Abstract
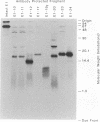
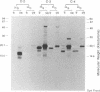
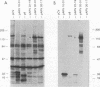
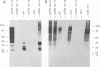
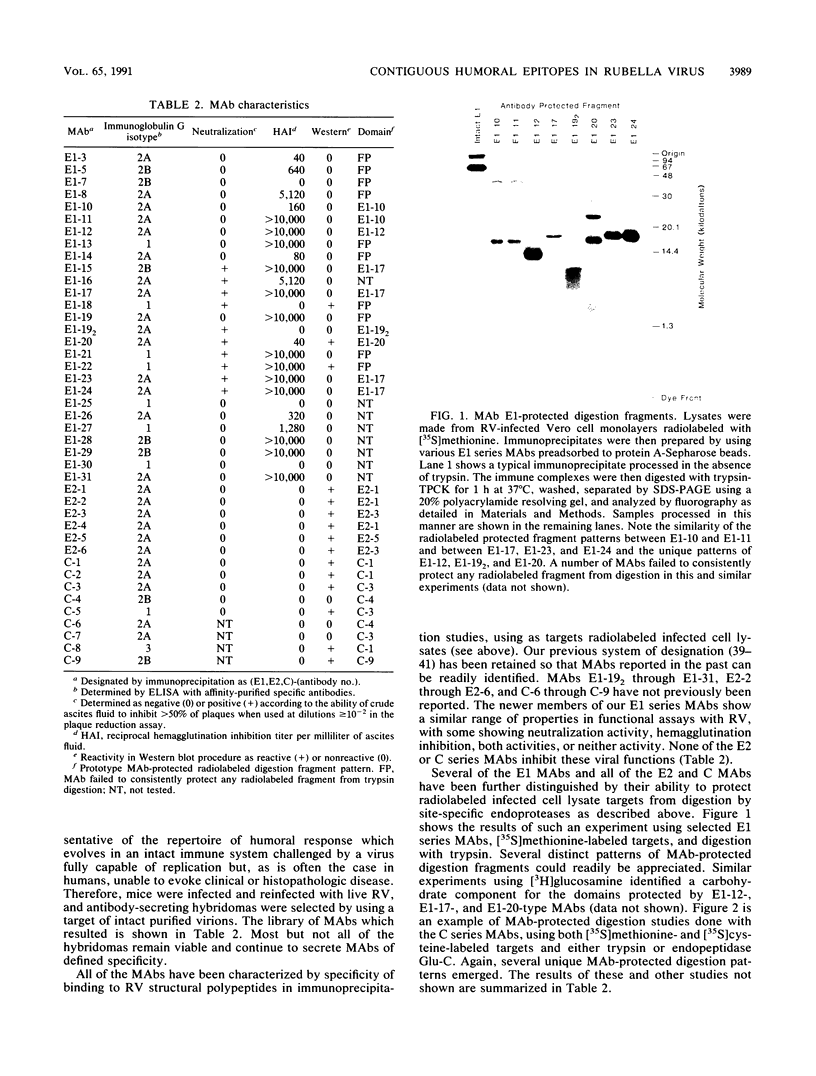
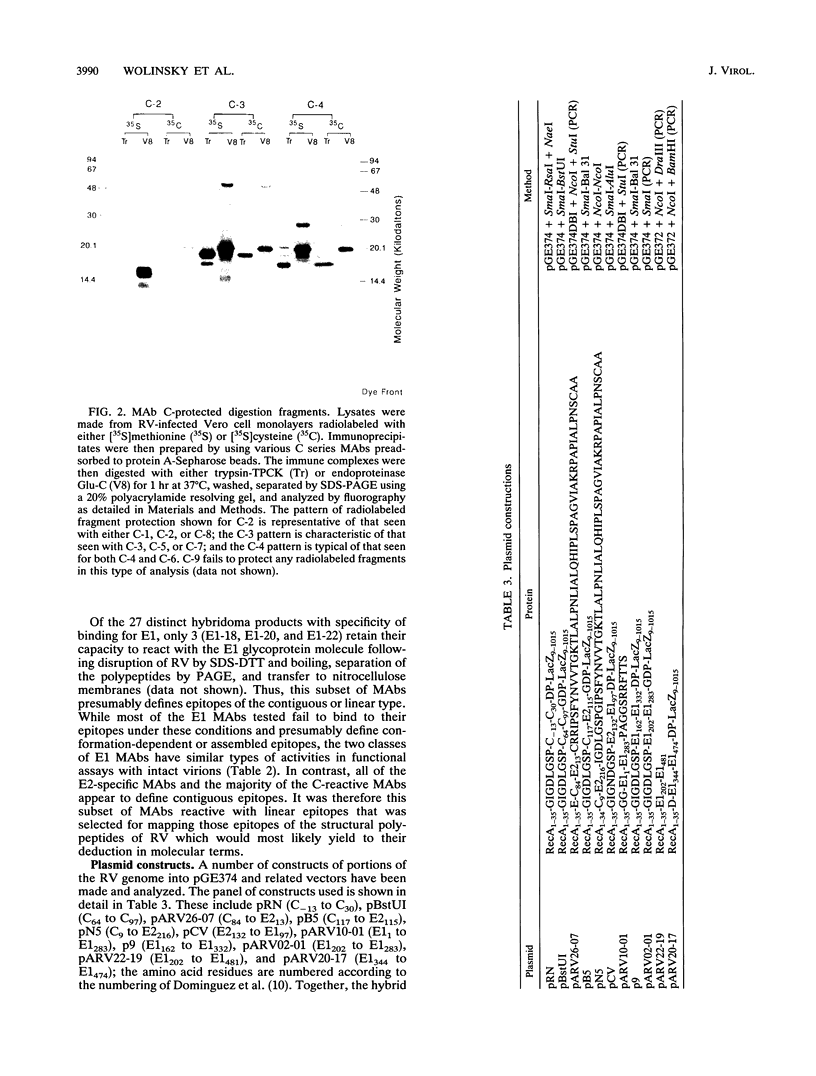
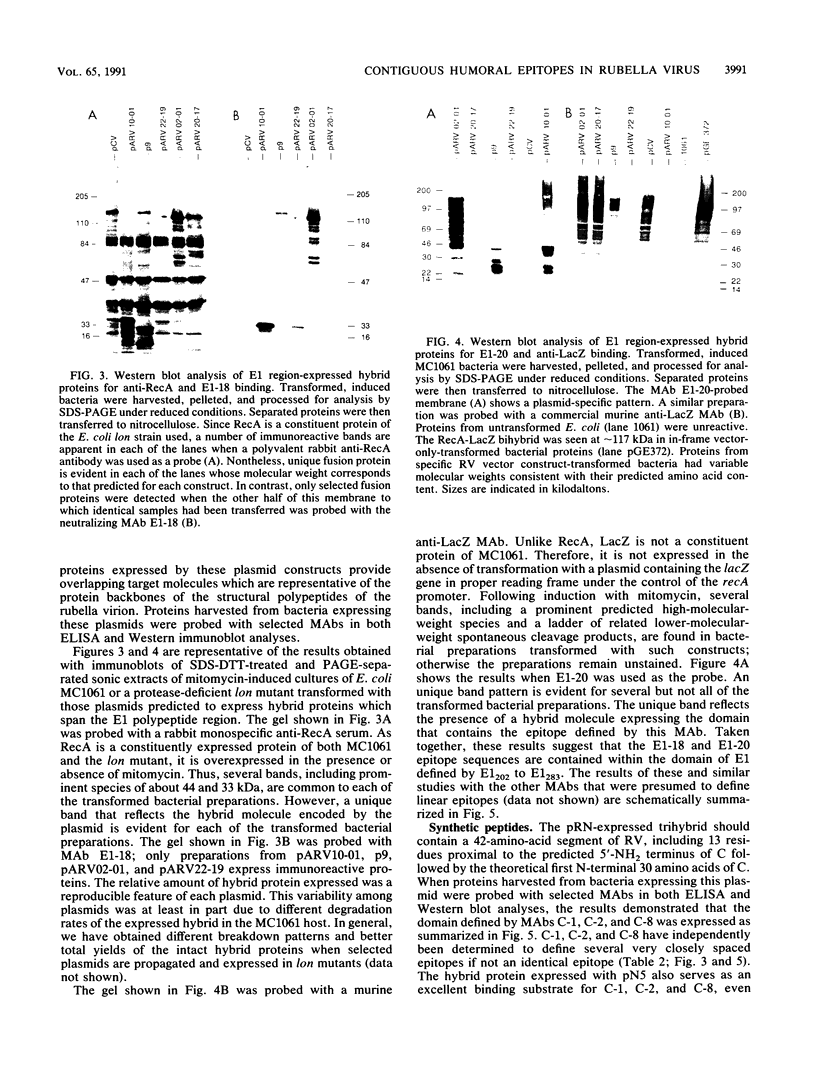
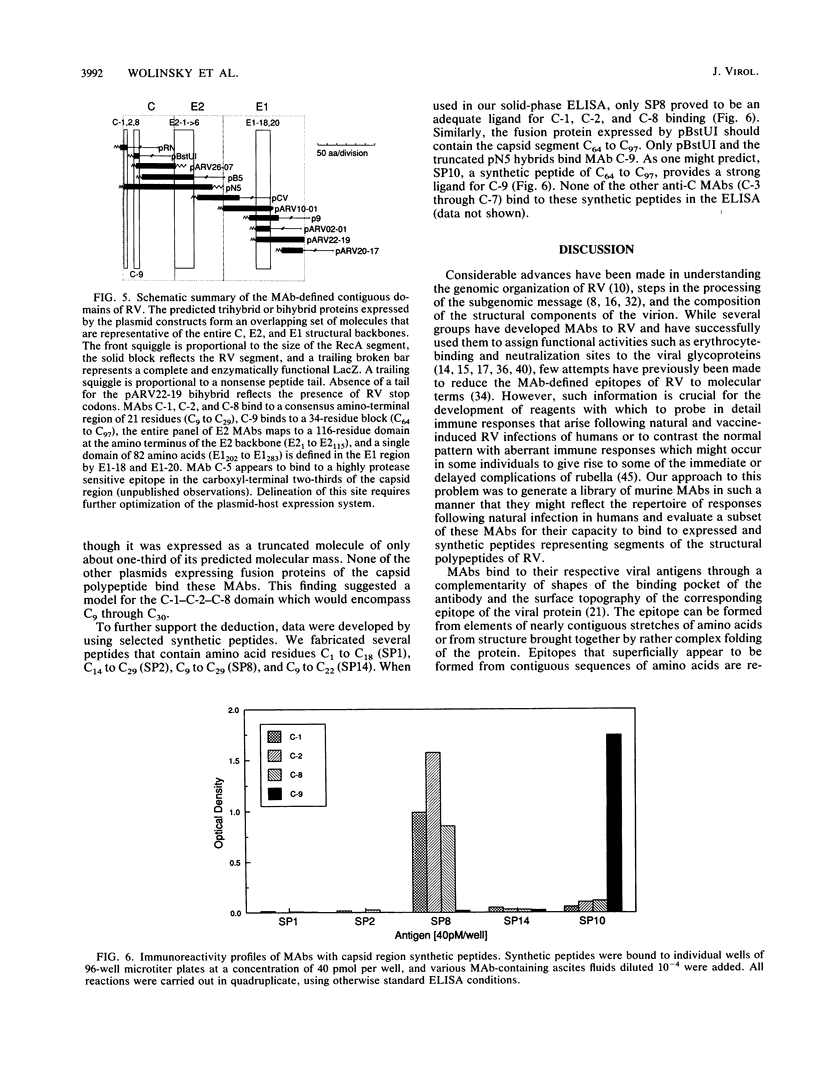
An expanded library of murine monoclonal antibodies (MAbs) was generated by infecting BALB/C mice with the Therien strain of rubella virus (RV) and selecting secreting hybrids by enzyme-linked immunosorbent assay (ELISA) using purified virion targets. A panel of plasmids containing specified RV cDNA fragments was also constructed by using a variety of strategies with pGE374- and pGE374-derived expression vectors. Hybrid RecA-RV-beta-galactosidase (LacZ)- or RecA-RV-truncated LacZ-containing proteins collectively representing the entire open reading frame of the structural proteins of RV were overexpressed in Escherichia coli. Bacterial lysates were then probed by ELISA with selected MAbs and by immunoblot following separation by electrophoresis under denaturing conditions. With this approach, MAbs that appeared to react with linear determinants defined epitopes localized within the following domains: MAbs C-1, C-2, and C-8 bind epitopes within the predicted amino-terminal 21 amino acids of the capsid region C9 to C29; MAb C-9 binds to a domain bounded by C64 and C97; MAbs E2-1 through E2-6 bind to the E2 glycoprotein backbone region from E2(1) to E2(115); MAbs E1-18 and E1-20 bind to the E1 glycoprotein region from E1(202) to E1(283). MAb E1-18 neutralizes RV infectivity; MAb E1-20 neutralizes infectivity and modestly inhibits hemagglutination. Analyses with selected synthetic peptides have confirmed several of the molecular domains deduced with the expressed proteins. These plasmid constructions and peptides have proven useful in beginning to unravel the molecular organization of several antigenic sites of this human pathogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden D. S., Westaway E. G. Rubella virus: structural and non-structural proteins. J Gen Virol. 1984 May;65(Pt 5):933–943. doi: 10.1099/0022-1317-65-5-933. [DOI] [PubMed] [Google Scholar]
- Bricker B. J., Snyder R. M., Fox J. W., Volk W. A., Wagner R. R. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus (New Jersey serotype): a method for preliminary mapping of epitopes. Virology. 1987 Dec;161(2):533–540. doi: 10.1016/0042-6822(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Caprioli R. M., Moore W. T. Continuous-flow fast atom bombardment mass spectrometry. Methods Enzymol. 1990;193:214–237. doi: 10.1016/0076-6879(90)93417-j. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Hui I., Chong P., Gillam S. Nucleotide sequence and in vitro expression of rubella virus 24S subgenomic messenger RNA encoding the structural proteins E1, E2 and C. Nucleic Acids Res. 1987 Apr 10;15(7):3041–3057. doi: 10.1093/nar/15.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., McDonald H., Gillam S. Expression of rubella virus cDNA coding for the structural proteins. Gene. 1988 May 15;65(1):23–30. doi: 10.1016/0378-1119(88)90413-1. [DOI] [PubMed] [Google Scholar]
- Dominguez G., Wang C. Y., Frey T. K. Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology. 1990 Jul;177(1):225–238. doi: 10.1016/0042-6822(90)90476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey T. K., Marr L. D., Hemphill M. L., Dominguez G. Molecular cloning and sequencing of the region of the rubella virus genome coding for glycoprotein E1. Virology. 1986 Oct 15;154(1):228–232. doi: 10.1016/0042-6822(86)90446-0. [DOI] [PubMed] [Google Scholar]
- Frey T. K., Marr L. D. Sequence of the region coding for virion proteins C and E2 and the carboxy terminus of the nonstructural proteins of rubella virus: comparison with alphaviruses. Gene. 1988;62(1):85–99. doi: 10.1016/0378-1119(88)90582-3. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gerna G., Revello M. G., Dovis M., Petruzzelli E., Achilli G., Percivalle E., Torsellini M. Synergistic neutralization of rubella virus by monoclonal antibodies to viral haemagglutinin. J Gen Virol. 1987 Jul;68(Pt 7):2007–2012. doi: 10.1099/0022-1317-68-7-2007. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Dorsett P. H. Rubella virus antigens: localization of epitopes involved in hemagglutination and neutralization by using monoclonal antibodies. J Virol. 1986 Mar;57(3):893–898. doi: 10.1128/jvi.57.3.893-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Terry L., Cohen A. Rubella virus haemagglutinin: association with a single virion glycoprotein. Arch Virol. 1985;84(3-4):207–215. doi: 10.1007/BF01378973. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Gillam S. In vitro and in vivo expression of rubella virus glycoprotein E2: the signal peptide is contained in the C-terminal region of capsid protein. Virology. 1989 Nov;173(1):241–250. doi: 10.1016/0042-6822(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Karounos D. G., Thomas J. W. Recognition of common islet antigen by autoantibodies from NOD mice and humans with IDDM. Diabetes. 1990 Sep;39(9):1085–1090. doi: 10.2337/diab.39.9.1085. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Perlman S., Weinstock G., DeVries J. R., Budzilowicz C., Weissemann J. M., Weiss S. R. Detection of a murine coronavirus nonstructural protein encoded in a downstream open reading frame. Virology. 1988 May;164(1):156–164. doi: 10.1016/0042-6822(88)90631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzi L., Rustici M., Corti M., Cusi M. G., Valensin P. E., Bracci L., Santucci A., Soldani P., Spreafico A., Neri P. Structure of rubella E1 glycoprotein epitopes established by multiple peptide synthesis. Arch Virol. 1990;110(3-4):271–276. doi: 10.1007/BF01311295. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Zheng D., Callahan L., Dave J. R., Liu T. Y. Rubella virus: mechanism of attenuation in the vaccine strain (HPV77). Virus Res. 1989 Jul;13(3):231–243. doi: 10.1016/0168-1702(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Nath A., Slagle B., Wolinsky J. S. Anti-idiotypic antibodies to rubella virus. Arch Virol. 1989;107(1-2):159–167. doi: 10.1007/BF01313888. [DOI] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Top Microbiol Immunol. 1989;145:127–135. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Payne L. G. Protein antigen-monoclonal antibody contact sites investigated by limited proteolysis of monoclonal antibody-bound antigen: protein "footprinting". Proc Natl Acad Sci U S A. 1988 Jan;85(1):1–5. doi: 10.1073/pnas.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H., Baron M. D. The E2 signal sequence of rubella virus remains part of the capsid protein and confers membrane association in vitro. J Virol. 1990 Nov;64(11):5500–5509. doi: 10.1128/jvi.64.11.5500-5509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Vidgren G., Ekstrand J., Hellman U., Kalkkinen N., Wernstedt C., Pettersson R. F. Nucleotide sequence of the rubella virus capsid protein gene reveals an unusually high G/C content. J Gen Virol. 1988 Mar;69(Pt 3):603–612. doi: 10.1099/0022-1317-69-3-603. [DOI] [PubMed] [Google Scholar]
- Terry G. M., Ho-Terry L., Londesborough P., Rees K. R. Localization of the rubella E1 epitopes. Arch Virol. 1988;98(3-4):189–197. doi: 10.1007/BF01322168. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Nadon F., Séguin C., Amarouch A., Payment P., Gillam S. E1 glycoprotein of rubella virus carries an epitope that binds a neutralizing antibody. J Virol Methods. 1985 Dec;12(3-4):243–250. doi: 10.1016/0166-0934(85)90135-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Hovi T. Structural proteins and subunits of rubella virus. J Virol. 1972 Jan;9(1):10–16. doi: 10.1128/jvi.9.1.10-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidgren G., Takkinen K., Kalkkinen N., Käriäinen L., Pettersson R. F. Nucleotide sequence of the genes coding for the membrane glycoproteins E1 and E2 of rubella virus. J Gen Virol. 1987 Sep;68(Pt 9):2347–2357. doi: 10.1099/0022-1317-68-9-2347. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. A model of the structural organization of rubella virions. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S133–S139. doi: 10.1093/clinids/7.supplement_1.s133. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Detailed immunologic analysis of the structural polypeptides of rubella virus using monoclonal antibodies. Virology. 1985 May;143(1):153–165. doi: 10.1016/0042-6822(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Immunochemical identification of rubella virus hemagglutinin. Virology. 1983 Apr 15;126(1):194–203. doi: 10.1016/0042-6822(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg R. A., Roberts M. A., Elliott L. B., Little L. M. Comparative evaluation of commercial rubella virus antibody kits. J Clin Microbiol. 1985 Feb;21(2):161–163. doi: 10.1128/jcm.21.2.161-163.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky J. S. Subacute sclerosing panencephalitis, progressive rubella panencephalitis, and multifocal leukoencephalopathy. Res Publ Assoc Res Nerv Ment Dis. 1990;68:259–268. [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mazancourt A., Waxham M. N., Nicolas J. C., Wolinsky J. S. Antibody response to the rubella virus structural proteins in infants with the congenital rubella syndrome. J Med Virol. 1986 Jun;19(2):111–122. doi: 10.1002/jmv.1890190203. [DOI] [PubMed] [Google Scholar]