Abstract
Ischemia- or toxin-induced acute kidney injury is generally thought to affect the cells of the proximal tubule, but it has been difficult to define the involvement of other tubular segments because of the widespread damage caused by ischemia/reperfusion or toxin-induced injury in experimental models. For evaluation of whether thick ascending limb (TAL)-specific epithelial injury results in acute kidney injury, a novel transgenic mouse model that expresses the herpes simplex virus 1 thymidine kinase gene under the direction of the TAL-specific Tamm-Horsfall protein promoter was generated. After administration of gancyclovir, these mice demonstrated apoptosis only in TAL cells, with little evidence of neutrophil infiltration. Compared with control mice, blood urea nitrogen and creatinine levels were at least five-fold higher in the transgenic mice, which also developed oliguria and impaired urinary concentrating ability. These findings suggest that acute injury targeted only to the TAL is sufficient to cause severe acute kidney injury in mice with features similar to those observed in humans.
Acute kidney injury (AKI), which contributes significantly to morbidity and mortality among hospitalized patients,1 is frequently multifactorial,2 with ischemia and nephrotoxins being the most common causes. Regardless of cause, histologic findings include dilated and flattened epithelium, loss of tubular epithelial cells, and the presence of Tamm-Horsfall protein (THP)-rich casts. Frank necrosis is not usually apparent, and apoptotic cells are consistently found in both ischemic and nephrotoxic forms of clinical AKI.3,4 At the cellular level, actin cytoskeletal abnormalities lead to loss of cell polarity and relocation of cell adhesion molecules.5,6 Endothelial dysfunction and inflammation are present, although morphologic changes are subtle.7,8
A key unanswered question in AKI is how the kidney protects itself from devastating losses of body fluids as a result of the failure of glomerular ultrafiltrate reabsorption. One proposed mechanism is tubuloglomerular feedback (TGF), whereby increased distal delivery of solutes to the macula densa results in feedback signals to the glomerulus to decrease GFR by afferent arteriole constriction. After injury, tubular epithelial cell reabsorption of sodium is impaired, which results in increased distal delivery of NaCl and subsequent activation of TGF. The decreased renal blood flow and GFR result in oliguria and, in severe cases, anuria. This regulatory mechanism was termed “acute renal success” by Thurau and Boylan.9
Reductions in tubular flow also result from cast formation, which further contributes to the pathogenesis of AKI. Casts consist of exfoliated viable renal tubular epithelial cells that aggregate in the tubular lumen and lead to tubular obstruction and subsequent backleak of glomerular ultrafiltrate into the bloodstream.10 This contributes to decreased oxygen delivery to an already relatively hypoxic outer medulla,11 resulting in profound depletion of ATP, accumulation of intracellular calcium, cytoskeletal degradation, accumulation of hypoxanthine, and generation of reactive oxygen species.1
Animal models of AKI usually rely on a single nephrotoxic insult (e.g., ischemia, glycerol, heavy metals, gentamicin)12 that results in widespread tubular necrosis primarily involving the late S3 segment of the proximal tubule with subtle changes seen in the thick ascending limb (TAL).13–15 Because these models are not able to delineate which tubular segments contribute to AKI, we developed a mouse whereby we targeted injury to the TAL epithelial cells using a herpes simplex virus I thymidine kinase gene (HSV1-tk) under the direction of the TAL-specific THP promoter to address specifically the question of whether TAL injury is sufficient to induce AKI.
RESULTS
HSV1tk Is Expressed in the TAL of THP-HSV1tk Transgenic Mice
A transgene containing the THP promoter, which directs gene expression specific to the TAL, upstream of HSV1-tk (THP-HSV1-tk) was constructed (Figure 1A). HSV1-tk gene expression was initially confirmed in vitro by the generation of HEK cells stably expressing the HSV1-tk transgene (Figure 1B). Transgenic mice were then generated by pronuclear injections of the THP-HSV1-tk transgene. Six independent F1 founders were obtained; however, only females were used for breeding and colony expansion, because males carrying the HSV1-tk transgene are sterile.16 Southern blotting revealed transmission of the THP-HSV1-tk transgene to subsequent generations with no apparent loss of copy number (data not shown). Transgenic positive mice had no obvious phenotypic abnormalities.
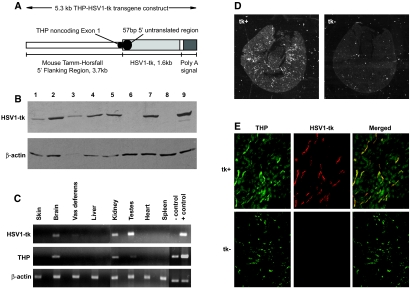
Figure 1.
(A) Schematic of 5.3-kb THP promoter HSV1-tk DNA transgene construct used for generation of mice. (B) Immunoblot for HSV1-tk revealed an expected size of 45 kD in six of seven clones screened (lanes 1 through 5 and 7). Lanes 8 and 9 contain HEK parental and HEK-HSV1-tk transiently transfected cell lysates, respectively. β-Actin was used as a loading control. A total of 30 μg of protein was loaded in each lane. (C) RT-PCR for THP, HSV1-tk, and β-actin in tissues from a transgenic-positive founder. THP and HSV1-tk were localized to the same tissues, including kidney, brain, and testes. For HSV1-tk and THP, negative and positive controls are PCR products from genomic tail DNA from a transgenic-negative and -positive mouse, respectively. (D) In situ hybridization shows the HSV1-tk transcript is distributed in a radial pattern in transgenic-positive kidneys and is absent in transgenic-negative kidneys (darkfield). (E) Immunofluorescence for THP, a specific marker for TAL, and HSV1-tk shows co-localization to the TAL in transgenic-positive kidneys. Transgenic-negative kidneys had a normal distribution of THP but lacked HSV1-tk expression. tk+, transgenic-positive kidney; tk−, transgenic-negative kidney. Magnifications: ×12.5 in D; ×200 in E.
HSV1-tk and THP mRNA transcripts exhibited identical tissue distributions limited to the kidney, testes, and brain, as verified by reverse transcriptase–PCR (RT-PCR; Figure 1C). Similarly, in situ hybridization confirmed the presence of HSV1-tk mRNA transcript within the cortical and outer medullary regions of the kidney (Figure 1D). Double immunofluorescence demonstrated that most cells expressing HSV1-tk protein expressed THP (Figure 1E). Notably, THP immunoreactivity predominated in the cytoplasm, whereas HSV1-tk protein was detected in both the cytoplasm and nuclei of TAL cells. Taken together, these data confirm that both HSV1-tk mRNA transcript and protein expression within the kidney are limited to the TAL.
Gancyclovir Induces Apoptosis in HSV1-tk–Expressing Cells
For first confirming that GCV efficiently induced apoptosis in vitro, HEK cells stably expressing HSV1-tk were treated with gancyclovir (GCV). As expected, addition of GCV to the tissue culture medium resulted in a concentration-dependent induction of apoptosis as shown by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL; Figure 2, A and B) and caspase-3 staining (data not shown).
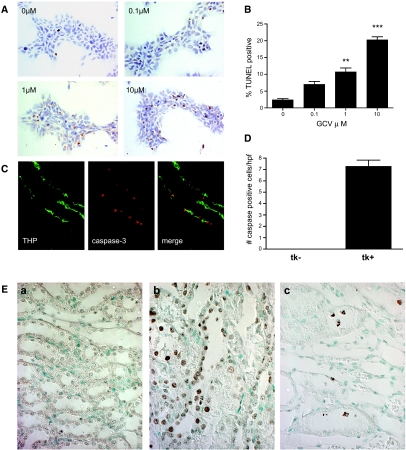
Figure 2.
Apoptosis studies. (A and B) In vitro studies. Representative fields from TUNEL assays on HEK-HSV1-tk stably transfected cells treated for 48 h with the doses of GCV indicated. (B) Quantitative analysis of percentage TUNEL positive. **P < 0.01 versus 0 μM; ***P < 0.001 versus 0 μM. (C) In vivo studies on GCV-treated kidneys co-stained for activated caspase-3 and THP. THP delineates TAL, and apoptosis was seen as nuclear staining in tubules that stain for THP. (D) Quantitative analysis of activated caspase-3. (E) TUNEL on kidney sections confirms the presence of apoptosis. (a) GCV-treated tk− kidneys had no evidence for TUNEL reactive cells. (b and c) GCV-treated tk+ kidneys show TUNEL-reactive cells in the tubular epithelia (b) as well as detached cells within tubular lumens (c; oil). Magnifications: ×630 in A and E, c; ×400 in C.
For verification of the induction of apoptosis in TAL cells, GCV was administered to 6- to 8-wk old transgenic-positive and littermate-negative control mice each weighing at least 20 g. After 6 to 8 d of GCV administration, activated caspase-3 was detected only in kidneys of transgenic-positive mice and was limited to cells of the TAL (average 7.3 ± 2.5 activated caspase-3–positive cells per high-power field; Figure 2, C and D). The presence of apoptosis was confirmed by TUNEL, which revealed TUNEL-positive cells in tubular epithelia as well as detached cells in the lumens of injured, dilated tubules (Figure 2E).
GCV-Treated Transgenic Mice Develop AKI Limited to the TAL
When the kidneys of GCV-treated HSV1-tk mice were examined microscopically, dilated tubules, flattened tubular epithelial cells, and intraluminal casts were observed in GCV-treated transgenic-positive kidneys and were present mainly within the cortical regions of the kidney (Figure 3A). Examination of the outer medullary regions revealed segmental nuclear dropout within the tubular epithelial cells, and some of the cells contained enlarged nuclei with associated chromatin clearing and prominent nucleoli (Figure 3A), indicative of active mitotic processes. No leukocyte infiltration was noted on hematoxylin- and eosin-stained slides, and this was confirmed by anti-granulocyte (anti–Gr-1) staining (Figure 3A), although numerous Gr-1 cells that were positive were observed in an ischemia-reperfusion kidney injury model (data not shown). When an injury score was applied to the kidneys, it was significantly higher in the HSV1-tk mice when compared with controls (Figure 3B). Loss of THP expression in HSV1-tk–positive cells as seen in double-immunofluorescence stains (Figure 4B) provided further evidence of injury.
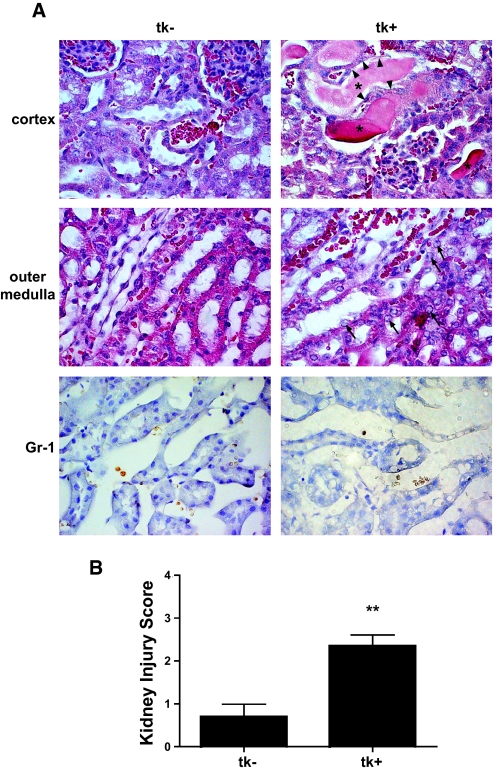
Figure 3.
(A) Histologic analysis of GCV-treated kidneys. Representative sections from a transgenic-positive kidney revealed dilated tubules in the cortex with evidence for nuclear dropout (arrowheads) and cast material present in tubular lumens (*). In the outer medulla, nuclei in damaged tubules were enlarged with prominent nucleoli (arrows). Representative cortex and outer medulla fields from a transgenic-negative kidney revealed normal architecture. Rouleaux formations were present in both transgenic-negative and -positive kidneys; however, no extravasation of red blood cells was seen to indicate endothelial injury. In sections stained for Gr-1, a marker for neutrophils, no leukocyte infiltration was seen on either injured transgenic-positive kidneys or uninjured transgenic-negative kidneys. (B) Kidney injury scoring (as described in the Concise Methods section) of GCV-treated tk− and tk+ kidneys revealed significantly higher injury scores in transgenic animals compared with controls (**P < 0.01). Magnification, ×630.
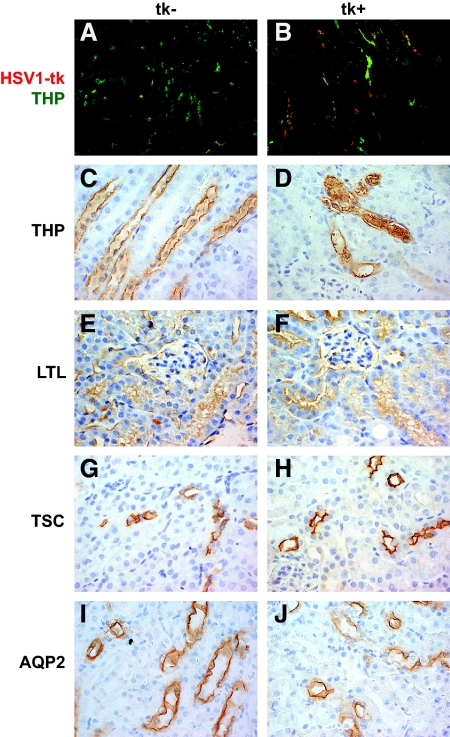
Figure 4.
Segment-specific staining for injury. (A and B). Co-immunofluorescence for THP and HSV1-tk shows normal THP and absent HSV1-tk in GCV-treated transgenic-negative kidneys (A). GCV-treated transgenic-positive kidneys revealed distorted THP expression with THP-rich casts and lack of co-localization with HSV1-tk (B). (C through J). IHC for nephron-specific markers. THP, Lotus tetragonolobus lectin (LTL), thiazide-sensitive co-transporter (TSC), aquaporin-2 (AQP2) represent markers specific for the TAL, proximal tubule, distal convoluted tubule, and collecting duct, respectively. Segment staining appeared intact in all tubular segments except in GCV-treated transgenic-positive kidneys stained for THP (D), which demonstrated tubular casts, tubular dilation, and flattened epithelium Magnification, ×630.
For verification that tubular injury was limited to the TAL, morphology of other nephron segments was examined in the presence of segment-specific markers. When GCV-treated transgenic-positive kidneys were stained for Lotus tetragonolobus lectin (specific for proximal tubule), thiazide-sensitive channel (specific for distal convoluted tubule), and aquaporin-2 (specific for collecting duct), no evidence of injury was found in these segments (Figure 4). Segment staining for THP (specific for TAL) revealed the presence of flattened epithelial cells, dilation of the tubular lumen, and THP-rich casts. These results further suggest that the apoptosis is limited and specific to the TAL.
GCV-Treated Transgenic Mice Develop Functional Evidence for Renal Failure
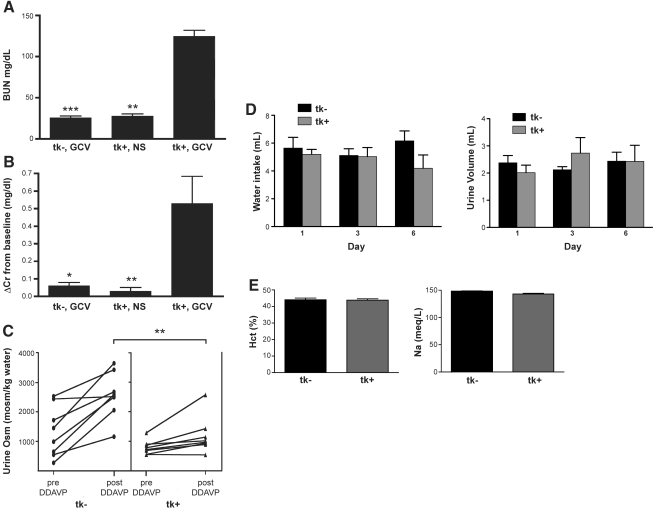
For determination of whether the histologic findings in the kidney correlated with decreased renal function, blood urea nitrogen (BUN) and creatinine were measured. As shown in Figure 5A, GCV-treated transgenic mice had statistically higher BUN levels compared with control mice. This decline in renal function was confirmed by a significant increase in serum creatinine values (Figure 5B).
Figure 5.
(A) Whole-blood BUN was significantly increased in GCV-treated transgenic-positive mice (tk+ GCV, n = 16) compared with controls. ***P < 0.001 versus tk− GCV (n = 14); **P < 0.01 versus +tk GCV (n = 7). (B) ΔCr was significantly increased in tk+ GCV mice (n = 7). *P < 0.05 versus tk− GCV (n = 7) and **P < 0.01 versus tk+ GCV (n = 6). HPLC Cr values obtained before injections was subtracted from Cr values obtained at the end of GCV injections to obtain ΔCr. (C) Urine osmolarity increased in both groups after administration of 20 ng of DDAVP intraperitoneal and water restriction; however, GCV-treated transgenic-positive mice were unable to concentrate their urine (1443 ± 509) to the same level achieved by GCV-treated transgenic control mice (2198 ± 459, **P = 0.003). (D) Balance studies for water intake and urine volume revealed no significant differences between the two groups (tk−, n = 5; tk+, n = 6). (E) Measurements of Hct and Na at the time of killing revealed values within the normal ranges (tk−, n = 13; tk+, n = 16).
Because the TAL plays a critical role in maintaining a hypertonic interstitium, which is required for the kidney's ability to concentrate urine, we assessed whether the urinary concentrating ability in GCV-injured mice was altered. Because mice maximally concentrate their urine after 1-desamino-8-d-arginine vasopressin (DDAVP) administration and water restriction, urine osmolarity (Uosm) from baseline morning spot urine was compared with spot urine obtained after administration of 20 ng of DDAVP and 4 h of water restriction. As shown in Figure 5C, all mice increased Uosm; however, GCV-treated transgenic-positive mice failed to concentrate their urine maximally (1443 ± 509 versus 2198 ± 459 mOsm/kg), consistent with a defect in TAL function. For verification that the difference in urinary concentrating ability between the mice was not due to differences in their volume status, balance studies were done during GCV administration. There were no significant differences in either water intake or urine volumes in GCV-injured mice as compared with control mice at baseline and at days 3 and 6 of GCV administration (Figure 5D). In addition, whole-blood measurements of hematocrit (Hct) and sodium (Na) were within the normal range and revealed no significant differences between the groups (Figure 5E). These data demonstrate functional evidence, suggesting that transgenic-positive mice develop AKI associated with defects in concentrating ability that are independent of hydration status and correlate with the abnormal histologic findings.
DISCUSSION
The mechanisms underlying the complex pathophysiology of AKI are incompletely understood. In this article, we describe a mouse model in which apoptotic injury is specifically targeted to the TAL cells of the kidney, thereby providing a novel tool for the investigation of pathologic mechanisms underlying AKI. Injury in these mice results in severely decreased GFR and diminished concentrating ability as well as histologic manifestations of AKI, suggesting that damaging the TAL is sufficient to result in severe AKI with many similarities to clinical AKI.
As opposed to mammalian thymidine kinases, HSV1-tk phosphorylates GCV, which can then be incorporated into the cellular genome, where it elicits premature chain termination and apoptotic injury. HSV1-tk has been successfully used to target apoptotic injury to a variety of cells in mice, including nonproliferating parietal cells of the gastric mucosa,17 proliferating lymphoid cells,17,18 and nonproliferating thyroid follicle cells.19 When HSV1-tk was expressed in nonproliferating gastric parietal cells, a loss of both mature postmitotic parietal cells as well as other terminally differentiated neighboring nonproliferating gastric epithelial cell lineages occurred after GCV administration.17 In contrast, in the present model, apoptosis was present only in the terminally differentiated nonproliferating TAL and not in neighboring epithelial, endothelial, or renal interstitial cells.
The widely used ischemia-reperfusion model lacks specificity and is characterized by widespread necrosis involving the S3 segment3 with associated inflammatory infiltration and vascular injury,20 which is rarely seen in clinical AKI. A model of radiocontrast injury, which results in medullary TAL necrosis and subsequent AKI in rats, has also been described.21 This model may be more clinically relevant; however, induction of injury is complicated and requires preconditioning with a combination of nonsteroidal anti-inflammatory drugs, nitric oxide inhibitors, and radiocontrast. The degree of injury is variable in this model, and it has not been firmly established in the mouse. In contrast to these models, which result predominantly in necrosis to the renal tubules, apoptosis is the predominant lesion seen in both ischemic- and nephrotoxic-induced clinical AKI.3 In our novel segment-specific model, apoptosis is the specific mechanism whereby injury is induced and the histologic pattern is that of dilated tubules and THP-rich cast formation. Thus, this model has many of the features seen in clinical AKI.
The mechanisms whereby GFR declines in tubular injury include activation of TGF as well as obstruction with tubular backleak.9 TGF should not be affected in the HSV1-tk model because injury is limited to the TAL and spares the macula densa22; however, to demonstrate conclusively that TGF activation is a major contributory factor would require tubular microperfusion studies. The presence of luminal casts suggests that tubular backleak from obstruction also contributes to AKI. Prerenal azotemia after GCV administration represents another potential cause for AKI in the HSV1-tk animals; however, this is highly unlikely because daily urine output was only slightly increased and Hct as well as Na values were relatively normal in both groups of mice.
Loss of urinary concentrating ability is one of the hallmarks of AKI.23 The TAL plays an important role in the kidney's capacity to concentrate urine because Na reabsorbed in the TAL is critical for maintaining the countercurrent exchange mechanism by generating a hypertonic medullary interstitium; therefore, loss of Na-K-2Cl co-transport in the TAL abolishes the medullary concentration gradient, resulting in an inability to concentrate urine even in the presence of vasopressin. Our GCV-injured transgenic mice exhibited defects in urinary concentration not corrected by the vasopressin analogue DDAVP, suggesting that these mice have lost their medullary concentration gradient secondary to failure of TAL Na transport.
The outer medullary location of the TAL makes this segment particularly vulnerable to injury, although notable morphologic changes may not be readily apparent.3 Our studies clearly show that targeting injury to the TAL cells results in AKI characterized by a severe decline in GFR, evidence of TAL cell apoptosis, cast formation, and defects in urinary concentration. As with clinical AKI, a disparity between the severe impairment of renal function and the somewhat modest histologic changes is also seen in our mice. These results are consistent with the observation in a morphometric analysis of human kidney allografts that the primary mechanism for the fall in GFR is due to afferent arteriolar constriction secondary to TGF.24 Thus, TAL injury may contribute a more central role to the pathogenesis of AKI than previously considered.
In summary, this novel transgenic mouse model of GCV-inducible selective TAL injury demonstrates that apoptotic injury initiated in and limited to the TAL results in significant functional abnormalities consistent with AKI. Furthermore, these mice exhibit several features reminiscent of clinical AKI. This is the first report of an inducible, segment-specific AKI mouse model and represents a unique approach to address the consequences of distal epithelial-specific injury. This novel transgenic mouse serves as a potentially useful model that can be used toward further investigation of the role of distal epithelial injury to AKI.
CONCISE METHODS
Antibodies and Other Materials
Primary antibodies included goat anti-THP (1:1000; Cayman Chemical, Ann Arbor, MI), rabbit anti–thiazide-sensitive channel (1:500; gift from Steve Hebert, Yale University, New Haven, CT), rabbit anti–aquaporin-2 (1:500; Alpha Diagnostic Int., San Antonio, TX), rabbit anti-cleaved caspase-3 (1:100; Cell Signaling, Danvers, MA), rabbit anti-mouse Gr-1 (1:100; Leinco Technologies, St. Louis, MO), mouse anti–β-actin (1:10000 Sigma, St. Louis, MO), and rabbit anti–HSV1-tk (1:100 to 1:1000; gift from William Summers, Yale University). Biotinylated Lotus tetragonolobus lectin was obtained from Vector Laboratories (Burlingame, CA). Secondary antibodies include Cy2 anti-goat and Cy3 anti-rabbit (1:200; Jackson ImmunoResearch, West Grove, PA), goat anti-rabbit horseradish peroxidase (1:5000; Jackson ImmunoResearch), and goat anti-mouse horseradish peroxidase (1:10000; Jackson ImmunoResearch). i-STAT EC-8+ cartridges (Abbott Laboratories, Abbott Park, IL) were used to measure BUN, Hct, and Na. Plasma creatinine was performed by HPLC analysis using the method of Dunn et al.25 with the following modifications: (1) Column was Zorbax 300-SCX, 5 μ, 2.1 × 150 mm; (2) mobile phase was 7.5 mM sodium acetate adjusted to pH 4.4 with acetic acid (total acetate approximately 15 mM) at a flow rate of 0.50 ml/min (isocratic); (3) column temperature was 40°C; and (4) sample injection volume was 3.0 μl.
THP-HSV1-tk Fusion Gene Construct
The mouse THP promoter (GenBank accession no. AF420599) containing the 3.7-kb segment of genomic DNA flanking the 5′ end of the mouse THP gene, a modified first noncoding exon (to contain Not1 and XhoI sites) of the THP gene and a portion of the first intron of the THP gene, was subcloned into pBluescript (provided by Peter Stricklett26). The HSV1-tk DNA segment (GenBank accession no. V00470; provided by James Goldenring17) contains 57 bp of 5′ untranslated sequence, the entire HSV1-tk coding region, and signals for polyadenylation. The HSV1-tk DNA segment was digested from its original vector using HindIII, blunted with Klenow (NEB, Ipswich, MA), then digested with BamH1 to release the 1.5-kb HSV1-tk insert. This insert was ligated into pcDNA3.1/Zeo(−) at the EcoRV and BamH1 sites. THP was excised from pBluescript using XbaI and XhoI and ligated into pcDNA3.1/Zeo(−) upstream of the HSV1-tk gene. This 5312-bp final transgene construct, designated THP-HSV1-tk, was excised from pcDNA3.1/Zeo(−) with PmeI and used for injection and production of transgenic mice in coordination with the Vanderbilt Transgenic Mouse/Embryonic Stem Cell Shared Resource (Figure 1A).
Generation of Stable Transfectants
HEK cells were transfected with the pcDNA3.1/Zeo(−) plasmid containing the HSV1-tk gene according to the manufacturer's protocol (Superfect; Qiagen, Valencia, CA). Stable transfectants were selected in medium containing 200 μg/ml zeocin and maintained at 100 μg/ml. Clones were screened by preparing cell lysates and immunoblotted for HSV1-tk (1:1000) or β-actin (1:10000) using standard SDS-PAGE and Western blot analysis. HSV1-tk–expressing cells were treated with varying dosages of GCV (0.1 to 50 μM) for 48 h. For detection of apoptosis, cells were fixed in 50% methanol/50% acetone, and TUNEL assay was performed according to the manufacturer's protocol (DeadEnd Colorimetric TUNEL System; Promega, Madison, WI). For kidney sections, TUNEL was performed by quenching deparaffinized sections with 3% H2O2 followed by boiling in citric acid and treating with proteinase K for 20 min at room temperature for antigen retrieval. Sections were then incubated in terminal deoxynucleotidyl transferase (Fisher Scientific, Pittsburgh, PA) and biotin-14-dATP (Invitrogen, Carlsbad, CA) for 1 h at room temperature. After washing in PBS, kidneys were developed with ABC kit (Vector Laboratories) and 3,3′-diaminobenzidine tablets (Sigma).
Generation of THP-HSV1-tk Transgenic Mice
The B6/D2 mouse strain was used for microinjections. Microinjections were performed by Dr. Catherine Pettepher (Vanderbilt Transgenic Mouse/Embryonic Stem Cell Shared Resource). DNA was obtained by tail biopsy, and founder mice were genotyped by PCR and Southern blotting. All subsequent litters were genotyped by PCR analysis.
PCR Primers
For RT-PCR and PCR genotyping of HSV1-tk, the primers 5′-TTTACGGGCTACTTGCCAAT-3′ and 5′-GTTATACAGGTCGCCGTTGG-3′ were used to amplify a 206-bp segment of the HSV1-tk gene. For THP, 5′-CTCAGGCAAACAGCCTCTTC-3′ and 5′-CCCCTGGGAGTTAGACACAA-3′ were used to amplify a 466-bp segment of the genomic THP promoter region. For β-actin, 5′-TCCTGTGGCATCCACGAAACT-3′ and 5′-GAAGCATTTGCGGTGGACGAT-3′ were used to amplify a 315-bp region of the β-actin gene.
GCV and DDAVP Administration
Transgenic mice and age-matched control littermates were housed in regular rodent cages and provided standard rodent food and water ad libitum. A subset of mice were housed in metabolic cages during GCV administration for balance studies (Hatteras Instruments, Cary, NC). GCV (Roche, Nutley, NJ) 50 mg/kg mouse or equivalent volume isotonic saline was administered by intraperitoneal injection twice daily for 6 to 8 d.
For concentration studies, DDAVP (Sigma-Aldrich) 20 ng intraperitoneally was administered after baseline spot urine was obtained. Spot urine was collected 3 to 4 h after DDAVP administration and water deprivation.
Kidney Histology
After GCV administration, mice were killed and kidneys were harvested and fixed in 4% DEPC-treated paraformaldehyde (Sigma-Aldrich). Kidneys were prepared in OCT (Tissue-Tek; Sakura Finetek, Torrance, CA) for frozen sectioning or paraffin embedded.
Kidney Injury Scoring
Histologic examinations were performed by a blinded renal pathologist. Histologic changes were quantified by calculation of the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilation as follows: 0, none; 1, ≤10%; 2, 11 to 25%; 3, 26 to 45%; 4, 46 to 75%; and 5, >76%. At least 10 fields (×200) were reviewed for each slide.
RT-PCR
Mice were killed by isoflurane inhalation, and tissues were snap-frozen in liquid nitrogen. Total RNA was isolated by Trizol reagent (Invitrogen), and 200 ng of DNAseI-treated (Ambion, Austin, TX) total RNA was used to generate cDNA for RT-PCR analysis (Superscript III; Invitrogen).
In Situ Hybridization
Before hybridization, 5-μm sections were deparaffinized, refixed in paraformaldehyde, treated with proteinase K (20 μg/ml), washed with PBS, refixed in 4% paraformaldehyde, and treated with triethanolamine plus acetic anhydride (0.25% vol/vol). Finally, sections were dehydrated in 100% ethanol. Antisense RNA was hybridized to the sections at 50 to 55°C for approximately 18 h as described previously.27 After hybridization, sections were washed at 50°C in 5× SSC + 10 mM β-mercaptoethanol. This was followed by a wash in 50% formamide, 2× SSC, and 100 mM β-mercaptoethanol for 60 min. After additional washes in 10 mM TRIS, 5 mM EDTA, and 500 mM NaCl (TEN), sections were treated with RNase (10 μg/ml), at 37°C for 30 min, followed by another wash in TEN (37°C). Sections were then washed twice in 2× SSC and then twice in 0.1× SSC (50°C). Slides were dehydrated with graded ethanols containing 300 mM ammonium acetate. For detection of the hybridized probe, slides were dipped in photo emulsion (Ilford K5, Knutsford, UK) diluted 1:1 with 2% glycerol/water and exposed for 7 d at 4°C. After development in Kodak D19, slides were counterstained with hematoxylin and eosin. Photomicrographs were taken using an upright Zeiss Axioimager.A1 microscope and AxioCam MRc5 camera using both bright- and darkfield optics.
Immunofluorescence and Immunohistochemistry
Fixed-frozen and paraffin-embedded 5-μm kidney sections were used for immunofluorescence and immunohistochemistry (IHC), respectively, with the exception of anti–Gr-1, in which fixed-frozen sections were used for IHC. Sections were washed three times in PBS, then blocked for 30 min at room temperature in 10% donkey serum. Sections were incubated in primary antibodies for at least one hour at room temperature, washed in PBS, then incubated in secondary antibodies for 30 min at room temperature. For IHC, sections were developed with DAB. Kidneys were visualized on a Zeiss AxioImager.A1 upright microscope, and images were captured using a Zeiss (Thornwood, NY) AxioCam MRc5 camera.
Statistical Analysis
Data were analyzed using Prism 4 (GraphPad Software, La Jolla, CA) for Macintosh software. For analysis of BUN and creatinine, one-way ANOVA using Kruskal-Wallis test and t test were used. For TUNEL analysis, the number of TUNEL-positive cells and total number of cells from 10 representative ×40 oil high-power fields were counted and reported as percentages. One-way ANOVA was used to calculate statistical significance. The numbers of activated caspase-3 cells were counted from a selection of 20 high-power fields taken from the corticomedullary region containing notable activated caspase-3 activity and reported as average ± SD.
DISCLOSURES
None.
Acknowledgments
This work was supported by the National Kidney Foundation (postdoctoral fellowship to M.B.S.), Veterans Administration Career Development Award (M.B.S.), DK39261-16 (M.D.B.), DK 69921 (R.Z.), DK51265 (R.H.), and Merit awards from the Department of Veterans Affairs (M.D.B., R.Z., and R.C.H.).
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Safirstein RL: Acute renal failure: From renal physiology to the renal transcriptome. Kidney Int Suppl S62–S66, 2004 [DOI] [PubMed]
- 4.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P: Activation of mitochondrial apoptotic pathways in human renal allografts after ischemia-reperfusion injury. Transplantation 76: 50–54, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Solez K, Morel-Maroger L, Sraer JD: The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58: 362–376, 1979 [PubMed] [Google Scholar]
- 6.Olsen TS, Hansen HE: Ultrastructure of medullary tubules in ischemic acute tubular necrosis and acute interstitial nephritis in man. APMIS 98: 1139–1148, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Kwon O, Phillips CL, Molitoris BA: Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am J Physiol Renal Physiol 282: F1012–F1019, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Thurau K, Boylan JW: Acute renal success: The unexpected logic of oliguria in acute renal failure. Am J Med 61: 308–315, 1976 [DOI] [PubMed] [Google Scholar]
- 10.Racusen LC, Fivush BA, Li YL, Slatnik I, Solez K: Dissociation of tubular cell detachment and tubular cell death in clinical and experimental “acute tubular necrosis.” Lab Invest 64: 546–556, 1991 [PubMed] [Google Scholar]
- 11.Brezis M, Rosen S: Hypoxia of the renal medulla: Its implications for disease. N Engl J Med 332: 647–655, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Dinour D, MBrezis M: Comprehensive Toxicology, Oxford, Elsevier, 1997
- 13.Brezis M, Shanley P, Silva P, Spokes K, Lear S, Epstein FH, Rosen S: Disparate mechanisms for hypoxic cell injury in different nephron segments: Studies in the isolated perfused rat kidney. J Clin Invest 76: 1796–1806, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megyesi J, Safirstein RL, Price PM: Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101: 777–782, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megyesi J, Andrade L, Vieira JM Jr, Safirstein RL, Price PM: Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int 60: 2164–2172, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Al-Shawi R, Burke J, Jones CT, Simons JP, Bishop JO: A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol 8: 4821–4828, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canfield V, West AB, Goldenring JR, Levenson R: Genetic ablation of parietal cells in transgenic mice: A new model for analyzing cell lineage relationships in the gastric mucosa. Proc Natl Acad Sci U S A 93: 2431–2435, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rindi G, Civallero M, Candusso ME, Marchetti A, Klersy C, Nano R, Leiter AB: Sudden onset of colitis after ablation of secretin-expressing lymphocytes in transgenic mice. Exp Biol Med (Maywood) 229: 826–834, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Wallace H, Ledent C, Vassart G, Bishop JO, al-Shawi R: Specific ablation of thyroid follicle cells in adult transgenic mice. Endocrinology 129: 3217–3226, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Lieberthal W, Nigam SK: Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol 278: F1–F12, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Heyman SN, Brezis M, Reubinoff CA, Greenfeld Z, Lechene C, Epstein FH, Rosen S: Acute renal failure with selective medullary injury in the rat. J Clin Invest 82: 401–412, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmann S, Metzger R, Bunnemann B: Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry 94: 517–523, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW: Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med 89: 47–50, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Alejandro V, Scandling JD Jr, Sibley RK, Dafoe D, Alfrey E, Deen W, Myers BD: Mechanisms of filtration failure during postischemic injury of the human kidney: A study of the reperfused renal allograft. J Clin Invest 95: 820–831, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K: Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Stricklett PK, Taylor D, Nelson RD, Kohan DE: Thick ascending limb-specific expression of Cre recombinase. Am J Physiol Renal Physiol 285: F33–F39, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Breyer MD, Jacobson HR, Davis LS, Breyer RM: In situ hybridization and localization of mRNA for the rabbit prostaglandin EP3 receptor. Kidney Int 44: 1372–1378, 1993 [DOI] [PubMed] [Google Scholar]