Abstract
Disorders of mineral and bone metabolism are prevalent in patients with chronic kidney disease (CKD). The recent National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend that blood calcium (Ca) be regularly measured in patients with stages 3 to 5 CKD. The Kidney Disease: Improving Global Outcomes (KDIGO) position states that the measurement of ionized Ca (iCa) is preferred and that if total Ca (tCa) concentration is used instead, then it should be adjusted in the setting of hypoalbuminemia. In 691 consecutive patients with stages 3 to 5 CKD, we compared the ability of noncorrected and albumin-corrected tCa concentration to identify low, normal, or high iCa concentration. The agreement between noncorrected or albumin-corrected tCa and iCa was only fair. The risk for underestimating ionized calcium was independently increased by a low total CO2 concentration when either noncorrected or albumin-corrected Ca was used and by a low albumin concentration only when noncorrected tCa was used. The risk for overestimating iCa was increased by a low albumin concentration only when albumin-corrected Ca was used. In conclusion, albumin-corrected tCa does not predict iCa better than noncorrected tCa. Moreover, both noncorrected and albumin-corrected tCa concentrations poorly predict hypo- or hypercalcemia in patients with CKD.
Disorders of mineral and bone metabolism are prevalent in patients with chronic kidney disease (CKD) and are an important cause of morbidity, in part via extraskeletal calcifications that have been found to be associated with increased cardiovascular mortality.1,2 Evaluation of disorders of bone and mineral metabolism is mandatory in patients with CKD.3 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines advise maintaining serum levels of total calcium (tCa), corrected for albumin level, within the “normal” range for the laboratory. Although the regulated variable is ionized Ca (iCa), they do not recommend measurement of iCa concentration because the reproducibility of iCa measurement was found to be worse than that of tCa and because, in current clinical practice, the technique is more time consuming, more expensive, and less easily available than that of tCa measurement.3 Finally, the guidelines recommend that tCa level be corrected for albumin and provide two distinct equations to perform the correction.
More recently, Kidney Disease: Improving Global Outcomes (KDIGO) published a position statement on the definition, evaluation, and classification of bone and mineral disorders.4 It is acknowledged that measurement of iCa is the preferred method for evaluating serum Ca and that, if total serum Ca concentration is used instead, it should be corrected for albumin when plasma albumin concentration is low. The position statement also acknowledges that it is necessary to assess the respective performances of albumin-corrected and noncorrected tCa concentrations in predicting the actual concentration of iCa.4 In this study, we compared the respective performances of noncorrected and albumin-corrected tCa in predicting low, normal, or high serum concentrations of iCa.
RESULTS
Demographic Data and Blood Ca Distribution
A total of 691 patients with stages 3 to 5 CKD were included in the study (Table 1). Using serum iCa as the reference, 554 patients had normal values, 109 had hypocalcemia, and 28 had hypercalcemia. Fourteen of those 28 patients with hypercalcemia were treated with oral Ca salts and/or active analog of vitamin D, and the diagnosis was excessive dosage. Three were treated with thiazide diuretics and one with lithium. The remaining 11, untreated, had a parathyroid hormone concentration that was either high or inappropriately normal; they were considered as having tertiary or primary hyperparathyroidism.
Table 1.
Demographic and biological characteristics of the study population
| Characteristic | Value | Normal Range |
|---|---|---|
| Female/male | 210/481 | |
| Age (yr; mean ± SD [range]) | 60 ± 15 (17 to 88) | |
| GFR (ml/min per 1.73 m2; mean ± SD [range]) | 31.2 ± 13.0 (5.1 to 59.8) | |
| iCa concentration (mmol/L; mean ± SD [range]) | 1.21 ± 0.07 (0.91 to 1.56) | 1.15 to 1.32 |
| Noncorrected tCa concentration (mmol/L; mean ± SD [range]) | 2.22 ± 0.14 (1.71 to 2.73) | 2.10 to 2.53 |
| Albumin-corrected calcium concentration 1 (mmol/L; mean ± SD [range]) | 2.23 ± 0.13 (1.75 to 2.73) | 2.10 to 2.53 |
| Albumin-corrected calcium concentration 2 (mmol/L; mean ± SD [range]) | 2.25 ± 0.13 (1.75 to 2.73) | 2.10 to 2.53 |
| Albumin concentration (g/L; mean ± SD [range]) | 39.6 ± 5.4 (13.3 to 59.0) | 35 to 50 |
| Albumin concentration <35 g/L (frequency [%]) | 108 (16) | |
| Total CO2 concentration (mmol/L; mean ± SD [range]) | 25.1 ± 3.3 (15.2 to 33.6) | 22 to 30 |
| Total CO2 concentration <22 mmol/L (frequency [%]) | 109 (16) | |
| Plasma phosphate concentration (mmol/L; mean ± SD [range]) | 1.14 ± 0.24 (0.56 to 2.70) | 0.82 to 1.40 |
Plasma albumin concentration ranged from 13.3 to 59.0 g/L. A total of 108 patients (15.6% of whole population) had a plasma albumin concentration <35 g/L (Table 2). The percentage of patients with low plasma albumin concentration significantly increased from stage 3 to stage 5 CKD (P = 0.004). Twenty-eight patients had a plasma albumin concentration <30 g/L; 21 of those 28 had a nephrotic syndrome, and seven had malnutrition. Nine patients had a plasma albumin concentration >50 g/L. In six, the most likely explanation was diuretic-induced extracellular volume contraction; in the remaining three, we did not find any reason for the moderately high value (52 to 53 g/L) of plasma albumin concentration.
Table 2.
Plasma albumin and total CO2 concentrations and distributions in the stages of CKD
| Parameter | CKD Stage
|
P | ||
|---|---|---|---|---|
| 3 | 4 | 5 | ||
| Whole population (n) | 356 | 254 | 81 | – |
| albumin (g/L) | 40.2 ± 5.1 | 39.0 ± 5.5 | 39.0 ± 5.7 | 0.007 |
| total CO2 (mmol/L) | 26.3 ± 2.9 | 24.3 ± 3.0 | 22.8 ± 3.4 | <0.001 |
| Plasma albumin <35 g/L (n) | 40 | 50 | 18 | |
| % of respective CKD stage | 11.2 | 19.7 | 22.2 | 0.004 |
| Total CO2 < 22 mmol/L (n) | 25 | 49 | 35 | |
| % of respective CKD stage | 7.0 | 19.3 | 43.2 | <0.001 |
A total of 109 patients (15.7% of whole population) had a low total CO2 (tCO2) concentration, <22 mmol/L (Table 2). The prevalence of low tCO2 concentration increased with the stage of CKD (P < 0.001).
Relationships between iCa, Noncorrected tCa, and Albumin-Corrected tCa Concentrations
The relations between noncorrected Ca, albumin-corrected Ca, and iCa concentrations, all expressed as z scores (see the Concise Methods section), are depicted in Figure 1. Regardless of correction for albumin concentration, individual points were widely scattered around the regression line, and the slope of the regression line was <0.5; the range of iCa values around the mean was more than twice as high as the range of either noncorrected or albumin-corrected Ca values. Standard regression analyses of these relationships showed only modest correlation between iCa and either noncorrected or albumin-corrected tCa concentrations.
Figure 1.
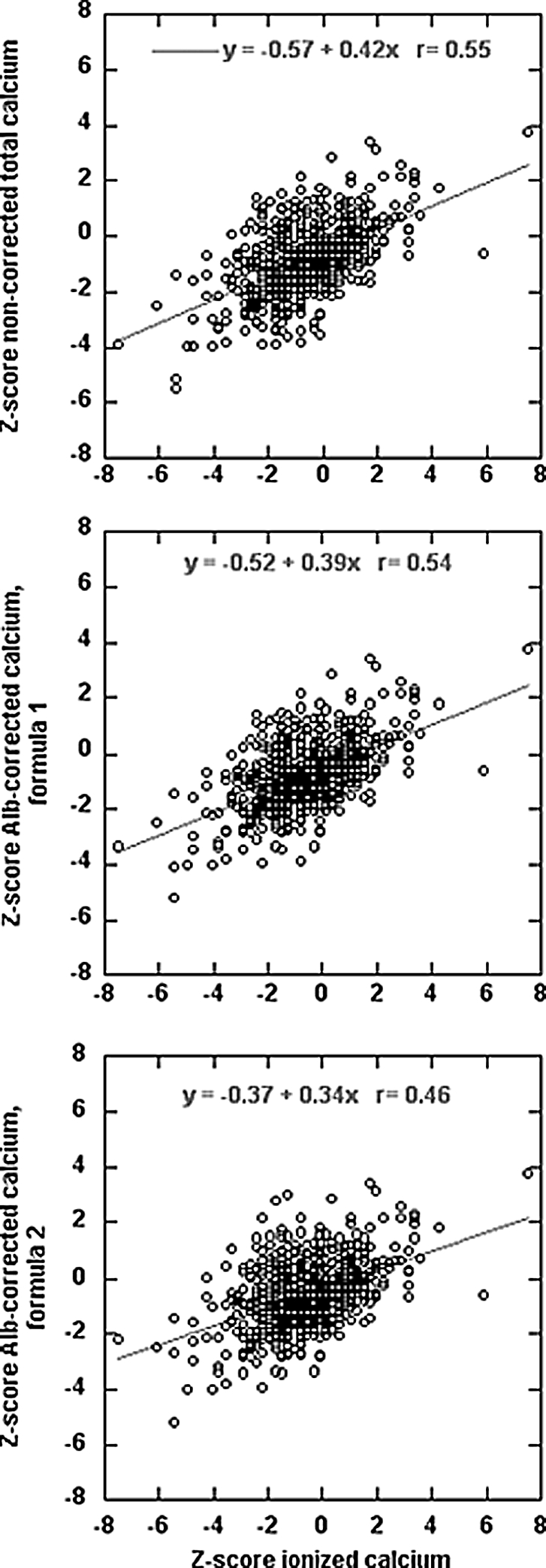
(A through C) Relationship between iCa concentration and noncorrected tCa (A); albumin-corrected tCa, formula 1 (B); and albumin-corrected tCa, formula 2 (C). All values are expressed as z scores (see the Concise Methods section).
Agreement among iCa, Noncorrected Ca, and Albumin-Corrected Ca Concentrations
The distribution of low, normal, and high calcium concentrations varied according to the estimator used; the number of patients with hypo- or hypercalcemia was lower when either noncorrected or albumin-corrected tCa concentrations were used instead of iCa (Table 3). As a whole, approximately 20% of the patients were not properly classified when either noncorrected or albumin-corrected tCa was used. The proportion of misclassification was particularly high in the patients with either low or high iCa concentration and did not differ whether noncorrected or albumin-corrected calcium was used. The low performance of tCa in predicting an abnormal iCa concentration was further confirmed by the values of the κ coefficient, showing only a fair agreement between tCa and iCa (Table 3).
Table 3.
Agreement between iCa concentration and the other estimators of blood calcium concentrationa
| iCa | Noncorrected tCa | Albumin-Corrected Ca, Formula 1 | Albumin-Corrected Ca, Formula 2 |
|---|---|---|---|
| Overall (n = 691) | |||
| agreement | 545 | 547 | 549 |
| underestimation | 76 | 69 | 58 |
| overestimation | 70 | 75 | 84 |
| κ coefficient (95% CI) | 0.29 (0.20 to 0.38) | 0.28 (0.19 to 0.37) | 0.25 (0.16 to 0.34) |
| Low (n = 109) | |||
| agreement | 44 | 39 | 32 |
| sensitivity/specificity (%) | 40.4/90.7 | 35.8/91.2 | 29.4/93.8 |
| High (n = 28) | |||
| agreement | 6 | 6 | 6 |
| sensitivity/specificity (%) | 21.4/99.2 | 21.4/99.2 | 21.4/98.8 |
| Normal (n = 554) | |||
| agreement | 495 | 502 | 511 |
| overestimation | 5 | 5 | 7 |
| underestimation | 54 | 47 | 36 |
CI, confidence interval.
As a consequence, the ability (sensitivity) to detect true hypocalcemia in our patients with CKD was 40.4, 35.8, and 29.4% using noncorrected, albumin-corrected tCa, formula 1 and albumin-corrected tCa formula 2, respectively. The diagnostic sensitivity for true hypercalcemia was homogeneously low (21.4%) when the three distinct estimators were applied. By contrast, the specificity of the various estimators exceeded 90% in each case.
To identify potential predictors of misclassification, we compared, as groups, patients with an agreement between noncorrected tCa and iCa values and patients without such agreement (when noncorrected Ca either over- or underestimated iCa; Supplemental Table 1). Taking patients with agreement between tCa and iCa as reference group, mean GFR values, tCO2, and albumin concentrations were significantly lower in patients in whom tCa underestimated iCa. By contrast, mean age was higher and mean albumin concentration was lower in patients in whom tCa overestimated iCa. No such difference was observed for plasma phosphate concentration.
Identification of Factors Associated with the Low Predictive Performance of tCa
Table 4 displays the factors identified as predicting misclassification (and odds ratio) when noncorrected tCa was used instead of iCa. We studied the variables that significantly differed between patients with and without agreement. In multivariate analysis, the risk for underestimation was significantly increased only by lower albumin and tCO2 concentrations, whereas none of the variables studied predicted the risk for overestimation. Because there was no difference in plasma phosphate concentration between patients with and without agreement, the logistic regression analysis did not include plasma phosphate as a factor.
Table 4.
Predictors and risk of under- or overestimation of iCa by noncorrected tCa: Univariate and multivariate logistic regression analysis
| Parameter | Underestimation
|
Overestimation
|
||
|---|---|---|---|---|
| Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | |
| Age (by 10 yr) | 0.86 (0.74 to 1.00)a | 0.93 (0.72 to 1.20) | 1.26 (1.05 to 1.51)a | 1.23 (0.54 to 2.78) |
| Albumin (g/L) | 0.93 (0.90 to 0.97)a | 0.94 (0.90 to 0.98)a | 0.92 (0.88 to 0.97)a | 0.94 (0.66 to 1.02) |
| tCO2 (mmol/L) | 0.79 (0.73 to 0.86)a | 0.72 (0.62 to 0.82)a | 1.02 (0.94 to 1.10) | 1.37 (0.91 to 2.06) |
| GFR (ml/min per 1.73 m2) | 0.98 (0.95 to 1.00)a | 1.02 (0.98 to 1.05) | 0.98 (0.91 to 1.05) | 0.95 (0.88 to 1.03) |
P < 0.05.
We separately tested venous pH, instead of tCO2 concentration, in the logistic regression model. A lower pH value was also an independent predictor of underestimation (data not shown); however, tCO2 concentration seemed to predict underestimation better than venous pH did: The maximum of likelihood corrected for the number of parameters included in the model was significantly better when tCO2 was incorporated in the model than when venous pH was (205 versus 218; P < 0.05).
We also performed the analysis after excluding patients with plasma albumin concentration <30 or >50 g/L (Supplemental Table 2). In those patients, plasma albumin concentration was not any more a risk factor for underestimation. This might indicate that patients with albumin concentration >30 g/L do not need correction for albumin.
To provide a more quantitative analysis and evaluate whether the results of the logistic regression could be the consequence of the arbitrary cutoffs for normal used in the logistic regression, we performed multivariate linear regression analysis to identify the determinants of noncorrected tCa, expressed as z score (Supplemental Table 3). The purpose was to identify which variables determined, besides iCa expressed as z score, how extreme tCa was compared with the normal range. The analysis included the same factors as in Table 4. When tCa underestimated iCa (z score tCa − z score iCa <0), iCa, albumin and tCO2 concentrations, and age were positively correlated with tCa: A lower tCa was independently predicted by lower iCa, plasma albumin or tCO2 concentrations, and younger age. When tCa overestimated iCa (difference in z scores >0), no variable but iCa correlated with tCa. The results of the linear regression analysis were thus consistent with those of the logistic regression analysis.
Tables 5 and 6 display the results of univariate and multivariate logistic regression analysis predicting under- or overestimation when albumin-corrected tCa, formula 1 or 2, respectively, was used instead of iCa. In multivariate analysis, the risk for underestimation was increased only by lower tCO2 concentration. The risk for overestimation was increased by lower albumin concentration. In multivariate linear regression analyses (Supplemental Tables 4 [dependent variable: z score albumin-corrected tCa, formula 1] and 5 [dependent variable: z score albumin-corrected tCa, formula 2]), iCa, age, and tCO2 concentration but not albumin significantly correlated with albumin-corrected tCa, formula 1 or 2, when albumin-corrected tCa underestimated iCa. When albumin-corrected tCa overestimated iCa, iCa positively correlated with albumin-corrected tCa, formulas 1 and 2, and albumin negatively correlated only with albumin-corrected tCa, formula 2.
Table 5.
Predictors and risk of under or overestimation of iCa by albumin-corrected tCa, formula 1: Univariate and multivariate logistic regression analysisa
| Parameter | Underestimation
|
Overestimation
|
||
|---|---|---|---|---|
| Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | |
| Age (by 10 yr) | 0.87 (0.74 to 1.01) | 0.89 (0.69 to 1.15) | 1.20 (1.00 to 1.42) | 1.10 (0.56 to 2.16) |
| Albumin (g/L) | 0.98 (0.93 to 1.02) | 1.04 (0.95 to 1.13) | 0.91 (0.87 to 0.95)b | 0.75 (0.63 to 0.90)b |
| tCO2 (mmol/L) | 0.79 (0.73 to 0.86)b | 0.72 (0.62 to 0.83)b | 0.99 (0.92 to 1.08) | 1.34 (0.95 to 1.91) |
| GFR (ml/min per 1.73 m2) | 0.98 (0.96 to 1.00)b | 1.02 (0.99 to 1.05) | 0.97 (0.91 to 1.03) | 0.94 (0.88 to 1.10) |
tCa value was corrected by formula 1 only in patients with plasma albumin concentration <35 g/L; in patients with plasma albumin concentration ≥35 g/L, the measured, noncorrected value was used in the analysis.
P < 0.05.
Table 6.
Predictors and risk of under- or overestimation of iCa by albumin-corrected tCa, formula 2: Univariate and multivariate logistic regression analysisa
| Parameter | Underestimation
|
Overestimation
|
||
|---|---|---|---|---|
| Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | Univariate Analysis (OR [95% CI]) | Multivariate Analysis (OR [95% CI]) | |
| Age (by 10 yr) | 0.91 (0.77 to 1.08) | 0.91 (0.70 to 1.18) | 1.23 (1.04 to 1.45) | 1.05 (0.56 to 1.98) |
| Albumin (g/L) | 1.04 (0.98 to 1.10) | 1.07 (0.89 to 1.18) | 0.88 (0.85 to 0.92)b | 0.73 (0.61 to 0.88)b |
| tCO2 (mmol/L) | 0.81 (0.75 to 0.89)b | 0.74 (0.64 to 0.85)b | 1.00 (0.94 to 1.08) | 1.42 (1.01 to 2.00) |
| GFR (ml/min per 1.73 m2) | 0.98 (0.96 to 1.00)b | 1.01 (0.98 to 1.05) | 0.96 (0.91 to 1.02) | 0.93 (0.87 to 1.00) |
tCa value was corrected by formula 2 only in patients with plasma albumin concentration <35 g/L; in patients with plasma albumin concentration ≥35 g/L, the measured, noncorrected value was used in the analysis.
P < 0.05.
DISCUSSION
The main observations in this study are that albumin-corrected tCa does not perform better than noncorrected tCa in predicting iCa abnormalities and that tCa displays a poor sensitivity for the diagnosis of either hypo- or hypercalcemia. This is mainly explained by the failure to take into account tCO2 concentration and overcorrection by the formulas correcting for albumin concentration.
The K/DOQI clinical practice guidelines recommend measurement of tCa because of poor reproducibility of iCa measurement, easier performance in clinical practice, and lower cost. In fact, reliable measurement of iCa is more demanding than that of tCa and requires appropriate sampling and handling. Blood should be sampled anaerobically, and the measurement should be performed as soon as possible to avoid loss of CO2 from the sample with subsequent changes in pH. When samples are handled in this way, the pH of the sample is in fact very close to the actual extracellular pH of the patient, and the day-to-day reproducibility of iCa measurement is quite good with a variation coefficient <2%, very similar to that of tCa.5–9 K/DOQI guidelines also recommend correction for albumin when plasma albumin concentration is low and provide two formulas for the correction.
In this study, the diagnostic performances of noncorrected and albumin-corrected tCa in predicting low, normal, or high values of iCa are rather low. Particularly, the performances in recognizing abnormalities of iCa concentration (hypo- or hypercalcemia) are dramatically weak: Approximately one third of patients with hypocalcemia only and one fifth of patients with hypercalcemia only had a correct diagnosis on the basis of the measurement of tCa.
By both logistic and linear regression analyses, we found that low albumin and tCO2 concentrations were independent risk factors of underestimation of blood Ca concentration when noncorrected tCa was measured. GFR, a significant risk factor for underestimation in univariate logistic analysis, was no more significant in multivariate analysis; the likely reason is that the prevalence of low tCO2 concentration increases with decreasing GFR, accounting for the association between GFR and the risk for underestimation. Low plasma pH is also an independent factor predicting underestimation; however, in our model, tCO2 concentration was a better predictor than plasma pH, suggesting that pH measurement is less reliable than tCO2 measurement. Younger age was also a factor of underestimation, in linear regression analysis only, but we unfortunately have no explanation to provide.
That low albumin and low tCO2 concentrations are independent risk factors for underestimation was expected because, in steady state, both factors decreased the amount of albumin-bound Ca without affecting the iCa concentration. Interestingly, when we analyzed the performance of noncorrected tCa, restricted to patients with albumin concentration between 30 and 50 g/L, plasma albumin concentration no longer predicted underestimation. Accordingly, when the performances of albumin-corrected tCa were evaluated, the sole independent risk factor of underestimation (besides age in linear regression analysis) was lower tCO2 concentration: Formulas 1 and 2 efficiently correct tCa concentration for low albumin concentration, but they did not improve prediction of iCa concentration because they did not take into account tCO2. In fact, the percentage of patients in whom noncorrected Ca or albumin-corrected Ca, formula 1 or 2, underestimated iCa was similar (Table 3). More generally speaking, these results suggest that a formula that does not correct tCa for both low albumin and low tCO2 concentrations fails to predict accurately the iCa concentration, at least when used in patients with stages 3 to 5 CKD.
Interestingly, low albumin concentration also was a risk factor of overestimation when formula 1 (in logistic regression only) or 2 (in both logistic and linear regressions) was used as estimators of blood Ca concentration. This indicates that both formulas may overcorrect Ca concentration.
Two recently published studies assessed the performance of albumin-corrected tCa in the diagnosis of blood Ca disturbances in dialysis patients.10,11 The authors of the first report, based on 50 patients, concluded that none of the published formulas improved the performance beyond that of noncorrected tCa.10 The authors of the second report, based on 34 patients, found, as in this study, that the use of albumin-corrected Ca led to an underestimation of the prevalence of hypocalcemia and an overestimation of the prevalence of hypercalcemia.11 Accordingly, the authors concluded that the use of albumin-corrected Ca may lead to inappropriate clinical decisions.
To our knowledge, ours is the first report on the performance of noncorrected and albumin-corrected tCa in a large cohort of patients with stages 3 to 5 CKD, excluding dialysis patients. As in the latter, we observed that correction for albumin did not improve the diagnostic performance of tCa. Likely, the main reason is that the correction formulas were developed in unselected patients, not specifically devoted to patients with CKD. That low tCO2 concentration was as prevalent in our population as low albumin concentration clearly is key to the observation that albumin-based formulas do not improve the prediction of abnormal iCa concentration. The consequence is that 60 to 70% of patients with CKD and hypocalcemia and almost 80% of patients with CKD and hypercalcemia would not receive an appropriate treatment in the absence of iCa measurement.
In conclusion, neither noncorrected nor albumin-corrected tCa seems to predict correctly low or high iCa concentrations in patients with stages 3 to 5 CKD. The main reason is that none of these estimators provides a correction for the prevalent metabolic acidosis, which increases the risk for underestimation, and that albumin-based correction formulas overcorrect tCa concentration, increasing the risk for overestimation; therefore, we propose not using albumin-corrected tCa in patients with CKD. An accurate assessment of blood Ca concentration requires the measurement of iCa at actual pH in patients with low tCO2 and/or plasma albumin concentrations.
CONCISE METHODS
Patients
From January 2000 to July 2006, 691 consecutive patients who had stages 3 to 5 CKD and were not yet on dialysis were studied at their enrollment in the NEPHROTEST Cohort. Kidney transplant recipients were excluded. The NEPHROTEST cohort is a prospective cohort including all adult patients who had CKD and underwent a yearly extensive check-up in two departments of physiology and nephrology in the Paris area. The NEPHROTEST cohort study was approved and sponsored by the French Ministry of Research and INSERM. All patients provided written informed consent for long-term handling of frozen blood-derived samples and for research use of prespecified clinical and biologic data. The study was conducted in accordance with good clinical practice guidelines.
Venous blood was collected in the morning after overnight fasting through an indwelling catheter inserted in a large vein of the forearm. Whenever possible, a tourniquet was not applied. When necessary, it was released at least 1 min before blood collection. Blood for Ca measurement was collected in a nonheparinized, dry tube. All other samples were collected in heparinized tubes. Samples were handled on ice until centrifugation within 30 min after sampling. Serum was anaerobically sampled from the tube for immediate measurement of iCa concentration. Plasma tCa and albumin concentrations were measured on the same day. The remaining plasma was aliquotted and kept frozen at −20°C until measurement.
For verification that collection in nonheparinized, dry tubes did not affect the pH of the sample, blood was also collected from 61 unselected consecutive patients, at the same time, on a self-filling blood sampler containing 60 IU of balanced heparin, transported on ice to the laboratory, and immediately assayed on the same gas analyzer. The mean difference in pH measured in the two samples was 0.01 ± 0.02 pH units, and the mean difference in Paco2 was 1.3 ± 3.1 mmHg. After blood sampling, renal clearance of (51)Cr-EDTA was determined as described previously.12–13
Analytical Methods
iCa concentration, pH and Pco2 were measured on an ABL 555 analyzer (Radiometer, Copenhagen, Denmark). The considered value of iCa concentration was that measured at the actual pH of the patient, not the calculated value, corrected for pH 7.4. Plasma tCa was measured by atomic absorption spectrometry (Model 3110; Perkin-Elmer, Norwalk, CT) and plasma albumin and phosphate by colorimetric methods (bromocresol green for albumin and phosphomolybdate complex for phosphate). Plasma tCO2 concentration was measured by a specific electrode. Coefficients of variation (between-assay) were 0.9 ± 0.2% for tCa and 1.1 ± 0.4% for iCa.
Calculations
Albumin-corrected Ca was calculated according to the two formulas provided in the K/DOQI guidelines3:
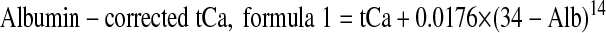 |
 |
where tCa is the plasma concentration of tCa, expressed in mmol/L, and Alb is the plasma concentration of albumin, expressed in g/L. Correction was performed only when plasma albumin concentration was <35 g/L.15
Statistical Analysis
Normally distributed variables were expressed as means ± SD and compared with one-way ANOVA; intergroup comparisons were performed by unpaired t test and Bonferroni correction for the level of significance. Categorical variables are presented as distribution (i.e., frequencies and percentages) and compared with χ2 test. Measured GFR was used as a continuous variable.
Each patient was classified into one of three categories (hypocalcemia, hypercalcemia, or normal Ca concentration) on the basis of iCa measurement. When the patient could be classified in the same category on the basis of tCa (either albumin corrected or noncorrected), both values were considered in agreement; otherwise, tCa either overestimated (high tCa and normal iCa concentrations, or normal tCa and low iCa concentrations) or underestimated (low tCa and normal iCa concentrations, or normal tCa and high iCa concentrations). The global performance of tCa to predict low, normal, or high iCa concentration was assessed using κ test.16 The sensitivity and specificity of each estimator to diagnose true hypo- or hypercalcemia was also calculated. Univariate and multivariate logistic regression analyses were performed to investigate the predictors of underestimation and overestimation.
For comparison of iCa and tCa (either albumin corrected or noncorrected), values were normalized by conversion to a z score allowing direct comparison of values that have different normal ranges. Z score calculations were performed as follows10: The lower and upper values of the normal range (1.15 and 1.32 mmol/L for iCa; 2.10 and 2.53 mmol/L for tCa) were treated as a 95% confidence interval and used to calculate mean and SD. Each measured value was then converted to a z score using the formula z score = (measured Ca − mean Ca)/SD.
The lack of agreement between tCa and iCa was separately quantified by the calculation of the difference between tCa and iCa, both expressed as z scores. A negative difference meant underestimation and a positive difference overestimation. Multivariate linear regression analyses were performed to identify the determinants of tCa (either noncorrected or albumin corrected) expressed as z score.
P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS software, version 9.1 (SAS institute, Cary, NC).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Dr. Tilman Drueke for critical reading of the manuscript. The NephroTest study was granted by a joined program from the French Ministry of Research and INSERM (Centre de Ressources biologiques cohorts et collections 2001), and supported by the Agence de Biomedecine. The NephroTest initiative was also sponsored by unrestricted grants from F. Hoffmann-La Roche Ltd. (M.F.) and Amgen France (J.R.).
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 3.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–201, 2003 [PubMed] [Google Scholar]
- 4.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boink AB, Buckley BM, Christiansen TF, Covington AK, Maas AH, Muller-Plathe O, Sachs C, Siggaard-Andersen O: Recommendation on sampling, transport, and storage for the determination of the concentration of ionized calcium in whole blood, plasma, and serum. IFC Scientific Division, Working Group on Ion-Selective Electrodes (WGSE). J Int Fed Clin Chem 4: 147–152, 1992 [PubMed] [Google Scholar]
- 6.Bowers GN Jr, Brassard C, Sena SF: Measurement of ionized calcium in serum with ion-selective electrodes: A mature technology that can meet the daily service needs. Clin Chem 32: 1437–1447, 1986 [PubMed] [Google Scholar]
- 7.Gouget B, Gourmelin Y, Blanchet F, Truchaud A: A reliable potentiometric measurement of ionized calcium and pH on the ICA2 Radiometer in clinical practice. Scand J Clin Lab Invest 49: 345–349, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Ladenson JH, Bowers GN Jr: Free calcium in serum. II. Rigor of homeostatic control, correlations with total serum calcium, and review of data on patients with disturbed calcium metabolism. Clin Chem 19: 575–582, 1973 [PubMed] [Google Scholar]
- 9.Muldowney FP, Freaney R, McMullin JP, Towers RP, Spillane A, O'Connor P, O'Donohoe P, Moloney M: Serum ionized calcium and parathyroid hormone in renal stone disease. Q J Med 45: 75–86, 1976 [PubMed] [Google Scholar]
- 10.Clase CM, Norman GL, Beecroft ML, Churchill DN: Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant 15: 1841–1846, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Goransson LG, Skadberg O, Bergrem H: Albumin-corrected or ionized calcium in renal failure? What to measure? Nephrol Dial Transplant 20: 2126–2129, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Brochner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30: 271–274, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Orrell DH: Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta 35: 483–489, 1971 [DOI] [PubMed] [Google Scholar]
- 15.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB: Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 351: 1548–1563, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics 33: 159–174, 1977 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


