Abstract
CYP4A11 arachidonic acid monooxygenase oxidizes endogenous arachidonic acid to 20-hydroxyeicosatetraenoic acid, a renal vasoconstrictor and natriuretic. Cyp4a deficiency causes hypertension in male mice, and a loss-of-function variant (T8590C) of CYP4A11 is associated with hypertension in white individuals. Hypertension and hypertensive renal disease are more common among black than white individuals, but the relationship between genetic variation at CYP4A11 and hypertension in black individuals is not known. This study tested the hypothesis that the CYP4A11 T8590C polymorphism is associated with higher BP or clinical outcomes in 732 black Americans with hypertensive renal disease participating in the African American Study of Kidney Disease (AASK). Men with the 8590CC genotype had significantly higher systolic BP (CC 156.5 ± 22.6 versus 148.4 ± 24.3 mmHg in CT and TT combined; P = 0.04) and pulse pressure (P = 0.04) at baseline; this association was not observed among women. In addition, this genotype was associated with higher systolic and diastolic BP at 36-mo follow-up among those randomly assigned to the lower BP arm of the AASK. Among all participants (or men but not women) with proteinuria, the 8590CC genotype was associated with an increased cumulative incidence of ESRD or death, controlling for randomization and clinical characteristics. In summary, the CYP4A11 8590CC genotype is associated with increased BP in black men with hypertensive nephrosclerosis and is associated with adverse clinical outcomes in those with baseline proteinuria. These data support a role for renal monooxygenases and 20-hydroxyeicosatetraenoic acid in the regulation of BP and renal function in men.
Hypertension is a significant independent risk for the development and progression of chronic kidney disease.1 Studies in men and women have demonstrated a strong, graded relationship between increasing hypertension severity and declining renal function.2 Differences in the incidence and progression of chronic kidney disease among ethnic groups have been reported,3–5 and black Americans are four times as likely to develop ESRD as white Americans.6 Animal7–9 and human studies10,11 support a causal role for genetic factors, but the genetic determinants of hypertension-associated renal disease in humans are largely unknown.
The CYP4A11 arachidonic acid monooxygenase, expressed in the human kidney, catalyzes the conversion of endogenous arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE). The 20-HETE metabolite regulates salt and water homeostasis at multiple sites within the kidney.12 Experimental models of hypertension indicate that 20-HETE can act in either a pro- or antihypertensive manner, depending on its site-specific expression in the renal vasculature or tubule, respectively.13–16 In previous work, we showed that knockout of Cyp4a14, a murine homologue of CYP4A11, caused gender-specific and androgen-sensitive hypertension.17 Several studies supported the involvement of 20-HETE, CYP4A, and omega hydroxylase activity in animal models of renal injury.18–20
In humans, we21 previously reported an association of a functional CYP4A11 variant, T8590C, characterized by reduced 20-HETE synthase activity, with hypertension in two independent white American cohorts. Mayer et al.22 also demonstrated an association of the T8590C variant with hypertension and systolic BP (SBP) in a German-based white population cohort. In our original study, we did not detect an association of CYP4A11 T8590C genotype with hypertension in an black American cohort, but the study was limited by a small sample size.
The primary goal of this study was to determine whether genetic variation in CYP4A11 (i.e., T8590C variant) influences hemodynamic indices or the progression of renal disease in hypertensive black Americans and whether gender-dependent genotype effects are detectable. To this end, we genotyped 732 participants of the African American Study of Kidney Disease (AASK), a trial designed to evaluate the effect of two different BP goals and three different treatment regimens on progression of hypertensive kidney disease. We assessed the effect of the CYP4A11 T8590C variant on BP and on clinical end points.
RESULTS
Baseline Characteristics
CYP4A11 T8590C genotype and allele frequencies were in Hardy-Weinberg equilibrium and similar to those reported previously in black individuals (Table 1).21 There were no significant differences among genotype groups in age, gender distribution, body mass index (BMI), baseline renal function, history of heart disease, or antihypertensive medication use. CYP4A11 T8590C genotype tended to be associated with diuretic use. In men, BMI correlated with diastolic BP (DBP; r = 0.197, P < 0.001) but not SBP. There was no relationship between BMI and SBP or DBP in women.
Table 1.
Patient characteristicsa
| Characteristic | TT (n = 345) | CT (n = 322) | CC (n = 65) | P |
|---|---|---|---|---|
| Age (yr) | 53.6 ± 10.9 | 53.9 ± 10.2 | 53.7 ± 10.9 | 0.90 |
| Women (%) | 40.0 | 41.0 | 35.4 | 0.70 |
| BMI (kg/m2) | 30.9 ± 6.3 | 31.4 ± 6.9 | 29.6 ± 6.3 | 0.12 |
| GFR (ml/min per 1.73 m2) | 47.6 ± 13.5 | 47.7 ± 13.9 | 46.1 ± 13.5 | 0.66 |
| Serum creatinine (mg/dl)b | 2.02 ± 0.73 | 1.98 ± 0.69 | 2.05 ± 0.79 | 0.60 |
| UP/Cr† | 0.32 ± 0.53 | 0.31 ± 0.49 | 0.27 ± 0.48 | 0.74 |
| History of heart disease (%) | 48.4 | 50.3 | 50.8 | 0.87 |
| Years of hypertension | 13.5 ± 10.3 | 13.9 ± 9.3 | 15.4 ± 11.3 | 0.38 |
| Antihypertensive meds | 2.5 ± 1.2 | 2.5 ± 1.1 | 2.7 ± 1.1 | 0.23 |
| Antihypertensive use (%) | ||||
| angiotensin-converting enzyme inhibitor | 44.6 | 38.9 | 35.6 | 0.21 |
| β blocker | 27.4 | 30.6 | 40.7 | 0.11 |
| calcium channel blocker | 65.8 | 60.5 | 71.2 | 0.18 |
| dihydropyridine calcium channel blocker | 48.2 | 43.6 | 54.2 | 0.24 |
| diuretic | 62.2 | 69.4 | 72.9 | 0.08 |
| Randomization to ramipril:metoprolol:amlodipine (%) | 43.8:38.3:18.0 | 39.4:41.0:19.6 | 27.7:43.1:29.2 | 0.10 |
| Randomization to low BP group (%) | 50.4 | 53.7 | 46.2 | 0.46 |
Data are means ± SD unless otherwise indicated. UP/Cr, urine protein to creatinine ratio at baseline.
To convert creatinine mg/dl to μ mol/L, multiply by 88.4. To convert urine protein g/d to mg/d, multiply by 1000.
Randomization to treatment arm or to level of BP control was similar among T8590C genotype groups (Table 1). Patients in the 8590CC genotype were more likely to have been randomly assigned to amlodipine (29.2 versus 18.7%; P = 0.04). This resulted from an imbalanced randomization to amlodipine of patients without baseline proteinuria (32.7% of CC versus 18.2% of CT and TT; P = 0.02); randomization to amlodipine was similar in the CC versus the CT and TT groups among patients with baseline proteinuria (18.8 versus 20.2%; P = 0.93).
Blood Pressure
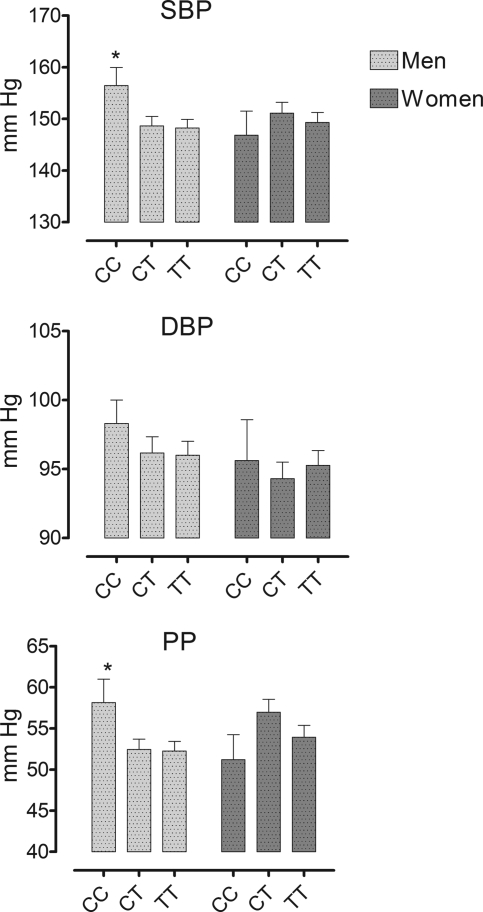
There was no effect of CYP4A11 T8590C genotype on baseline BP in the combined group. Analyzed separately in men and women, however, there was a significant effect of T8590C genotype BP in men, such that SBP and pulse pressure were higher in men with the 8590CC genotype compared with men with CT and TT genotypes (Figure 1).
Figure 1.
Relationship between CYP4A11 T8590C genotype and prerandomization SBP, DBP, and pulse pressure (PP) in men and women. *P < 0.05 versus CT and TT groups.
There was no difference in antihypertensive medication use among CYP4A11 genotypes at 3 or 36 mo (Table 2). At 3 mo after randomization, there was a significant relationship between CYP4A11 T8590C genotype and SBP in men and women combined who were randomly assigned to usual BP control. In patients who were randomly assigned to lower BP, SBP and DBP were significantly higher at 36 mo in men with the CYP4A11 8590CC genotype compared with men with CT and TT genotypes, whereas SBP was highest in women with the 8590CT genotype.
Table 2.
CYP4A11 genotype and BP during follow-upa
| Parameter | Genotype
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 3 Mo
|
36 Mo
|
|||||||
| TT(n = 345) | CT(n = 322) | CC(n = 65) | P | TT(n = 314) | CT(n = 292) | CC(n = 57) | P | |
| Usual BP | ||||||||
| medications, n | ||||||||
| men | 1.7 ± 1.3b | 2.1 ± 1.5 | 1.8 ± 1.2 | 0.18 | 2.1 ± 1.5b | 2.3 ± 1.7b | 2.0 ± 1.2b | 0.46 |
| women | 1.5 ± 1.3 | 1.3 ± 1.1b,c | 1.8 ± 1.5 | 0.32 | 2.0 ± 1.5 | 2.0 ± 1.5b | 2.4 ± 1.7 | 0.72 |
| all | 1.6 ± 1.3b | 1.8 ± 1.4b | 1.8 ± 1.3 | 0.48 | 2.1 ± 1.5b | 2.2 ± 1.6b | 2.1 ± 1.4 | 0.74 |
| SBP (mmHg) | ||||||||
| men | 139.1 ± 16.5b | 143.4 ± 16.1b | 146.3 ± 12.0b | 0.07 | 140.2 ± 13.7b | 142.3 ± 17.1b | 143.3 ± 9.0 | 0.58 |
| women | 139.4 ± 17.7b | 145.7 ± 19.0 | 145.3 ± 14.7b | 0.12 | 141.7 ± 15.9b | 143.9 ± 20.6b | 135.2 ± 12.5 | 0.36 |
| all | 139.3 ± 17.0b | 144.3 ± 17.3b,d | 145.9 ± 12.8b,d | 0.01 | 140.9 ± 14.7b | 142.9 ± 18.5b | 140.5 ± 10.9 | 0.54 |
| DBP (mmHg) | ||||||||
| men | 87.7 ± 9.8b | 89.4 ± 10.9b | 88.7 ± 11.5b | 0.53 | 86.8 ± 10.5b | 87.2 ± 10.5b | 87.1 ± 9.5 | 0.97 |
| women | 87.3 ± 11.2b | 84.8 ± 11.0† | 90.4 ± 10.8b | 0.21 | 84.8 ± 9.3b | 82.1 ± 11.6c | 87.2 ± 8.8b | 0.23 |
| all | 87.5 ± 10.4b | 87.7 ± 11.2b | 89.3 ± 11.1b | 0.66 | 85.9 ± 10.0b | 85.2 ± 11.2b | 87.1 ± 9.1 | 0.66 |
| Lower BP | ||||||||
| medications, n | ||||||||
| men | 2.5 ± 1.4 | 2.3 ± 1.1 | 2.2 ± 1.3 | 0.55 | 2.9 ± 1.5 | 3.1 ± 1.5 | 2.9 ± 1.2 | 0.57 |
| women | 1.7 ± 1.3c | 1.8 ± 1.3c | 1.5 ± 0.9 | 0.71 | 2.4 ± 1.6 | 2.7 ± 1.5 | 2.0 ± 1.1 | 0.31 |
| all | 2.2 ± 1.4 | 2.1 ± 1.2 | 1.9 ± 1.2 | 0.52 | 2.7 ± 1.5 | 2.9 ± 1.5 | 2.6 ± 1.2 | 0.36 |
| SBP (mmHg) | ||||||||
| men | 128.2 ± 17.2 | 126.8 ± 19.9 | 131.7 ± 20.2 | 0.55 | 124.6 ± 17.0 | 123.3 ± 17.0 | 139.1 ± 24.0d,e | 0.004 |
| women | 132.2 ± 23.6 | 138.9 ± 24.2c | 125.6 ± 15.2 | 0.10 | 126.4 ± 15.2 | 135.5 ± 22.8c,d | 124.1 ± 19.3 | 0.03 |
| all | 129.6 ± 19.7 | 132.0 ± 22.6 | 129.5 ± 18.5 | 0.52 | 125.2 ± 16.3 | 128.5 ± 20.5 | 133.5 ± 23.2 | 0.08 |
| DBP (mmHg) | ||||||||
| men | 80.2 ± 12.7 | 80.1 ± 12.9 | 80.0 ± 14.9 | 1.00 | 76.8 ± 10.6 | 75.9 ± 12.2 | 83.8 ± 16.4e | 0.048 |
| women | 81.3 ± 14.0 | 82.8 ± 14.2 | 78.9 ± 9.2 | 0.61 | 76.4 ± 10.4 | 78.6 ± 11.5 | 77.8 ± 9.8 | 0.57 |
| all | 80.5 ± 13.1 | 81.3 ± 13.5 | 79.6 ± 12.9 | 0.76 | 76.7 ± 10.5 | 77.1 ± 11.9 | 81.6 ± 14.4 | 0.13 |
Data are means ± SD.
P < 0.05 versus lower BP group.
P < 0.05 versus men.
P < 0.05 versus 8590TT.
P < 0.05 versus 8590CT.
Clinical End Points
Overall, there was no effect of CYP4A11 T8590C genotype on clinical end points (Table 3). This was true when analyzed separately in patients with and without baseline proteinuria. There was a NS trend toward a decreased incidence of GFR events in patients with the 8590CC genotype and without baseline proteinuria.
Table 3.
Number and rate of clinical eventsa
| Parameter | TT | CT | CC | P | P (CC versus CT + TT) |
|---|---|---|---|---|---|
| GFR | |||||
| total | 60 (17.4) | 59 (18.3) | 6 (9.2) | 0.20 | 0.08 |
| UP/Cr >0.22b | 34 (32.4) | 36 (34.6) | 5 (31.3) | 0.94 | 0.84 |
| ESRD | |||||
| total | 42 (12.2) | 47 (14.6) | 8 (12.3) | 0.64 | 0.81 |
| UP/Cr >0.22b | 33 (31.4) | 36 (34.6) | 7 (43.8) | 0.63 | 0.39 |
| Death | |||||
| total | 4 (1.2) | 3 (0.9) | 2 (3.1) | 0.35 | 0.19 |
| UP/Cr >0.22b | 1 (1.0) | 0 (0.0) | 1 (6.3) | 0.14 | 0.14 |
| ESRD or death | |||||
| total | 46 (13.3) | 50 (15.5) | 10 (15.4) | 0.71 | 0.83 |
| UP/Cr >0.22b | 34 (32.4) | 36 (34.6) | 8 (50.0) | 0.40 | 0.19 |
| GFR, ESRD or death | |||||
| total | 81 (23.5) | 79 (24.5) | 12 (18.5) | 0.57 | 0.32 |
| UP/Cr >0.22b | 49 (46.7) | 50 (48.1) | 9 (56.3) | 0.79 | 0.50 |
Data are n (%).
A baseline ratio of 0.22 corresponds approximately to proteinuria of 300 mg/d. For the end point death, the Fisher exact test was used because expected cell counts were <5.
Figure 2 shows the cumulative incidence of ESRD and death by CYP4A11 T8590C genotype group for all patients and for those with baseline proteinuria. There was no effect of genotype on the cumulative incidence of ESRD or death for all patients combined (P = 0.61). In a Cox proportional hazard regression, which included drug treatment group, urinary protein-to-creatinine ratio, and the presence or absence of electrocardiographic evidence of myocardial infarction, the P value for CYP4A11 T8590C was 0.06. Among patients with baseline proteinuria, CYP4A11 8590CC genotype was associated with an increased risk for ESRD and death over time (hazard ratio 3.16; P = 0.004), as were drug treatment group (hazard ratio 2.44; P = 0.04), urinary protein-to-creatinine ratio (P < 0.001), and the presence or absence of electrocardiographic evidence of previous myocardial infarction (P = 0.02). Baseline mean arterial pressure (MAP), BMI, BP group, and age were NS and were excluded from the model. When analyzed separately for men and women with proteinuria, the effect of CYP4A11 8590CC genotype on the cumulative incidence of ESRD or death was statistically significant for men (P = 0.003) but not for women (P = 0.20) by Cox regression analysis. When MAP at 36 mo, rather than BP group, was included in the model, CYP4A11 8590CC genotype (P = 0.007), baseline MAP (P = 0.005), drug treatment group (0.02), urinary protein-to-creatinine ratio (P = 0.001), and the presence or absence of electrocardiographic evidence of myocardial infarction (P = 0.04) significantly predicted progression to ESRD or death for men with proteinuria. MAP at 36 mo was NS. There was no effect of T8590C genotype on the cumulative incidence of the combined end point of GFR event, ESRD, or death either for all patients or for those with proteinuria (data not shown).
Figure 2.
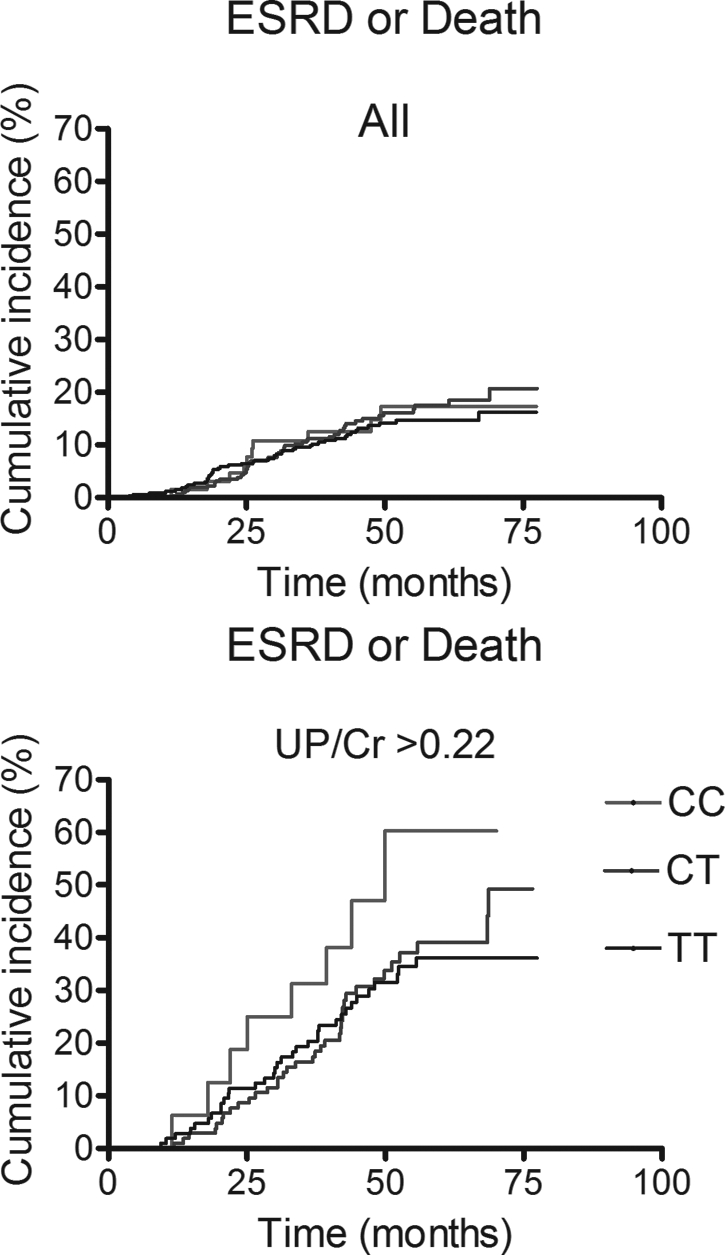
Product limit estimate of ESRD and death for all patients (above) and for those with a baseline urinary protein-to-creatinine ratio (UP/Cr) >0.22 (below).
DISCUSSION
CYP4A11 catalyzes the metabolism of arachidonic acid to 20-HETE in humans. A functional CYP4A11 C variant at nucleotide 8590, characterized by decreased 20-HETE synthase activity, has been associated with hypertension in three white populations: The Framingham Offspring Cohort, a Tennessee Cohort, and the MONitoring trends and determinants In Cardiovascular disease (MONICA) Augsburg echocardiographic substudy.21,22 Here, we report that the CYP4A11 8590CC genotype is associated with increased BP and with increased progression to ESRD or death in male hypertensive black Americans with mild to moderate renal disease and proteinuria.
In our previous report of an association of the CYP4A11 C allele with hypertension in the Framingham Offspring study cohort and in a Tennessee cohort, we modeled the C allele as a dominant allele, primarily because of the low frequency of the 8590CC genotype in these populations. In this study, the increased frequency of the 8590CC genotype in the AASK cohort together with the size of the cohort allowed us to explore other models of association with BP. The association of the CYP4A11 8590CC genotype with baseline SBP in men and with 36-mo SBP and DBP in men randomly assigned to low-salt intake is remarkably similar to that observed among white European participants in the MONICA Augsburd echocardiographic substudy.22 In that study, the investigators reported a recessive effect of the C allele on SBP in men and on SBP and DBP in men and women combined. As in the MONICA study, the degree of elevation of SBP at baseline in men with the 8590CC genotype (8 mmHg) and of SBP (16 mmHg) and DBP (7.5 mmHg) at 36 mo in men with the 8590CC genotype in the lower BP group is clinically significant in AASK.
The finding that the loss-of-function CYP4A11 8590C variant associates with increased SBP supports the concept that the effect of 20-HETE on tubular sodium transport predominates over its vasoconstrictor effect in determining sodium excretion and BP in humans. Tubular CYP4A11 deficiency is associated with salt-sensitive hypertension in rodent models.23 Humans with salt-sensitive hypertension demonstrate decreased urinary 20-HETE excretion in response to furosemide.24 Although participants in AASK were not specifically characterized according to the salt sensitivity of their BP, patients who carried a C allele tended to be taking a diuretic before randomization, suggesting the possibility that they were more likely to have salt-sensitive or volume-dependent hypertension.
We used a single-nucleotide polymorphism (SNP) association analysis to determine the relationship between the CYP4A11 gene and BP in the AASK population. Although it is possible that haplotype analysis can provide increased power to detect associations, this is not universal and depends on patterns of linkage disequilibrium among markers in the study population in general and between the studied SNP and functional variants.25 Fu et al.26 detected an association between the CYP4A11 gene and essential hypertension in Japanese men using haplotype analysis; however, the significance of the association depended on the inclusion of the CYP4A11 T8590C genotype, suggesting that the haplotype analysis afforded no advantage over the SNP analysis.
Genome-wide association (GWA) studies provide a particularly powerful new approach to identify genes involved in complex diseases such as hypertension. One such GWA study of 14,000 cases of seven common diseases identified 24 independent association signals at P < 5 × 10−7 with six common diseases but found no SNP associated with hypertension at that level of significance.27 The authors considered the possibilities that hypertension has fewer common risk alleles of larger effect size or that the study failed to detect susceptibility variants with large effect size because they were poorly tagged. In addition, hypertension may be more susceptible to the dilution effect resulting from the inclusion of individuals with hypertension among control subjects. The effects of multiple susceptibility alleles with small effect size and of confounding by misclassification can be minimized by studying homogeneous, precisely phenotyped populations such as the AASK cohort.
More dramatic than the effect of CYP4A11 T8590C variant on BP was the effect of the variant on the cumulative incidence of ESRD or death. As reported previously for the effect of ramipril versus amlodipine on decline in GFR,28 the association of ESRD or death with CYP4A11 genotype was observed only in patients who had elevated urinary protein excretion at baseline. It is known that proteinuria is associated with endothelial dysfunction and predicts risk for death as a result of cardiovascular disease.29 The association of the CYP4A11 8590CC genotype with ESRD and death could not be attributed to differences in baseline serum creatinine, GFR, or randomization to level of BP control or treatment among genotype groups, because these all were similar in patients with baseline proteinuria. Interestingly, genotype did not affect the cumulative incidence of GFR events or the combined end point of GFR event, ESRD, or death. On the basis of the magnitude of BP elevation associated with the 8590CC genotype, it is attractive to hypothesize that relative hypertension in patients of the 8590CC genotype group contributed to the increased incidence of ESRD or death. Although, as reported previously in AASK,30 randomization to usual or lower BP control did not affect the incidence of ESRD or death, baseline BP did predict progression to ESRD or death. Taken together with the finding that CYP4A11 8590CC genotype predicted progression to ESRD or death in men but not in women, the data suggest that the effect of the genotype on BP contributed to the effect of genotype on these outcomes in men.
The CYP4A11 8590CC genotype was associated with increased baseline SBP in men but not in women. This interactive effect of CYP4A11 genotype and gender is consistent with data from mice genetically deficient in Cyp4a14, the murine homologue of CYP4A11.17 Disruption of the Cyp4a14 gene causes a significantly greater increase in systolic and diastolic BP in male compared with female mice. In addition, castration-normalized BP and androgen replacement restore hypertension in male Cyp4a14 (−/−) mice, consistent with androgen-dependent regulation of CYP4A arachidonic acid monooxygenase. In the MONICA Augsburg echocardiographic substudy, homozygosity for CYP4A11 8590C allele was associated with increased SBP in men but not in women.22 Likewise, Fu et al.26 reported that the CYP4A11 T8590C polymorphism is associated with essential hypertension in Japanese men but not in Japanese women. In short, this study is the third to report a gender-specific association of the CYP4A11 T8590C genotype with BP.
In summary, we report the association of homozygosity for a functional variant of the CYP4A11 with increased baseline BP in black American men with hypertensive nephrosclerosis, as well as with an increased cumulative incidence of ESRD or death among those with baseline proteinuria. These data confirm an association between BP and the CYP4A11 gene previously reported in white cohorts and, thus, provide strong evidence of a role for the renal monooxygenases and 20-HETE in the regulation of BP in humans. If confirmed prospectively in other populations, then the data suggest that CYP4A11 genotype may be used to stratify patients with hypertensive renal disease and proteinuria according to their risk for ESRD and death.
CONCISE METHODS
The rationale for the AASK and details of the study design have been reported previously.30–32 In brief, participants were self-identified black Americans who had hypertension, were aged 18 to 70 yr, and had a GFR between 20 and 65 ml/min per 1.73 m2 and no other identified causes of renal insufficiency. Exclusion criteria were (1) diastolic BP <95 mmHg; (2) known history of diabetes; (3) urinary protein-to-creatinine ratio >2.5; (4) accelerated or malignant hypertension within 6 mo; (5) accelerated, malignant, or secondary hypertension; (6) evidence of non–hypertension-related causes of renal disease; (7) serious systemic disease including congestive heart failure; or (8) specific indication for or contraindication to a study drug or study procedure. The protocol was approved by the institutional review board at each center, and all participants gave written informed consent.30
The AASK used a 3 × 2 factorial design in which participants were randomly assigned to a usual MAP goal of 102 to 107 mmHg or to a low MAP goal of ≤92 mmHg and to treatment with one of three antihypertensive study drugs: A sustained-release β blocker (metoprolol), an angiotensin-converting enzyme inhibitor (ramipril), or a dihydropyridine calcium channel blocker (amlodipine). The primary composite clinical end point was prespecified as the time from randomization to any of the following: (1) GFR event, defined as a confirmed ≥50% or 25-ml/min per 1.73 m2 decline in GFR; (2) ESRD, defined as the need for replacement therapy; or (3) death.30,33,34 During the trial, the data safety and monitoring board recommended the termination of the amlodipine arm on the basis of a significant treatment effect in individuals with baseline proteinuria.30
Genotyping
Each of 732 participants from whom DNA was available were genotyped at the CYP4A11 T8590C (also referred to as F434S; rs1126742) locus using methods as described previously.21
Statistical Analysis
Data are presented as means ± SD unless otherwise noted. Continuous variables were compared using one-way ANOVA or an unpaired t test. Categorical variables were compared using χ2 testing or the Fisher exact test when the expected cell count was <5. A urinary protein-to-creatinine ratio of 0.22 (a value corresponding approximately to a threshold for clinically significant proteinuria of 300 mg/d) was used for subgroup analysis on the basis of the use of this cutoff in the original comparison of ramipril versus amlodipine after an interim analysis of AASK detected significant interactions between outcome variables and baseline proteinuria.28 Cumulative incidence rates for clinical end points were estimated using the product-limit method. Cox proportional hazard regression analysis was used to determine the effect of CYP4A11 genotype and other clinical factors on the cumulative incidence of ESRD or death. Variables were excluded using a backward conditional method. Analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL). This study was an ancillary study to AASK, and, as such, the analyses were not performed by the AASK Data Coordinating Center.
DISCLOSURES
E.P.D. is employed by BioVentures, Inc., a company that provides products and technical support for genotyping.
Acknowledgments
This work was funded by National Institutes of Health grants DK038226, DK048689, DK057867, HL065193, HL079184, HL004221, RR000071, and RR000095.
We thank Dr. Scott M. Williams for review of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J: Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 36: 646–661, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Jones CA, McQuillan GM, Kusek JW, Eberhardt MS, Herman WH, Coresh J, Salive M, Jones CP, Agodoa LY: Serum creatinine levels in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 32: 992–999, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Norris KC, Agodoa LY: Unraveling the racial disparities associated with kidney disease. Kidney Int 68: 914–924, 2005 [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005
- 7.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ: Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Shiozawa M, Provoost AP, van Dokkum RP, Majewski RR, Jacob HJ: Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Eikmans M, Aben JA, Koop K, Baelde HJ, de HE, Bruijn JA: Genetic factors in progressive renal disease: The good ones, the bad ones and the ugly ducklings. Nephrol Dial Transplant 21: 257–260, 2006 [DOI] [PubMed] [Google Scholar]
- 10.DeWan AT, Arnett DK, Atwood LD, Province MA, Lewis CE, Hunt SC, Eckfeldt J: A genome scan for renal function among hypertensives: The HyperGEN study. Am J Hum Genet 68: 136–144, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satko SG, Freedman BI, Moossavi S: Genetic factors in end-stage renal disease. Kidney Int Suppl S46–S49, 2005 [DOI] [PubMed]
- 12.McGiff JC, Quilley J: 20-HETE and the kidney: Resolution of old problems and new beginnings. Am J Physiol 277: R607–R623, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Carroll MA, McGiff JC: A new class of lipid mediators: Cytochrome P450 arachidonate metabolites. Thorax 55[Suppl 2]: S13–S16, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capdevila JH, Falck JR: The CYP P450 arachidonic acid monooxygenases: From cell signaling to blood pressure regulation. Biochem Biophys Res Commun 285: 571–576, 2001 [DOI] [PubMed] [Google Scholar]
- 15.McGiff JC, Quilley J: 20-Hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens 10: 231–237, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Roman RJ: P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Holla VR, Adas F, Imig JD, Zhao X, Price E Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH: Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A 98: 5211–5216, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisbee JC, Falck JR, Lombard JH: Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol 278: H1517–H1526, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Muller DN, Theuer J, Shagdarsuren E, Kaergel E, Honeck H, Park JK, Markovic M, Barbosa-Sicard E, Dechend R, Wellner M, Kirsch T, Fiebeler A, Rothe M, Haller H, Luft FC, Schunck WH: A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol 164: 521–532, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki T, Ishimoto T, Sakurai T, Yasuda Y, Taniguchi K, Doi M, Sato M, Roman RJ, Miyata N: Increased excretion of urinary 20-HETE in rats with cyclosporine-induced nephrotoxicity. J Pharmacol Sci 97: 132–137, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O'Donnell CJ, Brown NJ, Waterman MR, Capdevila JH: Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111: 63–69, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Mayer B, Lieb W, Gotz A, Konig IR, Aherrahrou Z, Thiemig A, Holmer S, Hengstenberg C, Doering A, Loewel H, Hense HW, Schunkert H, Erdmann J: Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension 46: 766–771, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Moreno C, Maier KG, Hoagland KM, Yu M, Roman RJ: Abnormal pressure-natriuresis in hypertension: Role of cytochrome P450 metabolites of arachidonic acid. Am J Hypertens 14: 90S–97S, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Laffer CL, Laniado-Schwartzman M, Wang MH, Nasjletti A, Elijovich F: Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic acid in human salt-sensitive versus salt-resistant hypertension. Circulation 107: 574–578, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Morris RW, Kaplan NL: On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genet Epidemiol 23: 221–233, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, Ozawa Y, Ma Y: A haplotype of the CYP4A11 gene associated with essential hypertension in Japanese men. J Hypertens 26: 453–461, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, III, Norris K, O'Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT Jr, Xu S: Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appel LJ, Middleton J, Miller ER, III, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O'Connor D, Ojo A, Phillips R, Sika M, Wright J Jr: Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Lewis J, Greene T, Appel L, Contreras G, Douglas J, Lash J, Toto R, Van LF, Wang X, Wright JT Jr: A comparison of iothalamate-GFR and serum creatinine-based outcomes: Acceleration in the rate of GFR decline in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol 15: 3175–3183, 2004 [DOI] [PubMed] [Google Scholar]