Abstract
The transition of oocytes from meiosis I (MI) to meiosis II (MII) requires partial cyclin B degradation to allow MI exit without S phase entry. Rapid reaccumulation of cyclin B allows direct progression into MII, producing a cytostatic factor (CSF)-arrested egg. It has been reported that dampened translation of the anaphase-promoting complex (APC) inhibitor Emi2 at MI allows partial APC activation and MI exit. We have detected active Emi2 translation at MI and show that Emi2 levels in MI are mainly controlled by regulated degradation. Emi2 degradation in MI depends not on Ca2+/calmodulin-dependent protein kinase II (CaMKII), but on Cdc2-mediated phosphorylation of multiple sites within Emi2. As in MII, this phosphorylation is antagonized by Mos-mediated recruitment of PP2A to Emi2. Higher Cdc2 kinase activity in MI than MII allows sufficient Emi2 phosphorylation to destabilize Emi2 in MI. At MI anaphase, APC-mediated degradation of cyclin B decreases Cdc2 activity, enabling Cdc2-mediated Emi2 phosphorylation to be successfully antagonized by Mos-mediated PP2A recruitment. These data suggest a model of APC autoinhibition mediated by stabilization of Emi2; Emi2 proteins accumulate at MI exit and inhibit APC activity sufficiently to prevent complete degradation of cyclin B, allowing MI exit while preventing interphase before MII entry.
INTRODUCTION
The process of vertebrate oocyte maturation, which produces a haploid gamete, is characterized by two consecutive M phases, meiosis I (MI) and meiosis II (MII), without an intervening interphase. To generate an egg competent for fertilization, the nascent oocyte must undergo entry into MI, transit from MI to MII, and finally, an arrest in metaphase of MII. Failure to complete any of these key cell cycle events prevents normal egg production. MI entry is driven by the Cdc2/cyclin B kinase, the molecular components of an activity known as maturation promoting factor (MPF; Masui, 2001; Doree and Hunt, 2002; Jones, 2004). In the well-characterized Xenopus oocyte system, progesterone treatment initiates the translation of several proteins that trigger maturation, including cyclin B and the Mos kinase (Frank-Vaillant et al., 1999). Mos facilitates MI entry through activation of the ERK–MAPK pathway, which promotes Cdc2 activation by antagonizing its inhibitory kinase, Myt1, and by enhancing the activity of its activating phosphatase, Cdc25 (Sagata et al., 1988; Palmer et al., 1998; Peter et al., 2002). An additional Cdc2 activator, Ringo, has also been implicated in MI entry; this protein both drives maximal Cdc2 activation and renders Cdc2 less susceptible than cyclin-bound Cdc2 to the inhibitory action of Myt1 kinase (Ferby et al., 1999; Karaiskou et al., 2001).
In addition to playing a role in MI, Mos kinase is a critical component of cytostatic factor (CSF), an activity required for arrest in MII (Masui and Markert, 1971; Lorca et al., 1993). Although Mos has been long known to act as a constituent of CSF (through activation of the MAPK pathway, as in MI; Sagata et al., 1989; Haccard et al., 1993), its precise mechanism of action was not clear. Recently, it was shown that Mos helps to maintain MII arrest by inhibiting degradation of substrates of the anaphase promoting complex (APC), including cyclin B and a key regulator of chromosome segregation, securin. This APC inhibitory activity of Mos is exerted through a known inhibitor of the APC, Emi2, or Erp1. In MII, Mos promotes both the stability and activity of Emi2; the ability of Emi2 to inhibit the APC is regulated through phosphorylation of its C-terminus by Cdc2 and Mos enhances Emi2 function by facilitating its PP2A-mediated dephosphorylation (Inoue et al., 2007; Nishiyama et al., 2007; Wu et al., 2007a). Moreover, Mos helps to maintain steady-state levels of Emi2 by promoting the dephosphorylation of multiple Cdc2 sites on Emi2, which trigger slow degradation of Emi2 when Cdc2/cyclin B kinase levels rise above a certain threshold. At fertilization, a transient increase in cellular Ca2+ level activates calmodulin-dependent protein kinase II (CaMKII), which primes Emi2 for docking of the Polo-like kinase 1 (Plx1 in Xenopus) and subsequent Plx1-mediated Emi2 phosphorylation. This creates a phosphodegron for the E3 ligase SCFβTrcp, leading to proteasomal degradation (Liu and Maller, 2005; Rauh et al., 2005; Hansen et al., 2006). When Emi2 is degraded, the APC is fully activated, releasing eggs from MII into the first embryonic cell cycle (Wu et al., 2007a).
In comparison to MI entry and the CSF-induced MII arrest, the MI–MII transition is not well understood. However, errors in this transition, including both inappropriate MI arrest and failure to enter MII after MI exit are not uncommon and can lead to parthenogenesis and/or teratoma formation if abnormal oocytes are not properly eliminated (Hashimoto et al., 1994; Eppig et al., 1996). It is generally accepted that Mos is required for the MI–MII transition (Kanki and Donoghue, 1991; Hashimoto et al., 1994; Dupre et al., 2002) because ablation of Mos translation clearly results in a failure of MII entry (and a consequent artificial interphase). Moreover, maintaining residual Cdc2 kinase activity at MI exit is necessary, as complete inhibition, either by chemical inhibitors or by overexpression of the inhibitory kinase Wee l promotes an artificial interphase (Iwabuchi et al., 2000). One unsettled question, however, concerns the role of the APC in the MI–MII transition. Early studies in Xenopus oocytes indicated that the APC was dispensable for this transition, as neither antibody neutralization of the APC nor overexpression of its natural inhibitor, Mad2, inhibited the first meiotic anaphase (Peter et al., 2001; Taieb et al., 2001). This idea was challenged more recently by the discovery that activation of the spindle assembly checkpoint (SAC), that targets the APC in MI, could lead to MI arrest (Homer et al., 2005). Moreover, in 2006, studies in both the Xenopus and murine oocyte systems demonstrated a requirement for Emi2 in the MI–MII transition and suggested that not only is the APC activated at MI anaphase, but also that its timely inhibition by Emi2 is required to promote entry into MII (Madgwick et al., 2006; Ohe et al., 2007). Although Emi2 protein has been remarkably difficult to detect in MI oocytes, it was shown that ablation of Emi2 message using antisense morpholino oligonucleotides could promote exit from MI into interphase. Moreover, overexpression of Emi2 in the immature oocyte will promote an MI arrest upon progesterone treatment. These findings argued that Emi2 is most likely present at the end of MI to prevent complete APC-mediated degradation of cyclin B, which would lead to parthenogenetic activation of the oocytes. Conversely, in order to prevent the MI arrest that would occur if cyclin B degradation were to be completely inhibited, Emi2 levels must be tightly controlled to allow only partial APC inhibition.
Though it was initially reported that Emi2 protein was present throughout oocyte maturation, multiple groups have subsequently determined that significant accumulation of Emi2 protein is prevented during MI (Liu et al., 2006; Ohe et al., 2007; Tung et al., 2007). One of these groups reported that Emi2 levels are kept appropriately low in MI through the dampening of Emi2 mRNA translation (Ohe et al., 2007). Rapid translation at the onset of MII would then allow efficient CSF arrest. However, Tung et al. (2007) found that Emi2 mRNA polyadenylation, which governs the timing of translation, was controlled by Cdc2 and began immediately after MI entry, though Emi2 protein did not accumulate until the onset of MII. In this study, we demonstrate that translation of Emi2 does indeed occur during MI and that regulation of Emi2 levels in MI is exerted mainly at the level of protein stability. Throughout MI, Emi2 protein undergoes continuous and rapid turnover. Interestingly, we demonstrate that the same degron that controls precipitous degradation of Emi2 at exit from MII also regulates the continuous degradation of Emi2 before MI exit (Rauh et al., 2005). Moreover, this degradation is required to prevent inappropriate MI arrest. However, unlike degradation at MII exit, MI Emi2 degradation does not appear to require Ca2+/CaMKII. Rather, phosphorylation of four sites in the Emi2 N-terminus (213/239/252/267) primes the protein for degradation through the degron site. Moreover, Mos facilitates MII entry, in large part, by promoting Emi2 stabilization through PP2A-mediated dephosphorylation of these sites, a pathway similar to that which controls slow Cdc2-mediated Emi2 degradation during MII. In concert with these observations, we have found that overall Cdc2/cyclin B kinase activity (and consequent Emi2 phosphorylation) is higher in MI than MII, providing an explanation for the instability and low abundance of Emi2 in MI that is necessary to avoid inappropriate MI arrest. Additionally, the reduction in Cdc2 kinase activity at MII, relative to MI, allows Mos/PP2A-mediated dephosphorylation of Emi2 to predominate, enhancing Emi2 stability, and allowing the prolonged arrest characteristic of MII.
MATERIALS AND METHODS
Cloning, Protein Expression, and mRNA Preparation
Emi2 mutants including S213A/T239A/T252A/T267A, T545/551A, DS32AA, and T195A were cloned as previously described (Wu et al., 2007b), as were the Myc6-tagged Emi2 open reading frame (ORF), including its own 3′-untranslated region (UTR; WT and DS32AA) in pCS2+ vector (Tung et al., 2007). For mRNA synthesis, Emi2 ORFs (Emi2 aa 489-651, Emi2 wild-type, Emi2 DS32AA, and Emi2 4A) were PCR amplified and subcloned into the NotI site of the pSP64T vector. Constructs were digested with XbaI, and mRNAs were produced using mCAP RNA capping kit (Stratagene, La Jolla, CA).
35S-labeled Emi2 proteins were generated using the TNT Quick-Coupled Transcription/Translation System (Promega, Madison, WI) in the presence of 35S-labeled methionine/cysteine (MP Biomedicals, Solon, OH).
Recombinant GST-Emi2 proteins (aa 489-651 T545/551A, aa 319-375, and aa 319-375 ST335AA) were prepared as previously described (Wu et al., 2007a).
To ablate translation, 20 μM morpholino was injected to oocytes. Mos morpholino (AAGGCATTGCTGTGTGACTCGCTGA) and inverted Mos morpholino (AGTCGCTCAGTGTGTCGTTACGGAA) were purchased from Gene Tools (Philomath, OR). Emi2 morpholino was prepared as previously described (Wu et al., 2007a).
Oocyte Injections and Lysate Preparation
Stage VI oocytes were treated with 2.8 U of liberase in OR-2 buffer (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5) for 1.5 h at room temperature, washed extensively with OR-2 buffer, and stored in OR-2 buffer with 10% fetal bovine serum and 0.5% gentamicin at 18°C. Oocyte lysate was made by crushing oocytes in oocyte lysis buffer (20 mM HEPES KOH, pH 7.5, 20 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM PMSF, and 5 ng/μl aprotinin/leupeptin). Lysate was clarified by centrifugation to remove insoluble material. For Western blot analysis, two oocytes equivalent was loaded to each lane. For autoradiography analysis, eight oocytes equivalent was loaded. To make MI extract, oocytes were treated with progesterone, and lysate was made immediately after Germinal Vesicle Breakdown (GVBD). To make MII extract, lysate was made 4 h after GVBD.
Immunoblot and Immunoprecipitation Analysis
The antibodies used for immunoblotting were as follows: mouse anti-cyclin B2 (Casaletto et al., 2005), rabbit anti-Emi2 (Tung et al., 2005), mouse anti-Cdc27 (BD Biosciences, San Diego, CA), mouse anti-PP2A (Upstate Biotechnology, Lake Placid, NY), rabbit anti-phospho-MAPK (Cell Signaling Technology, Beverly, MA), rabbit anti-phospho-Cdc2 (Cell Signaling Technology), mouse anti-Myc (Santa Cruz biotechnology, Santa Cruz, CA), and mouse anti-Rsk (Santa Cruz).
Myc-Emi2 was immunoprecipitated using anti-Myc-tag-Agarose (Molecular Biology Laboratory (Heidelberg, Germany). Lambda phosphatase (New England Biolabs, Ipswich, MA) treatment was performed according to the manufacturer's instructions.
HHI Kinase Assay
Oocytes lysate was made from five oocytes per sample and flash-frozen until processed. HI kinase reaction mix (15 μl; final concentrations: 10 mM HEPES KOH, pH 7.2, 5 mM MgCl2, 50 mM NaCl, 83 μM ATP, 4.2 mM DTT, 5 μg of histone HI) and 2 μCi [γ-32P]ATP were added to the extract, and the reaction was incubated at room temperature for 10 min. Samples were resolved by SDS-PAGE, and the bands corresponding to HHI were quantified with a phosphorimager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Tight Regulation of Emi2 Levels Is Critical for Ensuring a Smooth MI–MII Transition
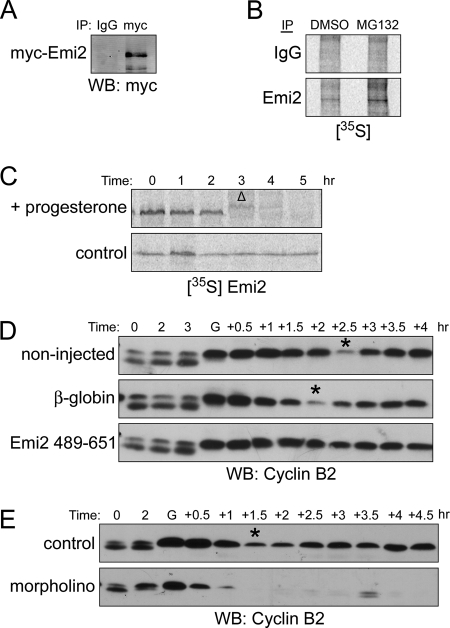
The very rapid accumulation of Emi2 observed upon transition of oocytes from MI into MII led us reexamine the notion that translation inhibition was the primary mechanism for restraining Emi2 abundance in MI. To monitor Emi2 translation, G2/prophase-arrested oocytes were injected with Myc-tagged Emi2 mRNA containing its own 3′-UTR, which is known to regulate endogenous Emi2 translation (Tung et al., 2007). After treatment with progesterone, oocytes were soaked in the proteasome inhibitor MG132 to inhibit any possible proteasomal degradation. Within 1 h after GVBD (oocytes typically reached anaphase of MI 1.5–2.5 h after visual GVBD), we were able to detect a significant accumulation of Emi2 protein (Figure 1A). Consistent with the idea that Emi2 could indeed be translated at MI, we found that newly synthesized endogenous Emi2 could be immunoprecipitated from MI oocytes that had been soaked in [35S]methionine/cysteine and treated with MG132 (Figure 1B). These data suggested that Emi2 translation could occur in MI, potentially resulting in sufficient Emi2 protein accumulation to prevent exit from MI (see below). These data also raised the possibility that controlled Emi2 degradation might be necessary at MI to prevent inappropriate Emi2 accumulation. To test this, we injected oocytes with 35S-labeled, in vitro–translated Emi2 and monitored its stability. As shown in Figure 1C, Emi2 was quickly degraded after GVBD in progesterone-treated oocytes, but remained stable in the untreated controls. To demonstrate the relevance of Emi2 regulation in the MI–MII transition, we injected oocytes with Emi2 (489-651) mRNA encoding a fragment of Emi2 known to be nondegradable in MII, but able to inhibit the APC (Wu et al., 2007b). In control oocytes (either uninjected or injected with β-globin control mRNA), cyclin B degradation, indicative of APC activation, occurred approximately 2 h after visual GVBD, though it was not complete (as is characteristic of the MI–MII boundary). Cyclin B then reaccumulated as oocytes progressed into MII. In contrast, expression of Emi2 (489-651) protein in oocytes led to an MI arrest, as evidenced by the maintenance of high cyclin B levels (Figure 1D). Conversely, ablation of Emi2 translation by injection of Emi2-directed antisense morpholino oligonucleotides led to complete and rapid degradation of cyclin B at MI exit, causing an inappropriate exit into interphase and a failure to reaccumulate cyclin B (Figure 1E). These data indicate that the partial inhibition of the APC (and the resulting partial degradation of cyclin B typical of the MI–MII transition) depends on Emi2. Strict control over Emi2 levels appears to be critical for the MI–MII transition, because either overexpression or underexpression results in abnormal maturation.
Figure 1.
Translation and degradation of Emi2 in MI. (A) Oocytes were injected with Myc6-Emi2–3′-UTR mRNA and incubated overnight (0.3 ng/oocyte). Oocytes were treated with progesterone and monitored visually for GVBD. One hour after GVBD, oocytes were lysed, and lysates were incubated with anti-Myc or IgG coupled to protein A Sepharose beads for 2 h at 4°C. The beads were retrieved, washed, and treated with lambda phosphatase before Western Blot analysis. (B) One hundred oocytes were treated with 200 μM Mg132 or DMSO in the presence of 400 μCi [35S]methionine/cysteine. One hour after GVBD, oocytes were lysed, and lysates were incubated with anti-Emi2 or IgG coupled to protein A Sepharose beads for 2 h at 4°C. The beads were retrieved, washed and treated with lambda phosphatase before analysis by autoradiography. (C) 35S-labeled Emi2 was injected into oocytes that were subsequently treated with or without progesterone. At the indicated times, lysates were made and analyzed by autoradiography after SDS-PAGE. GVBD was monitored visually. Δ, GVBD. (D) Oocytes were injected with either β-globin or Flag-Emi2 (489-651) mRNA appended with β-globin 3′-UTR (0.3 ng/oocyte). After overnight incubation, oocytes were treated with progesterone. At the indicated times, lysates were made and analyzed by Western blotting. Asterisks indicate the transition from MI to MII. GVBD was monitored visually. G, GVBD. (E) Oocytes were injected with either Emi2 morpholino (20 μM) or control morpholino (20 μM). After 1-h incubation, oocytes were treated with progesterone. At the indicated times, lysates were made and analyzed by Western blotting. Asterisks indicate the transition from MI to MII. GVBD was monitored visually. G, GVBD.
Emi2 Degradation in MI Is Mediated through Cdk-mediated Phosphorylation of S213/T239/T252/T267
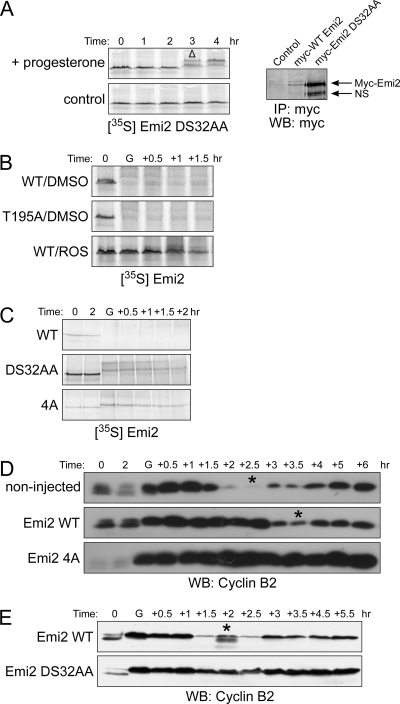
Rapid degradation of Emi2 is required for the release of eggs from MII arrest after fertilization by a well-characterized degradative pathway. To elucidate the mechanism underlying regulated Emi2 degradation in MI, we first wanted to know if the determinants were the same in MI and MII. As shown in Figure 2A (left panel), mutation of the previously identified degron at sites known to abrogate the required Plx-mediated phosphorylation that occurs in MII (changing D32 and S33 to alanine) also prevented Emi2 degradation in MI. Indeed, microinjection of this degron mutant Emi2 mRNA containing the Emi2 3′-UTR led to a significant accumulation of Emi2 protein only 1 h after GVBD, confirming the rapid synthesis of Emi2 at the time of passage through MI (Figure 2A, right panel). In contrast, mutation of the known site of CaMKII phosphorylation, T195, required for degradation at the time of fertilization, did not stabilize the protein, suggesting that CaMKII is not involved in the MI degradation pathway. We have also confirmed this conclusion using several CaMKII inhibitors, all of which failed to stabilize Emi2 in MI (data not shown). However, in the course of exploring this issue, we tested other kinase inhibitors and found that the CDK inhibitor, roscovitine, could fully stabilize Emi2, which strongly suggested a requirement for CDK activity (Cdc2 or Cdk2) in the MI Emi2 degradative pathway (Figure 2B).
Figure 2.
Emi2 degradation is mediated through Cdc2 phosphorylation on 213/239/252/267 sites. (A) Left, 35S-labeled DS32AA Emi2 protein was injected into oocytes, and samples were processed as in Figure 1C. GVBD was monitored visually. Δ, GVBD. Right, oocytes were injected with Myc6-Emi2-3′-UTR mRNA (wild-type or DS32AA). Samples were processed same as in Figure 1A. NS, nonspecific band. (B) Oocytes were injected with 35S-labeled Emi2 (WT or T195A). One hour after injection, oocytes were treated with progesterone in the presence or absence of 300 μM roscovitine. At the indicated times, lysates were made and analyzed by SDS-PAGE and autoradiography. GVBD was monitored visually. G, GVBD. (C) Oocytes were injected with 35S-labeled Emi2 (wild-type, DS32AA or 4A). One hour after protein injection, oocytes were treated with progesterone and samples were processed as in Figure 1B. (D) Oocytes were injected with Flag-Emi2 mRNA appended with β-globin 3′-UTR (wild-type or 4A; 0.3 ng/oocyte). After overnight incubation, oocytes were treated with progesterone, and samples were processed as in Figure 1D. (E) Oocyte were injected with Myc6-Emi2-3′-UTR mRNA (0.3 ng/oocyte) and incubated for 1 h. before progesterone treatment. Samples were processed as in Figure 1D.
We reported previously that when Cdc2 kinase activity exceeds a certain threshold, Emi2 undergoes slow degradation in MII, which is distinct from the precipitous degradation observed after fertilization. This slow degradation is triggered by phosphorylation of Emi2 by Cdc2 on four N-terminal sites in Emi2 (Wu et al., 2007a). To determine if these sites might also be relevant to MI Emi2 degradation, we generated 35S-labeled mutant Em2 in which the four Cdc2 phosphorylation sites had been mutated to alanine. As shown in Figure 2C, this mutant (hereafter referred to as 4A; note that these sites have been verified as Cdc2 sites previously; Wu et al., 2007a) was almost entirely stabilized in MI. To address the physiological relevance of phosphorylation at these four sites, we injected oocytes with either wild-type or 4A Emi2-encoding mRNA appended to the β-globin poly A tail (to abrogate any possible Emi2-specific translational control). Wild-type and 4A mutant Emi2-expressing mRNA accelerated GVBD, consistent with the existence of basal APC activity that limits cyclin B levels before MI entry (data not shown). Although expression of wild-type Emi2 somewhat delayed the MI–MII transition (most likely due to saturation of the Emi2 degradative pathway), expression of the 4A mutant Emi2 led to MI arrest, as evidenced by both a constant high level of cyclin B (Figure 2D) and phenotypic observation of the oocytes (data not shown). To further demonstrate that Emi2 degradation was indeed required for the onset of MI, we investigated the effects of replacing endogenous Emi2 mRNA with nondegradable Emi2. First, we monitored oocyte maturation after microinjection of oocytes with an antisense Emi2 morpholino oligonucleotide along with various levels of wild-type Emi2 mRNA appended to its own 3′-UTR (data not shown). With this approach, we were able to determine that the minimal level of wild-type Emi2 mRNA able to prevent the parthenogenesis resulting from ablation of Emi2 was 11 pg/oocyte. We then microinjected oocytes with the same amount of mRNA encoding nondegradable Emi2 (11 pg/oocyte) together with the Emi2-directed morpholino oligonucleotide. As shown in Figure 2E, the nondegradable Emi2 clearly prevented APC activation at MI anaphase, whereas wild-type Emi2 did not. These data are fully consistent with the notion that degradation of Emi2 is indeed required for the exit from MI.
Mos Promotes MII Entry by Promoting Stabilization of Emi2
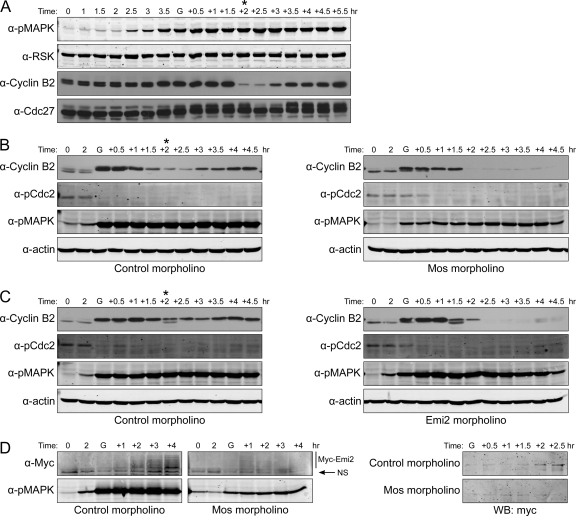
With the knowledge that Emi2 levels are controlled at the level of stability, we wanted to determine whether Mos controlled Emi2 stability in MI as it does in MII. As shown in Figure 3A, the Mos pathway is turned on soon after progesterone treatment, as indicated by activation of mitogen-activated protein kinase (MAPK; Figure 3A). Moreover, we could largely abrogate its activity by ablating its translation through morpholino oligonucleotide injection (on the basis of multiple experiments, we have routinely achieve a reduction in Mos activity of ∼75–95%, judging by the degree of MAPK phosphorylation). In the absence of Mos, oocytes were still able to initiate the maturation process (Figure 3B), though entry into MI (as indicated by GVBD) was significantly delayed (data not shown). However, oocytes lacking Mos were unable to transition appropriately to MII. Rather, oocytes exhibited complete cyclin B degradation, as was seen when Emi2 was ablated (Figure 3B). Conversely, when Emi2 expression was inhibited by injection with Emi2-directed morpholino oligonucleotides, Mos activity was unaffected, yet oocytes still failed to enter MII. These data demonstrate that the Mos–MAPK pathway itself is not sufficient to promote MII entry and strongly suggest that the role of Mos in promoting MII entry is mediated through Emi2. As Mos had been previously shown to regulate Emi2 stability in MII, we wanted to examine the effect of Mos on Emi2 stability at the end of MI. Accordingly, we again injected oocytes with Myc-tagged Emi2 mRNA appended to its own 3′-UTR, but then performed a second injection with either control or Mos-directed morpholino oligonucleotide. As shown in Figure 3D, loss of Mos resulted in a failure of Emi2 accumulation, most likely accounting for the failure in MII entry (note that samples in the right-hand panel of Figure 3D were treated with lambda phosphatase before SDS-PAGE to collapse the phosphorylated species into a single electrophoretic species). These data suggest that one crucial function of Mos in both blocking S phase initiation after MI and promoting entry into MII is to enable timely accumulation of Emi2, thereby allowing only partial, rather than full, cyclin B degradation.
Figure 3.
Mos ensures MII entry by promoting Emi2 stability. (A) Oocytes were treated with progesterone. At the indicated times, oocytes were lysed and analyzed by Western blotting. Asterisks indicate the transition from MI to MII. GVBD was monitored visually. G, GVBD. (B) Oocytes were injected with either Mos morpholino (20 μM) or control morpholino (20 μM). After 1-h incubation, oocytes were treated with progesterone. At the indicated times (G, GVBD), lysates were made and analyzed by Western blotting. In Mos morpholino injected oocytes in this experiment, phosphorylation of MAPK was reduced by 83%. Asterisks indicate the transition from MI to MII. GVBD was monitored visually. G, GVBD. (C) Oocytes were injected with either Emi2 morpholino (20 μM) or control morpholino (20 μM). Samples were taken at indicated times and analyzed by Western blotting. Asterisks indicate the transition from MI to MII. GVBD was monitored visually. G, GVBD. (D) Left, oocytes were first injected with Myc6-Emi2-3′-UTR mRNA (11 pg/oocyte). After overnight incubation, they were divided into two groups and injected with either control morpholino or Mos morpholino. In Mos morpholino injected oocytes in this experiment, phosphorylation of MAPK was reduced by 86%. One hour later, oocytes were treated with progesterone, and samples were taken at indicated times and analyzed by Western blotting. Right, before Western blot analysis, Myc-Emi2 was immunoprecipitated and treated with lambda phosphatase. GVBD was monitored visually. G, GVBD; NS, nonspecific band.
Differential Emi2 Stability in MI and MII Is Controlled by Different Levels of Cdc2 Activity
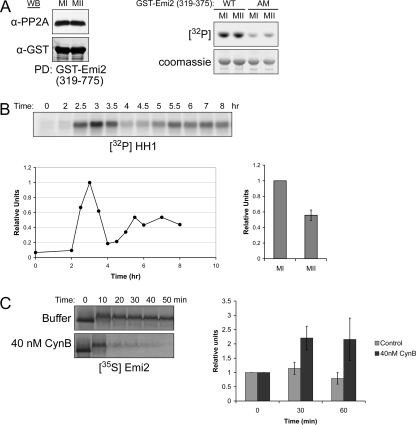
Taken together, our data suggested that Emi2 does not normally cause an arrest in MI because its levels are suppressed through Cdc2-mediated phosphorylation. Because our previous work demonstrated that this Cdc2-mediated pathway could also be operative during an MII arrest (Wu et al., 2007b), we were left with the perplexing question of why Emi2 was maintained at a level sufficient to produce an arrest in MII, but not in MI. We initially hypothesized that this difference might reflect differential activity of Mos or its effectors in MI and MII. From previous work, we knew that Mos could stabilize Emi2 by promoting its binding to PP2A (to catalyze dephosphorylation of the Cdc2 phosphorylation sites), and thus it was possible that decreased recruitment of PP2A to Emi2 in MI could enhance Emi2 turnover. To address this issue, we used the GST-Emi2 PP2A-binding domain (Emi2 aa 319-375) to retrieve PP2A from both MI and MII extracts (Wu et al., 2007a). As shown in Figure 4A, PP2A bound similarly to Emi2 in MI and MII. Consistent with these observations, Emi2-directed Rsk kinase activity, was also similar, based on in vitro kinase assays using Rsk immunoprecipitated from MI and MII extracts (Figure 4A, right). (A mutant lacking the Rsk phosphorylation site necessary for PP2A recruitment, AM, served as a negative control.) Alternatively, we considered the possibility that the magnitude of Cdc2 kinase activity might differ in these two consecutive phases. To address this, we treated oocytes with progesterone and followed the maturation process by withdrawing oocytes at different time points, preparing oocyte extract and then examining the activity of Cdc2 using histone HI as an exogenous substrate. Surprisingly, we consistently observed a twofold difference in histone HI-directed kinase activity between MI and MII, which had not been previously reported (Figure 4B). More importantly, we could fully recapitulate in MII extracts the rapid degradation of Emi2 observed in MI extracts by adding recombinant nondegradable cyclin B to induce Cdc2 kinase activity comparable to that observed in MI. Note that these extracts were also supplemented with a C-terminal nondegradable fragment of Emi2 to prevent the Cdc2-induced activation of the APC, which would promote degradation of endogenous cyclin B and thus down-regulate Cdc2 kinase activity as we previously reported (Wu et al., 2007b; Figure 4C). On the basis of these data, we conclude that the differential stability of Emi2 in MI and MII results from the combined facts that it is Cdc2 and not CaMKII that is the major determinant of Emi2 stability in MI and that Cdc2 kinase activity is sufficiently higher in MI than in MII to accelerate this degradative pathway (see model, Figure 5A). Whether there are other physiological consequences of differing kinase activity in MI and MII is an intriguing question that merits further investigation.
Figure 4.
Instability of Emi2 in MI results from high Cdc2 kinase activity. (A) Oocytes were treated with progesterone, and MI and MII lysates were prepared. Recombinant GST-Emi2 protein (aa 319-375), conjugated to glutathione Sepharose beads, was (left) incubated in either MI or MII extract for 1 h at 4°C. Beads were washed five times with PBS (supplemented with 300 mM NaCl and 0.1% Triton). The amount of bound PP2A was detected by immunoblotting. Right, Rsk kinase was immunoprecipitated from both MI and MII extracts, washed five times with PBS (supplemented with 300 mM NaCl and 0.1% Triton), and its activity was measured by in vitro kinase assay using recombinant GST-Emi2 (aa 489-651) wild-type or T545/551A as substrate. (B) Oocytes were treated with progesterone, and lysates were made at the indicated times. Cdc2 kinase activity was measured by in vitro HHI kinase assay followed by autoradiography (top). Results were quantified by phosphorimager (bottom, left). Quantification of the average Cdc2 kinase activity in MII relative to MI is shown on the bottom right; error bar, SD of three independent experiments. (C) Left, CSF extract supplemented with recombinant GST-Emi2 (aa 489-651) T545/551A mutant protein was treated with or without recombinant nondegradable cyclin B (40 nM). 35S-labeled Emi2 protein was added, and samples were taken at the indicated times and analyzed by SDS-PAGE and autoradiography. Right, Cdc2 kinase activity was measured by in vitro HHI kinase assay. Quantification of kinase activity was shown; error bar, SD of three independent experiments.
Figure 5.
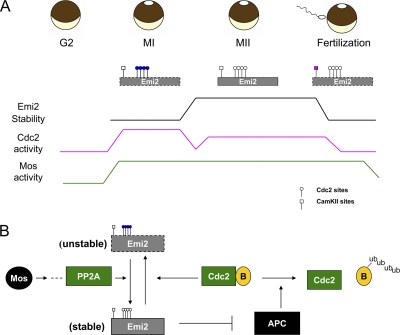
Regulation of Emi2 and the APC during the MI–MII transition. (A) Control of Emi2 stability during oocyte maturation. Emi2 stability is regulated by phosphorylation throughout the oocyte maturation process. On MI entry, high levels of Cdc2 kinase activity result in phosphorylation of four N-terminal sites (S213/T239/T252/T267) on Emi2, which leads to its degradation. Rapid degradation of Emi2 in MI is essential for MI exit, as its stabilization would lead to MI arrest. At the MI anaphase, Cdc2 kinase activity decreases as cyclin B is degraded by the APC. With Mos promoting its dephosphorylation, Emi2 is stabilized and accumulates, resulting in APC inhibition, which is necessary for S phase block and MII entry. In MII, Cdc2 kinase activity remains relatively low as compared with MI through an APC-mediated feedback loop we reported previously (Wu et al., 2007b). Emi2 is stable in MII, which is essential for CSF arrest. At fertilization, Emi2 is quickly degraded through the CaMKII mediated pathway, which allows full activation of APC and exit from MII. (B) Autoinhibitory regulation loop of APC. At MI anaphase onset, APC activation leads to degradation of cyclin B and a consequent decrease in Cdc2 Kinase activity. With the active Mos-PP2A pathway prevailing, Emi2 protein is dephosphorylated and stabilized, preventing the APC from completely degrading cyclin B; this is essential for the inhibition of S phase characteristic of the MI–MII transition.
DISCUSSION
It was previously reported that low Emi2 levels were maintained at MI through translational suppression. Rather, we report here that this control is exerted through Cdc2-regulated Emi2 degradation, which is required for exit from MI. Although some of the same determinants of Emi2 degradation appeared to operate in MI and MII, including a role for Mos in recruiting PP2A to Emi2, the absence of a role for CaMKII in controlling Emi2 stability in MI, coupled with the differential levels of Cdc2 kinase activity at these two cell cycle stages, appears to allow for subtle, but important differences in control of Emi2 abundance. These differences account, at least in part, for the ability of oocytes to exit MI, enter MII without an intervening interphase, and arrest for long periods in MII.
Cdc2 Kinase Activity in the Control of Emi2 Stability
Although we have not excluded the possibility that the translation of Emi2 is differentially regulated during MI and MII, it is clear that the rapid degradation of Emi2 is required to allow MI exit. This degradative process relies on the high Cdc2 kinase activity characteristic of MI. When Cdc2 kinase activity was raised in MII to mimic the higher levels found in MI, Emi2 was commensurately destabilized, consistent with this being a key determinant of Emi2 destabilization in MI. Although the Cdc2 kinase activity differed in MI and MII, we found that levels of cyclin B were very similar in MI and MII oocytes (Figure 3A; compare the 1.5- and 5.5-h time points). We speculate that the differing Cdc2 kinase activities at these two developmental stages could stem from higher levels of Ringo protein in MI than in MII (Gutierrez et al., 2006). Alternatively, regulation of Cdc2 by Cks proteins may also dictate differential kinase activity. It was reported that knockout of Cks2 in mouse promoted an MI arrest, though the reason for this was unclear (Spruck et al., 2003). It is possible that loss of Cks2 in the knockout lowered Cdc2 kinase activity in meiosis I, leading to inappropriate Emi2 stabilization. Finally, differential Wee1 levels may be another critical factor in determining Cdc2 activity (Kosaka et al., 2000). Because premature Wee1 expression arrests oocytes at MI, the MII-specific appearance of Wee1 could potentially lessen Emi2-directed Cdc2 kinase activity sufficiently to allow PP2A to prevail; this would maintain Emi2 in the stable and active configuration necessary for MII arrest.
Differential Control of the APC in MI and MII
In MII, when cyclin B levels rise through de novo synthesis, there is a feedback loop operative in which elevated Cdc2 kinase activity leads to Emi2 dissociation from the APC. This dissociation allows cyclin degradation sufficient to restore Cdc2 kinase activity to the baseline levels characteristic of CSF arrest. When Cdc2 kinase activity drops sufficiently, Emi2 reassociates with the APC, maintaining its inhibition. On the face of it, it is perplexing that all of the components critical for operation of this loop could potentially be present in MI, yet this feedback pathway does not appear to operate at this cell cycle stage. This is most likely due to the fact that the spindle checkpoint is operative in MI (Wassmann et al., 2003), but not during MII arrest (Tsurumi et al., 2004). This checkpoint results in profound APC inhibition until the metaphase I plate is formed, allowing constitutively high Cdc2 kinase activity and consequently rapid Emi2 turnover. Once the metaphase plate is formed and chromosomes are properly attached to the spindle, the checkpoint signal dissipates, leading to APC activation and cyclin B degradation. Only then is Emi2 able to accumulate, preventing complete degradation of cyclin B. This prevents interphase entry, allowing transition directly into MII.
Mos and the MI–MII Transition
Although Mos had been implicated in regulating the MI–MII transition, the mechanism was not clear. We have provided a distinct mechanism to explain the role of Mos in regulating this transition: Mos helps to stabilize Emi2 at the end of MI, thus maintaining Emi2 at levels that partially inhibit the APC, allowing incomplete cyclin B destruction. The ability of Mos to modulate Emi2 in MI appears to reside, as in MII, with the Rsk-mediated recruitment of PP2A to Emi2. Although Mos has been implicated in suppressing the Cdc2 inhibitor MytI at MI entry, because Mos-Rsk kinase activities appeared to be similar in MI and MII, Mos is unlikely to account for the observed differences in Cdc2 kinase activity between MI and MII (Palmer et al., 1998). In addition, similar Mos-mediated targeting of PP2A to Emi2 was observed in MI and MII. Thus, it is likely that similar phosphatase activities appear to be differentially effective given the different levels of antagonistic Cdc2 kinase activity. Taken together, these findings suggest that the smooth transition from MI to MII is a finely balanced process wherein higher Cdc2 kinase levels in MI than in MII renders Emi2 unstable in MI, but at the anaphase of MI, decreased kinase activity can be counterbalanced by Mos activity, which is critical to allow timely accumulation of Emi2 and the partial cyclin B degradation characteristic of the MI–MII transition.
An Autoinhibitory Loop Regulates APC Activity during the MI–MII Transition
Although exit from MI requires cyclin B degradation, residual cyclin B/Cdc2 kinase activity is known to be required for transiting from MI to MII. However, the mechanism underlying the delicate control of cyclin B degradation at MI–MII has not been clear. With the finding that Cdc2 and Mos coordinately control Emi2 stability, we now propose a model of APC-directed APC inhibition to ensure a smooth MI–MII transition. Before the onset of MI anaphase, Emi2 protein levels are held in check by Cdc2 kinase–mediated destabilization. APC activation then results in decreased Cdc2 kinase activity as cyclin B levels drop. With the Mos–PP2A pathway promoting Emi2 dephosphorylation, Emi2 can accumulate and effectively inhibit the APC. This leads to appropriately-timed stabilization of cyclin B and subsequent entry into MII without an intervening interphase. Although other regulatory pathways no doubt contribute to ensuring the smooth progression from MI to MII, this autoinhibitory regulation loop of APC plays critical role in regulating the meiotic transitions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant RO1 GM067225 to S.K.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-04-0417) on June 11, 2008.
REFERENCES
- Casaletto J. B., Nutt L. K., Wu Q., Moore J. D., Etkin L. D., Jackson P. K., Hunt T., Kornbluth S. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J. Cell Biol. 2005;169:61–71. doi: 10.1083/jcb.200411056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doree M., Hunt T. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 2002;115:2461–2464. doi: 10.1242/jcs.115.12.2461. [DOI] [PubMed] [Google Scholar]
- Dupre A., Jessus C., Ozon R., Haccard O. Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J. 2002;21:4026–4036. doi: 10.1093/emboj/cdf400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J. J., Wigglesworth K., Varnum D. S., Nadeau J. H. Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer Res. 1996;56:5047–5054. [PubMed] [Google Scholar]
- Ferby I., Blazquez M., Palmer A., Eritja R., Nebreda A. R. A novel p34(cdc2)-binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. Genes Dev. 1999;13:2177–2189. doi: 10.1101/gad.13.16.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Vaillant M., Jessus C., Ozon R., Maller J. L., Haccard O. Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. Mol. Biol. Cell. 1999;10:3279–3288. doi: 10.1091/mbc.10.10.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G. J., Vogtlin A., Castro A., Ferby I., Salvagiotto G., Ronai Z., Lorca T., Nebreda A. R. Meiotic regulation of the CDK activator RINGO/Speedy by ubiquitin-proteasome-mediated processing and degradation. Nat. Cell Biol. 2006;8:1084–1094. doi: 10.1038/ncb1472. [DOI] [PubMed] [Google Scholar]
- Haccard O., Sarcevic B., Lewellyn A., Hartley R., Roy L., Izumi T., Erikson E., Maller J. L. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Tung J. J., Jackson P. K. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Homer H. A., McDougall A., Levasseur M., Yallop K., Murdoch A. P., Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202–207. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D., Ohe M., Kanemori Y., Nobui T., Sagata N. A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature. 2007;446:1100–1104. doi: 10.1038/nature05688. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M., Ohsumi K., Yamamoto T. M., Sawada W., Kishimoto T. Residual Cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J. 2000;19:4513–4523. doi: 10.1093/emboj/19.17.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. T. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol. Hum. Reprod. 2004;10:1–5. doi: 10.1093/molehr/gah009. [DOI] [PubMed] [Google Scholar]
- Kanki J. P., Donoghue D. J. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc. Natl. Acad. Sci. USA. 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskou A., Perez L. H., Ferby I., Ozon R., Jessus C., Nebreda A. R. Differential regulation of Cdc2 and Cdk2 by RINGO and cyclins. J. Biol. Chem. 2001;276:36028–36034. doi: 10.1074/jbc.M104722200. [DOI] [PubMed] [Google Scholar]
- Kosaka Y., Yamaya M., Nakajoh K., Matsui T., Yanai M., Sasaki H. Prognosis of elderly patients with dysphagia in Japan. Gerontology. 2000;46:111–112. doi: 10.1159/000022144. [DOI] [PubMed] [Google Scholar]
- Liu J., Grimison B., Lewellyn A. L., Maller J. L. The anaphase-promoting complex/cyclosome inhibitor Emi2 is essential for meiotic but not mitotic cell cycles. J. Biol. Chem. 2006;281:34736–34741. doi: 10.1074/jbc.M606607200. [DOI] [PubMed] [Google Scholar]
- Liu J., Maller J. L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Lorca T., Cruzalegui F. H., Fesquet D., Cavadore J. C., Mery J., Means A., Doree M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Madgwick S., Hansen D. V., Levasseur M., Jackson P. K., Jones K. T. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol. 2006;174:791–801. doi: 10.1083/jcb.200604140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y. From oocyte maturation to the in vitro cell cycle: the history of discoveries of Maturation-Promoting Factor (MPF) and Cytostatic Factor (CSF) Differentiation. 2001;69:1–17. doi: 10.1046/j.1432-0436.2001.690101.x. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ohsumi K., Kishimoto T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- Ohe M., Inoue D., Kanemori Y., Sagata N. Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev. Biol. 2007;303:157–164. doi: 10.1016/j.ydbio.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Palmer A., Gavin A. C., Nebreda A. R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Castro A., Lorca T., Le Peuch C., Magnaghi-Jaulin L., Doree M., Labbe J. C. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 2001;3:83–87. doi: 10.1038/35050607. [DOI] [PubMed] [Google Scholar]
- Peter M., Labbe J. C., Doree M., Mandart E. A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. Development. 2002;129:2129–2139. doi: 10.1242/dev.129.9.2129. [DOI] [PubMed] [Google Scholar]
- Rauh N. R., Schmidt A., Bormann J., Nigg E. A., Mayer T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., de Miguel M. P., Smith A. P., Ryan A., Stein P., Schultz R. M., Lincoln A. J., Donovan P. J., Reed S. I. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science. 2003;300:647–650. doi: 10.1126/science.1084149. [DOI] [PubMed] [Google Scholar]
- Taieb F. E., Gross S. D., Lewellyn A. L., Maller J. L. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 2001;11:508–513. doi: 10.1016/s0960-9822(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Tsurumi C., Hoffmann S., Geley S., Graeser R., Polanski Z. The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J. Cell Biol. 2004;167:1037–1050. doi: 10.1083/jcb.200405165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. J., Hansen D. V., Ban K. H., Loktev A. V., Summers M. K., Adler J. R., 3rd, Jackson P. K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. J., Padmanabhan K., Hansen D. V., Richter J. D., Jackson P. K. Translational unmasking of Emi2 directs cytostatic factor arrest in meiosis II. Cell Cycle. 2007;6:725–731. doi: 10.4161/cc.6.6.3936. [DOI] [PubMed] [Google Scholar]
- Wassmann K., Niault T., Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr. Biol. 2003;13:1596–1608. doi: 10.1016/j.cub.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Hansen D. V., Guo Y., Wang M. Z., Tang W., Freel C. D., Tung J. J., Jackson P. K., Kornbluth S. Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc. Natl. Acad. Sci. USA. 2007a;104:16564–16569. doi: 10.1073/pnas.0707537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol. 2007b;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]