample were size fractionated on a 10% PAGE gel, electroblotted onto a nylon membrane, and probed with monoclonal antibody for pERK (1:200 dilution; Santa Cruz) and later stripped and reprobed for pan-ERK (1:1000; Santa Cruz). The immunosignal was detected on x-ray film (Bio-Rad) using Chemiluminescence reagent (Renaissance; NEN Life Science Products). X-rays were then digitized and the intensity of the pERK bands were quantified using Quantity-One (Bio-Rad) and normalized to the intensity of the total ERK bands. Data are reported as mean and sem.
Bio-plex phosphoprotein assay
HeLa cell lysates were prepared using the Bio-Plex cell lysis kit (Bio-Rad). Briefly, the cells were first washed with ice-cold wash buffer and then lysed in 100 μl lysis buffer. The lysates were centrifuged at 4500 × g for 20 min at 4 C, and the supernatant was stored at −20 C until further analysis. The total protein concentration was determined using the BCA protein assay kit (Pierce).
The phosphoproteins and total protein targets for ERK and Akt in HeLa cell lysates were determined using Bio-Plex premixed multiplex assay kit (Bio-Rad) according to the manufacturer’s instructions. Briefly, 50 μl of each sample (200 μg/ml concentration) was loaded in duplicate onto a prewet 96-well filter plate containing dye-coupled beads bound to antibodies specific for pERK and pAkt. Equal volume of cell lysis/assay buffer (1:1) was added to replace the cell lysate in blank wells and incubated overnight in a microplate shaker at 300 rpm at room temperature. The following day, unbound proteins were removed by three washes with wash buffer, followed by incubation with biotinylated detection antibodies (25 μl) specific for each target protein for 30 min at room temperature. The plate was vacuum filtered and washed three times with wash buffer and subsequently incubated with streptavidin-phosphatidylethanolamine (50 μl). The plate was rinsed three times with resuspension buffer, and the immunocomplex was suspended in 125 μl of resuspension buffer. The fluorescent signature of the beads was acquired using Bioplex manager software in a Bioplex suspension array system (Luminex 100 system; Bio-Rad). The total proteins for ERK and Akt were assayed similarly in a separate plate. Culture media from these studies was assayed for VEGF.
Statistical analysis
Statistical analysis was performed using a standard statistical package (SPSS, Chicago, IL). For experiments in which VEGF and the survival kinases were measured, a two-way ANOVA was used, coding for Dox dose and 17β-estradiol treatment as the two variables. Planned comparisons (t tests) were used to determine whether specific groups were significantly different from each other. Specific comparisons included Dox 10 vs. Dox 10 + 17β-estradiol and Dox 50 vs. Dox 50 + 17β-estradiol. In experiments in which a high and low Dox dose was used with varying estradiol concentrations and also for ERα antagonist studies, the data were analyzed by a one-way ANOVA with planned comparisons. Bar graphs shown are mean ± sem of a single representative of three independent experiments. Within each experiment, a single group consisted of six replicates. In all cases, group differences were considered significantly different when P ≤ 0.05.
Results
Growth factor regulation by 17β-estradiol under varying levels of ERα expression
Dose response effect of Dox on ERα expression.
Our previous studies indicated that 17β-estradiol treatment dramatically decreased growth factor synthesis in the reproductive senescent brain in which ERα expression was abnormally increased (33). To ascertain the effect of ERα overexpression on 17β-estradiol’s regulation on growth factors, this study used an engineered HeLa cell line and measured VEGF as a prototypic estrogen-regulated growth factor. HeLa cells, transfected with ERα under the control of tetracycline promoter (HTERα), were seeded on 6-well plates and treated, on 60% confluency, with a range (0, 0.1, 1, 10, 20, and 50 μg/ml) of Dox for 24 h. ERα expression was detected by Western blot and immunohistochemistry using a polyclonal antibody raised against the C-terminus epitope of the protein. As shown in Fig. 1Ai, a dose-dependent increase in ERα was observed in response to Dox. This was more prominent at the lower Dox doses (0–1). The intensity of the ER-α band was quantified for Dox 10 and Dox 50 groups which were the salient comparison groups for subsequent experiments. There was a significant increase in ERα expression at Dox 50 compared with Dox 10 (Fig. 1Aii).
Figure 1.
Dose-dependent ERα expression in HTERα cells. Ai, Western blot analysis of ERα protein expression in ERα-transfected HeLa (HTERα) cells treated with increasing doses of Dox for 24 h. Equal amount of protein (25 μg) was loaded in each lane, and the blot was probed with an ERα-specific antibody (ERα Ab16; Neomarkers, Fremont, CA). Quantitative analysis of the ERα bands at Dox 10 and Dox 50 indicates a small but significant increase in the expression of this receptor at Dox 50, compared with Dox 10 (Aii). B, Whereas no ERα immunoreactivity (using the MC20 antibody) was seen in the Dox 0 treatment (Bi), both cytoplasmic/membrane and nuclear receptors are present once cells are exposed to Dox. Furthermore, there is a dose-dependent increase in ERα-staining intensity between the 10 (Bii) and 50 (Biii) μg/ml Dox dose. No labeling was seen in cells that were not exposed to primary antibody (Biv). Bar, 40 μm. *, P < 0.05.
Whereas the difference between the optical density of the Western blot bands between the Dox 10 and Dox 50 groups is about 8%, the difference in Dox dose-induced ERα expression is more evident in the immunohistochemistry analysis. Whereas no staining was seen in the untreated HTERα cells (Fig. 1Bi), both the nuclear and cytoplasmic forms of ERα were seen at the 10 (Fig. 1Bii) and 50 μg/ml (Fig. 1Biii) Dox dose. Furthermore, whereas some ERα immunopositive cells were seen at the 10 μg/ml Dox (Fig. 1Bii), many more, brightly labeled cells were seen in the 50 μg/ml Dox dose (Fig. 1Biii).
17β-Estradiol-mediated regulation of VEGF as a function of ERα concentration.
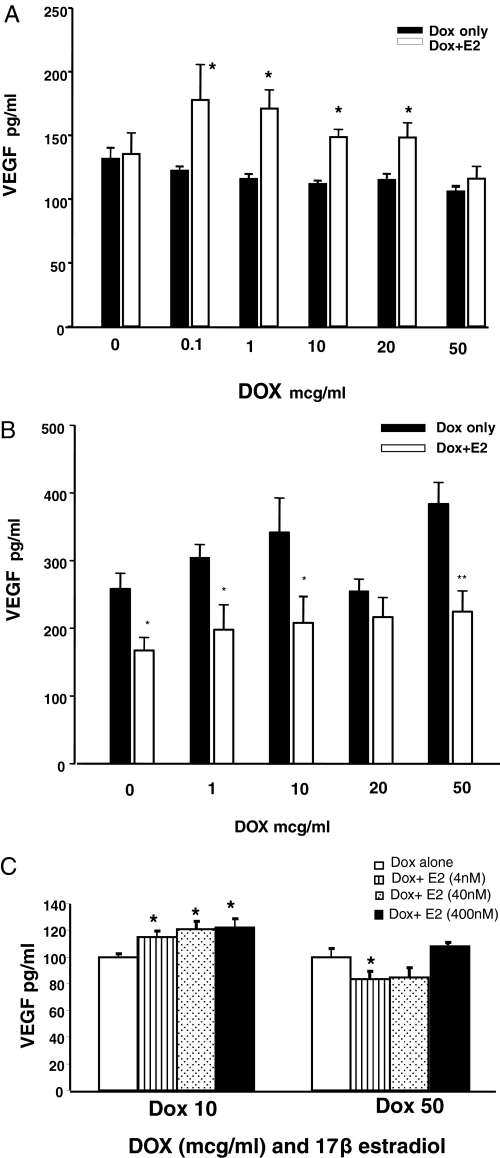
Media obtained from HTERα cells treated with Dox in the presence and absence of 17β-estradiol was assayed for VEGF by ELISA. As shown in Fig. 2Ai (black bars), there was a complex regulation of VEGF by 17β-estradiol as a function of increased ERα expression [interaction effect: F(5, 59): 3.17, P < 0.05]. Specifically, at low doses of Dox (hence low doses of ERα), estradiol increased VEGF release (Fig. 2A, white bars). However, at the high dose of Dox and, consequently, high ERα expression, there was a suppression of VEGF levels so that VEGF levels were no different from cells exposed to Dox but without estradiol (Fig. 2A, black bars). To determine whether the difference in media VEGF levels at low and high Dox doses is due to differences in synthesis or secretion, in a separate experiment. VEGF synthesis in protein lysates from high and low Dox-treated cells in the presence and absence of 17β-estradiol was assayed. There was a significant positive correlation between the VEGF levels in the media and lysates in Dox- (both high and low doses) and 17β-estradiol-treated cultures (+0.47, P < 0.05). 17β-Estradiol significantly increased growth factor levels in both cell lysates (20.9%) and media (9.9%) in low Dox-treated cultures and failed to increase VEGF in high-dose Dox exposed cultures.
Figure 2.
VEGF expression in HeLa cells. HTERα cells were treated with increasing doses of Dox in the presence (40 nm) and absence of 17β-estradiol for 24 h, and VEGF levels were measured by ELISA. A, In the absence of 17β-estradiol, Dox-dependent increase in ERα did not alter the level of VEGF secretion (black bars). 17β-Estradiol treatment significantly increased VEGF in the media at the low Dox doses (0.1–20 μg/ml) (white bars). However, at the high Dox dose (50 μg/ml), 17β-estradiol failed to increase VEGF. *, P < 0.05 (comparisons between vehicle and 17β-estradiol groups at a specific Dox dose). B, HeLa Tet-On cells transfected with an unrelated Dox-induced construct (Pitx2a) was used as a transfection control. Dox treatment did not cause any change in VEGF levels. 17β-Estradiol exposure failed to increase VEGF expression at any of the Dox doses. C, Dose-dependent effect of 17β-estradiol on VEGF release. HTERα cells exposed to low dose of Dox in the presence of increasing estradiol concentration (4, 40, and 400 nm) showed a dose-dependent increase in VEGF, but at high Dox dose, low dose of estradiol reduced VEGF and higher doses (40 and 400 nm) did not alter VEGF. Bars represent the VEGF levels at each dose of estradiol normalized to the baseline VEGF level for each dose of Dox. *, P < 0.05. In all cases, bars represent mean ± sem of a single representative experiment, with n = 6 for each treatment condition.
To establish that the biphasic regulation of VEGF was not a spurious interaction of Dox and 17β-estradiol, we used a control HeLa cell line that was transfected with the same Tet-On construct upstream of an unrelated gene (Pitx2a) (39). This control cell line was cultured under the same experimental conditions and exposed to the same range of Dox in the presence or absence of 17β-estradiol. Dox treatment did not have an effect on VEGF [F(4, 48): 2.45, P ≥ 0.05]. 17β-Estradiol failed to increase VEGF at all Dox doses (Fig. 2B) and in fact appeared to suppress VEGF expression at some doses [F(1, 48): 27.176, P < 0.05]. There was no interaction effect between Dox and 17β-estradiol in the control cell line [F(4, 48): 0.975, P ≥ 0.05].
ERα mediated regulation of VEGF as a function of estradiol concentration
To determine whether VEGF levels are regulated by a certain ratio of estradiol ligand and receptor, the next experiment tested VEGF expression in HTERa cells that were exposed to either low (10 μg/ml) or high (50 μg/ml) Dox with varying concentrations of estradiol (4, 40, and 400 nm). At the low dose of Dox (low ERα), VEGF levels were consistently increased with all estradiol doses (low ERα) [F(3, 22): 3.65, P < 0.05]. Interestingly, there was an overall effect of estradiol doses with the high Dox (high ERα) treatment as well (Fig. 2C) [F(3, 23): 4.89, P < 0.05]. At the lowest dose of estradiol (4 nm), VEGF expression was significantly reduced, compared with the Dox-only group, whereas at the middle and higher doses of estradiol (40 and 400 nm, respectively) VEGF expression was no different from baseline.
Receptor-mediated regulation of VEGF
Estradiol mediated VEGF release in HTERα cell line was further studied in the presence of the ER antagonist, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]1H-pyrazole dihydrochloride) (MPP; Tocris, Ellisville, MO), which is highly specific for ERα (40). HTERα cells were concurrently exposed to two different low doses of Dox (1 and 10 μg), in which VEGF expression was reliably increased by 17β-estradiol (see Fig. 3A), together with MPP (1 μm) in the presence and absence of 17β-estradiol for 24 h. MPP abolished the estrogen-mediated increase in VEGF at both doses of Dox (Fig. 3A) [F(3, 19): 48.78, P < 0.05], indicating that 17β-estradiol induction of growth factor at the low Dox dose is due to ERα expression.
Figure 3.
Receptor-mediated regulation of VEGF release. A, Concurrent exposure of HTERα cells to 17β-estradiol and MPP (ERα antagonist) at both the 1 and 10 μg Dox dose completely blocked 17β-estradiol mediated increase in VEGF. *, P < 0.05). Bars represent mean ± sem of a single representative experiment, with n = 6 for each treatment condition. B, RT-PCR indicated that the VEGF primers detected the appropriate size band (342 bp) for the VEGF gene, but there was no variation in the expression of VEGF mRNA as a result of 17β-estradiol treatment or increased Dox doses. Cyclophilin was also amplified from the same samples and used here to indicate that equal amount of RNA was amplified in each case.
Determination of molecular control of VEGF regulation by 17β-estradiol
To determine whether the ERα dose effect on VEGF expression is transcriptional, we analyzed VEGF mRNA expression in HTERα cells exposed to a range of Dox doses, with or without concurrent 17β-estradiol. Cells were harvested for RNA, and VEGF mRNA was determined by semiquantitative RT-PCR. There was no visible change in VEGF mRNA expression as a consequence of Dox or 17β-estradiol treatment (Fig. 3B), suggesting that 17β-estradiol regulation of VEGF protein in the media and in lysates was not due to transcriptional alteration.
17β-Estradiol regulates survival kinases as a function of ERα overexpression
Because VEGF activation and cell survival pathways are highly interdependent, we examined the expression of two major survival signaling kinases, pERK and the phosphorylated serine/threonine protein kinase (pAkt) in HTERα cells. Dox treatment did not alter ERK2 phosphorylation in the absence of 17β-estradiol. In the presence of 17β-estradiol, pERK2 was dramatically decreased in the high Dox dose (50 μg), compared with the low Dox dose (Fig. 4A), indicating that survival pathways are suppressed at high levels of receptor. This pattern of ERK suppression at high levels of Dox was confirmed by the dual laser-based fluorescence assay. Similar to the Western blot analysis, the fluorescent assay also indicated an interaction effect of Dox and estradiol on ERK2 phosphorylation [F(2, 28): 5.593, P < 0.05]. Estradiol significantly decreased phosphorylated ERK2 at high Dox (and therefore high ERα levels) (Fig. 4C). The fluorescent based assay indicated that 17β-estradiol differentially regulates Akt phosphorylation at low and high ERα levels [interaction effect, F(2, 25): 10.072, P < 0.05]. There is a robust increase in Akt phosphorylation with 17β-estradiol at low ERα levels, but phosphorylation of Akt by estradiol was attenuated at high ERα levels. Hence, the inability to increase VEGF expression in the high Dox (consequently high ERα) condition is associated with a critical loss of phosphorylated ERK2 and Akt expression.
Figure 4.
Regulatory effects of 17β-estradiol on survival kinase expression as a function of ERα concentration. A, Western blot analysis for expression of phosphorylated and total ERK-2 in lysates from HTERα cells treated with increasing amounts of Dox in the presence or absence of 17β-estradiol. Dox treatment alone had no significant effect on ERK2 activation. However, estrogen treatment significantly suppressed pERK2 at the high Dox dose (50 μg/ml). B, Bars represent group means ± sem of the relative OD of pERK2 normalized to total ERK (n = 6 for each treatment condition). *, P < 0.05. C, Dox/17β-estradiol-induced changes in ERK2 phosphorylation were confirmed with the Bio-Plex phosphoprotein assay, indicating again that 17β-estradiol decreased constitutive ERK2 phosphorylation at high ERα expression (n = 6, P < 0.05). D, Dox/17β-estradiol-induced changes in Akt phosphorylation was determined with the Bio-Plex phosphoprotein assay specific for this signaling protein. Bars represent mean ± sem of a single representative experiment, with n = 6 for each treatment condition. The graph represents mean ± sem of phospho-Akt normalized to Akt for each group. 17β-Estradiol increased Akt phosphorylation at low ERα concentrations, but the same dose of estradiol significantly suppressed Akt activation at high ERα expression. *, P < 0.05 (comparisons between vehicle and 17β-estradiol treatment for each Dox dose).
Discussion
The present study tested the hypothesis that ERα overexpression abolishes estradiol’s stimulatory effect on growth factor synthesis. Using the HTERα cell line in which ERα levels can be manipulated by exposure to Dox, we found that 17β-estradiol increased VEGF synthesis at low and moderate levels of ERα but failed to increase VEGF at high ERα levels. The study further confirmed high ER-α expression as the key player in VEGF modulation because of the lack of activational effect of estradiol at physiological and higher (pharmacological) concentrations. These data are reminiscent of our previous results in which high ERα expression in the olfactory bulbs of reproductive senescent females was associated with a loss of estradiol up-regulation on BDNF expression. Unlike the in vivo data, however, which are correlational, the present study directly ties ERα abundance to trophic support. These data also support our hypothesis that there may be a homeostatic dose of ERα necessary for the trophic effect of estrogen (41,42).
Recent studies have revealed a correlation between alterations/increases in ERα expression and disease pathologies in human and experimental animal models. Postmortem brains of Alzheimer’s disease patients show an increased expression of nuclear ERα in the vertical limb of the diagonal band of Broca (43) and also in the hypothalamus of both males and females (44). Similarly, high levels of ERα protein and mRNA have been reported in heart tissue from patients with dilated cardiomyopathy (45). Aging is accompanied by significant increases in ERα expression in the olfactory bulb in senescent animals (30) and hypothalamic neurons of aged female rats (36). Several recent studies also report that the localization of ERα is altered with age. In double-transgenic β-amyloid precursor protein-presenilin mice, nuclear ERα levels decreased in basal forebrain neurons (46). Similarly, in the aged human brain, an increased proportion of nuclear to cytoplasmic ERα was observed in the infundibular nucleus of the hypothalamus (44). Age-related increases in ERα have also been demonstrated in nonneural tissues such as human osteoblast cultures (47) and in the kidneys of female mice (48).
A growing body of evidence also indicates that transfected ERα overexpression potentiates growth inhibition. For example, 17β-estradiol inhibits the growth of ER-expressing HeLa cells (49), reduces the proliferation (50) and metastasis of an ER negative breast cancer cell line (MDA-MB-231) transfected with human ER, and decreases lung metastasis in athymic nude mice injected with ER-expressing cancer cells (51). A recent study demonstrated that overexpression of ERα receptor in the ECV304 and Ishikawa cell line inhibits growth through down-regulation of endothelin-1 and VEGF (52,53). The concept of a dose of ERα provides a plausible explanation for the aforementioned in vivo and in vitro studies, such that low to moderate levels of the receptor stimulate a trophic outcome whereas higher doses predict a more deleterious fate. The present study used VEGF analysis because it is a prototypic estrogen-inducible growth factor and demonstrated that 17β-estradiol increased VEGF levels when cells expressed low levels of ERα and failed to do so when ERα levels were increased. In addition, the present findings also have implications for the therapeutic potential of ER: first, it implies that the transfection of ERα in an ER-negative background can restore the potential for hormone responsiveness, and second, it suggests that the dose of the receptor limits estrogen’s growth-promoting action.
To determine the molecular switch that distinguishes high and low ERα levels in orchestrating 17β-estradiol’s effect on VEGF release, we examined this effect at a transcriptional level and at the protein level. Whereas studies have identified an estrogen response element in the VEGF gene (54) and shown that estrogen up-regulates VEGF mRNA in endometrial carcinoma cell lines (55) and human endometrium (56), no transcriptional changes were observed in the present study. However, protein levels were increased by estradiol in cell lysates of low Dox-exposed cultures, suggesting a posttranscriptional effect of estradiol on VEGF synthesis.
Selected members of the MAPK (ERK) and phosphatidylinositol 3-kinase (PI3-K) signaling (Akt) family play a central role in regulation of VEGF synthesis and release in ERα-positive and -negative cells. 17β-Estradiol treatment to the high ERα-expressing HTERα cells, in which VEGF is suppressed, also suppressed ERK phosphorylation. A precedent for this observation comes from studies in human myeloma cells in which the IL-6-induced increase in VEGF levels are correlated with increased pERK activity, with reduced VEGF secretion in the presence of an ERK inhibitor (57). Collectively, these studies suggest that inhibition of pERK is linearly related to growth factor regulation. The present data are also consistent with studies indicating that 17β-estradiol increases phosphorylation of both ERK1 and ERK2 in ERα-transfected immortalized hippocampal cells (58) and organotypic explants of the rat cerebral cortex (59). Interestingly, exposure to 16α,17-iodo-estradiol, an ERα-specific ligand-attenuated ERK phosphorylation in the same explant cultures (59,60), suggesting that specific activation of this receptor may be inhibitory to this pathway.
VEGF-dependent cell survival is mediated through activation of MAPK (61) and PI3-K signaling pathways (62). Akt, a major effector protein kinase in the PI3-K signaling, is regulated by steroid hormones (63,64), growth factors (65,66,67,68), and their receptors (69,70). MDA-MB-231 cells transfected with nuclear and membrane ERs showed increased Akt and decreased ERK phosphorylation in the presence of 17β-estradiol with slightly different temporal expression patterns between the receptors (41). ERα-mediated VEGF gene expression is shown to be strictly dependent on Akt pathway because in vivo administration of PI3-K inhibitors blocked E2-induced VEGF mRNA in rat uterus (71). In the present study, 17β-estradiol strongly enhanced Akt phosphorylation at low ERα expression and significantly attenuated Akt activation with increased ERα concentration in HTERα cells, and this was paralleled by decreased VEGF levels. Furthermore, the differential response in Akt phosphorylation in HTERα cells reinforces the concept of dose of ERα as a major regulator in estrogen-mediated nongenomic signaling. Although the present study does not allow us to determine whether ERK2 and Akt suppression precedes or follows VEGF regulation, it strongly indicates that 17β-estradiol treatment in the presence of high ERα levels is not cytoprotective.
In the present study, although both nuclear and nonnuclear receptors were increased by Dox treatment (see Fig 1, Bii and Biii), two observations suggest that the abundance of the nonnuclear receptor may be the critical checkpoint for estrogens trophic or nontrophic action. First, 17β-estradiol-mediated increases, and subsequent failure, to induce VEGF (due to receptor dose) was not accompanied by transcriptional regulation of VEGF, and second, at high ERα levels, 17β-estradiol strongly suppressed both ERK2 and Akt phosphorylation. The membrane ER is localized to caveolae or caveolae-like domains that play a crucial role in mediating membrane-associated receptor signaling and the downstream effectors in a variety of cells (72,73). Although the mechanism by which overexpression of this membrane receptor impacts the survival kinases is not clear, one possibility is that ERα overexpression may destabilize the caveolae, causing increased mobilization and endocytosis of this structure. Whereas estrogen signaling and membrane caveolar association has been well demonstrated in nonneuronal cells (72), emerging evidence indicates a similar mechanism of estrogen-induced caveolae-mediated signaling may occur in other cell types as well, notably in neuronal plasma membranes (74,75).
In conclusion, the dose-dependent effect of Dox on 17β-estradiol-mediated VEGF secretion in transfected HeLa cells suggests that the concentration of receptor is a critical checkpoint in estrogens growth-promoting effect. In view of the change in level and pattern of ERα expression in aging and brain pathologies, the present data strongly indicate that the concentration of ERα should be considered an important therapeutic target in estrogen replacement therapy for neural health.
Acknowledgments
The authors thank Dr. Steve Safe (Texas A&M University) for human ERα cDNA; Dr. Patrick Dunn (Texas A&M University) for pBluescript vector; Dr. Qize Wei (Kansas State University, Manhattan, KS) for Pitx2A cell line; and Dr. Wei-Jung Chen (Texas A&M Health Science Center) for advice on statistical analysis.
Footnotes
This work was supported by National Institutes of Health Grants AG 19515 and AG 027684 (to F.S.) and the National Institute of Environmental Health Sciences Grant P30-ES09106, which supported the core facilities used in the study.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: BDNF, Brain-derived neurotrophic factor; Dox, doxycycline; ER, estrogen receptor; HTERα, HeLa-Tet-On cell line stably transfected with ERα; MPP, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]1H-pyrazole dihydrochloride; p, phosphorylated; PI3-K, phosphatidylinositol 3-kinase; TRE, tetracycline-response element; VEGF, vascular endothelial growth factor.
References
- Simpkins JW, Singh M, Bishop J 1994 The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer’s disease. Neurobiol Aging 2:S195–S197 [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM 1998 Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18:1253–1258 [DOI] [PubMed] [Google Scholar]
- Sitges M, Leivas A, Heras M, Ferrer E, Roque M, Viles D, Roig E, Rivera F, Sanz G 2005 Short-term transdermal estradiol enhances nitric oxide synthase III and estrogen receptor mRNA expression in arteries of women with coronary artery disease. Int J Cardiol 105:74–79 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW 1994 Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644:305–312 [DOI] [PubMed] [Google Scholar]
- Nelson HD, Rizzo J, Harris E, Cauley J, Ensrud K, Bauer DC, Orwoll E 2002 Osteoporosis and fractures in postmenopausal women using estrogen. Arch Int Med 162:2278–2284 [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P 1986 Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139 [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-A 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 3:863–870 [DOI] [PubMed] [Google Scholar]
- Friend KE, Ang LW, Shupnik MA 1995 Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA 92:4367–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Lu B, Leygue E, Murphy LC 2003 Putative functional characteristics of human estrogen receptor-β isoforms. J Mol Endocrinol 30:13–29 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD 1994 Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci 14:459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD 1984 Gonadal hormones and brain development: implications for the genesis of sexual differentiation. Ann NY Acad Sci 435:101–111 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 1980 Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro. II. Morphological correlates and hormonal specificity. Brain Res 189:413–427 [DOI] [PubMed] [Google Scholar]
- Lustig RH, Sudol M, Pfaff DW, Federoff HJ 1991 Estrogenic regulation and sex dimorphism of growth-associated protein 43 kDa (GAP-43) messenger RNA in the rat. Brain Res 11:125–132 [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y 1981 Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res 213:422–426 [DOI] [PubMed] [Google Scholar]
- Stanley HF, Curtis A, Sheward WJ, Roberts JL, Fink G 1986 Prolactin messenger ribonucleic acid levels in the normal and hypogonadal mouse pituitary gland. Endocrinology 119:2422–2426 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW 1995 The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 136:2320–2324 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RCG, Toran-Allerand CD 1995 Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92:11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW 1992 Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol 116:23–39 [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Quirion R, Beaudet A 1990 Neurotensin regulation of endogenous acetylcholine release from rat cerebral cortex: effect of quinolinic acid lesions of the basal forebrain. J Neurochem 55:1397–1403 [DOI] [PubMed] [Google Scholar]
- Luine VN 1985 Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol 89:484–490 [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC 2002 Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115:547–558 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP 2001 Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci 21:6949–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG 2002 Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging 23:589–600 [DOI] [PubMed] [Google Scholar]
- Zhang Y-Q, Shi J, Rajakumar G, Day AL, Simpkins JW 1998 Effects of gender and estradiol treatment on focal brain ischemia. Brain Res 784:321–324 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Peeples KW, Marroquin OA 2000 Local and cortical effects of olfactory bulb lesions on trophic support and cholinergic function and their modulation by estrogen. J Neurobiol 45:61–74 [DOI] [PubMed] [Google Scholar]
- Ritz MF, Schmidt P, Mendelowitsch A 2002 17β-Estradiol effect on the extracellular concentration of amino acids in the glutamate excitotoxicity model in the rat. Neurochem Res 27:1677–1683 [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM 2005 Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms. J Endocrinol 185:11–17 [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Bottero D, Varricchio L, Nanayakkara M, Rotondi A, Auricchio F 2002 Sex steroid hormones act as growth factors. J Steroid Biochem Mol Biol 83:31–35 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1999 Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res 844:20–27 [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F 2000 Region- and peptide-specific regulation of the neurotrophins by estrogen. Mol Brain Res 85:77–84 [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW 2001 Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 14:1992–2002 [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ 2002 Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. Eur J Neurosci 22:2650–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F 2001 Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging 22:311–321 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A 2003 Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100:9614–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F 2003 Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging 24:733–743 [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC 2003 Stereologic analysis of estrogen receptor α (ERα) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol 466:409–421 [DOI] [PubMed] [Google Scholar]
- Nordell VL, Lewis DK, Bake S, Sohrabji F 2005 The neurotrophin receptor p75NTR mediates early anti-inflammatory effects of estrogen in the forebrain of young adult rats. BMC Neurosci 6:58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Lee M, Kim S-S, Ha J 2005 Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem 280:9963–9972 [DOI] [PubMed] [Google Scholar]
- Wei Q, Adelstein RS 2002 Pitx2a expression alters actin-myosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling. Mol Biol Cell 13:683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS 2002 Antagonists selective for estrogen receptor α. Endocrinology 143:941–947 [DOI] [PubMed] [Google Scholar]
- Sohrabji F 2005 Estrogen: a neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Ann NY Acad Sci 1052:75–90 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Bake S 2006 Age-related changes in neuroprotection: is estrogen pro-inflammatory for the reproductive senescent brain? Endocrine 29:191–197 [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF 2003 Increased neuronal metabolic activity and estrogen receptors in the vertical limb of the diagonal band of broca in Alzheimer’s disease: relation to sex and aging. Exp Neurol 183:159–172 [DOI] [PubMed] [Google Scholar]
- Hestiantoro A, Swaab DF 2004 Changes in estrogen receptor-α and -β in the infundibular nucleus of the human hypothalamus are related to the occurrence of Alzheimer’s disease neuropathology. J Clin Endocrinol Metab 89:1912–1925 [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V 2006 Estrogen receptor α up-regulation and redistribution in human heart failure. FASEB J 20:926–934 [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Roschier U, Puolivali J, Wang J, Miettinen R 2005 The effect of aging on the subcellular distribution of estrogen receptor-α in the cholinergic neurons of transgenic and wild-type mice. Eur J Neurosci 21:1437–1442 [DOI] [PubMed] [Google Scholar]
- Ankrom MA, Patterson JA, d'Avis PY, Vetter UK, Blackman MR, Sponseller PD, Tayback M, Robey PG, Shapiro JR, Fedarko NS 1998 Age-related changes in human oestrogen receptor α function and levels in osteoblasts. Biochem J 333(Pt 3):787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PK, Thakur MK 2004 Estrogen receptor α expression in mice kidney shows sex differences during aging. Biogerontology 5:375–381 [DOI] [PubMed] [Google Scholar]
- Maminta ML, Molteni A, Rosen ST 1991 Stable expression of the human estrogen receptor in HeLa cells by infection: effect of estrogen on cell proliferation and c-myc expression. Mol Cell Endocrinol 78:61–69 [DOI] [PubMed] [Google Scholar]
- Lazennec G, Katzenellenbogen BS 1999 Expression of human estrogen receptor using an efficient adenoviral gene delivery system is able to restore hormone-dependent features to estrogen receptor-negative breast carcinoma cells. Mol Cell Endocrinol 149:93–105 [DOI] [PubMed] [Google Scholar]
- Garcia M, Derocq D, Freiss G, Rochefort H 1992 Activation of estrogen receptor transfected into a receptor-negative breast cancer cell line decreases the metastatic and invasive potential of the cells. Proc Nat Acad Sci USA 89:11538–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upla P, Marjomaki V, Kankaanpaa P, Ivaska J, Hyypia T, van der Goot FG, Heino J 2004 Clustering induces a lateral redistribution of α2β1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol Biol Cell 15:625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE 2001 Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol 429:355–371 [PubMed] [Google Scholar]
- Hyder SM, Nawaz Z, Chiappetta C, Stancel GM 2000 Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 60:3183–3190 [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK 1993 Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod 48:1120–1128 [DOI] [PubMed] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN 1996 Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 81:1120–1128 [DOI] [PubMed] [Google Scholar]
- Giuliani N, Lunghi P, Morandi F, Colla S, Bonomini S, Hojden M, Rizzoli V, Bonati A 2004 Downmodulation of ERK protein kinase activity inhibits VEGF secretion by human myeloma cells and myeloma-induced angiogenesis. Leukemia 18:628–635 [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM 2003 Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology 144:832–838 [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Warren M, Toran-Allerand CD 1999 Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci 19:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo Jr G, Guan X, Frail DE, Toran-Allerand CD 2000 Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-α knock-out mice. J Neurosci 20:1694–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 1998 Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem 273:26722–26728 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E 1999 Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR 2004 Genomic and non-genomic effects of estrogens on endothelial cells. Steroids 69:537–542 [DOI] [PubMed] [Google Scholar]
- Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM 2006 Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine 29:199–207 [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM 2003 Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 23:11036–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK 2005 Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 27:9794–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frago LM, Paneda C, Argente J, Chowen JA 2005 Growth hormone-releasing peptide-6 increases insulin-like growth factor-I mRNA levels and activates Akt in RCA-6 cells as a model of neuropeptide Y neurones. J Neuroendocrinol 17:701–710 [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM 2005 Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19:2026–2028 [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA 2000 Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J Mol Neurosci 14:197–203 [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Kanetake H 2004 Fibroblast growth factor-2-mediated capillary morphogenesis of endothelial cells requires signals via Flt-1/vascular endothelial growth factor receptor-1: possible involvement of c-Akt. J Biol Chem 279:4007–4016 [DOI] [PubMed] [Google Scholar]
- Kazi AA, Koos RD 2007 Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 148:2363–2374 [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RGW, Shaul PW 2000 Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circulation Res 87:44–52 [DOI] [PubMed] [Google Scholar]
- Turi A, Kiss AL, Mullner N 2001 Estrogen down-regulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int 25:785–794 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly Jr ES, Nethrapalli IS, Tinnikov AA 2002 ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG 2007 Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27:9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]