Abstract
Serologic responses to T cell–dependent vaccinations are severely attenuated in patients with ESRD, but the reasons for this is unknown. In this study, a detailed analysis of antigen-specific T cell responses was performed. Patients on hemodialysis and age- and gender-matched healthy control subjects were vaccinated with hepatitis B surface antigen (HBsAg), antigen-specific CD4+ T cells were monitored at regular intervals with intracellular cytokine staining and proliferation assays. IL-2–and IFN-γ–producing CD4+ T cells were identified as either central or effector memory CD4+ T cells using antibodies directed against CD45RO and the chemokine receptor CCR7. Control subjects mounted a memory T cell response comprising both central and effector memory CD4+ T cells, with the central memory response occurring 1 wk before the effector memory response. IL-2+ HBsAg-specific memory CD4+ T cells were primarily detected within the effector population. Patients with ESRD showed a delayed response of IL-2–and IFN-γ–producing central memory CD4+ T cells, but their maximal responses were similar to those of control subjects. In contrast, patients with ESRD produced only 6.3% of the IL-2+ HBsAg-specific effector memory CD4+ T cells produced by control subjects (0.5 ± 0.2 × 104/L versus 8 ± 3.5 × 104/L; P < 0.001), and this impaired response correlated with antigen-specific T cell proliferation and anti-HBsAg IgG titers. In conclusion, the production of antigen-specific effector memory CD4+ T cells after vaccination, which is critical to achieve an adequate humoral response, is severely impaired in patients with ESRD.
Patients with ESRD show clinical signs of an immune defect characterized by an increased susceptibility for infections and a decreased immune response to T cell–dependent antigens (e.g., hepatitis B vaccination). The precise mechanisms responsible for this immune defect are unknown; however, changes in T cell subsets and/or function may contribute substantially to the impaired cellular immune response, because ESRD is associated with lymphopenia occurring in the B and T lymphocyte compartment.1–3 The available data on B cell function of patients with ESRD do not indicate an impaired function.4 The functional data on peripheral T cells in patients with ESRD are conflicting and obtained by using the unfractionated bulk of circulating T cells.1,3,5,6
Developments in T cell research and availability of various chemokine receptors, such as CCR7, have enabled a dissection of the T lymphocytes in different subsets.7,8 The major T cell compartments that can be distinguished are the naive T cells (Tnaive; antigen inexperienced) and the antigen experienced memory T cells. The memory compartment is further subdivided in the central memory T cells (Tcm), that home preferentially to lymphoid organs, and the effector memory T cells (Tem) that are able to react swiftly upon antigenic stimulation.7 After de novo antigen-specific stimulation, a linear development in T cell differentiation has been proposed, starting with antigen-specific Tnaive that develop into Tcm and finally Tem.7,9,10 Recently, we analyzed the composition of the circulating T cells in relation to renal function and showed a strong depletion of the Tnaive and CD4+ Tcm compartment.11,12
To gain further insight into the function of T cells in patients with uremia, it is necessary to identify the specific antigenic response on the single-cell level in the different T cell subsets. In addition, this response needs to be monitored in time, because kinetics may be different in healthy control subjects compared with patients with ESRD. Hepatitis B surface antigen (HBsAg) is a suitable antigen to use for such an analysis. The antibody response is T helper cell type 1 dependent,13 and intracellular IL-2 and IFN-γ production in the antigen-specific T cells can be reliably measured. In addition, patients with ESRD have a well-documented decreased responsiveness to the standard vaccination procedure.14,15 We postulated that a difference in the development of antigen-specific CD4+ T cells might underlie the impaired serologic response to HBsAg vaccination; therefore, in this study, we closely monitored the antigen-specific T cell response after vaccination for HBsAg and correlated our findings to the antibody titer obtained.
RESULTS
Kinetics of HBsAg-Specific Cytokine-Producing T Lymphocytes
In healthy control subjects, the maximal response (peak response) for IL-2+ T cells was observed in the Tnaive and Tcm subsets as early as 1 wk after the HBsAg booster and followed by a peak response at 2 wk in the Tem subset; however, delayed kinetics were observed for patients with ESRD, with the average peak responses in Tnaive, Tcm, and Tem subsets at 2 wk (Figure 1, A, C, E, and G). For IFN-γ antigen-specific T cells, the development in time differed from IL-2+ cells. In healthy control subjects, the peak response of IFN-γ+ HBsAg-specific T cells was at 2 wk in all T cell subsets. In addition, IFN-γ+ Tcm and Tnaive cells were not detectable in the circulation at 4 wk but reappeared at 12 wk. A similar profile was observed for patients with ESRD, with the exception of a delayed peak response at 4 wk in the Tcm subset (Figure 1, B, D, F, and H). The sequential development in time of IL-2+ and IFN-γ+ HBsAg-specific T cells within the defined T cell subsets was similar when expressed in total numbers of cells (data not shown).
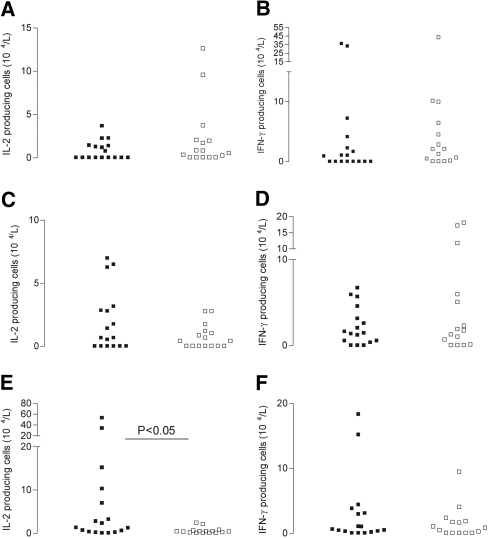
Figure 1.
Kinetics of HBsAg-specific IL-2–and IFN-γ–producing CD4+ T lymphocytes in the study population. PBMC were stimulated with αCD28 and αCD49d and HBsAg for 6 h in the presence of Brefeldin A for the last 5 h of incubation. Thereafter, the percentage of IL-2+ (A, C, E, and G) or IFN-γ+ (B, D, F, and H) cells was determined for the different T cell subsets. T cell subsets were defined by expression of cell surface markers CD4, CD45RO, and CCR7. The following subsets were defined Tnaive (CD4+CD45RO−CCR7+), Tcm cells (CD4+CD45RO+CCR7+), and Tem cells (CD4+CD45RO+CCR7−). ▪, Signals observed for healthy volunteers; □, signals observed for patients with ESRD. On the x axis, the various time intervals are depicted, and on the y axis, mean ± SEM percentages of HBsAg-specific cytokine-producing CD4+ (A and B), Tnaive (C and D), Tcm (E and F), and Tem (G and H) cells are displayed, respectively. *Significantly (P < 0.05) different from healthy volunteers.
Peak Responses and Absolute Numbers of HBsAg-Specific Cytokine-Producing T Lymphocytes
The peak response of HBsAg-specific T cells in every T cell subset was taken to compare the maximally achieved antigen-specific T cell responses after HBsAg vaccination for patients and control subjects. We previously showed that patients with ESRD differ from healthy control subjects in the absolute numbers and composition of their circulating T cell subsets.11 To compare the peak responses between patients and healthy control subjects, we took these observations into account and calculated absolute numbers of cytokine-producing cells. Of note, the average absolute number of total CD4+ T cells and differentiation within subsets did not significantly change during the study period in both patients and control subjects (data not shown). The average (±SEM) peak responses for patients with ESRD within the naive CD4+ compartment were not different from healthy volunteers and amounted to 2 ± 0.9 × 104/L and 6 ± 2.8 × 104/L for IL-2 (Figure 2A) and IFN-γ (Figure 2B), respectively. Also, no significant difference was observed when peak responses within the Tcm compartment were plotted. Numbers were 1 ± 0.2 × 104/L (patients with ESRD) versus 2 ± 0.6 × 104/L (healthy volunteers) and 4 ± 1.6 × 104/L versus 2 ± 0.5 × 104/L for IL-2 (Figure 2C) and IFN-γ (Figure 2D), respectively. Strikingly, decreased numbers were noticed for HBsAg-specific IL-2–producing (Figure 2E) Tem lymphocytes. Average numbers for HBsAg-specific IL-2–producing Tem cells amounted to 0.5 ± 0.2 × 104/L and 8 ± 3.5 × 104/L (P < 0.01) for patients with ESRD and healthy volunteers, respectively.
Figure 2.
Peak responses for HBsAg-specific IL-2–and IFN-γ–producing cells. Peak responses of absolute numbers of HBsAg-specific IL-2–and IFN-γ–producing naive (A and B), central memory (C and D), and effector memory (E and F) CD4+ T lymphocytes obtained within 4 wk after HBsAg vaccination. Absolute peak numbers of cells producing HBsAg-specific IL-2 and IFN-γ are displayed for healthy control subjects (▪) and patients with ESRD (□).
Polyclonal T Cell Stimulation
Peripheral blood mononuclear cells (PBMC) obtained at every point in time after vaccination were stimulated polyclonal with phytohemagglutinin (PHA). No significant changes in time were observed with respect to percentages of cytokine-positive cells. Significantly increased percentages of IL-2–producing cells were observed for patients with ESRD compared with healthy control subjects within the total CD4+, in particular the memory compartment (Figure 3A). Percentages of PHA-induced IFN-γ–producing cells were significantly higher in the total CD4+ compartment of patients with ESRD (Figure 3B).
Figure 3.
(A and B) PHA-induced IL-2–producing (A) and IFN-γ–producing (B) CD4+ T lymphocytes in study population. Similar as described in Figure 1, percentages of IL-2–producing (A) and IFN-γ–producing CD4+ T cells induced by PHA were determined. On the y axis, the percentages of cytokine producing cells (mean ± SEM) are displayed and on the x axis, the different CD4+ T cell subsets. ▪, signals observed for healthy volunteers; □, signals for patients with ESRD. *Significantly (P < 0.05) different from healthy volunteers.
Cytokine Production after Re-stimulation of HBsAg-Specific T Cells
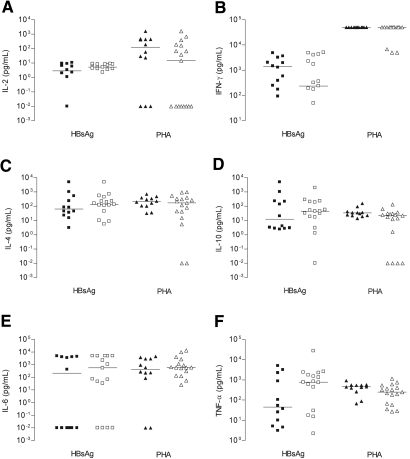
Type 2 cytokines, such as IL-4 and IL-10, were detected in both healthy volunteers and patients with ESRD at low levels compared with IFN-γ, confirming our observations with intracellular cytokine staining. IL-2 was detected at low levels, which is most likely caused by autocrine consumption by proliferating T cells. No significant differences were observed between patients and healthy controls (Figure 4).
Figure 4.
Levels of HBsAg-specific cytokines produced after re-stimulation. PBMC, obtained 2 wk after receipt of the last HBV booster vaccination, were stimulated with HBsAg (5 μg/ml; squares) or polyclonal with PHA (1 μg/ml; triangles). At day 6, the cells were re-stimulated for 48 h using fresh PBMC (1 × 105) with or without the various stimuli. The closed symbols represent healthy control subjects, and the open symbols represent the patients with ESRD.
HBsAg-Specific T Cell Proliferation
HBsAg-specific proliferation was significantly decreased when compared with healthy volunteers, but the maximal responses were observed at the same time point (i.e., 2 wk upon receipt of the last HBsAg vaccination; Figure 5). PHA-induced proliferation, expressed as stimulation indices, was not different (77.8 ± 12.8 for patients with ESRD versus 54 ± 8 for healthy individuals). HBsAg-specific proliferation was related only to responses observed for Tem CD4+ T cells (the Spearman rank correlation coefficient amounted to 0.613 [P < 0.01] and 0.425 [P < 0.05] for percentage of IL-2 and IFN-γ effector memory T lymphocytes, respectively).
Figure 5.
Kinetics of HBsAg-specific proliferation. At the various intervals, freshly isolated PBMC were stimulated with medium alone and HBsAg (5 μg/ml) for 6 d. At day 6, proliferation was determined by 3H-thymidine incorporation and is expressed as counts per minute (cpm). Subsequently, stimulation indices (SI) were calculated by dividing the HBsAg-induced proliferation by that induced by culture medium alone. On the x axis, various time intervals after the last HBV vaccination are depicted and on the y axis, proliferation expressed as SI. ▪, Responses for healthy volunteers; □, responses for patients with ESRD. *Significantly (P < 0.05) different from healthy volunteers.
Antibody Titers and Relation with Development of HBsAg-Specific T Cells
Average anti-HBsAg IgG titers amounted to 172 ± 61 IU/L for patients with ESRD and 33,936 ± 16,446 IU/L for healthy volunteers. Six of the 16 patients with ESRD did not acquire adequate antibody titers, and five patients achieved titers between 10 and 100 IU/L (Figure 6). These titers were related exclusively to the peak responses of IL-2+ HBsAg-specific Tem, expressed both as percentages (Figure 7A) of the total number of Tem and in absolute numbers (Figure 7B). Such a relation was observed within the group of patients and healthy control subjects separately and when combined. Anti-HBsAg IgG titers also correlated with the maximum antigen-specific proliferative response (R = 0.455, P = 0.015) obtained after vaccination.
Figure 6.
Anti-HBsAg titers. Four weeks after receipt of the last HBV vaccination, anti-HBsAg titers were measured. On the y axis, anti-HBs titers (IU/L) are depicted, and on the x axis, the healthy volunteers (▪) and patients with ESRD (□). The dotted lines represent the <10 IU/L (nonresponders) and >100 IU/L (good responders).
Figure 7.
(A and B) Peak responses for both percentages (A) and absolute numbers (B) of HBsAg-specific IL-2 effector memory CD4+ T cells correlate with anti-HBsAg titers. Peak responses for HBsAg-specific percentages and absolute numbers of IL-2–producing effector memory CD4+ T cells are associated with anti-HBsAg titers measured 4 wk after the last HBV vaccination. On the x axis, anti-HBs titers (IU/L) are depicted, and on the y axis, percentages (A) and absolute numbers (B) of HBsAg-specific IL-2–producing effector memory CD4+ T cells are displayed. ▪, Responses for healthy volunteers; □, responses for patients with ESRD. Spearman rank correlation was calculated using SPSS for Windows.
DISCUSSION
In this study, we analyzed the development and characteristics of vaccination-induced HBsAg-specific CD4+ T cells in patients with ESRD. The major observations include a delayed development of antigen-specific cells in the Tcm subset, defective or almost absent generation of antigen-specific IL-2 Tem, and decreased HBsAg-specific T cell proliferation.
The generation of IL-2+ HBsAg-specific T cells supports the hypothesis of a linear development in time of antigen-experienced T cells, with the peak of Tcm preceding the peak in Tem response. Patients with ESRD showed a similar sequence in time as healthy control subjects, but a delayed development of antigen-specific cells in the central memory compartment was observed. Development of antigen-specific memory T cells after vaccination involves many steps of which the underlying mechanisms are only partly unraveled. Antigen within the peripheral tissue needs to be delivered to the draining lymph node by dendritic cells and presented to antigen-specific T cells. These cells must reach the T cell–dependent areas within the lymph node by chemokine receptor–mediated trafficking, proliferate, and thereafter start recirculating again. All steps in this process may be affected in patients with ESRD but are poorly studied in humans thus far. Because the number of peripheral dendritic cells is decreased in patients with ESRD,16–18 one may speculate that the number of antigen-loaded dendritic cells migrating to the draining lymph nodes is decreased. Also, chemokine receptor expression on circulating T cells is changed in patients with ESRD, which may have functional consequences11; however, functional data on chemokine receptor–mediated migration are not available.
The most striking finding was the poor development of IL-2+ Tem in patients with ESRD, which was strongly associated with a low serologic response to HBsAg. Although the development of IFN-γ+ HBsAg-specific T cells in patients was different from that in healthy control subjects, no association was found between IFN-γ+ T cell development and serologic response. The unique relation between IL-2+ Tem and antibody titer was observed in healthy control subjects and patients, with patients with ESRD at the lower end of a seemingly continuous curve. This indicates that the generation of IL-2+ Tem is directly related to and possibly pivotal for the development of IgG-secreting plasma cells from antigen-specific B cells. A functional role of IL-2 secreted by antigen-specific T cells seems plausible, because IL-2 has been identified as an important cytokine in the final differentiation pathway of B cells into antibody secreting cells. The majority of memory B cells express the IL-2 receptor,19 and late in a primary immune response, the IL-2 secreted by antigen-activated T helper cells binds to high-affinity IL-2 receptors on antigen-activated B cells.20 IL-2 then acts on two crucial regulators of B cell development, the Pax5 gene–encoded transcription factor B cell lineage–specific activator protein and Blimp-1.21,22 Blimp-1 is a member of the zinc finger–containing family of DNA-binding proteins and is the master switch for the terminal differentiation of B cells into plasma cells.23 In accordance with these findings, the presence of T cells can be replaced by exogenous IL-2 in an in vitro model of antigen-specific T cell–dependent differentiation of B cells to plasma cells.24
Remarkably, recent data suggest that after vaccination for other antigens such as HIV (peptide pool to envelope clade A) and tetanus toxoid, the dominant response also consisted of IL-2+ memory T cells13; therefore, development of an IL-2 T cell memory response seems necessary for an adequate humoral response to vaccination. More specific, our data indicate that the generation of IL-2–producing Tem is a condition sine qua non for a serologic response after vaccination. This finding sheds new light on the observation in the past that administration of IL-2 is able to enhance the response to hepatitis B virus (HBV) vaccination in patients with ESRD.25
At this point, we can only speculate why the development of HBsAg-specific IL-2 Tem is so disturbed in patients with ESRD. In general, generation of memory T cells involves cytokines (e.g., IL-7, IL-15) and efficient stimulation by antigen-presenting cells. As stated before, the latter function may be negatively affected, and we observed lower serum concentrations of IL-7 in patients with ESRD.11 Of particular relevance to our data is a study that points to an important role for the co-stimulatory pathway B7-CD28. Blocking of this pathway with CTLA4-Ig in a mouse model of T cell stimulation by recall antigen resulted in absent generation of antigen-specific Tem but without affecting the Tcm response.26 In accordance with our findings, the IL-2 and not the IFN-γ response was almost exclusively affected. We recently described impaired maturation of monocyte-derived dendritic cells of patients with ESRD,27 which resulted in decreased expression of the B7 molecules (CD80 and CD86). Taken together, these findings suggest that dysfunctional dendritic cells in patients with ESRD may contribute to the selective absence of IL-2 antigen-specific Tem after vaccination. B lymphocytes might also be considered as a source of antigen-presenting cells, but whereas its intrinsic function does not seem to be impaired,28 the numbers of B lymphocytes present in the circulation are significantly decreased in patients with ESRD.11
The finding of normal and even increased cytokine production by T cell subsets after polyclonal stimulation is important. It shows that T cells from patients with ESRD are able to secrete cytokines to a similar extent as in healthy control subjects.
Finally, HBsAg -specific T cell proliferation was decreased in patients with ESRD, which can in part be explained by the low numbers of circulating IL-2+ Tem. This also explains the relation found between anti-HBsAg titer and HBsAg-specific T cell proliferation. Consistent with our previous findings,29 we observed normal polyclonal T cell proliferation in patients with ESRD, indicating no intrinsic defect in proliferative capacity.
In summary, this is the first article ascribing the aberrant humoral immune response to HBsAg in patients with ESRD to the defective development of antigen-specific IL-2–producing Tem. It is difficult to apply these findings to other antigens because we have studied only HBV vaccination; however, the same problems might also underlie the defective antibody response to other vaccination antigens, such as diphtheria and tetanus.30 Understanding the factors that are important for the development of IL-2–producing Tem may lead to improvement of current vaccination protocols.
CONCISE METHODS
Study Population
Sixteen patients who had ESRD and were receiving long-term intermittent hemodialysis and 17 age- and gender-matched healthy control subjects were included in the study (Table 1). Patients were vaccinated against HBV using the standard vaccination protocol with recombinant hepatitis B vaccine (HBVAXPRO; Sanofi Pasteur MSD NV, Amsterdam, Netherlands).31,32 Patients who used immunosuppressive agents, had autoimmune disease, had undergone kidney transplantation, or were positive for HIV and/or hepatitis C virus were excluded from the study. Healthy volunteers were vaccinated using a three-step (0, 1, and 6 mo) vaccination with only half of the dosage used for patients with ESRD per injection.
Table 1.
Study population characteristics
| Characteristic | Healthy Volunteers | Patients with ESRD |
|---|---|---|
| n | 17 | 16 |
| Gender (M/F) | 7/10 | 7/9 |
| Age (yr; mean ± SEM) | 43 ± 3.4 | 51 ± 3.2 |
| Primary kidney disease | ||
| diabetic nephropathy | 5 | |
| glomerulonephropathy | – | |
| hypertensive nephropathy | 3 | |
| polycystic kidney disease | 2 | |
| other | 6 |
Study Protocol
This study was approved by the Medical Ethical Committee of the Erasmus Medical Center (Rotterdam, Netherlands; Medical Ethical Committee no. 2005-265). It was conducted according to the principles of the Declaration of Helsinki and in compliance with International Conference on Harmonization/Good Clinical Practice regulations. Patients were followed up to 12 wk upon receipt of their last HBV vaccination step. At each interval (before and 1, 2, 4, and 12 wk after receiving the last booster), blood was drawn at the start of the hemodialysis procedure. PBMC were isolated, and plasma was stored at −20°C until analysis.
Isolation of PBMC
PBMC were isolated from 35 ml of heparinized venous blood samples27 and resuspended in RPMI 1640 (Gibco BRL, Paisley, Scotland) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 10% heat-inactivated AB+ pooled human serum (hereafter referred to as standard culture medium).
Intracellular Cytokine Staining
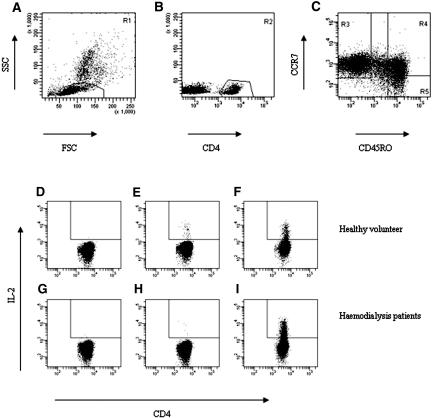
Intracellular cytokine staining was performed using co-stimulation alone (mixture of 1 μg/ml anti-CD28 and 1 μg/ml anti-CD49d) or in combination with a final concentration of 5 μg/ml HBsAg (Fitzgerald Industries Int., Concord, MA) or 1 μg/ml PHA (Murex Biotech Ltd., Kent, England) to stimulate PBMC.13,33 After permeabilization, cells were stained with either FITC-labeled IL-2 or FITC-labeled IFN-γ, and percentages of cytokine-positive cells were determined by analyzing the samples on the FACS Calibur (BD, Erebodegem, Belgium), selecting cells that have a typical lymphocyte scatter pattern and are CD4+ (Figure 8, A and B). Antigen-specific T cells were characterized by staining the cell surface with peridinin chlorophyll protein–labeled anti-CD4, allophycocyanin-labeled CD45RO (both from Becton Dickinson, Erebodegem, Belgium), and phycoerythrin-labeled CCR7 (R&D Systems Europe Ltd., Abingdon, UK) to dissect the different CD4+ T cell subsets.11 The different CD4+ T cell subsets (CCR7+CD45RO− [Tnaive], CCR7+CD45RO+ [Tcm], and CCR7−CD45RO+ [Tem]) were analyzed as shown in Figure 8C and described previously.11 A typical example of percentages of IL-2–producing CD4+ Tem cells is shown in Figure 8, D through I. HBsAg-specific responses could not be detected in the CD4+ T cell subsets of patients and control subjects who were not vaccinated with HBsAg (data not shown).
Figure 8.
Typical example of flowcytometric analysis of IL-2–producing Tem of healthy volunteer (D through F) and a hemodialysis patient (G through I) 2 wk after the last booster of HBV vaccination. A typical example of the flowcytometric analysis of cytokine-producing T cells. (A and B) The lymphocyte population (A) was gated first (R1), and subsequently the CD4+ T lymphocytes (R2) were gated within these lymphocytes (B). (C) Next, we identified the proportions of CD4+ T lymphocytes (R1+R2) in the various subsets using a dotplot of CCR7 versus CD45RO. Naive CD4+ T cells are displayed in region 3; central memory CD4+ T cells in region 4 and region 5 displays the effector memory CD4+ T population. (D through I) Next, the IL-2 signals observed for CD4+ Tem in presence of co-stimulation only (D and G), co-stimulation with HBsAg (E and H), and co-stimulation with PHA (F and I) are plotted for a healthy volunteer and a hemodialysis patient 2 wk after the last HBV vaccination. Percentages represent IL-2–producing cells.
Proliferation (3H-Thymidine Incorporation)
To determine antigen-specific proliferation, we used the protocol described by Verkade et al.27 Results are presented as stimulation indices, calculated by dividing the proliferation (in counts per minute) induced by the stimulus by that observed in absence of the stimulus (in counts per minute).
Antigen-Specific Cytokine Production
Two weeks after receiving the last vaccination for HBV, 1 × 105 PBMC/100 μl in a round bottom–shaped 96-well plate were stimulated for 6 d in the presence of recombinant HBsAg (5 μg/ml), PHA (1 μg/ml), or standard culture medium alone. On day 6, the supernatant was discarded and cells were re-stimulated by adding fresh autologous PBMC (1 × 105), with or without the same stimuli as used in the primary culture. Supernatants were collected 48 h after re-stimulation, and the levels of IL-2, -4, -6, and -10; IFN-γ; and TNF-α were determined by flowcytometry (cytometric bead array; BD).
Anti-HBsAg Titer
Anti-HBsAg IgG titers were determined 4 wk after receipt of last vaccination in plasma using the Enzyme Immuno Assay (AxSym; Abbott Diagnostics, Hoofddorp, Netherlands) according to the manufacturer's instructions.
Statistical Analysis
Statistical analyses were performed using SPSS 11.5 for Windows (SPSS, Chicago, IL). Data are mean ± SEM unless indicated otherwise. Comparison between patients with ESRD and healthy control subjects was done using the nonparametric Mann-Whitney U test. For testing whether relations between the different parameters exist, the nonparametric Spearman rank correlation was determined. P ≤ 0.05 were considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was financed by the Dutch Kidney Foundation (grant 1630008) and the Malpighi Foundation.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Kurz P, Kohler H, Meuer S, Hutteroth T, Meyer zum Buschenfelde KH: Impaired cellular immune responses in chronic renal failure: Evidence for a T cell defect. Kidney Int 29: 1209–1214, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Quadracci LJ, Ringden O, Krzymanski M: The effect of uremia and transplantation on lymphocyte subpopulations. Kidney Int 10: 179–184, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Raska K Jr, Raskova J, Shea SM, Frankel RM, Wood RH, Lifter J, Ghobrial I, Eisinger RP, Homer L: T cell subsets and cellular immunity in end-stage renal disease. Am J Med 75: 734–740, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy G, Kher V, Naik S: Low response to HBsAg vaccine in chronic renal failure patients is not due to intrinsic defect of B cells. Scand J Urol Nephrol 36: 377–382, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Libetta C, Rampino T, Dal Canton A: Polarization of T-helper lymphocytes toward the Th2 phenotype in uremic patients. Am J Kidney Dis 38: 286–295, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Sester U, Sester M, Hauk M, Kaul H, Kohler H, Girndt M: T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transplant 15: 1217–1223, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Geginat J, Lanzavecchia A: Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22: 745–763, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A: Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol 251: 167–171, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Seder RA, Ahmed R: Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 4: 835–842, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Litjens NH, van Druningen CJ, Betjes MG: Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol 118: 83–91, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND: Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int 70: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- 13.De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M: Vaccination in humans generates broad T cell cytokine responses. J Immunol 173: 5372–5380, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Crosnier J, Jungers P, Courouce AM, Laplanche A, Benhamou E, Degos F, Lacour B, Prunet P, Cerisier Y, Guesry P: Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: II, Haemodialysis patients. Lancet 1: 797–800, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Stevens CE, Alter HJ, Taylor PE, Zang EA, Harley EJ, Szmuness W: Hepatitis B vaccine in patients receiving hemodialysis. Immunogenicity and efficacy. N Engl J Med 311: 496–501, 1984 [DOI] [PubMed] [Google Scholar]
- 16.McKerrow KJ, Hawthorn RJ, Thompson W: An investigation of circulating and in situ lymphocyte subsets and Langerhans cells in the skin and cervix of patients with chronic renal failure. Br J Dermatol 120: 745–755, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Mettang T, Fritz P, Weber J, Machleidt C, Hubel E, Kiefer T, Kuhlmann U: Epidermal Langerhans cells in uremic patients on hemodialysis or continuous ambulatory peritoneal dialysis. Nephron 65: 278–283, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Hesselink DA, Betjes MG, Verkade MA, Athanassopoulos P, Baan CC, Weimar W: The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol Dial Transplant 20: 1868–1873, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi K, Cohen DI, Blackman M, Nielsen E, Ohara J, Hamaoka T, Koshland ME, Paul WE: Ig RNA expression in normal B cells stimulated with anti-IgM antibody and T cell-derived growth and differentiation factors. J Exp Med 160: 1736–1751, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinkenberger JL, Wallin JJ, Johnson KW, Koshland ME: An interleukin-2 signal relieves BSAP (Pax5)-mediated repression of the immunoglobulin J chain gene. Immunity 5: 377–386, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Turner CA Jr, Mack DH, Davis MM: Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 77: 297–306, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Kallies A, Nutt SL: Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol 19: 156–162, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Amu S, Tarkowski A, Dorner T, Bokarewa M, Brisslert M: The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scand J Immunol 66: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Bergamin F, Vincent IE, Summerfield A, McCullough KC: Essential role of antigen-presenting cell-derived BAFF for antibody responses. Eur J Immunol 37: 3122–3130, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Meuer SC, Dumann H, Meyer zum Buschenfelde KH, Kohler H: Low-dose interleukin-2 induces systemic immune responses against HBsAg in immunodeficient non-responders to hepatitis B vaccination. Lancet 1: 15–18, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL: Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol 177: 7698–7706, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Verkade MA, van Druningen CJ, Vaessen LM, Hesselink DA, Weimar W, Betjes MG: Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol Dial Transplant 22: 128–138, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Girndt M, Sester M, Sester U, Kaul H, Kohler H: Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl 78: 206–211, 2001 [DOI] [PubMed] [Google Scholar]
- 29.van Riemsdijk IC, Baan CC, Loonen EH, Knoop CJ, Navarro Betonico G, Niesters HG, Zietse R, Weimar W: T cells activate the tumor necrosis factor-alpha system during hemodialysis, resulting in tachyphylaxis. Kidney Int 59: 883–892, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Girndt M, Pietsch M, Kohler H: Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis 26: 454–460, 1995 [DOI] [PubMed] [Google Scholar]
- 31.DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, Kiaii M, Taylor PA, Levin A: Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: Earlier is better. Am J Kidney Dis 42: 1184–1192, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Propst T, Propst A, Lhotta K, Vogel W, Konig P: Reinforced intradermal hepatitis B vaccination in hemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. Am J Kidney Dis 32: 1041–1045, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Litjens NH, Huisman M, Baan CC, van Druningen CJ, Betjes MG: Hepatitis B vaccine-specific CD4(+) T cells can be detected and characterised at the single cell level: Limited usefulness of dendritic cells as signal enhancers. J Immunol Methods 330: 1–11, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.