Abstract
It is a matter of debate whether pancreas allografts independently contribute to renal allograft and patient survival in individuals who have type 1 diabetes and receive a simultaneous pancreas and kidney transplant (SPK). Using data from the Collaborative Transplant Study, we studied patients who had type 1 diabetes and were recipients of deceased-donor kidneys (DDK), living-donor kidneys (LDK), or SPK. We analyzed graft and patient survival rates with a maximum of 18 yr of follow-up. DDK recipients had inferior graft and patient survival compared with LDK and SPK recipients. LDK recipients had superior graft and patient survival rates initially, but SPK recipients demonstrated equal survival rates toward the end of follow-up. Multivariate analysis, adjusting for pretransplantation cardiovascular risk, showed that patient survival of SPK recipients was superior to that of LDK recipients beyond the 10th year after transplantation (hazard ratio 0.55; P = 0.005). In summary, the early survival advantage of LDK over SPK is lost during long-term follow-up, probably as a result of improved glycemic control in SPK recipients.
Simultaneous pancreas kidney transplantation (SPK) is believed to be the optimal treatment for patients who have type 1 diabetes and ESRD. After successful SPK, the majority of patients exhibit normoglycemia and normal or nearly normal levels of glycosylated hemoglobin, associated with an improved quality of life.1–3 The relative contribution of the pancreas allograft to the survival benefit after SPK, as compared with the benefit achieved by kidney transplantation alone, is unclear.4–8 In a recent analysis of SPK and kidney transplantation alone, comparing transplants in which one patient with diabetes received an SPK and a second patient with diabetes received the contralateral kidney from the same deceased donor but without a concurrent pancreas, there was no difference between the two groups of recipients in terms of kidney graft and patient survival up to 9 yr of follow-up.9 Whether microvascular lesions improve in patients who have type 1 diabetes and receive an SPK transplant is discussed controversially. In recipients of single pancreas transplants, regression of diabetic kidney lesions in the native kidneys has been documented after the fifth year of posttransplantation follow-up in a biopsy-controlled study.10,11 One would expect that with both an improvement of renal function and normalization of glucose metabolism in SPK recipients, morbidity and mortality should be reduced. It was suggested recently that, although patient mortality is increased in the early period after SPK transplantation, long-term patient survival might be improved and perhaps even superior to that in patients who have type 1 diabetes and receive a kidney transplant from a living donor (LDK)12; however, solid evidence in support of this hypothesis is lacking, and, to date, it is not clear whether long-term kidney allograft and patient survival in SPK recipients is superior to that of recipients of a kidney transplant alone.
Using the data of the Collaborative Transplant Study (CTS), we analyzed graft and patient survival in recipients who were treated with SPK or kidney transplantation alone, the latter separated according to whether the donor kidney originated from a deceased (DDK) or a living donor (LDK). We wished to address the question of whether glycemic control with a functioning pancreas allograft significantly contributes to prolonged kidney allograft and patient survival.
RESULTS
Transplants reported to the CTS from 1984 to 2000 were analyzed. All patients who were reported to the study center with type 1 diabetes and ESRD and received either a first SPK transplant from a deceased donor or a kidney transplant alone, from either a deceased donor (DDK) or a living donor (LDK), were included. Patients with pancreas after kidney transplantation were not considered in the study. Because SPK recipients are on average younger and the survival advantage associated with younger age would unduly influence the results of this comparative analysis, recipients who were older than 45 yr were excluded. Demographics of the patient population studied are shown in Table 1. Pretransplantation categorization of recipients by the transplant centers with respect to “overall risk as candidate for transplantation” and specifically with respect to “cardiovascular risk” showed advantages for SPK recipients. SPK recipients were more often categorized as “good risk recipients” (59.6%) as compared with LDK recipients (55.5%; P = 0.009) and DDK recipients (45.5%; P < 0.001). A total of 7.3% of SPK recipients were categorized before transplantation as being at high cardiovascular risk, as compared with 8.5% of LDK recipients (P = 0.17; NS) and 12.1% of DDK recipients (P < 0.001).
Table 1.
Characteristics of study populationa
| Characteristic | DDK (n = 5705) | LDK (n = 2190) | SPK (n = 3525) |
|---|---|---|---|
| Transplant year (n [%]) | |||
| 1984 to 1990 | 2944 (52) | 992 (45) | 850 (24) |
| 1991 to 1995 | 1727 (30) | 675 (31) | 1254 (36) |
| 1996 to 2000 | 1034 (18) | 523 (24) | 1421 (40) |
| Recipient gender (n [%]) | |||
| female | 2404 (42) | 1013 (46) | 1458 (41) |
| male | 3298 (58) | 1177 (54) | 2067 (59) |
| Recipient race (n [%]) | |||
| white | 4830 (90) | 1926 (90) | 3108 (97) |
| other | 544 (10) | 205 (10) | 92 (3) |
| Antibodies (% PRA; n [%]) | |||
| <10 | 4524 (90) | 1530 (93) | 2975 (94) |
| ≥10 | 529 (10) | 121 (7) | 205 (6) |
| HLA mismatches (n [%]) | |||
| 0 to 1 | 833 (15) | 646 (33) | 86 (3) |
| 2 to 4 | 3707 (69) | 1178 (60) | 1865 (56) |
| 5 to 6 | 858 (16) | 124 (6) | 1372 (41) |
| Recipient age (yr; mean ± SD) | 35.7 ± 5.9 | 34.0 ± 6.0 | 35.4 ± 5.7 |
| Donor age (yr; mean ± SD) | 33.0 ± 15.8 | 41.6 ± 12.9 | 28.0 ± 11.2 |
| Cold ischemia time (h; mean ± SD) | 22.0 ± 9.6 | 3.5 ± 8.4 | 11.8 ± 5.9 |
| General evaluation (n [%]) | |||
| good | 2071 (45) | 951 (56) | 1487 (60) |
| moderate | 1976 (43) | 632 (37) | 798 (32) |
| poor | 508 (11) | 129 (8) | 209 (8) |
| Cardiovascular risk (n [%]) | |||
| yes | 540 (12) | 143 (8) | 178 (7) |
| no | 3932 (88) | 1542 (92) | 2251 (93) |
Some values are missing as a result of incomplete reporting. For all characteristics, global P < 0.001. PRA, panel reactive antibodies.
Kidney Graft Survival
We analyzed two different time intervals because of improvements over time in pre- and postoperative management. Patients were separated into those who received a transplant between 1984 and 1990 and those who received a transplant between 1991 to 2000. Whereas during the period from 1984 to 1990 the vast majority of transplants in patients with type 1 diabetes were kidney transplants alone (either DDK or LDK), SPK transplants almost equaled the number of kidney transplants during the period from 1996 to 2000 (Table 1). After 10 yr of posttransplantation follow-up, 70 and 74% of SPK patients with a functioning kidney also had a functioning pancreas allograft during the first (1984 to 1990) and second (1991 to 2000) periods, respectively. A functioning pancreas allograft was defined as insulin-free normoglycemia (>95% of patients with a functioning pancreas transplant were reported in this category) or a clinically relevant reduction in insulin requirement (<5% of patients with a functioning graft were reported in this category).
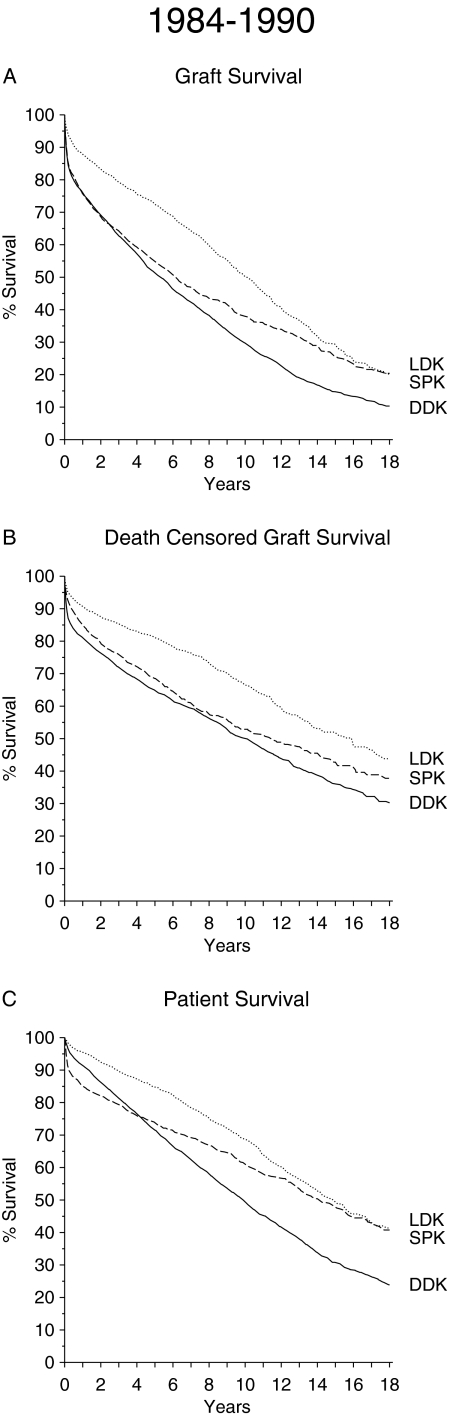
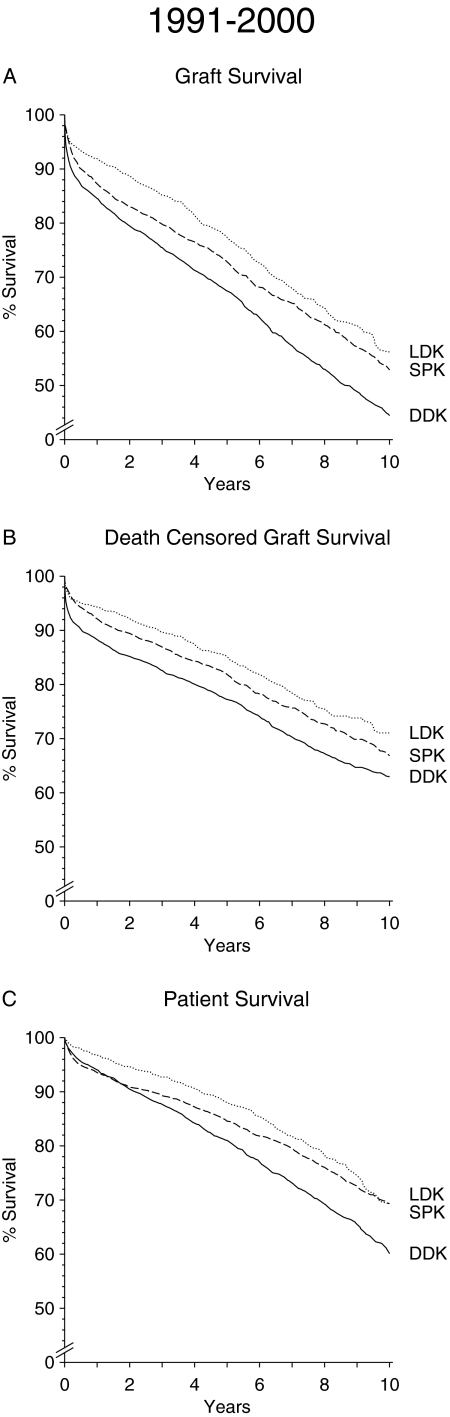
Kidney graft survival rates after DDK, LDK, and SPK are depicted in Figure 1A for the period from 1984 to 1990 and in Figure 2A for the period from 1991 to 2000. An inferior graft survival of DDK transplants as compared with LDK and SPK transplants is evident in both periods. LDK transplant shows superior early graft survival rate during the 1984 to 1990 as well as the 1991 to 2000 period. During the first period, SPK reached the level of graft survival of LDK toward the end of the 18-yr posttransplantation follow-up period. During the second period, from 1991 to 2000, the graft survival rates of SPK and LDK recipients merged after approximately 10 yr. The higher number of SPK graft losses within the first posttransplantation year in the period from 1984 to 1990 can be ascribed to a higher rate of technical problems related to inexperience and less efficient immunosuppression. In the second period, the SPK graft loss rate within the first posttransplantation year was much lower (1-yr SPK kidney graft survival 1984 to 1990 75.6%; 1991 to 2001 87.3%; P < 0.001).
Figure 1.
(A through C) Graft survival (A), death-censored graft survival (B), and patient survival (C) in patients with type 1 diabetes after DDK transplantation (n = 2944), LDK transplantation (n = 992), or SPK transplantation (n = 850) performed during the period from 1984 to 1990.
Figure 2.
(A through C) Graft survival (A), death-censored graft survival (B), and patient survival (C) in patients with type 1 diabetes after DDK transplantation (n = 2761), LDK transplantation (n = 1198), or SPK transplantation (n = 2675) performed during the period from 1991 to 2000.
The results of death-censored kidney graft survival are illustrated in Figures 1B and 2B. Multivariate analysis showed a superior survival rate for SPK compared with DDK only beyond 10 yr of posttransplantation follow-up during the transplant period from 1984 to 1990 (hazard ratio [HR] 0.64; P = 0.020; Table 2). When comparing LDK and SPK, there were no significant differences except that LDK grafts did better during the first years of follow-up in the period from 1984 to 1990.
Table 2.
Results of multivariate Cox regression analysisa
| Follow-up Year of Transplantation | SPK versus DDK
|
SPK versus LDK
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | P | n | HR | 95% CI | P | |
| Death-censored graft survival | ||||||||
| years 2 to 5 | ||||||||
| 1984 to 1990 | 2783 | 1.09 | 0.87 to 1.37 | 0.450 | 1435 | 2.36 | 1.70 to 3.27 | <0.001 |
| 1991 to 2000 | 4245 | 0.96 | 0.79 to 1.17 | 0.711 | 3038 | 1.32 | 1.00 to 1.74 | 0.052 |
| years 6 to 10 | ||||||||
| 1984 to 1990 | 1671 | 1.13 | 0.86 to 1.48 | 0.391 | 930 | 1.41 | 0.99 to 2.00 | 0.059 |
| 1991 to 2000 | 2395 | 1.00 | 0.79 to 1.26 | 0.991 | 1662 | 1.31 | 0.94 to 1.83 | 0.111 |
| years 11 to 18 | ||||||||
| 1984 to 1990 | 741 | 0.64 | 0.44 to 0.93 | 0.020 | 449 | 0.73 | 0.46 to 1.16 | 0.188 |
| Patient survival | ||||||||
| years 2 to 5 | ||||||||
| 1984 to 1990 | 2783 | 0.70 | 0.54 to 0.91 | 0.008 | 1435 | 1.34 | 0.93 to 1.94 | 0.120 |
| 1991 to 2000 | 4245 | 0.82 | 0.66 to 1.01 | 0.064 | 3038 | 1.31 | 0.96 to 1.79 | 0.085 |
| years 6 to 10 | ||||||||
| 1984 to 1990 | 1671 | 0.56 | 0.42 to 0.75 | <0.001 | 930 | 0.91 | 0.63 to 1.32 | 0.620 |
| 1991 to 2000 | 2395 | 0.64 | 0.51 to 0.82 | <0.001 | 1662 | 0.89 | 0.63 to 1.27 | 0.533 |
| years 11 to 18 | ||||||||
| 1984 to 1990 | 741 | 0.52 | 0.37 to 0.73 | <0.001 | 449 | 0.55 | 0.36 to 0.83 | 0.005 |
n = number of patients at beginning of follow up period.
Patient Survival
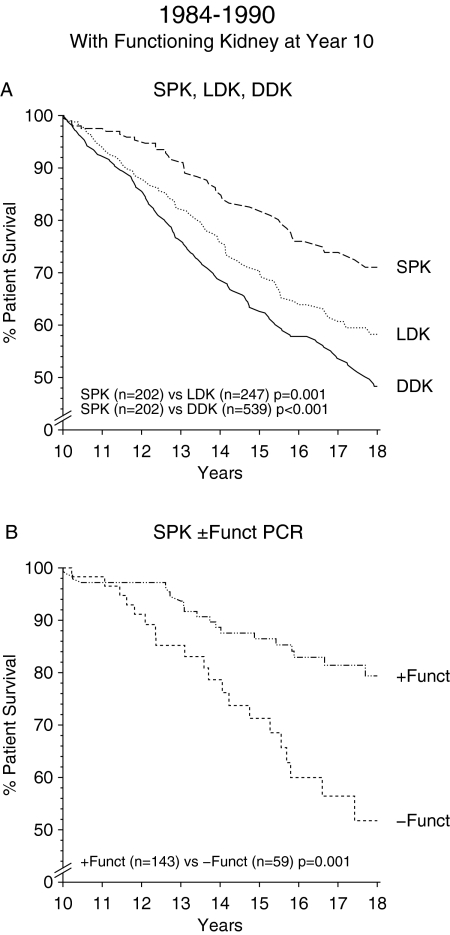
When long-term patient survival rather than kidney graft survival was analyzed, DDK recipients did strikingly worse than LDK or SPK recipients during both periods (Figures 1C and 2C). As in the analysis of kidney graft survival, in the period from 1984 to 1990, an early advantage of LDK compared with SPK faded away with prolonged follow-up. The results in the second period are even more striking. Patient survival in the two groups already merged after approximately 10 yr. By multivariate analysis, an increasing survival benefit over length of follow-up is seen in patients with SPK compared with DDK. For the transplant years 1991 to 2000, the HR decreased from 0.82 in the follow-up years 2 to 5 (P = 0.067) to 0.65 in the years 6 to 10 (P < 0.001; Table 2). When comparing LDK and SPK, multivariate analysis showed a trend toward an early survival disadvantage of SPK, which was no longer evident beyond the fifth year (Table 2). When patient survival beyond the 10th year was analyzed for recipients who had a functioning kidney graft 10 yr after transplantation, SPK recipients did significantly better than DDK or LDK recipients (Figure 3A). Furthermore, SPK patients who still had a functioning pancreas allograft beyond the 10th year had a markedly better survival compared with SPK patients who had lost their pancreas (P = 0.001; Figure 3B).
Figure 3.
(A) Patient survival after the 10th posttransplantation year for recipients with a functioning kidney allograft at year 10 after transplantation. (B) Patient survival after the 10th posttransplantation year for SPK recipients with a functioning kidney allograft, either with or without a functioning pancreas transplant. + Funct PCR, functioning pancreas allograft at year 10 after transplantation; − Funct PCR, no function of the pancreas allograft at year 10 after transplantation.
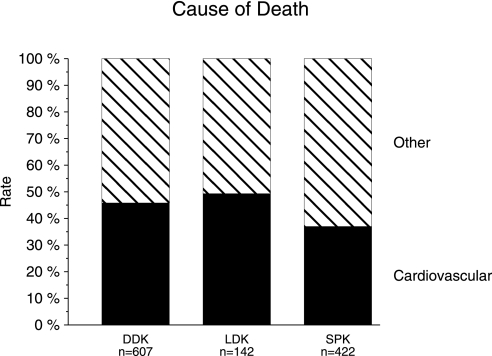
Cox regression analysis, in which the pretransplantation assessment of cardiovascular risk was appropriately considered, confirmed the superior survival rate of SPK compared with LDK recipients from years 10 to 18 (HR 0.55; P = 0.005; Table 2). It is widely known that patient survival is primarily a reflection of the cumulative rate of patient death from cardiovascular events. Cause of death information was collected prospectively in the CTS since 1990. Analysis of the recent era from 1991 to 2000 showed a lower percentage of cardiovascular death in recipients of SPK (37.0%) compared with DDK (45.8%) or LDK (49.3%; Figure 4). Multivariate logistic regression analysis including the same variables as those used in the Cox analysis confirmed that the rate of cardiovascular death was lower in recipients of SPK than DDK (relative risk 0.77; P = 0.049) or LDK (relative risk 0.56; P = 0.007).
Figure 4.
Cumulative cardiovascular death rate in patients with type 1 diabetes after DDK, LDK, or SPK transplantation performed during the period from 1991 to 2000.
DISCUSSION
It is debated to what extent pancreas allografts independently contribute to renal transplant and patient survival in patients with type 1 diabetes after SPK. Numerous studies have compared graft and patient survival in DDK and SPK recipients and found conflicting results.2–9,12,13 The differing outcomes can be attributed to different transplantation characteristics: In general, SPK transplantation is associated with more favorable donor characteristics, because most transplant centers accept only pancreas allografts obtained from young (i.e., younger than 40 yr), healthy donors. Furthermore, SPK recipients are on average younger and are in generally better physical condition. An approach to account for these confounders was recently published: Waki et al.9 compared the results of DDK and SPK from the same deceased donor. When the donor-specific variables were stratified in this manner, graft and patient survival did not differ between DDK and SPK; however, this approach did not stratify for the influence of recipient factors, and follow-up was short. We took a different approach. We analyzed outcomes separately in recipients of DDK, LDK, and SPK transplants performed during the periods 1984 to 1990 and 1991 to 2000. These two different periods were chosen because peri- and postoperative management improved in the past two decades, new immunosuppressive drugs were introduced, and surgical techniques were refined.14 As expected, patient and graft survival differed significantly between the two periods; however, during follow-up periods of 18 and 10 yr, respectively, graft and patient survival rates as well as the cumulative rates of cardiovascular death were significantly worse after DDK compared with LDK and SPK. During both periods, LDK was associated with the best initial graft and patient survival rates, but these merged with those of SPK. Most important, long-term follow-up beyond the 10th year after transplantation showed that patient survival was highest for SPK recipients. This was reflected by the lower cumulative cardiovascular death rate in recipients of SPK compared with recipients of DDK or LDK.
Several comments need to be made. Because we stratified recipient age by analyzing patients who were younger than 45 yr at the time of transplantation, recipient age in this analysis did not differ significantly among DDK, LDK, and SPK patients. Nevertheless, SPK recipients were more often categorized by the transplant centers as “good risk candidates for transplantation” and less often as recipients at “high cardiovascular risk,” factors that were appropriately considered in the Cox analysis. It is a limitation of this retrospective registry analysis that it is not possible to adjust for individual cardiovascular risk factors (e.g., hypertension, hyperlipidemia, and statin use; tobacco use; use of inhibitors of the renin angiotensin system). Most living donors were parents of LDK recipients; consequently, living donors were on average older compared with donors of DDK and SPK. Conversely, because of their genetic relationship, LDK transplants had a lower number of HLA mismatches, and because the donors were living donors, these transplants were associated with a shorter cold ischemia time. Thus, the variables donor age, HLA mismatches, and cold ischemia time were a priori unequally distributed among the study groups by definition; they were therefore unsuitable for inclusion in the Cox model because their inclusion would have produced erroneous results. In this context, it is important to consider that donor age has an important role in kidney transplantation when allografts from deceased donors are considered. In contrast, donor age has been shown to have a very small effect on the outcome of LDK transplants.15 Thus, older donor age does not explain the lower survival rate in LDK compared with SPK. LDK patients do even better than DDK patients despite a donor age advantage for DDK (Table 1), which also argues against a determining influence of donor age on the results of this analysis. Further support for the validity of this observation is shown in Figure 3B, in which survival rates of SPK patients are compared for two groups, depending on whether the pancreas transplant was still functioning after 10 yr and for whom the donor age criteria were equivalent. There was no evidence for a significant center effect when centers that performed all three types of transplants (DDK, LDK, and SPK) were considered. As mentioned before, the period of transplantation plays an important role with respect to the analysis of graft and patient survival. Allograft loss in the first year was more common between 1984 and 1990, especially in recipients of SPK, presumably related to less experience and inferior immunosuppression.14 The superior outcome of SPK patients was not associated with the type of immunosuppressive regimen (e.g., T cell–depleting induction therapy, use of tacrolimus versus cyclosporine, or use of mycophenolate mofetil versus azathioprine). It should be pointed out that, in the analysis of long-term survival (>10 yr; transplant years 1984 to 1990), most patients were on cyclosporine and azathioprine. Only 2 and 4% of these patients, respectively, were on maintenance immunosuppression at 10 yr with tacrolimus or mycophenolic acid. Type of immunosuppression was included as a covariate in the Cox regression model and not found to be a significant confounder. The frequency of administration of antirejection therapy during the first posttransplantation year was highest in the SPK group (40%) compared with DDK (31%) and LDK (25%). We cannot definitely exclude that more potent immunosuppression in SPK patients may have contributed to the better outcome.
Our results might be interpreted as documenting a beneficial effect of glycemic control on renal allograft and patient survival, supported by the finding of a reduced cardiovascular death rate in SPK recipients.16,17 In a biopsy-controlled study by Fioretto et al.,11 pancreas transplantation reversed diabetic glomerular lesions beyond the fifth year after transplantation. These results are in line with our data. The initially better graft survival in LDK compared with DDK and SPK can be attributed to a lower rate of ischemia reperfusion injury with LDK, a lower rate of delayed graft function, better HLA matching, a shorter time on pretransplantation dialysis, and a lower rate of technical problems. Our results suggest that the beneficial effect of normoglycemia influences renal function and has long-term effects that extend beyond the kidney. Previous studies documented that diabetic micro- and macrovascular lesions as well as visceral and peripheral neuropathy improved after successful pancreas transplantation.18–23 Although we cannot provide data on microvascular pathology because information on this factor was not collected by our registry, our results provide indirect support for an improved vascular state in SPK patients because the outcome measure “cardiovascular death rate,” which reflects systemic effects of normoglycemia, was substantially improved in recipients of SPK.
The results of this analysis are different from recent studies, in which no difference in outcome was found in comparisons of patients treated with SPK or kidney transplantation alone (mostly DDK).6,9,12 The main difference between those studies and ours is the length of the observation period; previous studies analyzed from 2 to a maximum of 9 yr of posttransplantation follow-up.9,12 Taking into account that it takes >5 yr (and probably up to 10 yr) to halt and reverse diabetic lesions,10,24 it is evident that differences in outcome between SPK and kidney transplantation alone can be evaluated in a valid manner only with >5 yr of posttransplantation follow-up. For the first time, we provide data on graft and patient survival for follow-up beyond the 10th posttransplantation year (for patients who received a transplant during the period from 1984 to 1990). A substantial benefit, in terms of both graft and patient survival, was found for SPK compared with DDK, and this benefit increased with the duration of posttransplantation follow-up. When comparing SPK with LDK, we found that an early graft and patient survival benefit of LDK was lost over time. With prolonged follow-up beyond 10 yr, SPK was found to be associated with significantly better patient survival compared with LDK. Our current data suggest that this survival benefit likely will apply also to patients who received a transplant from 1991 to 2000. The evolution of the survival curves indicates a similar trend as in the earlier observational period and suggests that the benefit of glycemic control conferred by a functioning pancreas will become apparent even earlier because of the better initial results of SPK in this second period.
CONCISE METHODS
Graft, death-censored graft, and patient survival rates were calculated according to the Kaplan-Meier method. To account for the potential influence of confounders, we conducted multivariate Cox regression analysis including the following covariates: Transplantation year; geographic region; recipient age, race, and gender; donor race and gender; type of immunosuppression; pretransplantation categorization of recipients by the transplant centers as “good risk for transplantation”; and pretransplantation categorization of recipients by the transplant centers as “high cardiovascular risk.” The covariates donor age, HLA mismatches, and cold ischemia time were consciously omitted because it would violate the principle of multivariate analysis to adjust for differences that are inherent a priori in these study populations. The follow-up period had to be partitioned into the intervals 2 to 5, 6 to 10, and 11 to 18 yr to meet the proportional hazards assumption of the Cox regression model. Differences among the patient groups with respect to the fraction of patients dying from cardiovascular death were analyzed by logistic regression including the same covariates as those used in the Cox regression model. The backstep elimination algorithm was used to exclude NS confounders. The software packages R (version 2.5; The R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) and SPSS (version 15.0; SPSS, Chicago, IL) were used. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
We thank the centers participating in the CTS for generous and selfless support.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Robertson RP, Kendall D, Teuscher A, Sutherland D: Long-term metabolic control with pancreatic transplantation. Transplant Proc 26: 386–387, 1994 [PubMed] [Google Scholar]
- 2.Robertson RP, Sutherland DE, Kendall DM, Teuscher AU, Gruessner RW, Gruessner A: Metabolic characterization of long-term successful pancreas transplants in type I diabetes. J Investig Med 44: 549–555, 1996 [PubMed] [Google Scholar]
- 3.Drognitz O, Benz S, Pfeffer F, Fischer C, Makowiec F, Schareck W, Hopt UT: Long-term follow-up of 78 simultaneous pancreas-kidney transplants at a single-center institution in Europe. Transplantation 78: 1802–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Meier-Kriesche HU, Hanson JA, Leichtman A, Magee JC, Cibrik D, Wolfe RA, Port FK, Agodoa L, Kaufman DB, Kaplan B: The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 71: 82–90, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Becker BN, Brazy PC, Becker YT, Odorico JS, Pintar TJ, Collins BH, Pirsch JD, Leverson GE, Heisey DM, Sollinger HW: Simultaneous pancreas-kidney transplantation reduces excess mortality in type 1 diabetic patients with end-stage renal disease. Kidney Int 57: 2129–2135, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bunnapradist S, Cho YW, Cecka JM, Wilkinson A, Danovitch GM: Kidney allograft and patient survival in type I diabetic recipients of cadaveric kidney alone versus simultaneous pancreas kidney transplants: A multivariate analysis of the UNOS database. J Am Soc Nephrol 14: 208–213, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Israni AK, Feldman HI, Propert KJ, Leonard M, Mange KC: Impact of simultaneous kidney-pancreas transplant and timing of transplant on kidney allograft survival. Am J Transplant 5: 374–382, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Smets YF, Westendorp RG, van der Pijl JW, de Charro FT, Ringers J, de Fijter JW, Lemkes HH: Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet 353: 1915–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Waki K, Terasaki PI: Kidney graft and patient survival with and without a simultaneous pancreas utilizing contralateral kidneys from the same donor. Diabetes Care 29: 1670–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Fioretto P, Mauer SM, Bilous RW, Goetz FC, Sutherland DE, Steffes MW: Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet 342: 1193–1196, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Reddy KS, Stablein D, Taranto S, Stratta RJ, Johnston TD, Waid TH, McKeown JW, Lucas BA, Ranjan D: Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 41: 464–470, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Robertson RP, Holohan TV, Genuth S: Therapeutic controversy: Pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 83: 1868–1874, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE: Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 231: 269–275, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.University of Heidelberg: Collaborative Transplant Study. Available at: http://www.ctstransplant.org. Accessed March 1, 2008
- 16.Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM: Effect of glycemic control on early diabetic renal lesions: A 5-year randomized controlled clinical trial of insulin-dependent diabetic kidney transplant recipients. JAMA 272: 600–606, 1994 [PubMed] [Google Scholar]
- 17.Bohman SO, Tyden G, Wilczek H, Lundgren G, Jaremko G, Gunnarsson R, Ostman J, Groth CG: Prevention of kidney graft diabetic nephropathy by pancreas transplantation in man. Diabetes 34: 306–308, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Hathaway DK, Abell T, Cardoso S, Hartwig MS, el Gebely S, Gaber AO: Improvement in autonomic and gastric function following pancreas-kidney versus kidney-alone transplantation and the correlation with quality of life. Transplantation 57: 816–822, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Allen RD, Al-Harbi IS, Morris JG, Clouston PD, O'Connell PJ, Chapman JR, Nankivell BJ: Diabetic neuropathy after pancreas transplantation: Determinants of recovery. Transplantation 63: 830–838, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Navarro X, Sutherland DE, Kennedy WR: Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol 42: 727–736, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Wicks MN, Hathaway DK, Shokouh-Amiri MH, Elmer DS, McCulley R, Burlew B, Gaber AO: Sustained improvement in cardiac function 24 months following pancreas-kidney transplant. Transplant Proc 30: 333–334, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Cheung AT, Perez RV, Chen PC: Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: A computer-assisted intravital microscopy study on the conjunctival microcirculation. Transplantation 68: 927–932, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Najarian JS, Kaufman DB, Fryd DS, McHugh L, Mauer SM, Ramsay RC, Kennedy WR, Navarro X, Goetz FC, Sutherland DE: Long-term survival following kidney transplantation in 100 type I diabetic patients. Transplantation 47: 106–113, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Fioretto P, Kim Y, Mauer M: Diabetic nephropathy as a model of reversibility of established renal lesions. Curr Opin Nephrol Hypertens 7: 489–494, 1998 [DOI] [PubMed] [Google Scholar]