Abstract
Unlike most fenestrated capillary endothelial cells, adult glomerular endothelial cells (GEnC) are generally thought to lack diaphragms at their fenestrae, but this remains controversial. In this study, morphologic and immunocytochemical analyses demonstrated that, except for a small fraction, GEnC of adult rats lacked diaphragmed fenestrae, which contain the transmembrane glycoprotein PV-1. In contrast, the GEnC in embryonic rats exhibited diaphragmed fenestrae and expressed PV-1 protein. The luminal surface of the fenestral diaphragm possesses a high density of anionic sites, thereby compensating for the functional immaturity of the embryonic glomerular filtration barrier. In addition, GEnC with diaphragmed fenestrae and PV-1 expression were significantly increased in adult rats with Thy-1.1 nephritis, presumably reflecting a process of restorative remodeling of the glomerular capillary tuft after injury; therefore, the reappearance of PV-1 expression and diaphragmed fenestrae may serve as a marker of glomerular capillary remodeling.
Capillary endothelial cells are morphologically and biochemically specialized to maintain the specific functions of individual organs, and a number of studies have investigated the relationship between their ultrastructural features and their permeability.1,2 In the capillary, where transendothelial exchange vigorously takes place, numerous transcellular pores or fenestrae exist to form “sieve plates” at this highly attenuated portion of endothelial cells.3 Such fenestrated endothelial cells are found in the endocrine and exocrine glands, intestinal villi, choroid plexus, liver sinusoid, kidney (both glomerular and peritubular capillaries), and so forth.4,5
Fenestrated capillaries with diaphragmed fenestrae show a remarkably high permeability to water and other small hydrophilic solutes6; however, their permeability to plasma proteins does not exceed that of the nonfenestrated capillaries or continuous capillaries.7 The impermeability of the fenestrated capillary to plasma proteins is attributed to the existence of fenestral diaphragms and endothelial basement membrane.1,6 Each fenestral diaphragm morphologically consists of a central mesh (or knob) and several fibrils radiating from mesh to fenestral rim.8,9 Among the several regions of endothelial cell surface, the luminal surface of fenestral diaphragm possesses the highest density of anionic sites, which is derived mainly from heparan sulfate proteoglycans.10–13 These anionic sites presumably contribute to the impermeability of the fenestrated capillary to anionic plasma proteins.
Caveolae and transendothelial channels are also involved in transcapillary exchanges1,14 and are furnished with stomatal diaphragms at their orifices in fenestrated capillaries.15,16 Stomatal diaphragms are morphologically similar to fenestral ones, but the former do not possess anionic sites.11,12 Caveolae exist in various cell types,17 but stomatal diaphragms are found only in the caveolae of specific endothelial cell types. These facts suggest that stomatal diaphragms of caveolae may play a specific role in specific endothelial cell types, although their exact functions remain unknown.
Fenestral and stomatal diaphragms have a common structural component, PV-1, a type II transmembrane glycoprotein that forms a homodimer in vivo.18–20 In cultured endothelial cells lacking both PV-1 and diaphragms, treatment with phorbol myristate ester induces expression of PV-1 and de novo formation of the fenestral and stomatal diaphragms.21 Moreover, knockdown of PV-1 expression using an small interference RNA approach prevents the formation of stomatal diaphragms and the formation of fenestrae and transendothelial channels in the cultured endothelial cells treated with phorbol myristate ester.21 These findings strongly suggest that PV-1 is an essential molecule to the formation of both stomatal and fenestral diaphragms.17
In this article, we demonstrate that (1) glomerular endothelial cells (GEnC) in rat adult kidney, apart from a small fraction, do not furnish diaphragms with their fenestrae and caveolae; (2) most GEnC in the immature glomeruli of rat embryos have diaphragmed fenestrae and caveolae; and (3) the number of GEnC with diaphragmed fenestrae and caveolae is increased in the glomeruli of Thy-1.1 nephritis rats. Finally, we discuss the physiologic significance of the diaphragmed fenestrae in GEnC.
RESULTS
GEnC of Normal Mature Glomeruli in Adult Rats
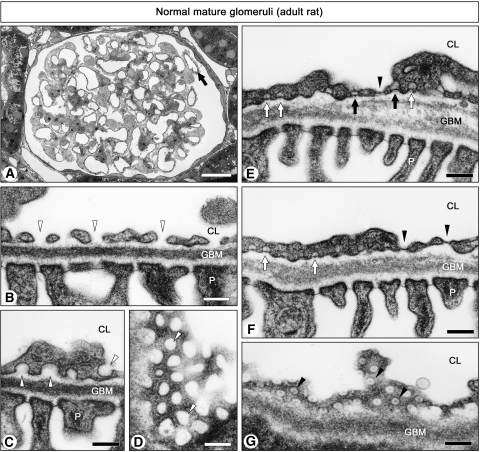
Most GEnC exhibited nondiaphragmed fenestrae and caveolae in mature glomeruli of normal 15-wk-old rats, as reported previously22,23 (Figure 1, A through D), but a small part of GEnC exhibited diaphragms at almost all of their fenestrae and caveolae (Figure 1, E through G). We found these GEnC with diaphragmed fenestrae in 2.0 ± 0.2% (n = 3) of total glomerular capillary cross-sections with transmission electron microscopy (TEM). In the GEnC with diaphragmed fenestrae and caveolae, transendothelial channels with two kinds of diaphragm (luminal and abluminal) were also observed (Figure 1E). Diaphragmed fenestrae (50 to 60 nm in diameter) were more uniform in shape and size than nondiaphragmed ones (60 to 160 nm in largest diameter; Figure 1, D and G).
Figure 1.
Ultrastructure of GEnC in mature glomeruli. In normal glomerulus of 15-wk-old rat (A), most GEnC exhibit nondiaphragmed fenestrae (arrowheads in B and D) and caveolae (arrowheads in C); however, only a small number of GEnC exhibit diaphragmed fenestrae (closed arrowheads in E through G), diaphragmed caveolae (open arrow in E and F), and transendothelial channels with two diaphragms (closed arrows in E). In A, the GEnC with diaphragms exist in the capillary cross-sections denoted by the arrow. D and G show grazing sections of nondiaphragmed and diaphragmed fenestrae, respectively. All of the micrographs except for A are of the same magnification. CL, capillary lumen; P, podocyte. Bar = 20 μm in A; 200 nm in B through G.
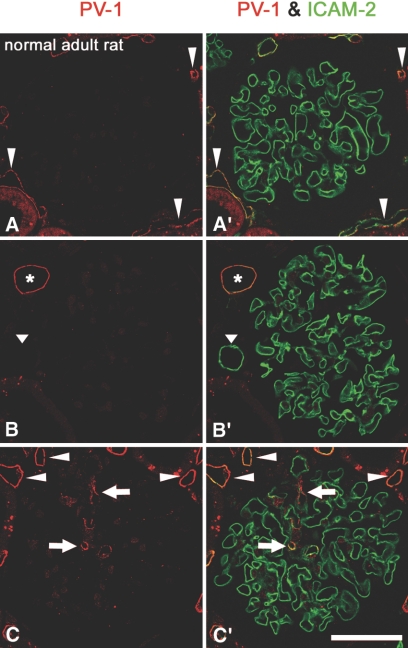
Double-immunofluorescence staining for PV-1 and intercellular adhesion molecule-2 (ICAM-2), which is a useful positional marker for endothelial cells including GEnC,24 showed that most GEnC do not exhibit the immunoreactivity for PV-1 in normal 15-wk-old rats (Figure 2, A, A′, B, and B′); however, we found the GEnC exhibiting PV-1 immunoreactivity in 1.6 ± 0.2% (n = 3) of total glomerular capillary cross-sections which were visualized by the anti–ICAM-2 antibody, as observed in TEM samples (Figure 2, C and C′). Immunoreactivity for PV-1 was also found in endothelial cells of peritubular capillary, vasa recta, and intrarenal vein.
Figure 2.
Protein expression of PV-1 in GEnC of mature glomeruli. In normal glomeruli of 15-wk-old rat, most GEnC, which are visualized with the anti–ICAM-2 antibody, show no immunoreactivity for PV-1 (red; A, A′, B, and B′). Only a small number of GEnC exhibit immunoreactivity for PV-1 (arrows in C and C′). Endothelial cells of peritubular capillaries (arrowheads in A, A′, C and C′) and intrarenal vein (asterisk in B and B′) show immunoreactivity for PV-1; however, those of intrarenal artery do not (arrowhead in B and B′). Bar = 50 μm.
GEnC of Immature Glomeruli in Rat Embryos
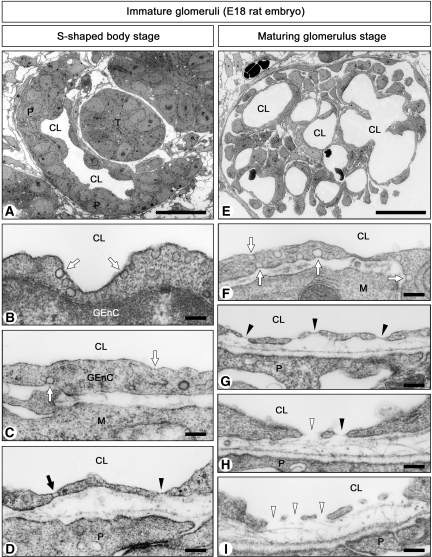
We next observed the GEnC of immature glomeruli in day 18 rat embryos, where all stages of glomerulogenesis except for the completely mature one were found. In the S-shaped body stage, a simple capillary reticulum was situated in the vascular cleft and was close to the columnar epithelial layer of immature podocytes via minute basement membrane (Figure 3A). GEnC from this stage were continuous and had diaphragmed caveolae on both their luminal and abluminal surfaces (Figure 3, B and C). In rare cases, diaphragmed fenestrae and transendothelial channels with two stomatal diaphragms were seen (Figure 3D).
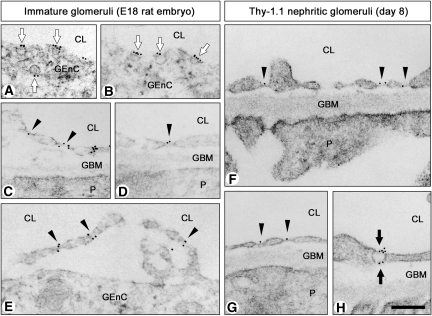
Figure 3.
Ultrastructure of GEnC in immature glomeruli. (A through D) Late S-shaped body stage in embryonic day 18 (E18) rat embryos. Glomeruli contain simple reticulum of capillary (A). GEnC often exhibit diaphragmed caveolae on both their luminal and abluminal surfaces (arrows in B and C) but rarely possess diaphragmed fenestrae (arrowhead in D) and transendothelial channels with two diaphragms (arrow in D). Nondiaphragmed fenestrae and caveolae are not found at this stage. (E through I) Maturing glomerulus stage in E18 rat embryos. Glomeruli contain multiple capillary loops and primitive loose mesangium (E). GEnC exhibit diaphragmed caveolae (arrows in F) and diaphragmed fenestrae (arrows in G). They also exhibit nondiaphragmed fenestrae (arrowheads in I). In some GEnC, diaphragmed and nondiaphragmed fenestrae coexist (H). M, mesangial cell; T, tubule. Bar = 25 μm in A and E; 200 nm in B through D and F through I.
In the capillary loop stage, each glomerular capillary loop protruded toward Bowman's space, and podocytes developed foot processes and filtration slits. Diaphragmed fenestrae and transendothelial channels were occasionally found, and most caveolae were furnished with stomatal diaphragms.
In the maturing glomerulus stage, capillary reticulum became more complicated, and primitive mesangium became arborized (Figure 3E). GEnC had increased numbers of fenestrae and transendothelial channels. In some GEnC, all of the fenestrae and caveolae were furnished with diaphragms (Figure 3, F and G), whereas in others, diaphragmed and nondiaphragmed fenestrae and caveolae were observed to coexist within the same individual cells (Figure 3H). We also found GEnC whose fenestrae and caveolae were virtually devoid of diaphragms (Figure 3I).
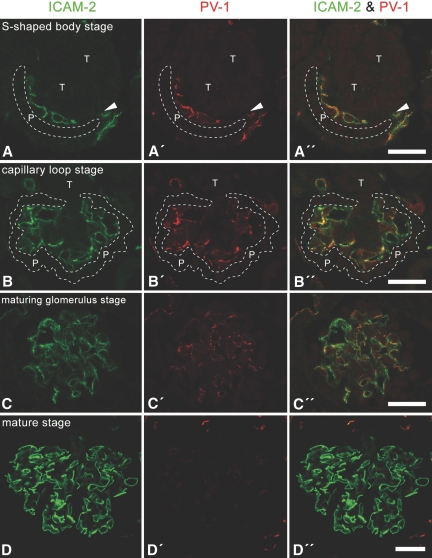
Immunoreactivity for PV-1 was detected in immature glomeruli from the S-shaped body stage to the maturing glomerulus stage and was co-localized with that for ICAM-2 (Figure 4). We further confirmed that PV-1 was predominantly localized at the stomatal and fenestral diaphragms of GEnC in immature glomeruli by the use of immunogold electron microscopy (Figure 5, A through D). Immunogold labelings for PV-1 were also observed at the fenestral diaphragms of pored domes, which form a fenestrated cytoplasmic sheet protruding into capillary lumen (Figure 5E).
Figure 4.
Protein expression of PV-1 in GEnC of immature glomeruli. In the S-shaped body (A through A″), capillary loop (B through B″), and maturing glomerulus (C through C″) stages, the immunoreactivity for PV-1 (red) is found in almost all GEnC, which are visualized by the anti–ICAM-2 antibody (green); however, most of the GEnC in adult rat do not exhibit immunoreactivity for PV-1 (D through D″). Arrowheads, vascular cleft. Bar = 25 μm.
Figure 5.
Localization of PV-1 in GEnC of immature and Thy-1.1 nephritic glomeruli. Localization of PV-1 was examined in GEnC by the use of immunogold electron microscopy. (A through E) E18 rat embryos. Immunogold labelings for PV-1 are predominantly localized at stomatal diaphragms of caveolae (arrows in A and B) and fenestral diaphragms (arrowheads in C and D). Immunogold labelings for PV-1 are also localized at the fenestral diaphragms of pored dome, which form a fenestrated cytoplasmic sheet that protrudes into capillary lumen (arrowheads in E). (A and B) S-shaped body stage. (C through E) Maturing glomerulus stage. (F through H) Thy-1.1 nephritis rats (day 8). Immunogold labelings for PV-1 are predominantly localized at fenestral diaphragms (arrowheads in F and G) and stomatal diaphragms of transendothelial channel (arrows in H). All of the micrographs are of the same magnification. Bar = 200 nm.
GEnC in Thy-1.1 Nephritis Rats
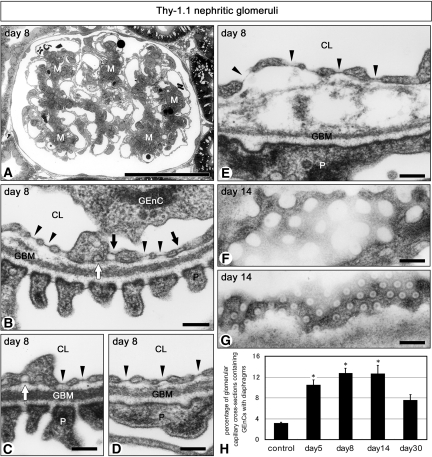
Reconstructive remodeling of injured glomerular capillary tuft can be observed in Thy-1.1 nephritic glomeruli as shown in our previous study,24,25 then we examined the GEnC in the recovery phase of this nephritis (5 to 30 d after the antibody injection). Enlargement of mesangium including proliferated mesangial cells and increased matrices was observed from day 5 to day 14 in Thy-1.1 nephritis rats (Figure 6A). TEM quantification showed that the percentage of the glomerular capillary cross-sections containing the GEnC with diaphragmed fenestrae was three to four times larger in Thy-1.1 nephritic glomeruli than in control 6-wk-old ones (Figure 6H). In the case of GEnC with diaphragmed fenestrae, almost all of their fenestrae and caveolae were furnished with diaphragms in some cells, whereas in others, diaphragmed and nondiaphragmed fenestrae and caveolae were observed to coexist within the same individual cells. Transendothelial channels were also found in both types of GEnC. Furthermore, some GEnC with diaphragmed fenestrae seemed to be morphologically intact and adhered to glomerular basement membrane (GBM; Figure 6, B through D), but others were detached from GBM (Figure 6E). Diaphragmed fenestrae (50 to 60 nm in diameter) were more uniform in shape and size than nondiaphragmed ones (60 to 160 nm in largest diameter), as shown in normal mature glomeruli (Figure 6, F and G).
Figure 6.
Ultrastructure of GEnC in Thy-1.1 nephritic glomeruli. (A) Mesangium (M) is enlarged by proliferated mesangial cells and increased matrices at day 8. Diaphragmed fenestrae (arrowheads in B through E), diaphragmed caveolae (open arrows in B and C), and transendothelial channels with two diaphragms (closed arrows in B) are frequently found in GEnC. GEnC with diaphragms seem to adhere to GBM normally in some sites (B through D) but are separated from GBM in other sites (E). Grazing sections of nondiaphragmed (F) and diaphragmed (G) fenestrae are shown. All of the micrographs except for A are of the same magnification. (H) The percentages of glomerular capillary cross-sections containing the GEnC with diaphragms are significantly large in all of the Thy-1.1 nephritis groups except for day 30, in comparison with control (control 3.1 ± 0.2%; day 5 10.5 ± 0.9%; day 8 12.7 ± 1.0%; day 14 12.6 ± 1.6%; day 30 7.5 ± 1.1%; n = 3 in each group). Bar = 50 μm in A; 200 nm in B through G. *P < 0.05 versus control.
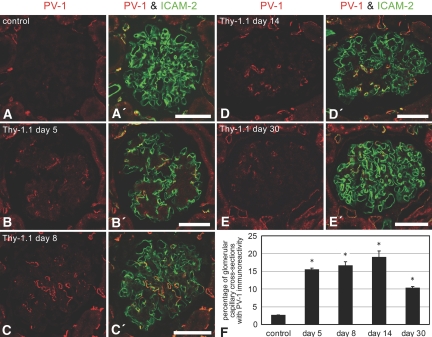
Double-immunofluorescence staining for PV-1 and ICAM-2 showed that the percentage of the glomerular capillary cross-sections with PV-1 immunoreactivity was three to six times larger in Thy-1.1 nephritic glomeruli than in control 6-wk-old ones (Figure 7). We confirmed that PV-1 was also specifically localized at the stomatal and fenestral diaphragms of GEnC in Thy-1.1 nephritic glomeruli by the use of immunogold electron microscopy (Figure 5, F through H).
Figure 7.
Protein expression of PV-1 in GEnC of Thy-1.1 nephritic glomeruli. Double-immunofluorescence staining for PV-1 (red) and ICAM-2 (green) reveals that PV-1–expressing GEnC are frequently found in Thy-1.1 nephritic glomeruli, especially from day 5 to day 14 (A through E and A′ through E′). (F) The percentages of PV-1–positive glomerular capillary cross-sections are significantly large in all of the Thy-1.1 nephritis groups, in comparison with control (control 2.6 ± 0.1%; day 5 15.4 ± 0.4%; day 8 16.5 ± 1.2%; day 14 18.9 ± 1.8%; day 30 10.2 ± 0.5%; n = 3 in each group). Bar = 50 μm. *P < 0.05 versus control.
DISCUSSION
GEnC in adult kidney are frequently regarded to lack the diaphragms, but the issue is still controversial.26,27 In this study, we demonstrate that GEnC, apart from a small fraction, do not possess diaphragms at their fenestrae and caveolae in adult kidney by precise TEM observation, in addition to the immunocytochemical demonstration of absence of PV-1, the only known component of endothelial diaphragm. These results support the previous morphologic studies.22,23
Rostgaard and Qvortrup28 clearly demonstrated filamentous sieve plugs, which presumably consist of proteoglycans and contribute to impermeability of glomerular capillary wall to plasma proteins at nondiaphragmed fenestrae of GEnC. For visualization of the sieve plug, they used a new fixative containing oxygen-carrying blood substitute and a contrast enhancement method with K3[Fe(CN)6], tannic acid, and uranyl acetate. In this study, we were able to improve visibility of endothelial diaphragms containing PV-1 by the use of tannic acid and uranyl acetate as contrast enhancers; however, our simple method was not capable of visualizing the sieve plug at nondiaphragmed fenestrae of GEnC.
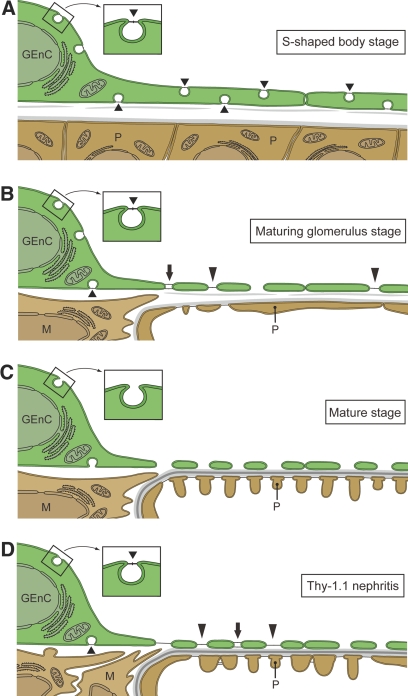
We furthermore showed that the GEnC with diaphragmed fenestrae and caveolae frequently appeared in embryonic and Thy-1.1 nephritic kidneys, as summarized in Figure 8. The GEnC form the S-shaped body stage exhibited diaphragmed caveolae but rarely diaphragmed fenestrae and transendothelial channels, indicating that the most part of the immunofluorescence signals for PV-1 in these GEnC represent the stomatal diaphragms of caveolae. We frequently found diaphragmed fenestrae at the maturing glomerulus stage, and some previous researchers also reported that diaphragmed fenestrae were found in the GEnC of immature glomeruli.23,29,30 Our detailed observations further revealed that the diaphragmed and nondiaphragmed fenestrae coexist in some individual cells at the maturing glomerulus stage, in which fenestrae are vigorously formed. During glomerular development at least, nondiaphragmed fenestrae may hence be formed by the disappearance of diaphragms from existing diaphragmed fenestrae.
Figure 8.
Diagram showing the state of fenestrae and caveolae in GEnC of immature and Thy-1.1 nephritic glomeruli. (A) In the S-shaped body stage, GEnC possess a relatively large number of diaphragmed caveolae (short arrowheads). Nondiaphragmed fenestrae and caveolae are not found in this stage. (B) In the maturing glomerulus stage, GEnC frequently exhibit transendothelial channels with two diaphragms (arrow), diaphragmed fenestrae (long arrowheads), and nondiaphragmed fenestrae. (C) In completely mature stage, diaphragms are diminished from fenestrae and caveolae in most GEnC. (D) In Thy-1.1 nephritic glomeruli, the GEnC with diaphragmed fenestrae and caveolae are significantly increased in comparison with those of normal mature glomeruli.
In matured glomeruli, permselectivity of capillary wall to plasma proteins is controlled by endothelial glycocalyx, GBM, and podocyte slit diaphragms.27,31–34 In fenestrated capillary, fenestral diaphragms are believed to contribute to permselectivity of capillary wall to plasma proteins, because fenestral diaphragms possess abundant anionic sites on their luminal surface and these anionic sites presumably repel plasma proteins.1,11,12 Fenestral diaphragms in immature glomeruli also carry abundant anionic charges, as seen in other endothelial cell types.23 It is thus conjectured that fenestral diaphragms may compensate for the functional immaturity of developing glomerular filtration barrier in embryonic kidney; however, for verification of this hypothesis, glomerular permselectivity in PV-1-null mutant mouse embryos will need to be evaluated in future research.
Fenestral diaphragms are probably involved in determining the size of fenestrae in a certain small range, because the diameter of diaphragmed fenestrae was smaller than that of nondiaphragmed ones in immature glomeruli, as shown here. The disappearance of diaphragms from fenestrae and the concomitant enlargement of fenestral diameter are linked to the increase of glomerular filtration coefficient (Kf). These changes thus contribute to efficient production of a considerable amount of primary urine in mature glomeruli. Similar changes are also seen in the developing liver. Liver sinusoidal endothelial cells (LSEnC) typically have large nondiaphragmed fenestrae in normal adult liver, whereas, in embryonic liver, LSEnC exhibit smaller diaphragmed fenestrae.35 Such larger nondiaphragmed fenestrae in LSEnC of adult liver enhance the exposure of hepatocytes to circulating blood and permit more efficient clearance of chylomicron remnants.
The reemergence of the GEnC with diaphragms in Thy-1.1 nephritic glomeruli presumably reflects a process of reconstruction of the glomerular capillary tuft in injured glomerulus. Glomerular capillary loops destroyed in Thy-1.1 nephritis undergo restorative reconstruction in an intussusceptive angiogenesis manner, as shown in our previous reports.24,25 Reconstructing capillaries in Thy-1.1 nephritic glomeruli are likely to recapitulate the following processes as seen in glomerulogenesis: The diaphragmed fenestrae are first formed, then the expression of PV-1 is downregulated, and finally the diaphragms disappear from the fenestrae. Reemergence of diaphragmed fenestrae has also been reported in the LSEnC of cirrhotic liver, in which the organization of the hepatocyte and sinusoidal network undergo remodeling.36
Vascular endothelial growth factor (VEGF), which is secreted from podocytes in mature glomeruli, plays a crucial role in the formation and maintenance of fenestrae in GEnC, as well as in various endothelial cell types.37–40 VEGF has also been reported to upregulate the expression of PV-1 mRNA and protein in human endothelial cell41,42; however, most GEnC in mature glomeruli do not express PV-1, as reconfirmed in this study. This inconsistence presumably indicates that the intracellular signaling pathways for the formation of fenestrae and the upregulation of PV-1 are not completely identical. Upregulation of PV-1 requires the activation of phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase.41,42 VEGF–phosphatidylinositol 3-kinase–p38 mitogen-activated protein kinase pathway is a candidate for the mechanisms involved in the upregulation of PV-1 in the GEnC of immature and Thy-1.1 nephritic glomeruli. In most GEnC without diaphragms, however, this pathway may be inactivated in adult mature glomeruli by some yet unknown mechanisms. Conversely, the formation of fenestrae requires the thinning of the cell periphery by depolymerization of actin filaments,43,44 but the pathway linking VEGF and the depolymerization of actin filaments in GEnC remains to be elucidated.
In conclusion, the GEnC in mature glomeruli, except for a small fraction, exhibit nondiaphragmed fenestrae and caveolae. During glomerulogenesis, diaphragmed fenestrae and caveolae, which exist in most GEnC of immature kidney, are replaced by nondiaphragmed ones. Furthermore, the reemergence of diaphragmed fenestrae and caveolae in injured (Thy-1.1 nephritic) glomeruli presumably reflects the postinjury remodeling of the glomerular capillary tuft.
CONCISE METHODS
Antibodies
Chicken polyclonal anti-rat PV-1 antibody was raised against the last 12 amino acid residues of the extracellular C terminus of the rat PV-1.18 Production of mouse monoclonal anti-rat ICAM-2 antibody (clone D12) was carried out as described previously.24 TRITC-conjugated donkey anti-chick IgY F(ab′)2 fragment and FITC-conjugated donkey anti-mouse IgG F(ab′)2 fragment were from Jackson ImmunoResearch Laboratories (West Grove, PA). Colloidal gold–conjugated goat anti-chick IgY was from British BioCell (Cardiff, UK).
Animals
In all of the experiments, we used Wistar rats obtained form Charles River Japan (Kanagawa, Japan). For induction of Thy-1.1 nephritis, we intravenously injected mouse monoclonal anti-rat Thy-1.1 antibody (clone E30, 100 μg/kg) into 6-wk-old male Wistar rats, as reported previously.45 All of the procedures performed on laboratory animals were approved by the institutional animal care committee of Juntendo University School of Medicine, and all of the animal experiments were carried out in compliance with the guidelines for animal experimentation of Juntendo University School of Medicine.
Transmission Electron Microscopy
Rat kidneys were perfused with 2.5% glutaraldehyde fixative buffered with 0.1 M phosphate buffer (PB) under anesthesia with pentobarbital. Fixed samples were processed by modified cold dehydration method, as reported previously.46 In brief, samples were successively immersed in 0.4% OsO4 in 0.1 M PB for 1 h, 2% low molecular weight tannic acid in 0.05 M maleate buffer for 4 h, and 1% uranyl acetate in 0.05 M maleate buffer for 3 h. Samples were then dehydrated with a graded series of acetone at 0 to −30°C before embedding in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a JEM1230 transmission electron microscope (JEOL, Tokyo, Japan).
Immunofluorescence and Immunogold Labeling
Rat kidneys were perfused with 4% paraformaldehyde fixative buffered with 0.1 M PB under anesthesia with pentobarbital. Fixed samples were immersed successively in PBS containing 10, 15, and 20% sucrose; frozen in OCT compound (Sakura Finetechnical, Tokyo, Japan); and then cut into 5-μm-thick cryosections. After mounting on glass slides, the sections were incubated for 2 h at room temperature or for 12 h at 4°C with the primary antibodies diluted 1:100 with 1% BSA in PBS. Subsequently, the sections were incubated for 1 h at room temperature with fluorescence dye–or colloidal-gold–conjugated secondary antibodies diluted 1:100 to 1:200 with 1% BSA in PBS. Fluorescence specimens were observed with a LSM510 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany). Immunogold-labeled specimens were further dehydrated and embedded in Epon 812. Ultrathin gold sections were observed with a JEM1230. Peritubular capillary endothelial cells were available for good positive control sites. As the negative control experiment, the primary antibodies were omitted from the incubation solution.
Statistical Analysis
We quantified the percentage of the glomerular capillary cross-sections containing the GEnC with diaphragms in the TEM samples. Moreover, we quantified the percentage of the glomerular capillary cross-sections containing PV-1-expressing GEnC in the double-immunolabeling samples for PV-1 and ICAM-2. The anti–ICAM-2 antibody was used to visualize all of the glomerular capillary cross-sections, which were recognized as circular or oval linear signals. In both quantifications, we selected the glomerular cross-sections that were expected to pass through the center of the glomerulus and examined 15 to 20 of these sections per animal. Three rats were examined in each group. Values are presented as means ± SEM. Differences were tested using ANOVA followed by the Bonferroni test as post hoc test. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by a Grant-in-aid from the Ministry of Education, Science, Sport and Culture of Japan (16390247 to H.K.). R.S. is supported by National Institutes of Health grant HL083249.
We thank Mr. Koichi Ikarashi for skillful technical assistance in electron microscopy.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Fenestrated Glomerular Capillaries Are Unique,” on pages 1439–1440.
REFERENCES
- 1.Simionescu N: Cellular aspects of transcapillary exchange. Physiol Rev 63: 1536–1579, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Simionescu M: Structural, biochemical and functional differentiation of the vascular endothelium. In: Morphogenesis of Endothelium, edited by Risau W, Rubanyi GM, Amsterdam, Harwood Academic Publishers, 2000, pp 1–22
- 3.Roberts WG, Palade GE: Endothelial fenestrae and fenestral diaphragms. In: Morphogenesis of Endothelium, edited by Risau W, Rubanyi GM, Amsterdam, Harwood Academic Publishers, 2000, pp 23–42
- 4.Rhodin JA: The diaphragm of capillary endothelial fenestrations. J Ultrastruct Res 6: 171–185, 1962 [DOI] [PubMed] [Google Scholar]
- 5.Stan RV: Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med 11: 621–643, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levick JR, Smaje LH: An analysis of the permeability of a fenestra. Microvasc Res 33: 233–256, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Renkin EM, Curry FE: Transport of water and solutes across capillary endothelium. In: Transport Organs, edited by Giebisch G, Tosteson DC, Ussing HH, Berlin, Springer-Verlag, 1979, pp 1–45
- 8.Bearer EL, Orci L: Endothelial fenestral diaphragms: A quick-freeze, deep-etch study. J Cell Biol 100: 418–428, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maul GG: Structure and formation of pores in fenestrated capillaries. J Ultrastruct Res 36: 768–782, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Simionescu M, Simionescu N, Palade GE: Differentiated microdomains on the luminal surface of capillary endothelium: Distribution of lectin receptors. J Cell Biol 94: 406–413, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simionescu M, Simionescu N, Silbert J, Palade GE: Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J Cell Biol 90: 614–621, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simionescu N, Simionescu M, Palade GE: Differentiated microdomains on the luminal surface of the capillary endothelium. I. Preferential distribution of anionic sites. J Cell Biol 90: 605–613, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milici AJ, L'Hernault N, Palade GE: Surface densities of diaphragmed fenestrae and transendothelial channels in different murine capillary beds. Circ Res 56: 709–717, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP: Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem 276: 48619–48622, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Stan RV: Structure and function of endothelial caveolae. Microsc Res Tech 57: 350–364, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Bruns RR, Palade GE: Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol 37: 244–276, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stan RV: Structure of caveolae. Biochim Biophys Acta 1746: 334–348, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Stan RV, Ghitescu L, Jacobson BS, Palade GE: Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J Cell Biol 145: 1189–1198, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stan RV, Kubitza M, Palade GE: PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A 96: 13203–13207, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stan RV: Multiple PV1 dimers reside in the same stomatal or fenestral diaphragm. Am J Physiol Heart Circ Physiol 286: H1347–H1353, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Stan RV, Tkachenko E, Niesman IR: PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol Biol Cell 15: 3615–3630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pease DC: Electron microscopy of the vascular bed of the kidney cortex. Anat Rec 121: 701–721, 1955 [DOI] [PubMed] [Google Scholar]
- 23.Reeves WH, Kanwar YS, Farquhar MG: Assembly of the glomerular filtration surface: Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol 85: 735–753, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notoya M, Shinosaki T, Kobayashi T, Sakai T, Kurihara H: Intussusceptive capillary growth is required for glomerular repair in rat Thy-1.1 nephritis. Kidney Int 63: 1365–1373, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ichimura K, Kurihara H, Sakai T: Involvement of mesangial cells expressing α-smooth muscle actin during restorative glomerular remodeling in Thy-1.1 nephritis. J Histochem Cytochem 54: 1291–1301, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ballermann BJ: Contribution of the endothelium to the glomerular permselectivity barrier in health and disease. Nephron Physiol 106: 19–25, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ballermann BJ, Stan RV: Resolved: Capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol 18: 2432–2438, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Rostgaard J, Qvortrup K: Sieve plugs in fenestrae of glomerular capillaries: Site of the filtration barrier? Cells Tissues Organs 170: 132–138, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Bergelin IS, Karlsson BW: Functional structure of the glomerular filtration barrier and the proximal tubuli in the developing foetal and neonatal pig kidney. Anat Embryol (Berl) 148: 223–234, 1975 [DOI] [PubMed] [Google Scholar]
- 30.Friis C: Postnatal development of the pig kidney: Ultrastucure of the glomerulus and the proximal tubule. J Anat 130: 513–526, 1980 [PMC free article] [PubMed] [Google Scholar]
- 31.Jeansson M, Haraldsson B: Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol 14: 1756–1765, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Jeansson M, Haraldsson B: Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol 290: F111–F116, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW: Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol 18: 2885–2893, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Tryggvason K, Wartiovaara J: Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Bankston PW, Pino RM: The development of the sinusoids of fetal rat liver: Morphology of endothelial cells, Kupffer cells, and the transmural migration of blood cells into the sinusoids. Am J Anat 159: 1–15, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Braet F, Wisse E: Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp Hepatol 1: 1, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitamoto Y, Tokunaga H, Tomita K: Vascular endothelial growth factor is an essential molecule for mouse kidney development: Glomerulogenesis and nephrogenesis. J Clin Invest 99: 2351–2357, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W: Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 140: 947–959, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts WG, Palade GE: Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108: 2369–2379, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Strickland LA, Jubb AM, Hongo JA, Zhong F, Burwick J, Fu L, Frantz GD, Koeppen H: Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF). J Pathol 206: 466–475, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Carson-Walter EB, Hampton J, Shue E, Geynisman DM, Pillai PK, Sathanoori R, Madden SL, Hamilton RL, Walter KA: Plasmalemmal vesicle associated protein-1 is a novel marker implicated in brain tumor angiogenesis. Clin Cancer Res 11: 7643–7650, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Ioannidou S, Deinhardt K, Miotla J, Bradley J, Cheung E, Samuelsson S, Ng YS, Shima DT: An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc Natl Acad Sci U S A 103: 16770–16775, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braet F, Spector I, De Zanger R, Wisse E: A novel structure involved in the formation of liver endothelial cell fenestrae revealed by using the actin inhibitor misakinolide. Proc Natl Acad Sci U S A 95: 13635–13640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinosaki T, Notoya M, Nomura Y, Miyai I, Kobayashi T, Kurihara H: Glomerular epithelial cell injury accelerates the progression of antibody-induced mesangial proliferative nephritis. Exp Nephrol 10: 245–258, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Ichimura K, Kurihara H, Sakai T: Actin filament organization of foot processes in vertebrate glomerular podocytes. Cell Tissue Res 329: 541–557, 2007 [DOI] [PubMed] [Google Scholar]