Abstract
Hemoglobin (Hb) serves as the main oxygen transporter in erythrocytes, but it is also expressed in nonhematopoietic organs, where it serves an unknown function. In this study, microarray and proteomic analyses demonstrated Hb expression in the kidney. Rat kidneys were perfused extensively with saline, and glomeruli were isolated by several techniques (sieving, manual dissection, and laser capture-microdissection). Reverse transcriptase–PCR revealed glomerular α- and β-globin expression, and immunoblotting demonstrated expression of the protein. In situ hybridization studies showed expression of the globin subunits in the mesangium, and immunostaining confirmed this localization of Hb. Furthermore, globin mRNA expression was detected in primary cultures of rat mesangial cells but not in cultured glomerular endothelial or epithelial cells. For investigation of Hb function in mesangial cells, the SV40-MES13 murine mesangial cell line was transfected with a vector expressing α- and β-globins; this overexpression reduced production of hydrogen peroxide–induced intracellular radical oxygen species and enhanced cell viability against oxidative stress. In summary, Hb is expressed by rat mesangial cells, and its potential functions may include antioxidative defense.
Hemoglobin (Hb) facilitates the transport of oxygen in the erythrocytes of the circulatory system. The Hb molecule is an assembly of four globular protein subunits. In adult humans, the most common type is Hb A, consisting of two α and two β subunits noncovalently bound (α2β2). Each subunit has a molecular weight of approximately 17,000 Da, for a total molecular weight of the tetramer of approximately 68,000 Da.1 Myoglobin is another member of the conventional globin family. Myoglobin stores oxygen temporally and enhances oxygen diffusion to the mitochondria in the cardiac and striated myocytes.2
Although blood flow to the kidney is high, the presence of oxygen shunt diffusion between arterial and venous vessels that run in close parallel contact keeps renal tissue oxygen tensions comparatively low. As a consequence, the kidney is very sensitive to changes in oxygen delivery. Thus, chronic hypoxia of the kidney as a final common pathway in end-stage kidney injury has been the focus of intensive research.3,4 To investigate pathomechanisms of chronically hypoxic kidneys, we studied expression profiles of genes and proteins. To our surprise, we observed upregulation of Hb in the hypoxic kidney.
In this study, we applied multiple methods, such as reverse transcriptase–PCR (RT-PCR), immunoblotting, in situ hybridization, and immunohistochemical analysis, to confirm Hb expression in the normal rat kidney. Interestingly, we found that Hb in the rat kidney was localized to glomerular mesangial cells, although the tubulointerstitium, not the glomerulus, is presumably a main target of chronic hypoxia injury. To clarify a biologic role of Hb in mesangial cells, we performed in vitro overexpression studies.
RESULTS
Hb Expression Was Detected in the Kidney
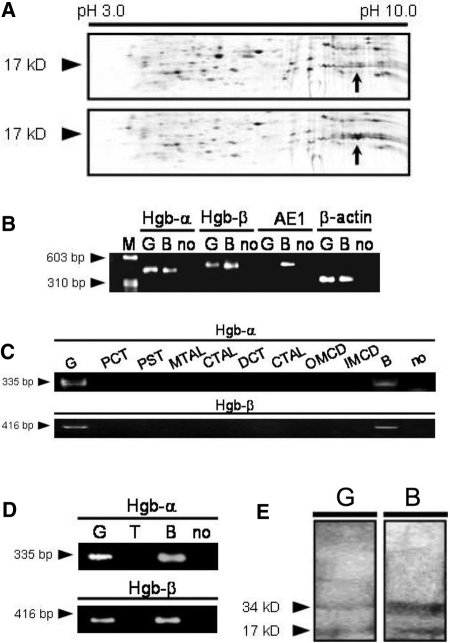
To study expression profiles of genes and proteins in the kidney under normoxic and hypoxic conditions, we induced chronic hypoxia in the rat kidney by constricting the left main renal artery via a U-shaped silver clip for 7 d and performed microarray analysis and proteomic studies with the kidneys. Our microarray analysis revealed expression of rat α- and β-globin genes, which was transiently upregulated during chronic hypoxia of the kidney (Table 1). Our proteomic studies also identified β-globin in the kidney, which showed a 6.42-fold increase in the kidney rendered to chronic hypoxia (Figure 1A). It should be noted that we tried to avoid contamination of blood by careful perfusion with saline at the harvest in these experiments.
Table 1.
Microarray analysis for the rat kidney under chronic hypoxia
| Gene (Affymetrix Gene No.) | Control | Day 3 | Day 7 |
|---|---|---|---|
| Hb α1 (AI577319) | 814.5 | 1699.5 | 362.0 |
| Hb α1 (AI179404) | 1849.0 | 2588.0 | 1622.0 |
| Hb α1 (AI237401) | 209.0 | 283.5 | 162.5 |
| Hb β (NM_033234) | 813.5 | 2100.0 | 756.5 |
| Hb β (X05080) | 142.5 | 572.0 | 106.5 |
| Hb β (BI287300) | 222.5 | 1026.5 | 153.5 |
Figure 1.
Expression of globin in the rat kidney glomeruli. (A) Two-dimensional differential in gel electrophoresis of proteins sampled from chronic hypoxia kidney was analyzed. The arrows represent one of the spots that were increased by hypoxia with respect to the control rat group with sham operation (top) and the rat group with renal artery stenosis for 7 d (bottom). Subsequent proteomic studies identified a significant increase in β-globin in the kidney rendered to chronic hypoxia. (B) Expression of globins in the normal rat glomeruli obtained by sieving was confirmed by RT-PCR. Arrowheads indicate the predicted size of the PCR product by each primer pair for cDNA (not genomic DNA). Sequence-specific primers: Hb-α, rat α-globin (429 bp); Hb-β, rat β-globin (444 bp); AE1, mouse/rat erythrocyte anion exchanger (467 bp); β-actin (306 bp). (C and D) Furthermore, expression of globins in the manually dissected (C) and the laser capture–microdissected (D) rat nephron segments was confirmed by RT-PCR. Arrowheads indicate the predicted size of the PCR product by each primer pair for cDNA (not genomic DNA). Sequence-specific primers: Hb-α, rat α-globin (335 bp); Hb-β, rat β-globin (416 bp). (E) Immunoblot analyses for the detection of Hb in isolated normal rat glomeruli (80 μg of total protein) confirmed Hb protein expression. Rat blood lysate served as a positive control. M, marker, ϕX174/HaeIII; G, glomeruli; B, blood; no, no template; PCT, proximal convoluted tubule; PST, proximal straight tubule; MTAL, medullary thick ascending limb; CTAL, cortical thick ascending limb; DCT, distal convoluted tubule; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct; T, tubules.
Hb Is Expressed in Glomeruli of the Normal Rat Kidney
To confirm Hb expression and determine its localization in the normal rat kidney, we isolated rat glomeruli by the conventional sieving method after intensive in situ renal perfusion with saline and performed RT-PCR to evaluate the expression of the α- and β-globin genes. PCR products corresponding to α- and β-globin, the globin mRNA expressed by erythroid precursors in adult mammals, were found in isolated glomeruli (Figure 1B). To confirm purity of glomeruli and lack of contaminating erythroid cells obtained by the sieving, we also performed RT-PCR of an abundant-in-erythrocyte gene, AE1,5,6 and showed that glomeruli did not express AE1. The absence of the PCR products generated by primers specific for the AE1 gene transcript strongly suggested that globin gene expression in rat glomeruli was not the result of erythroid cell contamination. In addition, the PCR products were subjected to DNA sequencing to verify that they were indeed the specific amplified products of globin gene transcripts. The sequencing results were compared with those in GenBank using NCBI BLAST nucleotide engine and showed complete matching with the full length of the corresponding globin mRNA (data not shown).
The hand-dissected method (microdissection) has been established to isolate structures of interest from the kidney with cellular heterogeneity. We performed RT-PCR using cDNA obtained from manually dissected nephron compartments of glomeruli and every tubular segment and confirmed expression of the globin genes only in glomeruli (Figure 1C). The glomeruli showed no expression of AE1 mRNA (data not shown), excluding a possibility of intraglomerular erythrocyte contamination. We also used laser capture microdissection, which has been developed to allow effective and precise isolation of structures, including glomerular tuft, from kidney sections.7 We examined gene expression by RT-PCR in rat glomeruli and tubules sampled with this technique and obtained the same results (Figure 1D).
Immunoblotting was performed to determine whether Hb subunits are expressed by rat glomeruli at the protein level. Using polyclonal antibodies to Hb, Hb protein was detected in lysates from glomeruli isolated from saline-perfused normal rat kidneys by the differential sieving (Figure 1E). Two bands observed at approximately 17,000 and 34,000 Da presumably consisted of monomer and heterodimer of globins, respectively. Not surprisingly, much higher levels of globins were detected in rat blood lysate compared with glomeruli lysate.
In Situ Hybridization Studies Revealed Hb Expression in the Mesangium
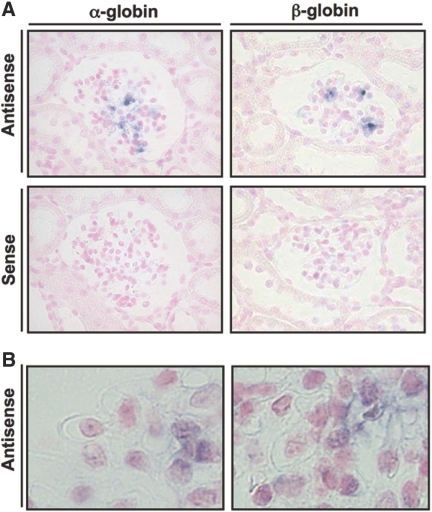
To refine the localization of Hb expression in rat glomeruli, we performed in situ hybridization studies using antisense RNA probe specific for rat α- and β-globin subunits (Figure 2A). Higher magnification images demonstrated that each globin signal was localized to the mesangial region (Figure 2B). As described in the previous section, the rat kidney was perfused intensively to exclude contamination of erythrocytes strictly before fixation and sectioning. These results of in situ hybridization studies with each globin subunit and control probes suggested that globin genes were expressed in rat glomeruli, especially in the mesangial region.
Figure 2.
In situ hybridization studies showed globin subunit mRNA expression in the mesangial region. (A) Hybridization using antisense probe evidenced expression of α- and β-globin genes in rat glomeruli. Of note, the signals were localized in the mesangial region. Specificity of hybridization was confirmed using sense probes, which resulted in complete lack of signal of both globins. (B) Higher magnification images showed localization of these antisense signals of each globin to the cytosol of mesangial cells. Magnifications: ×400 in A; ×600 in B.
Immunofluorescence Analysis Revealed Hb Expression in the Mesangium
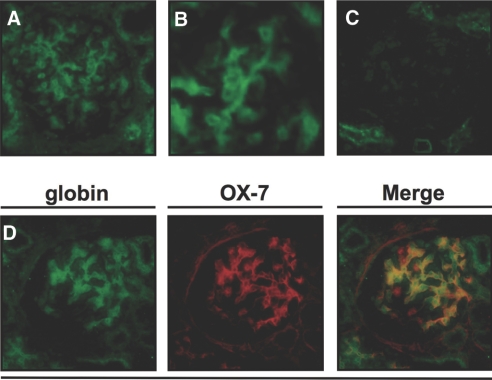
To confirm localization of Hb in the normal rat kidney at the protein level, we performed immunofluorescence studies of saline-perfused rat kidneys with antibody to Hb and revealed that Hb was expressed in the mesangial region (Figure 3A). Higher magnification image confirmed the immunostaining of Hb in mesangial cells (Figure 3B). Omission of the primary antibody in the same immunofluorescence system served as a negative control (Figure 3C). Double-immunofluorescence analysis showed that Hb staining pattern was co-localized with that of OX-7, a marker of rat mesangial cells (Figure 3D). The results indicated that Hb protein was localized in glomerular mesangial cells but not in the other glomerular cell types.
Figure 3.
Double-immunofluorescence studies of rat kidney showed localization of Hb protein in mesangial region. (A) Immunofluorescence of the normal rat kidney with antibody to Hb was observed in the glomerular mesangial region in green. (B) Higher magnification image showed immunofluorescence in mesangial cells. (C) Immunofluorescence without primary antibody revealed negligible signals in the glomerulus. (D) Immunofluorescence of the normal rat kidney with antibody to α-globin subunit also showed some glomerular cells in green. Double immunostaining with OX-7, a marker of rat mesangial cells, demonstrated that Hb was expressed by mesangial cells. Magnifications: ×400 in A, C, and D; ×600 in B.
Hb mRNA Is Expressed by Primary Cultured Rat Mesangial Cells
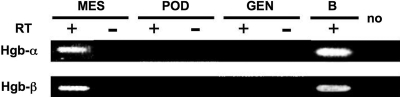
To verify our in vivo findings of Hb expression in rat mesangial cells, we performed in vitro studies using several kinds of cultured rat glomerular cells. Primary cultured rat mesangial cells expressed α- and β-globin mRNA, but rat glomerular endothelial or epithelial cell did not (Figure 4). Interestingly, the primary cultured mesangial cells showed gene expression of Hb mRNA only from passages 2 to 4 (data not shown).
Figure 4.
Rat cultured mesangial cells express globin subunit mRNA. Expression of globins in several cultured rat glomerular cells, such as primary cultured rat mesangial cells, podocytes, and endothelial cells, was confirmed by RT-PCR. MES, mesangial cells; POD, podocytes; GEN, endothelial cells; B, rat blood. Sequence-specific primers: Hb-α, rat α-globin (335 bp); Hb-β, rat β-globin (416 bp).
Next, we investigated whether stimuli associated with chronic hypoxia could upregulate globin gene expression in mesangial cells in vitro. After primary cultured rat mesangial cells were subjected to anoxia (in oxygen of 0.2%) or cultured with angiotensin II (AngII) of 10 μM or hydrogen peroxide (H2O2) of 100 μM for 12 h, expression of each globin gene was evaluated by quantitative RT-PCR. Fold changes of α- and β-globin mRNA levels compared with control were 1.06 ± 0.12- (P = 0.84) and 1.77 ± 0.43-fold (P = 0.20) in anoxia, 2.07 ± 0.49- (P = 0.12) and 1.78 ± 0.20-fold (P = 0.16) when exposed to AngII, and 3.16 ± 2.23- (P = 0.37) and 1.02 ± 0.54-fold (P = 0.98) when exposed to H2O2, respectively (n = 5 for each, representative results of four independent experiments). Incubation in anoxia or with AngII at the same concentration for 24 h produced essentially the same results (data not shown).
Overexpression of Hb in Mesangial Cells Ameliorated Oxidative Stress
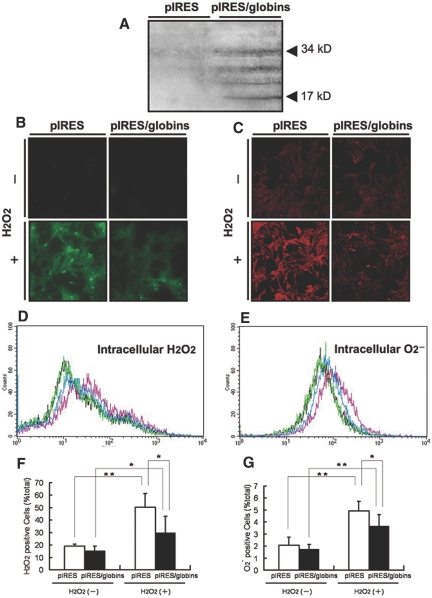
Hb acts as tetramer of two different subunits. To investigate a biologic role of Hb in mesangial cells, we overexpressed two subunits in cultured cells. Our immunoblotting analysis confirmed overexpression of α- and β-globin in a murine mesangial cell line, SV40-MES13, by transient transfection with this construct (Figure 5A).
Figure 5.
Transfection of murine mesangial cells with the globin-expressing vector suppressed intracellular ROS generation. (A) The construct pIRES/α-globin/β-globin was designed to express effectively both α- and β-globin subunits in transfected cells. Immunoblotting with rabbit polyclonal anti-Hb antibody identified overexpressed globin protein in lysate of murine mesangial cells, SV40-MES13, transfected with this vector. Control and Hb-overexpressing cells were stained with fluorogenic probes to detect intracellular H2O2 and superoxide anion and exposed to extracellular H2O2 of 1000 μM for 2 min. (B and C) Immunocytochemistry confirmed that the intracellular generation of H2O2 (B) and superoxide anion (C) was suppressed in Hb-overexpressing cells. (D and E) Intracellular levels of H2O2 (D) and superoxide anion (E) in these cells were estimated by flow cytometry analysis. Although those levels in control (pink histogram) and transfected cells (blue) exposed to extracellular stimuli were higher as total than control (black) and transfected cells (green) with no exposures, respectively, Hb-overexpressing cells included a lower level of cellular ROS as total than control cells when exposed to extracellular H2O2. (F and G) Quantitative analyses confirmed that the intracellular generation of H2O2 (F) and superoxide anion (G) was inhibited in Hb-overexpressing cells (▪) more than in control cells (□; P < 0.05). *P < 0.05; **P < 0.01.
Next, we investigated whether Hb in the nonerythroid cells has novel functions instead of facilitation of oxygen transport. We hypothesized that nonerythroid Hb could show antioxidant properties and estimated intracellular levels of reactive oxygen species (ROS) generated by exposing cells to H2O2 of 1000 μM using fluorogenic probes specific for H2O2 and superoxide anion. Immunofluorescence analyses demonstrated that intracellular generation of both radicals was partially inhibited in cultured murine mesangial cells, SV40-MES13, by globin-expressing vector transfection (Figure 5, B and C). On the basis of a flow cytometry system, histogram analysis revealed that the transfection reduced the number of each ROS-positive mesangial cell compared with transfection with the empty vector as a control (Figure 5, D and E). Percentage per total cell count with high levels of each ROS in cells overexpressing α- and β-globins was significantly lower than that in cells transfected with the control vector (P < 0.05; n = 6 for each group; Figure 5, F and G).
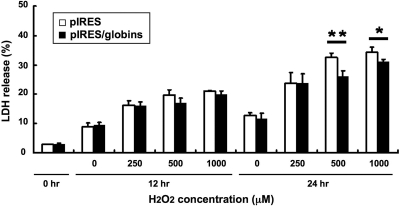
We also evaluated cell viability under oxidative stress by the lactate dehydrogenase (LDH) release assay. SV40-MES13 cells overexpressing α- and β-globins showed 31.1 ± 0.8% of LDH release ratio, whereas those with the empty vector showed 34.3 ± 1.7% (P < 0.05; n = 6 for each group) when the cells were exposed to H2O2 of 1000 μM for 24 h (Figure 6). Improvement of mesangial cell viability by transfection of the globin-overexpressing vector was also observed when cells were exposed to 500 μM H2O2 for 24 h (P < 0.01). Taken together, these results suggested that globin overexpression in mesangial cells has a protective effect in ROS scavenging.
Figure 6.
Transfection with the globin-expressing vector improved cell viability against oxidative stress. SV40-MES13 viability when exposed to H2O2 of 0, 250, 500, or 1000 μM for 24 h as extracellular oxidative stress was evaluated by the LDH release assay. The assay showed that SV40-MES13 cells transfected with the globin-expressing vector (▪) were more resistant against H2O2 stimuli of 500 and 1000 μM for 24 h than cells transfected with the control vector (□). *P < 0.05; **P < 0.01.
DISCUSSION
Our microarray analysis and proteomic studies gave us an important clue on in situ expression of Hb in the kidney and stimulated us to pursue this old but new subject. We provided the first evidence that Hb is expressed in mesangial cells of the kidney. We confirmed Hb expression in isolated rat glomeruli by RT-PCR and immunoblotting analysis. Mesangial localization of globin mRNA and Hb protein was demonstrated by in situ hybridization and immunofluorescence staining of the rat kidney. This finding was also supported by studies with cultured rat mesangial cells.
A long-standing notion that Hb gene can be expressed only in the cells of erythroid lineage was challenged in a recent study, in which the treatment with LPS and IFN-γ led to the activation of the β-globin gene in murine macrophages.8 Moreover, two groups independently demonstrated that Hb was expressed in alveolar epithelial type II cells of the lung.5,9 Pituitary of the neonatal rat is another site in which expression of globin subunit mRNA has been reported.10 It should be noted that low expression of α- and β-globin mRNA has been reported in a variety of nonhematopoietic tissues and cell lines(http://www.ncbi.nlm.nih.gov/sites/entrez?db=unigene).Therein, UniGene's expressed sequence tag (EST) profile suggested that Rattus norvegicus Hba-a1 (Rn.107334) and Hbb (Rn.216394) genes were expressed in multiple body sites, including the kidney. Furthermore, recent progress in DNA microarray profiling unveiled upregulated expression of globin in human cardiac ventricular tissue after ischemia-reperfusion.11 Microarray analysis of the kidney by other groups also showed local expression of globin genes.12,13 Although they did not rule out a possibility of contamination of cells of erythroid lineage as strictly as we did in this study, the results support our findings of Hb expression in mesangial cells.
The immunoblotting of the perfused normal rat glomeruli showed not only the monomer but also homodimer or heterodimer forms with the corresponding molecular weights of the Hb protein, even after detergent and heat denaturation. The similar phenomenon was observed in the current transfection study of cultured murine mesangial cells with the globin-expressing vector and also in the previous report about pulmonary Hb expression.5 This might be explained by excessively stable conformation of Hb molecule, although it is unknown whether this phenomenon reflects excessive levels of the globin subunits.
We confirmed mesangial expression of Hb in primary cultured mesangial cells. Of note, serial passage of glomerular mesangial cells affects gene expression profiles.14,15 We found that only passages 2 through 4 mesangial cells expressed Hb, suggesting that phenotypic changes associated with serial passage lead to loss of Hb expression. In support of this notion, SV40-MES13, a mesangial cell line, was devoid of Hb expression.
Although the tubulointerstitium is considered to be a main target of chronic hypoxia, we originally discovered upregulation of globin expression in the mesangium on the basis of microarray and proteomic analyses on chronic hypoxic rat kidney. Chronic ischemia of the kidney is known to be associated with glomerular changes known as ischemic obsolescence, and recent studies showed that a novel marker of ischemic stress, 6A3–5, was shown to be upregulated in mesangial cells of human renal biopsies with various kidney diseases as well as in mesangial cells of experimental animals subjected to hypoxia.16 Our in vitro studies clarified an antioxidative role of Hb, and oxidative stress participates in mesangial injury. Thus, we speculate that mesangial cells express Hb as an antioxidative defense. Our experiments in vitro using anoxia, AngII, and H2O2 failed to show regulation of the globin gene expression by these stimuli under the current experimental conditions, although these stimuli were “not chronic.” The exact regulatory mechanism of Hb expression in the kidney is a subject for future studies.
A biologic function of Hb expressed at nonerythroid sites remains to be elucidated. Although oxygen transport is widely known and probably the most important function, Hb may also have a variety of other functions. These include a molecular heat transducer by virtue of its oxygenation–deoxygenation cycle, Hb oxidation, enzymatic activities, the alteration of red blood cell metabolism, and drug interactions.17 In this study, we demonstrated for the first time that the antioxidant effect is one of the essential functions of Hb expressed by nonerythroid cells. Hb is also thought to be involved in the protection of cells against nitrosative stress.18,19 In addition, recent studies showed that Hb can detoxify highly oxidizing radicals, yielding the ferric state,20 and that the H2O2 removal ability by Hb was larger than that by the glutathione peroxidase-glutathione reductase system.21 Our results also suggested that superoxide anion as well as H2O2 can be scavenged by Hb.
Neuroglobin and cytoglobin, two other members of the vertebrate globin family, also have protective functions, including oxygen sensing and scavenging.22 Cytoglobin is present in almost all organs and tissues, including the kidney, whereas neuroglobin is predominantly expressed in the nervous system. Even though their amino acid and gene sequences are distinct from each other, their functional structure has striking similarities and serves as an antioxidative protein.22,23 These findings support our data about the antioxidant effect of mesangial Hb.
It remains unknown why mesangial cells but not the other glomerular cells express Hb, and the physiologic significance of the observed expression of mesangial Hb with antioxidant effect also remains to be elucidated. Oxidative stress induced by the generation of free radicals is a major inciting mechanism of renal injury, leading to renal cell damage followed by mesangial cell proliferation, fibrosis, and ultimate glomerulosclerosis.4,24 Exposure of mesangial cells to a diabetic environment such as high glucose or free fatty acids increases ROS generation.25,26 Also, AngII induces oxidative stress in mesangial cells through NADPH activation.27,28 Cells, however, possess antioxidant defense systems that include ROS degrading molecules, such as uric acid, ascorbic acid, and glutathione, and antioxidant enzymes, such as catalase, glutathione peroxidase, and superoxide dismutases. Our results raise a possibility that globin may physiologically act in mesangial cells as one of the endogenous antioxidant defensive proteins.
In conclusion, for the first time, our results herein demonstrated that Hb is expressed by rat mesangial cells and plays a cytoprotective role against oxidative insults. These data provide a significant impact not only in globin biology but also in the understanding of various renal pathogenesis associated with oxidative stress.
CONCISE METHODS
Animal Experiment
All experiments were conducted in accordance with the Guide for Animal Experimentation, Faculty of Medicine, University of Tokyo (Tokyo, Japan). Male Sprague-Dawley rats weighing 160 to 180 g were purchased from Nippon Bio-Supply Center (Saitama, Japan). All rats were housed in individual cages in a temperature- and light-controlled environment in an accredited animal care facility.
Experimental chronic renal ischemia was induced by unilateral stenosis of the left main artery by placing a U-shaped silver clip (0.23 μm internal diameter) around the left renal artery, and kidneys were harvested for microarray or proteomic analysis at days 3 or 7. For glomerular isolation, kidneys were harvested after extensive perfusion with saline, followed by the conventional differential sieving.29
Microarray and Proteomics
Total RNA from cortical tissues from each rat was isolated with ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. The Affymetrix Rat 230A oligonucleotide array sets (Affymetrix, Santa Clara, CA) were used to compare gene expression profiles of baseline, 3-d, and 7-d group rats. The data obtained were analyzed with Affymetrix Microarray Suite 5.0. These three time points were microarrayed as experimental duplicate. Details of the microarray analysis will be described elsewhere (Kojima and Nangaku, manuscript in submission).
Details of the proteomic studies are reported separately.30 In brief, kidneys were perfused extensively with saline to minimize blood contamination. Three kidney samples from the rat group with sham operation and the rat group with renal artery occlusion were homogenized together in lysis buffer for protein analysis. Isoelectric focusing was performed at 5000 V for 15 h. For SDS gel electrophoresis, a 10 to 18% SDS gradient gel was prepared. Protein patterns in the gel were analyzed using the PDQuest software (Bio-Rad, Hercules, CA). For protein identification, gel pieces containing the desired protein spots were excised for digestion, followed by analysis with AXIMA-CFR matrix-assisted laser desorption ionization time of flight mass spectrometer (Shimadzu Biotech, Kyoto, Japan). The search for protein identity on the basis of the peptide mass fingerprint was performed with Mascot search software (Matrix Science, Boston, MA).
RT-PCR
Total RNA was isolated from kidney tissue or cells using ISOGEN. One microgram of total RNA was reverse-transcribed into cDNA using Superscript II (Invitrogen, Carlsbad, CA). The PCR was performed using the following conditions: 94°C for 5 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 30 s. For sequencing analyses, oligonucleotide primer pairs were designed to amplify the individual full length of open reading frame of rat α- and β-globin subunits. Otherwise, oligonucleotide primer pairs specific for various globin gene transcripts were carefully designed to amplify the individual sequences among the closely homologous globin genes, and every PCR product would contain intron sequences when derived from amplification of contaminating genomic DNA.5 The primers used are shown in Table 2. The PCR product was then visualized by 2% agarose gel electrophoresis. For quantitative RT-PCR, 1 μL of cDNA was added to SYBR Green PCR Master Mix (Qiagen, Hilden, Germany) and subjected to PCR amplification in the iCycler system (Bio-Rad) with β-actin gene as an internal control.
Table 2.
PCR primers
| α-globin (full length, 429 bp) |
| forward: 5′-ATG GTG CTC TCT GCA GAT GA-3′ |
| reverse: 5′-TTA ACG GTA CTT GGA GGT CA-3′ |
| β-globin (full length, 444 bp) |
| forward: 5′-ATG GTG CAC CTA ACT GAT GCT G-3′ |
| reverse: 5′-TTA GTG GTA CTT GTG AGC CA-3′ |
| α-globin (335 bp) |
| forward: 5′-CTC TCT GGG GAA GAC AAA AGC AAC-3′ |
| reverse: 5′-GGT GGC TAG CCA AGG TCA CCA GCA-3′ |
| β-globin (416 bp) |
| forward: 5′-TGA ACC CTG ATG ATG TTG GTG GCG AGG-3′ |
| reverse: 5′-AAG ACA AGA GCA GGA AAA GAG GTT TAG-3′ |
| AE1 (467 bp) |
| forward: 5′-TGG CTG CTG TCA TCT TCA TCT AC-3′ |
| reverse: 5′-TTT GGG CTT CAT CAC AAC AGG-3′ |
| β-actin (306 bp) |
| forward: 5′-CTT TCT ACA ATG AGC TGC GTG-3′ |
| reverse: 5′-TCA TGA GGT AGT CTG TCA GG-3′ |
DNA Sequencing
Selected PCR amplicons were gel-purified by DNA extraction kit (Roche Applied Science, Basel, Switzerland), subcloned into pGEM-T Easy vector (Promega, Madison, WI), and subjected to DNA sequencing using the universal primers SP6 and T7. Sequences were analyzed using the NCBI BLAST engine and DNASIS software 3.7 (Hitachi Software Engineering, Tokyo, Japan).
Manual Microdissection and Laser Capture Microdissection
Manual microdissection of individual nephron segments was performed as described previously.31 In addition to the glomeruli, the following nephron tubular segments were microdissected: Proximal convoluted tubules; proximal straight tubules; medullary and cortical thick ascending limb of Henle's loop; distal convoluted tubules; and cortical, outer medullary, and inner medullary collecting ducts.
For laser capture microdissection, frozen sections of kidney at 8 μm were prepared and mounted on glass slides. A HistoGene LCM Frozen Section Staining Kit (Arcturus Engineering, Mountain View, CA) was used for fixation. First, the sections were rehydrated in 75% ethanol and distilled water for 30 s. After staining with HistoGene staining solution for 20 s, they were washed with distilled water for 30 s and dehydrated in 75, 95, and 100% ethanol for 30 s each, followed by xylene for 5 min, and air-dried. The regions of glomeruli was then dissected using an AutoPix LCM System (Arcturus Engineering) and dropped immediately onto the CapSure HS LCM Caps (Arcturus Engineering).
Immunoblotting
Glomerular tissue and cultured cell lysate were examined for SDS-PAGE followed by immunoblotting. The protein was separated by electrophoresis on a 4 to 20% gradient gel (Daiichi Pure Chemicals, Tokyo, Japan), followed by electrotransfer to polyvinylidene difluoride membranes (GE Healthcare Bio-Sciences, Buckinghamshire, UK). Transfer membranes were blocked with 5% nonfat milk in TBS with 0.01% Tween-20 for 30 min at room temperature. The membrane was incubated with the anti-Hb antibody (Dako, Glostrup, Denmark). Then, horseradish peroxidase–conjugated anti-rabbit IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, GE Healthcare Bio-Sciences).
In Situ Hybridization
The rat kidneys were dissected after perfusion, fixed, embedded in paraffin by proprietary procedures, and sectioned at 6 μm. Single-stranded sense and antisense probes were generated by in vitro transcription from the cDNA encoding rat α-globin chain (BC059150), nucleotides 33 to 461 (428 bp), and β-globin chain (NM_033234), nucleotides 48 to 491 (444 bp), which were labeled with digoxigenin using the DIG RNA Labeling Mix (Roche Applied Science). Hybridization was performed with probes at a concentration of 300 ng/ml at 60°C for 16 h. Anti-DIG AP conjugate (Roche Applied Science) was used as the detection antibody, and coloring reactions were performed with NBT/BCIP solution (Sigma-Aldrich, St. Louis, MO). The sections were counterstained with Kernechtrot stain solution (Mutoh Chemical, Tokyo, Japan), dehydrated, and then mounted with Malinol (Mutoh Chemical).
Immunofluorescence Studies
Immunostaining of the rat intensively saline-perfused kidneys was performed with rabbit polyclonal anti-rat Hb antibody (AbD Serotec, Oxford, UK) followed by incubation with FITC-conjugated swine anti-rabbit IgG (Dako). Double immunofluorescence studies of frozen sections was performed with a rabbit polyclonal antibody to α-globin subunit (Santa Cruz Biotechnology, Santa Cruz, CA) and a mAb, OX-7, to mesangial cells. The sections were incubated with the antibodies, followed by incubation with Alexa546 goat anti-mouse IgG (Molecular Probes, Eugene, OR) and FITC-conjugated swine anti-rabbit IgG (Dako).
Cell Culture
Primary cultured rat mesangial cells were obtained by sieving rat glomeruli and were cultured in DMEM (Nissui Seiyaku, Tokyo, Japan) with 25 mM HEPES (Sigma-Aldrich), 20% FBS (SAFH Biosciences, Lenexa, KS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.01 mM nonessential amino acid.32 The rat podocyte was a gift of Dr. Hidetake Kurihara (Juntendo University, Tokyo, Japan)33 and was cultured in 1:1 mixture of DMEM/F-12 medium with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1% Insulin-Trans-Cel-X (Life Technologies BioChemical, Grand Island, NY). Experiments were performed under growth-restricted condition in which podocytes are fully differentiated and quiescent, resembling the in vivo phenotype as described previously.34 The rat endothelial cell was a gift of Dr. Stephen Adler (New York Medical College, Valhalla, NY).35 In brief, after glomeruli were isolated by sieving and cultured in 1:1 mixture of hepatoma G2 conditioned MDCB 107/K1 medium, outgrowth with the appearance of endothelial cells was cloned and characterized by immunostaining and from biochemical aspects as described previously.36 Cells were cultured in RPMI 1640 (Nissui Seiyaku) with 2000 mg/L glucose, 10% FBS, and 10% NuSerum (BD Biosciences, Bedford, MA). The SV40-MES13 cell was obtained from American Type Culture Collection (Manassas, VA) and was cultured in 3:1 mixture of high-glucose DMEM/F12 medium with 14 mM HEPES, 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated in humidified 95% air with 5% carbon dioxide at 37°C. Stimulation of primary cultured mesangial cells was performed using H2O2 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at a concentration of 100 μM, AngII (Calbiochem, La Jolla, CA) at a concentration of 10 μM, or anaerocult A mini (Merck, Darmstadt, Germany), which reduces the oxygen content to 0.2% within 1 h, under serum-free conditions.
Construction of pIRES/Globins Vector and Transfection
To construct an optimal vector expressing both α- and β-globin subunits in mesangial cell, we used pIRES (Clontech Palo Alto, CA) vector that allows expression of two genes of interest by cloning them into multiple cloning sites (MCS) A and B. Both MCS are located on either side of the internal ribosome entry site (IRES) from the encephalomyocarditis virus, which allows translation of two consecutive open reading frames from the same messenger RNA.
Total RNA from human blood was reverse-transcribed, and the resulting cDNA was amplified in the PCR using several pairs of oligonucleotide primers designed according to open reading frame coding for hemoglobin α chain (NM_000558) and β chain (NM_000518). The PCR primer pair used was α-globin (5′-CCG CTC GAG ACC ATG GTG CTG TCT CCT GCC GAC AAG-3′, 5′-CCG GAA TTC TTA ACG GTA TTT GGA GGT CAG CAC GG-3′) and β-globin (5′-GCT CTA GAA CCA TGC ATC TGA CTC CTG AGG AG-3′, 5′-TGC GGT CGA CTT AGT ACT TGT GGG CCA GGG C-3′), both of which included Kozak's sequence.37 Each PCR fragment was subcloned into pGEM-T Easy vector (pGEM-T/α-globin and pGEM-T/β-globin). pGEM-T/α-globin was then digested with EcoRI and inserted into the same enzyme site of MCS A in pIRES (pIRES/α-globin), whereas β-globin fragment was digested with XbaI and inserted into the same enzyme site of multicloning site B in pIRES/α-globin (pIRES/α-globin/β-globin). Each construct was verified by the DNA sequencing and purified using a plasmid purification kit (Roche Applied Science). This final construct, pIRES/α-globin/β-globin, was transiently transfected to SV40-MES13 cells using Lipofectamine 2000 reagents (Invitrogen) according to the manufacturer's protocol.
Cellular ROS Analysis
After being extensively washed by PBS, SV40-MES13 cells were treated with 10 μM of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydroflurescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes) and 0.5 μM MitoSOX Red (Molecular Probes) for 30 and 10 min in the dark to detect intracellular H2O2 and superoxide anion, respectively, challenged for H2O2 of 1000 μM for 2 min, and trypsinized. First, to evaluate the intracellular ROS generation, immunofluorescence technique was applied using microscopy BIOREVO BZ-9000 (Keyence, Osaka, Japan). Second, this ROS production was analyzed using a FACScalibur (Becton Dickinson, Mountain View, CA) equipped with a 488-nm argonion laser and the software CELL Quest Pro (Becton Dickinson). CM-H2DCFDA and MitoSOX Red fluorescence were analyzed in the FL-1 (530 nm) and FL-2 (585 nm) channel, respectively. Forward and side scatter were used to gate out cellular fragments, and data were collected for 10,000 cells per sample.
Cell Viability Assay
Biologic significance of cellular Hb as antioxidant was further explored by exposing the cells to 250 to 1000 μM H2O2 for 12 to 24 h after transfection of pIRES/globins under serum-free conditions. Cell viability was evaluated by measurement of LDH in the cultured medium and in the cell lysate (Kainos Laboratories, Tokyo, Japan) as described previously.38 Percentage of cell lysis was calculated from the amount of LDH in the medium divided by the total amount of LDH in the medium and cell lysate.
Statistical Analysis
All data are reported as means ± SD. Statistical analyses were performed using the paired t test. Differences with P < 0.05 were considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science to M.N. (19390228) and to R.I. (19590939).
We appreciate Drs. Makiko Koike-Matsumoto, Nobuaki Eto (Kirin Pharma, Co. Ltd.), Koei Yamada (Kichijoji Ekimae Clinic), and Toshio Miyata (Tokai University) for valuable advice.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Hemoglobin in the Kidney: Breaking with Traditional Dogma,” on pages 1440–1441.
REFERENCES
- 1.Hardison RC: A brief history of hemoglobins: Plant, animal, protist, and bacteria. Proc Natl Acad Sci U S A 93: 5675–5679, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittenberg BA, Wittenberg JB: Transport of oxygen in muscle. Annu Rev Physiol 51: 857–878, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C: Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl S46–S51, 2005 [DOI] [PubMed]
- 4.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Newton DA, Rao KM, Dluhy RA, Baatz JE: Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem 281: 5668–5676, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Passow H: Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol 103: 61–203, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA: Laser capture microdissection. Science 274: 998–1001, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Zeng M, Stamler JS: Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci U S A 96: 6643–6647, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran M, Chen H, Chen Z, Liu L: Hemoglobin is expressed in alveolar epithelial type II cells. Biochem Biophys Res Commun 333: 1348–1352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leffers H, Navarro VM, Nielsen JE, Mayen A, Pinilla L, Dalgaard M, Malagon MM, Castano JP, Skakkebaek NE, Aguilar E, Tena-Sempere M: Increased expression of alpha- and beta-globin mRNAs at the pituitary following exposure to estrogen during the critical period of neonatal sex differentiation in the rat. J Steroid Biochem Mol Biol 99: 33–43, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Arab S, Konstantinov IE, Boscarino C, Cukerman E, Mori A, Li J, Liu PP, Redington AN, Coles JG: Early gene expression profiles during intraoperative myocardial ischemia-reperfusion in cardiac surgery. J Thorac Cardiovasc Surg 134: 74–81, 81.e1–81.e2, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TJ, Chen R, Zhang SL, Liu F, Brezniceanu ML, Whiteside CI, Fantus IG, Ingelfinger JR, Hamet P, Chan JS: Upregulation of osteopontin gene expression in diabetic rat proximal tubular cells revealed by microarray profiling. Kidney Int 69: 1005–1015, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Eikmans M, Roos-van Groningen MC, Sijpkens YW, Ehrchen J, Roth J, Baelde HJ, Bajema IM, de Fijter JW, de Heer E, Bruijn JA: Expression of surfactant protein-C, S100A8, S100A9, and B cell markers in renal allografts: Investigation of the prognostic value. J Am Soc Nephrol 16: 3771–3786, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Schnaper HW, Kopp JB, Poncelet AC, Hubchak SC, Stetler-Stevenson WG, Klotman PE, Kleinman HK: Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. J Cell Sci 109: 2521–2528, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Elger M, Drenckhahn D, Nobiling R, Mundel P, Kriz W: Cultured rat mesangial cells contain smooth muscle alpha-actin not found in vivo. Am J Pathol 142: 497–509, 1993 [PMC free article] [PubMed] [Google Scholar]
- 16.Garin G, Badid C, McGregor B, Vincent M, Guerret S, Zibara K, Hurlstone A, Laville M, McGregor JL: Ischemia induces early expression of a new transcription factor (6A3–5) in kidney vascular smooth muscle cells: Studies in rat and human renal pathology. Am J Pathol 163: 2485–2494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardison R: Hemoglobins from bacteria to man: Evolution of different patterns of gene expression. J Exp Biol 201: 1099–1117, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hausladen A, Gow AJ, Stamler JS: Nitrosative stress: Metabolic pathway involving the flavohemoglobin. Proc Natl Acad Sci U S A 95: 14100–14105, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner PR, Gardner AM, Martin LA, Salzman AL: Nitric oxide dioxygenase: An enzymic function for flavohemoglobin. Proc Natl Acad Sci U S A 95: 10378–10383, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein S, Samuni A: Intra- and intermolecular oxidation of oxymyoglobin and oxyhemoglobin induced by hydroxyl and carbonate radicals. Free Radic Biol Med 39: 511–519, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Masuoka N, Kodama H, Abe T, Wang DH, Nakano T: Characterization of hydrogen peroxide removal reaction by hemoglobin in the presence of reduced pyridine nucleotides. Biochim Biophys Acta 1637: 46–54, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Pesce A, Bolognesi M, Bocedi A, Ascenzi P, Dewilde S, Moens L, Hankeln T, Burmester T: Neuroglobin and cytoglobin: Fresh blood for the vertebrate globin family. EMBO Rep 3: 1146–1151, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S: Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett 410: 146–151, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pautz A, Franzen R, Dorsch S, Boddinghaus B, Briner VA, Pfeilschifter J, Huwiler A: Cross-talk between nitric oxide and superoxide determines ceramide formation and apoptosis in glomerular cells. Kidney Int 61: 790–796, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H: High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Shah AM: ROS generation by nonphagocytic NADPH oxidase: Potential relevance in diabetic nephropathy. J Am Soc Nephrol 14[Suppl 3]: S221–S226, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kobori H, Nangaku M, Navar LG, Nishiyama A: The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Jaimes EA, Galceran JM, Raij L: Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int 54: 775–784, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Salant DJ, Darby C, Couser WG: Experimental membranous glomerulonephritis in rats: Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest 66: 71–81, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son D, Kojima I, Inagi R, Matsumoto M, Fujita T, Nangaku M: Chronic hypoxia aggravates renal injury via suppression of Cu/Zn-SOD: A proteomic analysis. Am J Physiol Renal Physiol 294: F62–F72, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Tanemoto M, Vanoye CG, Dong K, Welch R, Abe T, Hebert SC, Xu JZ: Rat homolog of sulfonylurea receptor 2B determines glibenclamide sensitivity of ROMK2 in Xenopus laevis oocyte. Am J Physiol Renal Physiol 278: F659–F666, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Okuda T, Yamashita N, Kurokawa K: Angiotensin II and vasopressin stimulate calcium-activated chloride conductance in rat mesangial cells. J Clin Invest 78: 1443–1448, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaoita E, Kurihara H, Sakai T, Ohshiro K, Yamamoto T: Phenotypic modulation of parietal epithelial cells of Bowman's capsule in culture. Cell Tissue Res 304: 339–349, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Eto N, Wada T, Inagi R, Takano H, Shimizu A, Kato H, Kurihara H, Kawachi H, Shankland SJ, Fujita T, Nangaku M: Podocyte protection by darbepoetin: Preservation of the cytoskeleton and nephrin expression. Kidney Int 72: 455–463, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Adler S, Eng B: Integrin receptors and function on cultured glomerular endothelial cells. Kidney Int 44: 278–284, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Miyata T, Inagi R, Kurokawa K, Adler S, Fujita T, Nangaku M: Hypoxia-induced apoptosis in cultured glomerular endothelial cells: Involvement of mitochondrial pathways. Kidney Int 64: 2020–2032, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kozak M: Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. EMBO J 16: 2482–2492, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nangaku M, Quigg RJ, Shankland SJ, Okada N, Johnson RJ, Couser WG: Overexpression of Crry protects mesangial cells from complement-mediated injury. J Am Soc Nephrol 8: 223–233, 1997 [DOI] [PubMed] [Google Scholar]