Abstract
It is unknown whether chronic kidney disease (CKD) is associated with nonalcoholic fatty liver disease among patients with type 2 diabetes. We followed 1760 outpatients with type 2 diabetes and normal or near-normal kidney function and without overt proteinuria for 6.5 yr for the occurrence of CKD (defined as overt proteinuria and/or estimated GFR <60 ml/min per 1.73 m2). During follow-up, 547 participants developed incident CKD. Nonalcoholic fatty liver disease, diagnosed by liver ultrasound and exclusion of other common causes of chronic liver disease, was associated with a moderately increased risk for CKD (hazard ratio 1.69; 95% confidence interval 1.3 to 2.6; P < 0.001). Adjustments for gender, age, body mass index, waist circumference, BP, smoking, diabetes duration, glycosylated hemoglobin, lipids, baseline estimated GFR, microalbuminuria, and medications (hypoglycemic, lipid-lowering, antihypertensive, or antiplatelet drugs) did not appreciably attenuate this association (hazard ratio 1.49; 95% confidence interval 1.1 to 2.2; P < 0.01). In conclusion, our findings suggest that nonalcoholic fatty liver disease is associated with an increased incidence of CKD in individuals with type 2 diabetes, independent of numerous baseline confounding factors.
Chronic kidney disease (CKD) in type 2 diabetes is a major problem for patients and health care systems. CKD often progresses to ESRD with its attendant complications. Patients with diabetes and ESRD are now accepted for renal replacement therapy in steadily increasing numbers and currently account for more than one third to one half of new patients in some countries.1–3 The treatment of earlier stages of nephropathy in diabetes is effective in slowing the progression toward ESRD.1–6 Thus, the early detection of precursors and risk factors for CKD is very important.
Nonalcoholic fatty liver disease (NAFLD), in its whole spectrum of disease ranging from simple steatosis to steatohepatitis and cirrhosis, is one of the most common causes of chronic liver disease in clinical practice.7–9 NAFLD prevalence has been estimated to be in the 15 to 30% range in the general population in various countries7–11 and is almost certainly increasing. Compared with individuals without diabetes, patients with type 2 diabetes seem to be at increased risk for developing NAFLD and certainly have a higher risk for developing fibrosis and cirrhosis.7–9 It has been estimated that approximately 70 to 75% of patients with type 2 diabetes have some form of NAFLD.9,12,13 Moreover, growing evidence suggests that NAFLD may be linked to an increased risk for cardiovascular disease (CVD), especially in the population with type 2 diabetes.13–15
Currently, there is a dearth of information on the association between NAFLD and risk for developing CKD in type 2 diabetes. The impact of NAFLD on the incidence of CKD deserves particular attention in view of the potential implications for screening/surveillance strategies in the growing number of patients with NAFLD. The aim of this study was to assess whether NAFLD is associated with an increased incidence of CKD in a large cohort of individuals with type 2 diabetes.
RESULTS
As shown in Figure 1, 1827 outpatients with type 2 diabetes and normal or near-normal kidney function and without overt proteinuria at baseline were eligible for the study after exclusion of those with secondary causes of chronic liver disease (alcohol, viral hepatitis, medications) and those who had a history of malignancy and CVD. The ascertainment at the end of the 6.5-yr follow-up period for the eligible cohort was 96.2%. Participants who attended the follow-up examinations (n = 1760) were essentially similar to those who were initially eligible (n = 1827) and to those who did not attend the follow-up examinations (n = 67) in terms of demographic variables, estimated GFR (eGFR), and NAFLD status; 1760 participants were included in the final analysis.
Figure 1.
Details of the study design. 1760 participants attending the follow-up examinations were included in the final analysis.
During follow-up, 547 participants developed incident CKD (i.e., approximately 4.5% of participants progressed every year to eGFR <60 ml/min per 1.73 m2 and/or overt proteinuria); their mean ± SD eGFR at follow-up was 55 ± 12 ml/min per 1.73 m2. Of these, seven developed ESRD requiring chronic dialysis, 428 developed CKD without overt proteinuria, and 112 developed overt proteinuria, irrespective of eGFR, at follow-up.
The baseline characteristics of the cohort by CKD status at follow-up are shown in Table 1. At baseline, patients who developed CKD at follow-up were older, were more centrally obese, were more likely to be male, and had both longer diabetes duration and greater frequency of microalbuminuria than those who did not. They also had higher systolic BP (SBP), glycosylated hemoglobin (HbA1c), triglycerides, and liver enzymes and lower HDL cholesterol and eGFR levels. Diastolic BP, smoking status, and LDL cholesterol did not significantly differ between the groups. The proportion using antihypertensive drugs was higher in those who progressed to CKD, whereas the proportion using antiplatelet or lipid-lowering drugs was essentially similar in both groups. Notably, the frequency of NAFLD was remarkably greater in those who developed CKD at follow-up than in those who did not.
Table 1.
Baseline characteristics of the cohort with diabetes by CKD status at follow-upa
| Variables | No CKD at Follow-up(n = 1213) | CKD at Follow-up(n = 547) | P |
|---|---|---|---|
| Gender (% men) | 60 | 63 | 0.001 |
| Age (yr) | 57 ± 3 | 60 ± 4 | 0.001 |
| BMI (kg/m2) | 26 ± 3 | 27 ± 3 | 0.001 |
| Waist circumference (cm) | 92 ± 11 | 96 ± 13 | 0.001 |
| Duration of diabetes (yr) | 12 ± 3 | 14 ± 3 | 0.001 |
| Oral hypoglycemic drugs (%) | 61 | 63 | 0.400 |
| Insulin only (%) | 16 | 20 | 0.001 |
| Antihypertensive users (%) | 63 | 71 | 0.001 |
| Aspirin users (%) | 49 | 48 | 0.800 |
| Lipid-lowering users (%) | 36 | 37 | 0.600 |
| Current smokers (%) | 22 | 23 | 0.700 |
| SBP (mmHg) | 127 ± 12 | 130 ± 15 | 0.001 |
| DBP (mmHg) | 82 ± 11 | 83 ± 13 | 0.200 |
| HbA1c (%) | 6.9 ± 0.8 | 7.3 ± 1.0 | 0.001 |
| Triglycerides (mmol/L) | 1.34 ± 0.70 | 1.60 ± 1.00 | 0.001 |
| HDL cholesterol (mmol/L) | 1.40 ± 0.30 | 1.33 ± 0.40 | 0.001 |
| LDL cholesterol (mmol/L) | 3.33 ± 0.40 | 3.31 ± 0.40 | 0.700 |
| eGFR (ml/min per 1.73 m2) | 96 ± 7 | 92 ± 10 | 0.001 |
| Microalbuminuria (%) | 24 | 43 | 0.001 |
| AST (U/L) | 19 ± 4 | 23 ± 10 | 0.001 |
| ALT (U/L) | 21 ± 7 | 28 ± 11 | 0.001 |
| GGT (U/L) | 20 ± 8 | 28 ± 12 | 0.001 |
| NAFLD (%) | 63 | 96 | 0.001 |
Data are means ± SD or frequencies. Differences are assessed by the unpaired t test (for continuous variables) and by the χ2 test (for categorical measures). Cohort size n = 1760. ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic BP; GGT, γ -glutamyl transferase.
As expected,12 patients with NAFLD were older, were more centrally obese, were more likely to be male, and had both longer diabetes duration and greater frequency of microalbuminuria than their counterparts without NAFLD. They also had lower eGFR and HDL cholesterol levels and higher SBP, triglycerides, HbA1c, and liver enzymes, although the vast majority (88%) of patients with NAFLD had serum alanine aminotransferase concentrations within the reference range (data not shown).
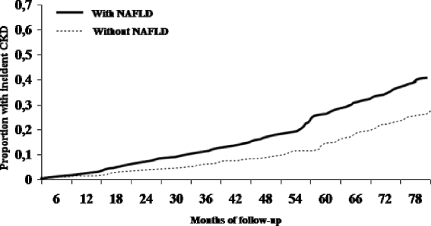
The cumulative proportions of participants who developed CKD at follow-up by NAFLD status are shown in Figure 2. The difference between the groups was significant (P = 0.002 by the log-rank test).
Figure 2.
Cumulative proportions of patients who had type 2 diabetes and developed CKD during follow-up, stratified by NAFLD status.
In univariate regression analysis (Table 2), NAFLD was associated with an increased risk for CKD. Male gender, older age, SBP, hypertension treatment, diabetes duration, insulin treatment, HbA1c, triglycerides, alanine aminotransferase, smoking, microalbuminuria, and lower baseline eGFR were also associated with increased CKD risk, whereas body mass index (BMI), LDL cholesterol, and lipid-lowering and antiplatelet treatments were not.
Table 2.
Univariate and multivariate Cox regression analyses showing associations of NAFLD and other factors with incident CKD among adults with type 2 diabetesa
| Parameter | Univariate | Multivariate
|
|
|---|---|---|---|
| Model 1 | Model 2 | ||
| NAFLD (yes versus no) | |||
| HR (95% CI) | 1.69 (1.30 to 2.60) | 1.60 (1.20 to 2.50) | 1.49 (1.10 to 2.20) |
| P | 0.001 | 0.001 | 0.010 |
| Age (per year) | |||
| HR (95% CI) | 1.06 (1.03 to 1.11) | 1.05 (1.04 to 1.10) | 1.07 (1.04 to 1.12) |
| P | 0.001 | 0.001 | 0.001 |
| Gender (male versus female) | |||
| HR (95% CI) | 1.31 (1.30 to 2.20) | 1.30 (1.20 to 2.10) | 1.13 (0.90 to 1.80) |
| P | 0.001 | 0.001 | 0.200 |
| Smoking (yes versus no) | |||
| HR (95% CI) | 1.36 (1.20 to 1.90) | 1.34 (1.20 to 1.90) | 1.30 (1.00 to 1.70) |
| P | 0.001 | 0.001 | 0.050 |
| BMI (per unit) | |||
| HR (95% CI) | 1.15 (0.90 to 1.30) | 1.14 (0.90 to 1.30) | 1.10 (0.90 to 1.20) |
| P | 0.300 | 0.500 | 0.600 |
| SBP (per unit) | |||
| HR (95% CI) | 1.80 (1.30 to 2.70) | 1.78 (1.30 to 2.70) | 1.75 (1.10 to 2.60) |
| P | 0.001 | 0.001 | 0.010 |
| LDL cholesterol (per unit) | |||
| HR (95% CI) | 1.11 (0.80 to 1.40) | 1.10 (0.80 to 1.40) | 1.10 (0.80 to 1.30) |
| P | 0.600 | 0.600 | 0.300 |
| Triglycerides (per unit) | |||
| HR (95% CI) | 1.38 (1.10 to 1.80) | 1.29 (1.00 to 1.50) | 1.08 (0.70 to 1.40) |
| P | 0.001 | 0.010 | 0.300 |
| HbA1c (per unit) | |||
| HR (95% CI) | 1.42 (1.20 to 3.00) | 1.40 (1.10 to 2.90) | 1.31 (1.00 to 1.90) |
| P | 0.001 | 0.001 | 0.050 |
| Diabetes duration (per year) | |||
| HR (95% CI) | 1.19 (1.20 to 2.40) | 1.18 (1.20 to 2.40) | 1.20 (1.20 to 2.60) |
| P | 0.001 | 0.001 | 0.001 |
| Microalbuminuria (yes versus no) | |||
| HR (95% CI) | 1.88 (1.40 to 2.90) | 1.86 (1.40 to 2.90) | 1.73 (1.10 to 2.60) |
| P | 0.001 | 0.001 | 0.010 |
| eGFR (per unit) | |||
| HR (95% CI) | 0.67 (0.50 to 0.90) | 0.69 (0.50 to 0.90) | 0.72 (0.50 to 0.90) |
| P | 0.001 | 0.001 | 0.001 |
| ALT (per unit) | |||
| HR (95% CI) | 1.34 (1.10 to 2.10) | 1.31 (1.00 to 1.80) | 1.15 (0.80 to 1.40) |
| P | 0.001 | 0.010 | 0.500 |
| Insulin treatment (yes versus no) | |||
| HR (95% CI) | 1.59 (1.20 to 2.60) | 1.52 (1.20 to 2.50) | 1.22 (0.90 to 2.00) |
| P | 0.001 | 0.400 | 0.200 |
| Antihypertensive treatment (yes versus no) | |||
| HR (95% CI) | 1.99 (1.40 to 2.90) | 1.97 (1.40 to 2.90) | 1.95 (1.40 to 2.80) |
| P | 0.001 | 0.001 | 0.001 |
| Lipid-lowering treatment (yes versus no) | |||
| HR (95% CI) | 1.12 (0.80 to 1.80) | 1.00 (0.80 to 1.70) | 1.00 (0.70 to 1.40) |
| P | 0.500 | 0.600 | 0.800 |
| Antiplatelet treatment (yes versus no) | |||
| HR (95% CI) | 1.18 (0.90 to 1.60) | 1.14 (0.90 to 1.60) | 1.00 (0.80 to 1.30) |
| P | 0.300 | 0.500 | 0.800 |
Data are HR (95% CI) by Cox regression analysis. Multivariate model 1: Adjusted for age and gender. Multivariate model 2: Further adjusted for BMI, waist circumference, SBP, diabetes duration, HbA1c, baseline eGFR, microalbuminuria, LDL cholesterol, triglycerides, smoking history, and current use of medications (hypoglycemic, lipid-lowering, antihypertensive, or antiplatelet drugs).
In multivariate regression analysis (Table 2), the association between NAFLD and CKD was little affected by adjustment for gender and age (model 1). The results remained essentially unchanged when the association between NAFLD and CKD was examined in subgroups stratified by gender and age (data not shown). Additional adjustments for numerous baseline confounding factors did not alter the significant relationship between NAFLD and CKD (model 2). Of note, other variables independently associated with the development of CKD were older age, smoking, diabetes duration, HbA1c, SBP, hypertension treatment, microalbuminuria, and lower baseline eGFR.
Similar results were found for each of the components of the renal outcome: overt proteinuria and eGFR <60 ml/min per 1.73 m2, separately (multiple-adjusted HR 1.45 [95% confidence interval (CI) 1.05 to 2.6] and 1.57 [95% CI 1.2 to 2.5], respectively; P < 0.05 to 0.01). The results of the fully adjusted regression model remained unchanged when we additionally adjusted for daily alcohol consumption or when participants who were light to moderate drinkers (n = 368) were excluded from analysis. Also in this case, NAFLD was independently associated with an increased incidence of CKD (HR 1.52; 95% CI 1.2 to 2.4; P = 0.014).
DISCUSSION
This is the first prospective study specifically aimed at assessing the association between NAFLD and incident CKD during a 6.5-yr follow-up in a large cohort of adults with type 2 diabetes.
Our major finding was that NAFLD, as diagnosed by patient history, blood sampling, and characteristic sonographic features, is associated with a moderately increased risk for CKD in a population with type 2 diabetes. The annual cumulative incidence rate of CKD in our cohort (approximately 4.5% per year) was comparable to that previously described in other Italian and European populations with diabetes and similar baseline characteristics (2.4 to 8.5% of patients who progressed every year to CKD).16,17 Notably, in our study, the association between NAFLD and CKD seems to be independent of numerous baseline confounding factors, such as age, gender, adiposity, diabetes duration, glycemic control, lipids, smoking, baseline eGFR, microalbuminuria, hypertension, and medications use.
Our findings are corroborated by a recent study of 10,337 healthy Korean men followed for approximately 3.5 yr, showing that mildly elevated serum γ-glutamyl transferase concentrations, as surrogate markers of NAFLD,7–9 are associated with an increased risk for CKD (defined as overt proteinuria and/or eGFR <60 ml/min per 1.73 m2) independent of age, baseline eGFR, metabolic syndrome features, insulin resistance, C-reactive protein, smoking, and alcohol consumption.18
The underlying biologic mechanisms by which NAFLD may increase the risk for CKD in type 2 diabetes are poorly understood. The most obvious explanation for our findings is that the higher risk for CKD in patients with NAFLD simply reflects the coexistence of underlying known risk factors; however, because in our study NAFLD was associated with an increased risk for CKD independent of numerous baseline risk factors, it is conceivable that NAFLD may confer an excess risk over and above the risk expected as a result of the underlying known risk factors. This suggests that NAFLD not only is a marker of CKD in type 2 diabetes but also may be involved in its pathogenesis.
The possible molecular mediators linking NAFLD and CKD may include the release of some pathogenic factors from the liver, including elevated advanced glycated end products, increased reactive oxygen species, elevated C-reactive protein, TNF-α, TGF-β1, and other proinflammatory cytokines. Importantly, several studies have shown that these potential mediators of vascular and/or renal injury are remarkably higher in diabetic/obese patients with NAFLD than in those without15,19–28 and are thought to be pathogenic factors for the progression of CKD.1–3,6,19 Consistent with the hypothesis that liver inflammation (or other liver-derived factors) in NAFLD may play a role in the development and progression of CKD, Cheng et al.29 reported that in a large cohort of individuals with type 2 diabetes those with chronic hepatitis B virus infection were more likely to develop ESRD than those who were not infected with hepatitis B virus. Finally, NAFLD may worsen whole-body insulin resistance and hyperglycemia,7,15 which may in turn contribute to the progression of CKD.1,2,6,19,23,30,31 This notion is supported by the observation, in this study, that the HbA1c was higher in patients with NAFLD than in those without (7.2 ± 1.0 versus 6.7 ± 0.6%; P < 0.001).
The potential implications of our findings for patient care are that in people with type 2 diabetes, the casual detection of NAFLD during ultrasound examination should alert clinicians to the coexistence of other chronic diabetic complications (including CKD and CVD) warranting evaluation and treatment as much as the risk for advancing liver disease. Thus, identifying people with NAFLD would highlight a subgroup of individuals who have type 2 diabetes and should be targeted with more intensive therapy to decrease the risk for developing CKD and CVD events.
Our study has several strengths, including the large number of participants, the complete nature of the data set, the ability to adjust for multiple potential confounders, and the ultrasound diagnosis of NAFLD for all participants. In addition, our patients were free of diagnosed CVD and cirrhosis (thus excluding also patients with hepatorenal syndrome); the evaluation of patients with such complications would almost certainly have confounded interpretation of the data.
Despite the comprehensive nature of the data set, there are some limitations to our study. First, we used an eGFR instead of a directly measured GFR to define CKD. A recent review reported that current GFR estimates had greater inaccuracy in populations without known CKD than in those with the disease.32 Nonetheless, current GFR estimates facilitate the detection, evaluation, and management of CKD, and many organizations recommend the use of equations that estimate GFR for the evaluation of renal function in large epidemiologic studies and in clinical practice.32,33 Second, NAFLD diagnosis was based on ultrasound imaging and exclusion of other, secondary causes of chronic liver disease but was not confirmed by liver biopsy. It is known that none of the radiologic features can distinguish between nonalcoholic steatohepatitis and other forms of NAFLD and that only liver biopsy can assess the severity of damage and the prognosis7–9,15; however, liver biopsy would be impossible to perform routinely in a large epidemiologic study. Moreover, liver ultrasonography is by far the most common way of diagnosing NAFLD in clinical practice. It has a sensitivity of 89% and a specificity of 95% in detecting moderate and severe steatosis,7–9 but this sensitivity is reduced when hepatic fat infiltration upon liver biopsy is <33%.34 Thus, although some nondifferential misclassification of NAFLD on the basis of ultrasonography is likely (some of the control subjects could have underlying NAFLD, despite normal serum liver enzymes and a negative ultrasonography), this limitation would serve to attenuate the magnitude of our effect measures toward null; thus, our results can probably be considered as conservative estimates of the relationship between NAFLD and CKD risk. Finally, whether these observations can also be extended to nonwhite ethnic groups remains to be determined.
Because liver biopsies were not available in this study, we cannot exclude the possibility of a differential relationship between histologic severity of NAFLD and CKD risk. Recent indirect evidence substantiates this possibility, showing a strong, graded relationship between carotid atherosclerosis and the severity of NAFLD histology35 and a significant increase in cardiovascular mortality, particularly in patients with nonalcoholic steatohepatitis.36,37
It is not known whether improving NAFLD will ultimately prevent the progression of CKD; however, it is notable that interventions that are known to be effective in preventing or delaying the progression of CKD in type 2 diabetes, including weight reduction,38–40 and treatments with angiotensin receptor blockers (losartan)41 or insulin-sensitizing agents (glitazones or metformin),20,42–44 may possibly improve NAFLD.
In conclusion, this large prospective study suggests that NAFLD is associated with an increased incidence of CKD in individuals with type 2 diabetes independent of a large number of baseline confounding factors. Further prospective studies are required to confirm the reproducibility of these results.
CONCISE METHODS
Study participants were recruited from the Valpolicella Heart Diabetes Study cohort, a prospective observational study designed primarily to evaluate associations between type 2 diabetes and incidence of chronic vascular complications.13,14 Details on the study design and recruitment methods are summarized in Figure 1. Briefly, we enrolled all of the outpatients who had type 2 diabetes and regularly attended our adult diabetes clinic in the period January to December 2000 after excluding (1) patients with secondary causes of chronic liver disease (e.g., alcohol abuse, viral hepatitis, medications) as ascertained by patient history and blood testing and imaging; (2) those who had a history of malignancy and CVD (e.g., angina, myocardial infarction, ischemic stroke, symptomatic peripheral artery disease, coronary/peripheral revascularization procedures); and (3) those who had overt proteinuria, had eGFR <60 ml/min per 1.73 m2, or were receiving medical treatment for current kidney disease at the time of their initial examinations.
Ultimately, 1760 adults with type 2 diabetes (approximately 56% of the whole sample of patients who attended our clinic) were included in this analysis and were observed for the development of incident CKD during a follow-up of 6.5 yr (through December 31, 2006; range 7 to 79 mo). All participants were periodically seen (every 3 to 6 mo) for routine medical examinations of glycemic control and chronic complications of diabetes. All participants gave their informed consent. The local ethics committee approved the study protocol.
At baseline, BMI was calculated by dividing weight in kilograms (measured to the nearest 1 kg with the patient unclothed) by height (measured with a fixed stadiometer) in meters squared. Waist circumference was measured at the level of the umbilicus. BP was assessed in triplicate with a standard mercury manometer. Information on daily alcohol consumption, smoking status, and use of medications was obtained from all participants by questionnaire.13 Most participants were abstainers (78%) or drank minimally (alcohol consumption <20 g/d; 12% of total); only approximately 10% of participants drank moderately (from 20 to 60 g/d).
Venous blood was drawn in the morning after an overnight fast. Serum liver enzymes, lipids, creatinine, and other biochemical blood measurements were determined by automatic colorimetric methods (DAX 96; Bayer Diagnostics, Milan, Italy). Most participants had serum liver enzymes within the reference ranges in our laboratory, which for aminotransferases were 10 to 35 U/L for women and 10 to 50 U/L for men. No participants had seropositivity for viral hepatitis B and C. LDL cholesterol was calculated by Friedewald's formula. HbA1c was measured using an HPLC analyzer (HA-8140; Menarini Diagnostics, Florence, Italy). Kidney function was estimated using the simplified Modification of Diet in Renal Disease (MDRD) Study equation that is defined as eGFR = 186.3 × (serum creatinine−1.154) × (age−0.203) × 1.212 (if black) × 0.742 (if female).32,33 Urinary albumin excretion rate was measured from an early morning urine sample as the albumin-to-creatinine ratio by an immunonephelometric method; microalbuminuria and macroalbuminuria (overt proteinuria) were present when urinary albumin excretion was 30 to 299 μg/mg creatinine and ≥300 μg/mg creatinine, respectively.45 For this study, CKD was defined as eGFR <60 ml/min per 1.73 m2 and/or overt proteinuria.32,33 Both of these outcome measures were confirmed for all participants on a least two consecutive occasions and then periodically repeated during follow-up. Liver function tests were not systematically repeated for the whole cohort of participants to ascertain the development/progression of liver disease.
Hepatic ultrasonography scanning was performed on all participants by an experienced radiologist, who was blinded to participants’ details. Diagnosis of hepatic steatosis was made on the basis of characteristic sonographic features: evidence of diffuse hyperechogenicity of liver relative to kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures.7–9,12–14 The intraobserver variability for the ultrasound diagnosis of steatosis was within 3%.46
Data are means ± SD unless otherwise indicated. Skewed variables (serum triglycerides) were logarithmically transformed to improve normality before analysis. The unpaired t test and the χ2 test with Yates correction for continuity were used to analyze the differences among the characteristics of the participants at the time of enrollment in relation to their future development of CKD. Event curves by NAFLD status are based on Kaplan-Meier analysis. We also used the Cox proportional-hazards model to calculate the adjusted hazard ratios (HR) in the model for CKD. For prediction of incident CKD, men and women were combined and first-order interaction terms for gender-by-NAFLD interactions on risk for CKD were examined. Because the interactions were not statistically significant, a gender-pooled multivariate Cox regression analysis was used. In the fully adjusted regression model, gender, age, BMI, waist circumference, SBP, diabetes duration, HbA1c, LDL cholesterol, triglycerides, baseline eGFR, microalbuminuria, smoking, and medications use were included as baseline covariates. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Mogensen CE, Cooper ME: Diabetic renal disease: From recent studies to improved clinical practice. Diabet Med 21: 4–17, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Rossing P: Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia 49: 11–19, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Rossing P: Diabetic nephropathy: Worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep 6: 479–483, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ: Treating hypertension in the patient with overt diabetic nephropathy. Semin Nephrol 27: 182–194, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC: Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev 4: CD006257, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fioretto P, Bruseghin M, Berto I, Gallina P, Manzato E, Mussap M: Renal protection in diabetes: Role of glycemic control. J Am Soc Nephrol 17[Suppl 2]: S86–S89, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Angulo P, Lindor KD: Nonalcoholic fatty liver disease. CMAJ 172: 899–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day CP: Nonalcoholic fatty liver disease: Current concepts and management strategies. Clin Med 6: 19–25, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolman KG, Fonseca V, Dalpiaz A, Tan MH: Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30: 734–743, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S: Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos Nutrition and Liver Study. Hepatology 42: 44–52, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH: Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40: 1387–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G: Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G: Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54: 3541–3546, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G: Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 30: 2119–2121, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Targher G: Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet Med 24: 1–6, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Pagano G, Cavallo-Perin P: Progression to overt nephropathy in type 2 diabetes. The Casale Monferrato Study. Diabetes Care 26: 2150–2155, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Parving HH: Diabetic nephropathy: Prevention and treatment. Kidney Int 60: 2041–2055, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS: Gamma-glutamyltransferase as a predictor of chronic kidney disease in non-hypertensive and non-diabetic Korean men. Clin Chem 53: 71–77, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Tan AL, Forbes JM, Cooper ME: AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol 27: 130–143, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, De Fronzo R, Bannayan GA, Schenker S, Cusi K: A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Alessi MC, Bastelica D, Mavri A, Morange P, Berthet B, Grino M, Juhan-Vague I: Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol 23: 1262–1268, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Bertolini L, Scala L, Zenari L, Lippi G, Franchini M, Arcaro G: Plasma PAI-1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care 30: e31–e32, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Targher G: Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. J Hepatol 45: 879–881, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M: The pathobiology of diabetic complications. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi S: The liver is the main site for metabolism of circulating advanced glycation end products. J Hepatol 36: 123–125, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H: Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int 26: 39–45, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Chalasani N, Deeg MA, Crabb DW: Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99: 1497–1502, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP: Immune response towards lipid peroxidation products as a predictor of progression of nonalcoholic fatty liver disease to advanced fibrosis. Gut 54: 987–993, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen AY, Kong AP, Wong VW, So WY, Chan HL, Ho CS, Lam CW, Tam JS, Chow CC, Cockram CS, Chan JC, Tong PC: Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia 49: 1777–1784, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Groop PH, Forsblom C, Thomas MC: Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1: 100–110, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Sarafidis PA, Ruilope LM: Insulin resistance, hyperinsulinemia, and renal injury: Mechanisms and implications. Am J Nephrol 26: 232–244, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ: The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123: 745–750, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G: Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 29: 1325–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P: The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 129: 113–121, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S: Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K: Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE: Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology 39: 1647–1654, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI: Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K: Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 40: 1222–1225, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G: A randomized controlled trial of metformin versus vitamin E or prescriptive diet in non-alcoholic fatty liver disease. Am J Gastroenterol 100: 1082–1090, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Neuschwander-Tetri BA, Brunt E, Wehmeier K, Oliver D, Bacon B: Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H: Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW, American Diabetes Association: Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G: Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: Role of visceral fat accumulation. Diabetes Care 27: 1498–1500, 2004 [DOI] [PubMed] [Google Scholar]