Abstract
This study investigated the effect of treatment with 6-dimethylaminopurine (6-DMAP) following fusion on in vitro development of porcine nuclear transfer (NT) embryos. Frozen thawed ear skin cells were transferred into the perivitelline space of enucleated oocytes. Reconstructed oocytes were fused and activated with electric pulse in 0.3M mannitol supplemented with either 0.1 or 1.0 mM CaCl2. In each calcium concentration, activated oocytes were divided into three groups. Two groups of them were exposed to either ionomycin (I) + 6-DMAP or 6-DMAP alone. In experiment 2, fused NT embryos in 0.3M mannitol containing 1.0 mM CaCl2 were exposed to 6-DMAP either immediately or 20 min after fusion/activation. For 0.1 mM CaCl2, oocytes activated with either I + 6-DMAP or 6-DMAP alone showed a higher (P<0.05) developmental rate to the blastocyst stage than those activated with an electric pulse alone (26.7 and 22.5 vs. 12.5%). For 1.0 mM CaCl2, oocytes activated with either I + 6-DMAP or 6-DMAP alone showed significantly higher (P<0.05) developmental rate to the blastocyst stage (35.6 and 28.3 vs. 19.8%). Developmental rate to the blastocyst stage was (P<0.05) increased in NT embryos activated with 6-DMAP 20 min after fusion. 6-DMAP made a higher and wider Ca2+ transient compared to that induced by electric pulses (Figure 3). The fluctuation lasted during the time that oocytes were cultured in 6-DMAP.
Regardless of Ca2+ concentration in fusion medium, activation with 6-DMAP following electric pulses supported more development of porcine NT embryos. Activation of NT embryos with 6-DMAP after fusion in the presence of 1.0 mM CaCl2 could support better developmental rate to the blastocyst stage.
Introduction
Although nuclear transfer (NT) has successfully produced cloned piglets, the development to blastocyst and term is still low. Activation of the NT embryos is one of the key factors to improve the developmental ability of porcine NT embryos. Electric pulses or combined chemicals such as Ca-ionophore/6-DMAP (Cibelli et al., 1998; De Sousa et al., 1999), ionomycin/6-DMAP (Wells et al., 1999), or cycloheximide/cytochalasin B (Zakhartchenko et al., 1999) have been used to activate NT embryos. Matured oocytes have been used as recipient oocytes in NT. They are generally arrested at metaphase II stage and cannot resume meiosis without fertilization or artificial stimuli. It is essential to understand the activation of oocytes for the success of animal cloning by nuclear transfer. The first cloned pigs from somatic cells were produced by serial nuclear transfer strategy in which the second recipient oocyte was a zygote derived from in vivo (Polejava et al., 2000). Onishi et al. (2000) produced cloned piglets derived from in vivo matured oocytes oocytes by using an electric pulse for fusion and activation. Chemical activation of in vitro matured oocytes has also produced cloned piglets. In that study, calcium ionophore and 6-dimethylaminopurine (6-DMAP) were used to activate NT embryos (Betthauser et al. 2000). The electric pulse has been frequently used for the activation of NT embryos (Lai et al., 2002, Betthauser et al., 2002), but better development could be obtained by using the additional treatment of chemicals such as ionomycin and 6-DMAP. The electric stimulation of oocytes induces a single transient rise in intracellular calcium concentration (Swann and Ozil, 1994). Although a single calcium transient can induce the resumption of second meiosis, long lasting calcium oscillations produced by repetitive electric pulses facilitate pronucleus formation and later embryonic development, even in oocytes soon after ovulation that are resistant to parthenogenetic activation (Swann and Ozil, 1994; Ozil and Swann, 1995; Zhu et al., 2002). The treatment of activated oocytes with 6-DMAP can deplete MPF (maturation promoting factor) and keep it low longer (Grupen et al., 2002). Therefore, in this study we compared additional activation strategies with chemicals such as ionomycin and 6-DMAP, either in combination or alone, and then monitored calcium levels following activation.
Materials and Methods
In vitro maturation of oocytes
Prepubertal gilt ovaries were collected at a local abattoir and transported to the laboratory in 0.9% NaCl solution at 30 to 35 °C. Cumulus-oocytes complexes (COCs) were aspirated from 3 to 6 mm diameter antral follicles by using a 10 mL disposable syringe with an 18-gauge needle. COCs with an evenly distributed cytoplasm and at least three compact layers of cumulus cells were selected and washed three times in TL-Hepes supplemented with 0.1% (w/v) polyvinyl alcohol (PVA). Fifty to 70 oocytes were transferred into 500 μL of maturation medium (TCM-199; Gibco-BRL, Grand Island, NY, USA) that had been covered with mineral oil in a four-well multidish (Nunc, Roskilde, Denmark) per well. Oocytes were matured for 42 to 44 h at 38.5 °C under 5% CO2 in air. The TCM-199 was supplemented with PVA (0.1%), D-glucose (3.05 mM), sodium pyruvate (0.91mM), cysteine (0.57 mM), lutenizing hormone (0.5 μg/mL), follicle stimulating hormone (0.5 μg/mL), epidermal growth factor (10 ng/mL), penicillin G (75 μg/mL), and streptomycin (50 μg/mL).
Parthenogenetic activation
Oocytes matured for 42-44 h were denuded from cumulus cells by vigorous vortexing for 5 min in TL-Hepes supplemented with 0.1% PVA and 0.1% hyaluronidase. Cumulus-free oocytes with the first polar body were placed between 0.2-mm diameter platinum electrodes, 1 mm apart in activation medium. The medium used for activation was 0.3 M mannitol, supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. For activation, 2 DC pulses (1 sec interval) of 1.2 kV/cm for 30 μs were applied, by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA).
Estimation of calcium changes
Fura-2 AM and pluronic F-127 were from Molecular Probes (Eugene, OR). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO). Changes in the [Ca2+]i were estimated by using the Ca2+ indicator dye fura-2. Oocytes were incubated in the presence of 2 μM of the acetoxymethyl ester (AM) form of the dye together with 0.02% pluronic F-127 in TL-HEPES. After the dye was loaded, an oocyte was placed in the fusion medium or PZM-3, and then electric pulse was applied or chemicals, such as 6-DMAP and CH, were added. Fluroscence recording were performed by using a Photo scan-2 photon-counting fluorescence microscope system (Nikon Corp., Tokyo, Japan). Excitation wavelengths of 340 and 380 nm were alternated by a rotating chopper disk. The emitted fluorescence intensity was measured at 510 nm with a photomultiplier tube after background subtraction. [Ca2+]i is presented as ratio values of the 340/380 nm excitation intensities; ration of 1.2 and 6.5 correspond to 65 nM and 602 nM Ca2+, respectively (Machaty et al., 1997).
Preparation of donor cells
Ear skin cells were obtained from 4-day-old transgenic pig transduced with enhanced green fluorescent protein (eGFP) recombinant retrovirus (Cabot et al., 2001). The tissue was cut into small pieces with fine scissors. Cells were incubated for 30 min at 39 °C in PBS containing 0.05% trypsin and 0.02 mM EDTA (all chemicals, unless noted otherwise, were from Sigma Chemical Company, St. Louis, MO, USA), and the suspension was centrifuged at 300 × g for 10 min. The cell pellet was resuspended and cultured in Dulbecco's Modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with fetal calf serum (15%), penicillin G (75 μg/mL) and streptomycin (50 μg/mL). The cells were passaged three times, and then frozen by using DMEM supplemented with 10% dimethylsulfoxide (DMSO). To be used as donor cells in nuclear transfer, cells were thawed and cultured until they reached confluence. Before nuclear transfer, cells were treated with 0.05% trypsin and 0.02 mM EDTA for single-cell isolation.
Production of nuclear transfer embryos
After maturation, cumulus cells were freed by vigorous vortexing for 5 min in TL-Hepes supplemented with 0.1% PVA and 0.1% hyaluronidase. Enucleation and injection were carried out in TCM-199 supplemented with Hepes, 0.3% Bovine Serum Albumin, and 7.5 μg/mL cytochalasin B at 38 °C. Denuded oocytes with the first polar body were enucleated by aspirating the first polar body and neighboring cytoplasm in the micromanipulation medium with a glass pipette 30 μm in diameter. Round glossy cells were selected as donor cells and introduced into the perivitelline space of the enucleated recipient oocytes through the hole made at enucleation. Enucleated and injected oocytes were placed between 0.2-mm diameter platinum electrodes, 1 mm apart in fusion and activation medium. The medium used for fusion and activation was 0.3 M mannitol, supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. For fusion and activation, 2 DC pulses (1 sec interval) of 1.2 kV/cm for 30 μs were applied, by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA).
In vitro culture of embryos
After fusion/activation treatment, embryos were washed and transferred into 500 μL of each culture medium covered with mineral oil in a four-well multidish. Basic culture medium was PZM-3 (Im et al, 2004). The culture environment was 5% CO2 in air at 38.5 °C. NT embryos and parthenogenetically activated oocytes were evaluated for the cleavage rate on Day 3. Percent blastocyst and the number of nuclei in the blastocyst were determined on Day 6. Blasocysts were stained with 5 μg/mL of bisbenzmide (Hoechst 33342) to determine the number of nuclei in the blastocysts and evaluated by using an epifluorescent microscope (Nikon Melville, NY, USA).
Experimental design
Each experiment was replicated four or six times. Experiment 1 was carried out to investigate the development of electrically activated oocytes according to calcium concentration in fusion medium and activation methods were investigated. Fusion medium supplemented with either 0.1 or 1.0 mM Ca2+ was used. Treatments were E: two DC pulses of 120volts for 30μs, I: 10 μM ionomycin for 5min, D: 2mM 6- DMAP for 3h. In experiment 2, the development of porcine NT embryos activated with 6-DMAP at different times after fusion was investigated. NT embryos were activated with 6-DMAP either immediately after fusion pulses or after the completion of fusion. In experiment 3, calcium changes in oocytes activated with E or E + 6-DMAP in fusion medium were monitored.
Statistical analysis
To determine the statistical significance between treatment effects of the fusion rate, cleavage rate, developmental rate to the blastocyst stage, and the number of nuclei, all data were subjected to a Generalized Linear Model procedure (PROC-GLM) of the Statistical Analysis System (SAS Institute, Cary, NC, USA). Differences among treatment means were determined by using the Duncan's multiple range-test or t-test. All data were expressed as Least Square (LS) mean ± SEM. Differences among treatment effects were considered significant at P<0.05.
Results
Development of activated oocytes and NT embryos
In experiment 1, E+I+D showed a significantly higher cleavage rate (81.4 vs. 69.1 and 67.6%) than that of E or E+D, but there was no significant difference for the blastocyst rate (26.7 vs. 22.5%) between activation methods in the fusion medium supplemented with 0.1 mM calcium. For 1.0 mM calcium in fusion medium, there was no significant difference in cleavage rate, but E+D showed a higher blastocyst rate (35.6 vs. 19.8 and 28.3%) compared to E or E+I+D. The oocytes activated by electric pulses alone in the fusion medium supplemented with 1.0 mM calcium showed a higher blastocyst rate compared to 0.1 mM (Table 2). In experiment 2, we activated NT embryos with 6-DMAP for 2 h either immediately or 20 min following electric pulses for fusion. There was no significant difference for cleavage rate between treatments, but NT embryos activated with 6-DMAP 20 min following electric pulses for fusion showed a higher developmental rate to the blastocyst (19.9 vs. 12.3%) (Table 4).
Table 2.
Effect of 6-DMAP treatment time after fusion on the development of porcine nuclear transfer embryos
| Activation | No.(%) of fused oocytes | No.(%) of oocytes developed to | Nuclei No. | |
|---|---|---|---|---|
| ≥2cell | Blastocyst | |||
| C | 71/89(78.8±3.50) | 54/71(73.5±1.84)b | 7/71(10.0±1.23)b | 27.6±1.67 |
| D1 | 67/86(76.8±3.27) | 54/67(79.0±4.84)a | 8/67(12.3±0.70)b | 26.0±1.66 |
| D2 | 82/102(79.9±1.81) | 67/82(80.3±5.48)a | 15/82(19.9±2.66)a | 26.9±1.76) |
p<0.05 for treatments with different superscripts(SAS, GLM).
C: fusion and activation were induced simultaneously by using electric pulses only.
D1: fused embryos were treated with 6-DMAP immediately after applying electric pulses.
D2: fused embryos were treated with 6-DMAP 20min after fusion.
Monitoring changes of [Ca2+]i in the activated oocytes
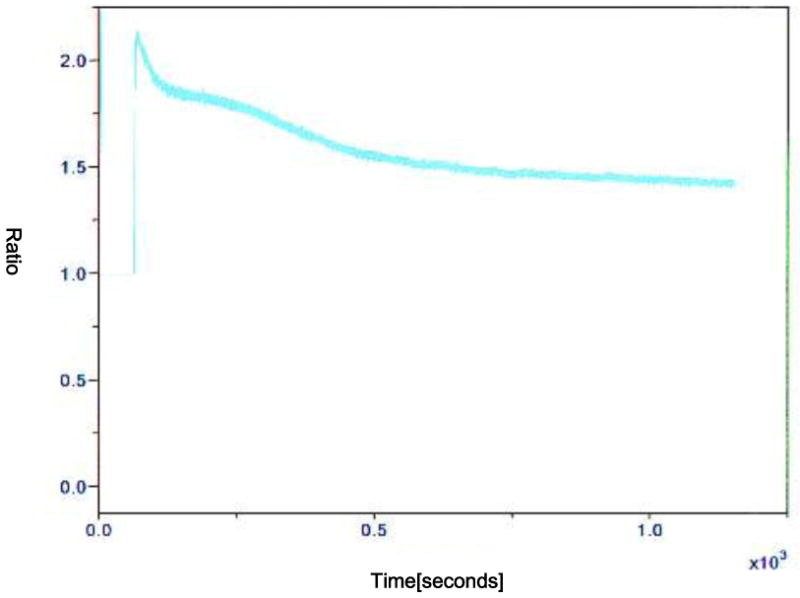
Activation by using one or two electric pulses showed a Ca2+ transient. Two consecutive pulses induced a higher and longer transient than one pulse (Figure 1 and 2). Also, 6-DMAP made a higher and wider transient compared to that induced by electric pulses (Figure 3). The fluctuation lasted during the time that oocytes were cultured in 6-DMAP. The oscillation started immediately following 6-DMAP administration, and the number of Ca2+ spikes per oocyte was 2. Characteristically, the second transient in each oocyte showed a wider amplitude than first one. The oscillation generally stopped after 2 transients, and Ca2+ drifted gradually downward and continued during the incubation time.
FIG. 1.
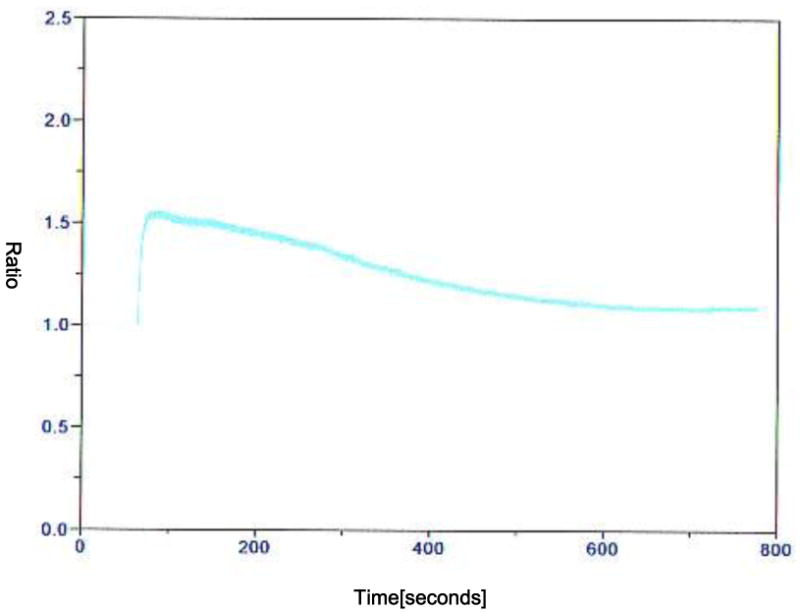
Representative recording of [Ca2+]i in a oocyte treated with a DC pulse of 1.2 kV/cm for 30 μs by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA). The recording was done in 0.3 M mannitol supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. The y-axis is the fluorescence ratio.
FIG. 2.
Representative recording of [Ca2+]i in a oocyte treated with 2 DC pulse (1 sec interval) of 1.2 kV/cm for 30 μs by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA). The recording was done in 0.3 M mannitol supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. The y-axis is the fluorescence ratio.
FIG. 3.

Representative recording of [Ca2+]i in a oocyte which was activated with 2 DC pulse (1 sec interval) of 1.2 kV/cm for 30 μs by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA), and then treated with 2 mM 6-DMAP immediately after the electric pulse. The recording was done in 0.3 M mannitol supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. The y-axis is the fluorescence ratio.
Discussion
Since the first successful cloned pig production by using somatic cells (Polejaeva et al., 2000), many cloned piglets have been born by the somatic cell NT technique. In most NT studies in pig, the fused NT embryos have been exposed to additional activation such as electric pulse, calcium ionophore A23187/cycloheximide or ionomycin/6-DMAP following fusion (Betthauser et al., 2000; Cheong et al., 2000; Koo et al., 2000). It has been reported that successful oocyte activation was affected by oocytes source (in vivo or in vitro), maturation time, electric field strength at fusion, and treatment time of chemical agents (Kühholzer et al., 2000; Grupen et al., 1999, 2000; Cheong et al., 2000; Koo et al., 2000). The initiation of mammalian oocyte activation is induced by an increase in intracellular free calcium concentration, which is triggered by an influx of extra cellular calcium (Sun et al., 1992). The rise in [Ca2+]i in oocytes is the pivotal signal in parthenogenetically activated or NT oocytes like fertilization in all mammalian oocytes. To alter intracellular calcium level of nuclear transfer embryos, NT embryos have been treated with electric pulse or chemicals.
In this study, oocytes activated in the medium containing 1.0 mM CaCl2 showed a significantly higher blastocyst formation rate than those fused/activated in the medium containing 0.1 mM CaCl2. This result indicates that calcium influx induced by electric pulse is transient and insufficient for the development of activated oocytes, and also consistent with previous result (Cheong et al. 2002) that reconstructed oocytes fused/activated in a medium containing 10 fold higher calcium concentration (1.0 mM) showed a higher development than those in a medium containing 0.1 mM CaCl2. In our study, an additional activation of ionomycin+6-DMAP or 6-DMAP alone increased blastocyst formation rates of activated oocytes. For 0.1 mM CaCl2, activated oocytes exposed to ionomycin+6-DMAP showed a higher blastocyst formation rate compared to those exposed to 6-DMAP alone. However, activated oocytes treated with 6-DMAP after fusion in medium containing 1.0 mM CaCl2 showed a significantly (P<0.05) higher blastocyst formation rate compared to those treated with ionomycin+6-DMAP. It shows that calcium concentration at fusion in the medium is important for activation and subsequent development of activated oocytes. It was suggested that the level of calcium stimulation could affect activation and development of oocytes, and insufficient or excessive calcium stimulation could decrease development of parthenogenetic oocytes (Collas et al., 1993). This results showed that a calcium concentration of 1.0 mM in fusion medium is enough to activate oocytes and for their subsequent development, however an additional activation, which could maintain the elevated calcium level in the cytoplasm of embryos following activation, is still needed for better subsequent development of activated oocytes.
For the development of NT embryos, 6-DMAP treatment 20 min after fusion supported a significantly higher blastocyst formation rate compared to electric pulse alone. However, NT embryos with an additional activation of 6-DMAP immediately after fusion did not show a significantly higher developmental rate than those fused/activated by electric pulse alone. This result showed that the timing of activation of NT embryos could affect the development to the blastocyst stage. Tani et al. (2001) reported that the direct exposure of donor chromosomes to nonactivated oocytes is effective for the reprogramming of bovine somatic cell nuclei, but activated cytoplasm does not reprogram the nucleus. Eleven to 29% of activated bovine oocytes receiving a donor cell stopped developing at the 8-cell stage, but 21 to 50% of nonactivated oocytes receiving donor cells irrespectively of cell cycles developed into blastocyst stage embryo. Yin et al. (2003) also reported that more reconstructed porcine oocytes developed to the blastocyst stage in the delayed activation condition than in the simultaneously activation condition. These reports indicated that the timing of activation is important for reprogramming of transferred donor nucleus and subsequent development to term in porcine NT (Yin et al., 2003; Kawahara et al., 2005).
In this study, we monitored changes of [Ca2+]i in oocytes activated with electric pulse, or E + 6-DMAP. Only a single Ca2+ transient was observed after an electric pulse and consecutive two pulses showed a single higher and longer transient than that of one pulse (Figure 1 and 2). A higher and wider transient was induced by additional activation of 6-DMAP compared to electric pulses (Figure 3), and the fluctuation lasted during the time that oocytes were in 6-DMAP. The single treatment with calcium ionophore, ionomycin or electric pulse induces meiotic resumption by the inactivation of histone H1 kinase, followed by reactivation of kinase, whereas repetitive calcium stimuli by electric pulses delays the reactivation. The persistent calcium level in the oocyte cytoplasm inactivates CSF, by which MPF is inactivated. Once MPF is inactivated, the protein synthesis/phosphorylation inhibitor treatment prevents regeneration of CSF (Soloy et al., 1997; Swann and Lai, 1997; Collas et al., 1993; Presicce and Yang, 1994a, b). It is also known that the protein kinase inhibitor, 6-DMAP prevents occurrence of GVBD by inhibiting phosphotyrosine dephosphorylation of p34cdc2 without any effects on protein synthesis (Jessus et. al., 1991).
Our result showed that 6-DMAP induced higher and wider Ca2+ transients in comparison with that produced by electric pulses. It means that 6-DMAP could activate oocytes by changing [Ca2+]i in oocytes as well as ihibiting phosphotyrosine dephosphorylation. These results indicated that additional activation by 6-DMAP following fusion could maintain the fluctuation line of calcium level and increase subsequent development of porcine preimplantation NT embryos.
FIG. 4.

Representative recording of [Ca2+]i in a oocyte which was activated with 2 DC pulse (1 sec interval) of 1.2 kV/cm for 30 μs by using a BTX-Cell Manipulator 200 (BTX, San Diego, CA, USA), and then treated with 2 mM 6-DMAP 20min after electric pulse. The recording was done in 0.3 M mannitol supplemented with 1.0 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. The y-axis is the fluorescence ratio.
Table 1.
Effect of calcium concentration in fusion medium and activation methods on the development of electrically activated porcine oocytes
| Calcium conc. (mM) | Activation method | No. of oocytes treated | No.(%±SE) of oocytes developed to | Nuclei No. | |
|---|---|---|---|---|---|
| ≥2cell | Blastocyst | ||||
| E | 103 | 73(69.1±2.02) bc | 13(12.5±1.74) c | 29.3±3.50 | |
| 0.1 | E+I+D | 106 | 86(81.4±3.75) a | 29(26.7±2.47) b | 25.0±1.75 |
| E+D | 105 | 72(67.6±2.43) c | 22(22.5±3.20) b | 25.4±2.36 | |
|
| |||||
| E | 120 | 96(77.8±3.42) | 19(19.8±1.95) b | 25.6±1.77 | |
| 1.0 | E+I+D | 112 | 95(84.2±3.16) | 31(28.3±1.41) b | 25.0±1.76 |
| E+D | 111 | 93(83.0±3.59) | 39(35.6±1.67) a | 23.5±1.65 | |
p<0.05 for treatments with different superscripts(SAS, GLM).
E: electric pulse, I: ionomycin, D: 6-DMAP.
Acknowledgments
This research was supported by a grant from NIH NCRR R01 RR13438 and Food for the 21st Century.
References
- Betthauser J, Forsberg E, Augenstein M, Childs L, Eilertsen K, Enos J, Forsythe T, Golueke P, Jurgella G, Koppang R, Lesmeister T, Mallon K, Mell G, Misica P, Pace M, Pfister-Genskow M, Strelchenko N, Voelker G, Watt S, Tompson S, Bishop M. Production of cloned pigs from in vitro systems. Nat Biotechnol. 2000;18:1055–1059. doi: 10.1038/80242. [DOI] [PubMed] [Google Scholar]
- Cabot RC, Kuholzer B, Chen AW, Lai L, Park KW, Chong KY, Schatten G, Murphy CN, Abeydeera LR, Day BN, Prather RS. Transgenic pigs produced using oocytes infected with retroviral vector. Anim Biotechnol. 2001;12:205–214. doi: 10.1081/ABIO-100108347. [DOI] [PubMed] [Google Scholar]
- Cheong HT, Ikeda K, Martinez Diaz MA, Katagiri S, Takahashi Y. Development of reconstituted pig embryos by nuclear transfer of cultured cumulus cells. Reprod Fertil Dev. 2000;12:15–20. doi: 10.1071/rd00051. [DOI] [PubMed] [Google Scholar]
- Cheong HT, Park KW, Im GS, Lai L, Sun QY, Day BN, Prather RS. Effect of elevated Ca(2+) concentration in fusion/activation medium on the fusion and development of porcine fetal fibroblast nuclear transfer embryos. Mol Reprod Dev. 2002;61:488–92. doi: 10.1002/mrd.10110. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Pounce de leon A, Robl JM. Cloed transgenic calves produced from non-quiescent fetal fibroblasts. Science. 1998;280:1256–1258. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- Collas P, Fissore R, Robl JM, Sullivan EJ, Barnes FL. Electrically induced calcium elevation, activation, and parthenogenetic development of bovine oocytes. Mol Reprod Dev. 1993;34:212–23. doi: 10.1002/mrd.1080340214. [DOI] [PubMed] [Google Scholar]
- De Sousa PA, Winger Q, Hill JR, Jones K, Watson AJ, Westhusin ME. Reprogramming of fibroblast nuclei after transfer into bovine oocytes. Cloning. 1999;1:63–69. doi: 10.1089/15204559950020102. [DOI] [PubMed] [Google Scholar]
- Grupen CG, Mau JC, McIlfatrick SM, Maddocks S, Nottle MB. Effect of 6-dimethylaminopurine on electrically activated in vitro matured porcine oocytes. Mol Reprod Dev. 2002;62:387–96. doi: 10.1002/mrd.10126. [DOI] [PubMed] [Google Scholar]
- Grupen CG, Verma PJ, Du ZT, McIlfatrick SM, Ashman RJ, Nottle MB. Activation of in vivo- and in vitro-derived porcine oocytes by using multiple electrical pulses. Reprod Fertil Dev. 1999;11:457–62. doi: 10.1071/rd00033. [DOI] [PubMed] [Google Scholar]
- Im GS, Lai L, Liu Z, Hao Y, Wax D, Bonk A, Prather RS. In vitro development of preimplantation porcine nuclear transfer embryos cultured in different media and gas atmospheres. Theriogenology. 2004;61:1125–1135. doi: 10.1016/j.theriogenology.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Jessus C, Rime H, Haccard O, Van Lint, Goris J, Merlevede W. Tyrosine phosphorylation of p34cdc2 and p42 during meiotic maturation of Xenopus oocyte. Antagonistic action of okadaic and 6-DMAP. Development. 1991;111:813–820. doi: 10.1242/dev.111.3.813. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Wakai T, Yamanaka K, Kobayashi J, Sugimura S, Shimizu T, Matumoto H, Kim JH, Sasada H, Sato E. Caffeine promotes premature chromosome condensation formation and in vitro development in porcine reconstructed embryos via a high level of maturation promoting factor activity during nuclear transfer. Reproduction. 2005;130:351–357. doi: 10.1530/rep.1.00644. [DOI] [PubMed] [Google Scholar]
- Koo DB, Kang YK, Choi YH, Park JS, Han SK, Park IY, Kim SU, Lee KK, Son DS, Chang WK, Han YM. In vitro development of reconstructed porcine oocytes after somatic cell nuclear transfer. Biol Reprod. 2000;63:986–92. doi: 10.1095/biolreprod63.4.986. [DOI] [PubMed] [Google Scholar]
- Kühholzer B, Tao T, Macháty Z, Hawley B, Greenstein J, Day BN, Prather RS. Production of transgenic porcine blastocysts by nuclear transfer. Mol Reprod Dev. 2000;56:145–148. doi: 10.1002/(SICI)1098-2795(200006)56:2<145::AID-MRD4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lai L, Colber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3 galactosyltransferase knockout pigs by nuclear transfer. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Machaty Z, Wang WH, Day BN, Prather RS. Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biol Repord. 1997;57:1123–1127. doi: 10.1095/biolreprod57.5.1123. [DOI] [PubMed] [Google Scholar]
- Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry ACF. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Swann K. Stimulation of repetitive calcium transients in mouse eggs. J Physiol. 1995;483:331–346. doi: 10.1113/jphysiol.1995.sp020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Presicce GA, Yang X. Nuclear dynamics of parthenogenesis of bovine oocytes matured for 20 and 40 hours and activated with combined ethanol and cycloheximide treatment. Mol Reprod Dev. 1994a;37:61–68. doi: 10.1002/mrd.1080370109. [DOI] [PubMed] [Google Scholar]
- Presicce GA, Yang X. Parthenogenetic development of bovine oocytes matured for 24hr and activated by ethanol and cycloheximide. Mol Reprod Dev. 1994b;38:380–385. doi: 10.1002/mrd.1080380405. [DOI] [PubMed] [Google Scholar]
- Soloy E, Kauka J, Viuff D, Simith SD, Calleson SD, Greve T. Time course of pronuclear deoxyribonucleic acid synthesis in parthenogenetically activated bovine oocytes. Biol Reprod. 1997;57:27–35. doi: 10.1095/biolreprod57.1.27. [DOI] [PubMed] [Google Scholar]
- Sun FZ, Hoyland J, Huang X, Mason W, Moor RM. A comparison of intracellular changes in porcine eggs after fertilization and electroactivation. Development. 1992;115:947–956. doi: 10.1242/dev.115.4.947. [DOI] [PubMed] [Google Scholar]
- Swan K, Lai FA. A novel signaling mechanism for generating Ca2+ oscillations at fertilization in mammals. Bioassays. 1997;19:371–378. doi: 10.1002/bies.950190504. [DOI] [PubMed] [Google Scholar]
- Swann K, Ozil JP. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:183–222. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001;64:324–330. doi: 10.1095/biolreprod64.1.324. [DOI] [PubMed] [Google Scholar]
- Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer cultured adult mural granulose cells. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Cho SK, Park MR, Im YJ, Park JJ, Bhak JS, Kwon DN, Jun SH, Kim NH, Kim JH. Nuclear remodeling and the developmental potential of nuclear transferred porcine oocytes under delayed-activated conditions. Zygote. 2003;11:167–174. doi: 10.1017/s096719940300220x. [DOI] [PubMed] [Google Scholar]
- Zakhartchenko V, Durcova-Hills G, Stojkovic M, Schernthaner W, Prelle K, Steinborn R, Muller M, Brem G, Wolf E. Effects of serum starvation and re-cloning on the efficiency of nuclear transfer using bovine fetal fibroblasts. J Reprod Fertil. 1999;115:325–331. doi: 10.1530/jrf.0.1150325. [DOI] [PubMed] [Google Scholar]
- Zhu J, Telfer EE, Fletcher J, Springbett A, Dobrinsky JR, De Sousa PA, Wilmut I. Improvement of an electrical activation protocol for porcine oocytes. Biol Reprod. 2002;66:635–41. doi: 10.1095/biolreprod66.3.635. [DOI] [PubMed] [Google Scholar]


