Abstract
Membrane type-1 matrix metalloproteinase (MT1-MMP) drives cell invasion through three-dimensional (3-D) extracellular matrix (ECM) barriers dominated by type I collagen or fibrin. Based largely on analyses of its impact on cell function under two-dimensional culture conditions, MT1-MMP is categorized as a multifunctional molecule with 1) a structurally distinct, N-terminal catalytic domain; 2) a C-terminal hemopexin domain that regulates substrate recognition as well as conformation; and 3) a type I transmembrane domain whose cytosolic tail controls protease trafficking and signaling cascades. The MT1-MMP domains that subserve cell trafficking through 3-D ECM barriers in vitro or in vivo, however, remain largely undefined. Herein, we demonstrate that collagen-invasive activity is not confined strictly to the catalytic, hemopexin, transmembrane, or cytosolic domain sequences of MT1-MMP. Indeed, even a secreted collagenase supports invasion when tethered to the cell surface in the absence of the MT1-MMP hemopexin, transmembrane, and cytosolic tail domains. By contrast, the ability of MT1-MMP to support fibrin-invasive activity diverges from collagenolytic potential, and alternatively, it requires the specific participation of MT-MMP catalytic and hemopexin domains. Hence, the tissue-invasive properties of MT1-MMP are unexpectedly embedded within distinct, but parsimonious, sequences that serve to tether the requisite matrix-degradative activity to the surface of migrating cells.
INTRODUCTION
Normal as well as neoplastic cells traverse interstitial tissues by mobilizing proteolytic enzymes that dissolve intervening structural barriers that are dominated by cross-linked networks of either type I collagen or fibrin (Hiraoka et al., 1998; Chun et al., 2004; Sabeh et al., 2004; Hotary et al., 2006; Itoh and Seiki, 2006). Although multiple proteinases have been implicated in tissue-invasive processes, recent studies suggest that the membrane type-1 matrix metalloproteinase (MT1-MMP) plays a critical role in conferring cells with the ability to remodel and penetrate extracellular matrix (ECM) barriers in vivo (Chun et al., 2004; Sabeh et al., 2004; Filippov et al., 2005; Hotary et al., 2006; Itoh and Seiki, 2006). Nonetheless, the critical structural and functional properties that imbue MT1-MMP with proinvasive activity and that distinguish it from the bulk of the >500 proteinases encoded in the mammalian genome remain largely undefined (Puente et al., 2003).
Similar in its overall domain structure to the larger family of secreted MMPs, the MT1-MMP zymogen is composed of a propeptide domain, a Zn-containing catalytic domain, a flexible linker peptide, and a hemopexin-like domain near its carboxy terminus (Itoh and Seiki, 2006). In contrast to the secreted MMPs, however, the MT1-MMP hemopexin domain is extended to include a glutamic acid-rich stem region that connects the extracellular face of the proteinase to a single-pass transmembrane segment that terminates in a short, 20-amino acid cytosolic tail (Itoh and Seiki, 2006). In its membrane-tethered configuration, studies to date have largely focused on the ability of MT1-MMP to bind and process the secreted metalloproteinase zymogens, MMP-2 and MMP-13, to catalytically active forms (Sato et al., 2005; Itoh and Seiki, 2006). The MT1-MMP catalytic domain displays, however, an unusually broad substrate specificity that not only allows the proteinase to hydrolyze directly multiple ECM components but also to cleave integrins, adhesion molecules, surface proteoglycans, and receptors as well as trigger signal transduction cascades (Sato et al., 2005; Itoh and Seiki, 2006; Basile et al., 2007; Nyalendo et al., 2007). Moreover, in a manner that further distinguishes MT1-MMP from secreted MMP family members, the MT1-MMP cytosolic tail has been proposed to control protease endocytosis and recycling, distribution at the cell surface, and interaction with intracellular signaling molecules (Itoh and Seiki, 2006; Nyalendo et al., 2007; D'Alessio et al., 2008).
As new insight into MT1-MMP structure and function has been brought to bear, increasingly complex schemes have been proposed to underlie the protease's impact on cell behavior (Itoh et al., 2006; Itoh and Seiki, 2006; Lafleur et al., 2006; Nyalendo et al., 2007; D'Alessio et al., 2008). Nonetheless, the majority of these studies have been restricted to analyses of cell–matrix interactions that take place under two-dimensional (2-D) culture conditions that either fail to recapitulate three-dimensional (3-D) ECM environments or with matrix composites devoid of the physiological cross-links that characterize normal tissues (Chun et al., 2006; Hotary et al., 2006; Yamada and Cukierman, 2007). Herein, we characterize MT1-MMP deletion mutants and chimeric constructs that serve to identify the key domains that underlie pericellular proteolysis, substrate specificity, and 3-D tissue-invasive activity in vitro and in vivo. Unexpectedly, by expressing active, membrane-tethered forms of a secreted collagenase on the surface of invasion-incompetent host cells, we find that collagen-invasive activity can be generated de novo independently of a specific requirement for the MT1-MMP catalytic, hemopexin, type I transmembrane, or cytosolic tail domains. By contrast, the structural rules underlying fibrin-invasive activity are more stringent in terms of requiring the participation of both MT-MMP–specific catalytic and hemopexin domains. These studies establish a new, and heretofore unpredicted, molecular basis for constructing the membrane-tethered, proteolytic machinery that arms mammalian cells with tissue-invasive activity in the 3-D setting.
MATERIALS AND METHODS
Cell Culture and Transfection
COS-1 and HT1080 cells were routinely maintained in complete DMEM supplemented with 10% fetal bovine serum (FBS). Cells were transfected transiently with FuGENE6 (Roche Diagnostics, Indianapolis, IN) according to manufacturer's instructions.
Construction of Expression Plasmids
Full-length MT1-MMP, hemagglutinin (HA)-tagged MT1-MMP, MT3-MMP, MMP-2, MMP-13, soluble MT1-MMP (MT1ΔTM), cytosolic tail truncated mutant MT1-MMP (MT1ΔCT), and catalytically inactive MT1-MMP (E/A; Glu240→A) were described previously (Hotary et al., 2000; Yana and Weiss, 2000; Chun et al., 2004). MT1ΔIS-2 (Pro163-Gly170 deleted) and MT1ΔpolyE (Glu519-Glu532 deleted) were constructed using the overlap extension method. Using overlapping primers with polymerase chain reaction (PCR) combination methods described previously (Pei and Weiss, 1995), MT1/MT3HPX was generated by combining fragments of MT1-MMP (Met1-Gly284) and MT3-MMP (Pro292-Val607). MT1/MMP-1CAT, MT1/MMP-1CATΔCT, and MT1/MMP-1CATΔTM were generated by combining HFC+10 (Met1-Tyr270) (Pei and Weiss, 1995) with fragments of MT1-MMP (Gly284-Val582), MT1ΔCT (Gly284-Arg563), or MT1ΔTM (Gly284-Gly535), respectively. MT1-MT6 · GPI was constructed by combining fragments of MT1-MMP (Met1-Gly535) and MT6-MMP (Asp531-Arg562) (Kojima et al., 2000). MT1-IL2R · CT was generated by combining MT1-MMP (Met1-Arg563) with the cytosolic tail of IL-2Rα (Ser257-Ile272) (Nakahara et al., 1997), whereas MT1-IL2R · TM was constructed by combining an MT1-MMP fusion construct between MT1-MMP (Met1-Arg535) and the transmembrane domain of IL2R (Nakahara et al., 1997) with the MT1-MMP cytosolic tail (Arg563-Val582). MT1ΔPEX (deleted Cys318-Gly535) was obtained from D. Q. Pei (University of Minnesota). MT1/MMP-1CATΔPEX was generated from MT1/MMP-1CAT by deleting the PEX domain of MT1 (Cys318-Gly535); MT1ΔPEXΔCT and MT1ΔPEX-MT6 · GPI were generated from MT1ΔCT and MT1-MT6 · GPI accordingly. All MT1-MMP mutants were subcloned into pCR3.1 vector for expression studies.
Collagen, Gelatin, and Fibrin Degradation Assays
Collagen gel films (∼100 μg/cm2) or fibrin films (∼150 μg/cm2) were labeled with Alexa Fluor-594 (Invitrogen, Carlsbad, CA) as described previously (Hotary et al., 2002; Sabeh et al., 2004). COS cells (5 × 104) were cultured atop the collagen films for 3 d in DMEM/10% FBS at either 37 or 25°C, and fluorescence images of labeled collagen were captured by laser confocal microscopy (Sabeh et al., 2004). Collagen degradation products were quantified by hydroxyproline release (Sabeh et al., 2004). Subjacent gelatin degradation was monitored as described previously (Yana and Weiss, 2000).
Invasion Assays
Collagen and fibrin invasion assays were performed as described previously (Hotary et al., 2000, 2002). In brief, 1 × 105 COS cells, suspended in DMEM/10% FBS, were added to the upper well of 24-mm Transwell dishes (Corning, Corning, NY) that contained either a 3-D gel of wild-type or r/r type I collagen (1 ml final volume of 2.2 mg/ml; Hotary et al., 2003) or cross-linked fibrin (1 ml final volume of 3.0 mg/ml). After a 3-d culture period, the number of invasive foci was counted in randomly selected high-powered fields (hpf) (Sabeh et al., 2004).
Gelatin Zymography, Western Blotting, and Antibodies
Gelatin zymography and Western blot analysis (using rabbit polyclonal antisera against the MT1-MMP hemopexin or catalytic domains) were performed as described previously (Lehti et al., 2000; Yana and Weiss, 2000). For MT1-MMP trafficking and cell surface localization studies, anti-HA antibody (12CA5; Roche Diagnostics) was tagged with an Alexa Fluor-488 antibody labeling kit (Invitrogen) according to the manufacturer's protocol.
Chick Chorioallantoic Membrane (CAM) Invasion Assays
COS cell invasion in vivo was assessed using the 11-d-old chick embryo CAM as described previously (Sabeh et al., 2004). COS cells (2 × 105/assay) were labeled with Fluoresbrite carboxylate microspheres (45 nm in diameter; Polysciences, Warrington, PA), and the percentage of invading cells (Inv) was quantified in three or more randomly selected fields (ImageQuant; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Depth of invasion from the CAM surface (invasion front [IF]) was defined as the leading front of three or more invading cells in randomly selected fields (1 U = 200 μm).
Cell Surface Biotinylation, MT1-MMP Internalization, and Recycling
Cell surface MT1-MMP expression was determined after surface biotinylation and immunoprecipitation as described previously (Yana and Weiss, 2000). For MT1-MMP internalization, cells were incubated for 1 h on ice in the presence of 0.5 mg/ml NHS-SS-biotin (Pierce Chemical, Rockford, IL) (Fabbri et al., 1999). Labeled cells were then washed and incubated at 37°C for the times indicated to allow for internalization. Samples were then washed and treated successively (20 min at 4°C) with a reducing solution (42 mM glutathione, 75 mM NaCl, 1 mM EDTA, 1% bovine serum albumin, and 75 mM NaOH) to strip biotinylated proteins from the cell surface. After a final wash, cells were lysed in 10 mM Tris, pH 7.2, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, and 1% Nonidet P-40. Biotinylated proteins were captured with streptavidin-Sepharose beads (Pierce Chemical), and recovered complexes were resolved under reducing conditions by SDS-PAGE followed by Western blot analysis (Fabbri et al., 1999). The total pool of biotinylated MT1-MMP was determined in samples where the incubation step with the reducing solution was omitted. For recycling studies, biotinylated surface proteins were internalized at 37°C, the cells washed in reducing buffer and the pool of internalized MT1-MMP allowed to recycle to the cell surface over a 60-min culture period at 37°C in duplicate samples. At the end of the recycling period, one of the two samples was reduced to quantify the amount of protein that remained intercellular, whereas the other sample was left unreduced to determine the total MT1-MMP pool size. The percentage of MT1-MMP internalized or externalized was estimated by densitometric analysis of immunoblots (Yana and Weiss, 2000).
To visualize trafficking of either HA-tagged MT1-MMP or MT1ΔCT in transfected COS cells, cell surface proteases were labeled with Alexa Fluor-tagged anti-HA antibody in the presence of 25 μg/ml Texas (TX) Red-transferrin (Tf) (Invitrogen) for 1 h at 4°C. After a 40-min incubation at 37°C, cells were fixed and processed for confocal imaging.
Small Interfering RNA (siRNA) Electroporation and Reverse Transcription (RT)-PCR Analysis
The antisense strand of siRNA was targeted against a 21-nt MT1-MMP sequence (5′-AAC AGG CAA AGC TGA TGC AGA-3′; nt 228–248). The nucleotide sequence was scrambled to generate an siRNA control sequence (5′-AAG TGA TCA AGC ACC GAA GAG-3′). Human (h)MMP-1 was targeted using 21-nt sequences (5′-AAG ATG TGG ACT TAG TCC AGA-3′). siRNA oligonucleotides (QIAGEN, Valencia, CA) were introduced into tumor cells (50–100 nM) using a nucleofector kit and electroporation (Amaxa Biosystems, Gaithersburg, MD) as described previously (Sabeh et al., 2004). Total RNA was isolated using TRIzol reagent (Invitrogen). RT-PCR was performed with 1 μg of total RNA and 10 μM of specific primers (Hotary et al., 2002) by using One-Step RT-PCR System reagent (Invitrogen).
RESULTS
MT1-MMP Uniquely Confers Tissue-invasive Activity In Vitro and In Vivo
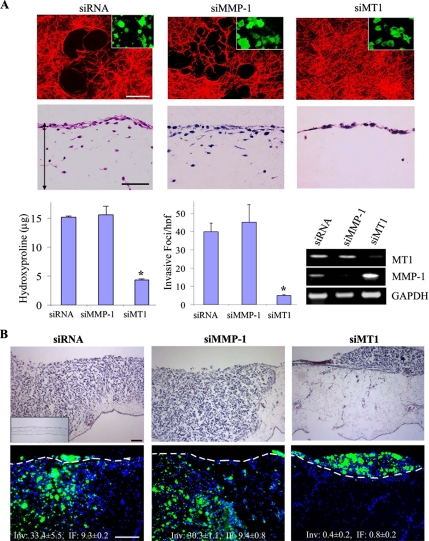
HT-1080 fibrosarcoma cells express multiple proteolytic activities as a consequence of their ability to synthesize members of all four major classes of proteinases (Sabeh et al., 2004; Hotary et al., 2006). Consequently, when cultured atop an interlocking network of type I collagen fibrils, HT-1080 cells display subjacent collagenolytic activity and express a collagen-invasive phenotype (Figure 1A, left-hand column). The invasive activity of HT-1080 cells is not limited to homogenous connective tissue constructs and can likewise be extended to more complex ECM barriers such as the CAM of the live chick (Figure 1B, left-hand column). Nonetheless, despite the ability of the fibrosarcoma cells to mobilize a broad repertoire of proteinases, HT-1080–dependent collagenolytic as well as tissue-invasive activities—in vitro or in vivo—are inhibited almost completely after MT1-MMP silencing (Figure 1, A and B, right-hand columns). The invasive activity of MT1-MMP siRNA-silenced cells is restored after transfection with an siRNA-resistant form of MT1-MMP (Supplemental Figure 1). By contrast, the siRNA-dependent targeting of MMP-1, a secreted collagenase previously implicated in invasive events (Jiang et al., 2005; Wyatt et al., 2005), neither affected collagenolysis nor invasion in either the in vitro or in vivo settings (Figure 1, A and B, middle columns). Proteinase- or MT1-MMP–independent mechanisms for traversing ECM constructs (Wolf et al., 2003; Wolf et al., 2007), a phenotype commonly observed when pepsin-extracted, rather than native, type I collagen is used as substrate (Sabeh et al., 2004), are not detected under any of the conditions studied (Figure 1, A and B).
Figure 1.
MT1-MMP confers tissue-invasive activity of tumor cells in vitro and in vivo. (A) HT-1080 cells were cotransfected with GFP and scrambled (siRNA), MT1-MMP(siMT1)–, or MMP-1(siMMP-1)–specific siRNA. Transfectants were then cultured for 3 d atop type I collagen films labeled with Alexa Fluor-594 (red) to assess subjacent proteolysis (representative laser confocal micrographs are shown in the first row) or 3-D type I collagen gels to monitor invasive activity (hematoxylin and eosin [H&E]-stained cross sections shown in the second row with the double-headed arrows demarcating the upper and lower boundaries of the collagen gels). Insets display the GFP-labeled transfectants (green) adherent to the underlying collagen film (bar, 50 μm). Collagenolytic activity is quantified by hydroxyproline release (mean μg ± SEM; n = 3), whereas invasion is expressed as the number of invasive foci/hpf (mean ± SEM of 5 randomly selected cross sections in a single representative experiment of 3 or more performed). MT1-MMP and MMP-1 expression in each of the transfectants was examined by RT-PCR (bottom right). (B) HT1080 cell transfectants were labeled with fluorescent nanobeads (green) and seeded atop the CAM of 11-d-old chicks. After a 3-d incubation period, cross sections were prepared and stained with H&E (top row; inset, CAM without HT-1080 cells) or treated with the nuclear stain 4,6-diamidino-2-phenylindole (DAPI) (blue), and then they were examined by fluorescence microscopy (bottom row). The dashed line shown in each image represents the outline of the CAM surface. Invasion is quantified as the number of HT1080 cells that cross the CAM surface (mean Inv ± SEM of 3 or more experiments) and average depth of the leading front of the invasive cells (mean IF ± SEM). Bar, 100 μm. *p < 0.01.
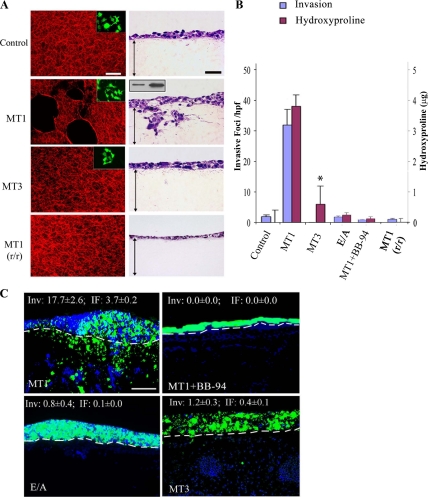
Given the multiplicity of proteases expressed by HT-1080 cells that might complement MT1-MMP activity, the ability of the protease to confer collagenolytic- and invasion-null COS cells with pericellular proteolytic activity was assessed. After transient transfection with wild-type (wt) MT1-MMP (at protein levels lower than those found on the surface of HT-1080 cells; Figure 2A, inset), COS cells degrade type I collagen, invade 3-D type I collagen gels, and traverse the CAM surface (Figure 2, A–C). Proteolysis, as well as invasion in vitro or in vivo, is blocked completely by the peptidomimetic MMP inhibitor, BB-94 (Sabeh et al., 2004) (Figure 2, B and C). Although a catalytic mutant of MT1-MMP devoid of proteolytic activity [i.e., MT1-MMP harboring an E240→A point mutation in its catalytic domain; MT1-MMP(E/A)] can trigger 2-D motility and cell signaling (Cao et al., 2004; D'Alessio et al., 2008), this construct is unable to support collagenolytic or invasive activity (Figure 2, A–C). MT1-MMP might conceivably regulate invasive activity independently of its collagenolytic activity (e.g., by cleaving integrins, CD44, or syndecans) (Itoh and Seiki, 2006), but MT1-MMP–expressing COS cells cultured atop collagenase-resistant r/r collagen (Hotary et al., 2003) are unable to degrade or invade a 3-D gel of the mutant collagen fibrils (Figure 2, A and B). Furthermore, despite the ability of MT1-MMP to process either the MMP-2 or MMP-13 zymogen to active collagenases (Itoh and Seiki, 2006; Gioia et al., 2007), COS cells engineered to coexpress either MT1-MMP and MMP-2, or MT1-MMP and MMP-13, did not increase their collagenolytic or invasive activities relative to MT1-MMP alone (Supplemental Figure 2). Recent studies have assigned a similar collagenolytic and invasive activity to MT3-MMP (Jiang and Pei, 2003; Shi et al., 2008), but COS cells transfected with wt MT3-MMP display limited type I collagen degradative activity and fail to confer tissue-invasive activity in vitro or in vivo (Figure 2, A–C). Likewise, COS cells cotransfected with MT3-MMP and MMP-2, although able to process the MMP zymogen to its active form, fail to increase collagenolytic or invasive activity (Supplemental Figure 2).
Figure 2.
MT1-MMP–mediated collagen degradation regulates invasive activity. (A) COS cells expressing MT1-MMP or MT3-MMP were cultured for 3 d atop a film of Fluor-594–labeled, acid-extracted wild-type or r/r collagen. Collagenolysis was visualized by confocal laser microscopy (left column; bar, 50 μm; insets, GFP-labeled COS transfectants). Alternatively, the cells were cultured atop a 3-D gel of wild-type or r/r collagen for 3 d, and H&E-stained cross sections were prepared (right column; bar, 50 μm). Double-headed arrows demarcate the upper and lower boundaries of the collagen gel. Inset shows cell surface MT1-MMP protein levels in 1 × 106 COS cells (left) transfected with MT1-MMP (>80% transfection efficiency) relative to HT-1080 cells as assessed by Western blot. (B) Collagen degradation and the invasive activity of cells expressing MT1-MMP (MT1), MT3-MMP (MT3), catalytically inactive MT1-MMP E/A (E/A), or MT1-MMP in the presence of BB-94 are expressed as mean micrograms of hydroxyproline ± SEM of three or more experiments, and mean number of invasive foci/hfp ± SEM of five randomly selected cross sections in a single representative experiment of three or more performed. (C) CAM invasion by COS cells expressing MT1-MMP, MT1-MMP in the presence of BB-94, MT1-MMP E/A, or MT3-MMP. Nanobead-labeled COS cells fluoresce green, whereas DAPI-stained chick and COS cell nuclei are shown in blue. Bar, 100 μm.
MT1-MMP Drives Invasion by Functioning as a Pericellular Collagenase
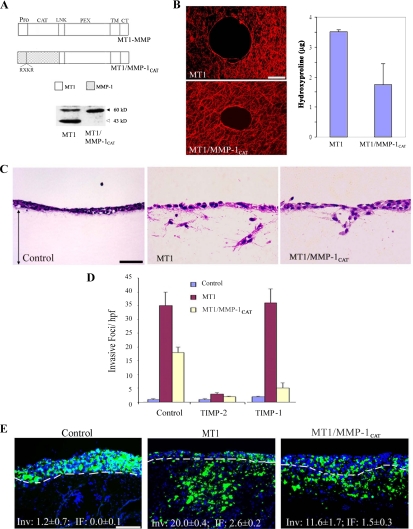
Despite the fact that collagenolytic activity is a critical component of the invasion-promoting behavior of MT1-MMP, the catalytic domain of the proteinase displays a broad and unique substrate repertoire that extends beyond collagenous targets (Pei and Weiss, 1996; d'Ortho et al., 1997; Kridel et al., 2002; Tam et al., 2004b). To determine whether tissue-invasive activity is specific to the MT1-MMP catalytic domain, a domain exchange was engineered with that of the “classical” collagenase, MMP-1 (Brinckerhoff and Matrisian, 2002), and the hybrid proteinase expressed in COS cells. As shown in Figure 3A, Western blot analysis of surface-biotinylated proteins confirms MT1/MMP-1CAT expression at the cell surface. Unlike wt MT1-MMP, and consistent with a change in enzymic properties, the chimeric construct does not undergo autocatalytic processing (i.e., the generation of the 43 kDa form of wt MT1-MMP; Figure 3A) (Osenkowski et al., 2005). Nonetheless, the MT1/MMP-1CAT chimera maintains significant type I collagenolytic activity (Figure 3B), and of note, confers the transfected COS cells with collagen-invasive activity in vitro (Figure 3, C and D). As predicted with a shift in the catalytic properties of the chimera, whereas MT1-MMP–mediated invasion is blocked by the endogenous MMP inhibitor, tissue inhibitor of metalloproteinases (TIMP)-2, but not by TIMP-1 (Sabeh et al., 2004), the MT1/MMP-1CAT chimera is equally sensitive to both TIMP-2 and TIMP-1 (Figure 3D). Finally, in accordance with the type I collagen-invasive properties displayed by MT1/MMP-1CAT in vitro, the chimera-transfected COS cells display an invasive phenotype in the CAM system (Figure 3E).
Figure 3.
A tethered collagenase mimics MT1-MMP activity. (A) Schematic diagram of MT1-MMP and the MT1/MMP-1CAT chimera display the locations of the pro-, catalytic (CAT), linker (LNK), hemopexin (PEX), transmembrane (TM) and cytoplasmic (CT) domains of the respective proteinases. The furin-recognition sequence inserted in the MMP-1 predomain is shown for the chimeric protein and depicted as RXKR (Arg-X-Lys-Arg). Western blot analysis of cell surface expression of MT1-MMP and MT1/MMP-1CAT was assessed after surface biotinylation and immunoprecipitation (the level of MT1/MMP-1CAT is 55% of total wt MT1-MMP as quantified by ImageQuant 5.2). The active and autodegraded forms of wild-type MT1-MMP migrate as ∼60- and ∼43-kDa bands (marked with black and gray arrows, respectively). The MT1/MMP-1CAT appears only as a ∼56-kDa band in a manner consistent with the inability of the MMP-1 catalytic domain to cleave within the MT1-MMP hinge region (Osenkowski et al., 2005). (B) Pericellular collagenolysis of MT1-MMP- and MT1/MMP-1CAT–expressing COS cells as assessed by confocal laser microscopy (bar, 50 μm) and hydroxyproline release, respectively. Results for collagen degradation are expressed as the mean micrograms of hydroxyproline ± SEM of three experiments. (C and D) Type I collagen gel invasion by control-, MT1-MMP-, or MT1/MMP-1CAT–transfected COS cells was assessed in the absence or presence of either TIMP-1 (2 μg/ml) or TIMP-2 (2 μg/ml). Representative cross sections of H&E-stained gels are shown in C (bar, 50 μm), with invasive activity quantified in D. Results are expressed as the mean ± SEM of three or more experiments. (E) Fluorescent micrographs of CAM cross sections after culture with control-, MT1-MMP- or MT1/MMP-1CAT–transfected COS cells for 3 d. Results are representative of three or more experiments performed. Bar, 100 μm.
Hemopexin Domain-directed Regulation of MT1-MMP Activity
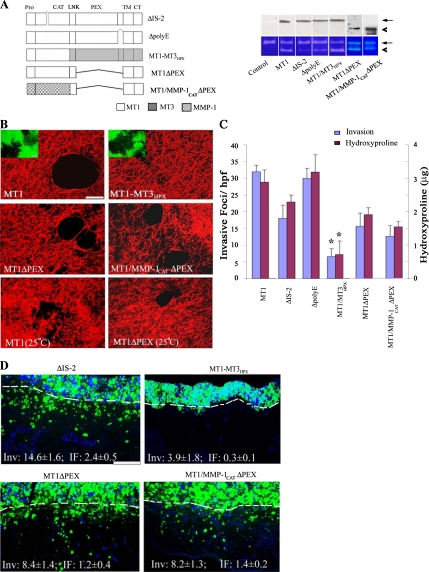
Relative to the secreted collagenases, the extracellular domain of MT1-MMP contains at least three distinct structural characteristics, i.e., i) an IS-2 domain which comprises an 8-amino acid insert between the βII and the βIII strands of the proteinase catalytic domain, ii) a glutamic acid-rich sequence found at the extreme C-terminus of the MT1-MMP ectodomain (i.e., the poly-E box) and iii) a structurally distinct hemopexin domain (Ohuchi et al., 1997; English et al., 2001; Lehti et al., 2002; Tam et al., 2002; Tam et al., 2004a; Wang et al., 2004; Itoh et al., 2006). To first characterize the potential functional role of either the IS-2 insert or poly-E box in MT1-MMP-dependent activity, the respective domains were deleted, and the ability of the mutant enzymes to support collagen degradation or invasion assessed. As reported, the IS-2-deletion mutant (ΔIS-2) displays a significant defect in its ability to activate MMP-2 (Figure 4A) (English et al., 2001). Nonetheless, COS cells expressing the IS-2-deletion mutant retain the bulk of their type I collagen degradative and invasive activities both in vitro (Figure 4, B and C) and in vivo (Figure 4D). Similarly, although the poly-E box has been postulated to play a role in MT1-MMP–dependent collagenolytic activity (Ohuchi et al., 1997), the ΔpolyE-box mutant maintains MMP-2–processing activity (Figure 4A) and expresses full collagenolytic activity (Figure 4, B and C) as well as invasive activity (Inv) in vitro (Figure 4C) and in vivo (Inv, 14.2 ± 1.5 and IF, 21 ± 0.6; mean ± 1 SD, n = 3).
Figure 4.
Domain-dependent regulation of MT1-MMP–mediated collagenolysis and invasive activities. (A) Schematic diagram of MT1-MMP mutants, ΔIS-2, ΔpolyE, MT1-MMP3HPX, MT1ΔPEX, and MT1/MMP-1CATΔPEX. Deleted domains are marked by the symbol “⋀,” whereas MT1-MMP, MT3-MMP, and MMP-1 domains are shaded white, gray, and patterned, respectively. COS cells were transfected with control-, MT1-MMP-, ΔIS-2-, ΔpolyE, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX expression vectors. Expression levels of membrane-localized MT1-MMP or MT1-MMP variants were determined by Western blot analysis after surface biotinylation (top; arrow and arrowhead mark position of active forms of the proteinase). The cell surface expression levels of MT1-MMP variants were 109, 113, 133, 103, and 167% of MT1-MMP, respectively. The processing of exogenous MMP-2 by the transfected cells was monitored by gelatin zymography (bottom). The pro- and active forms of MMP-2 are marked by arrow and arrowhead, respectively. (B) Laser confocal micrographs of pericellular collagenolytic (bar, 50 μm) or gelatinolytic (insets; bar, 50 μm) activity expressed by COS cells transfected with control, MT1-MMP, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX expression vectors and cultured at 37°C. In the bottom two panels, the pericellular collagenolytic activity expressed by COS cells transfected with MT1-MMP or MT1ΔPEX cultured at 25°C is confirmed. (C) Invasion and collagenolytic activities were quantified as invasive foci/hpf (mean ± SEM of 5 randomly selected fields in a single representative experiment of 5 performed) and as micrograms of hydroxyproline released as described (mean ± SEM; n = 3). *p < 0.1 versus MT1. (D) Fluorescent micrographs of CAM cross sections after a 3-d culture with ΔIS-2-, MT1-MT3HPX, MT1ΔPEX, or MT1/MMP-1CATΔPEX transfectants. The control values for MT1-MMP–transfected COS cells are Inv, 20.0 ± 0.4 and IF, 2.6 ± 0.2 (results representative of 3 or more experiments; see Figure 3E). Results shown are representative of three or more experiments performed. Bar, 100 μm.
Recent studies have proposed that the collagenolytic activity of MT1-MMP is dependent on an intact hemopexin domain that supports proteinase homodimerization as well as heterodimerization, binds type I collagen and expresses triple-helicase activity (Mori et al., 2002; Hurst et al., 2004; Wang et al., 2004; Itoh et al., 2006; Lafleur et al., 2006). Indeed, when the hemopexin domain of MT1-MMP is replaced with that of MT3-MMP (i.e., an MT-MMP largely devoid of type I collagenase activity; see above), the chimeric construct (termed MT1/MT3HPX) efficiently processes the MMP-2 zymogen and expresses gelatinolytic activity, but no longer mounts a collagenolytic response (Figure 4, A–C). Coincident with the decrease in type I collagenolytic activity, MT1/MT3HPX predictably confers COS cells minimal collagen-invasive activity in vitro or in vivo (Figure 4, C and D). Although these data support a specific requirement for the MT1-MMP hemopexin domain in regulating collagenolytic activity, hemopexin domains have been reported to exert dominant-negative effects on MT-MMP catalytic activity (Wang et al., 2004; Atkinson et al., 2006). As such, the MT1-MMP hemopexin domain was deleted (while maintaining the MT1-MMP stalk as well as the transmembrane and cytosolic domains; termed MT1ΔPEX), and the functional activity of the membrane-tethered, mutant enzyme was assessed. Unexpectedly, MT1ΔPEX-transfected COS cells continue to display significant collagenolytic activity (Figure 4, B and C). The ability of hemopexin-deleted MT1-MMP to degrade type I collagen cannot be ascribed to partial unwinding of the type I collagen triple helix at 37°C because collagenolytic activity is similarly detected at 25°C (Figure 4B) (Lee et al., 2006). Furthermore, despite the absence of the hemopexin domain, the mutant proteinase retains >50% of the tissue-invasive potential of the wt enzyme, both in vitro and in vivo (Figure 4, C and D). Finally, the ability to maintain significant collagenolytic and invasive activities in the absence of the MT1-MMP hemopexin domain is not restricted to the wild-type catalytic domain. Likewise, when the catalytic domain of MT1-MMP is replaced with that of MMP-1 and the MT1-MMP hemopexin domain of the chimera deleted (i.e., MT1/MMP-1CATΔPEX), degradative and invasive activities are maintained (Figure 4, C and D).
Regulation of MT1-MMP Proteolytic Activity at the Cell Surface
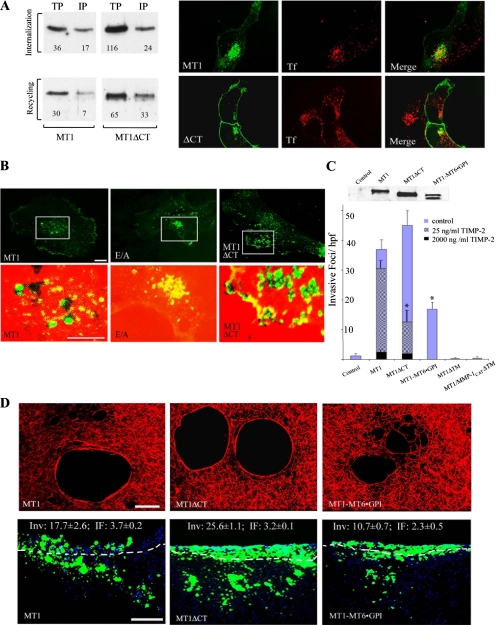
MT1-MMP is tethered to the cell surface by a single-pass transmembrane domain that terminates in a 20-amino acid cytosolic tail that has been reported to contain structural information critical to proteinase trafficking as well as proteinase localization to invadopodia at the cell surface (Nakahara et al., 1997; Lehti et al., 2000; Uekita et al., 2001; Itoh and Seiki, 2006; Nyalendo et al., 2007). Consistent with recent studies that have identified endocytotic motifs in the MT1-MMP tail (Uekita et al., 2001; Anilkumar et al., 2005; Itoh and Seiki, 2006), the surface-localized, HA-tagged wild-type protease (labeled with fluorescent anti-HA antibody at time 0 at 4°C) is internalized rapidly into a transferrin (TF)-positive recycling compartment during a 40-min chase at 37°C (Figure 5A). After surface biotinylation, ∼50% of MT1-MMP is internalized, with almost 80% of the protease recycled to the cell surface as described previously (Figure 5A) (Itoh and Seiki, 2006). By contrast, tail-deleted MT1-MMP (MT1ΔCT) is internalized at markedly reduced rates (i.e., ∼20% of the surface biotinylated enzyme is endocytosed), whereas exocytosis proceeds at rates comparable with those observed with the wild-type enzyme (i.e., ∼50% recycled; Figure 5A). Despite the marked effects that deleting the cytosolic tail exerts on MT1-MMP internalization, COS cells transfected with HA-tagged MT1ΔCT and cultured on a substratum of heat-denatured collagen (i.e., gelatin), a matrix derivative that allows for the rapid visualization of proteolytic zones, concentrate degradative activity in MT1-MMP–positive foci indistinguishable from those generated by the HA-tagged wild-type enzyme (Figure 5B). Furthermore, both subjacent type I collagenolysis and the invasive activity of MT1-MMP are retained in vitro and in vivo regardless of whether the cytosolic tail is deleted (Figure 5, C and D) or replaced with that of the IL2 receptor (MT1-IL2R · CT) (Supplemental Figure 3). In similar manner, COS cells expressing the MT1/MMP-1CAT chimera or MT1ΔPEX constructs in which the respective cytosolic tails have been deleted also express collagenolytic and proinvasive activities (Supplemental Figure 3). Noteworthy, however, is the fact that the invasive activity of the internalization-defective MT1ΔCT mutant demonstrates a heightened sensitivity to inhibition by exogenous TIMP-2, presumably as a consequence of the inability of the tail-deleted construct to regenerate itself as an active proteinase (i.e., by dissociating the reversible inhibitor away from the MT1-MMP catalytic domain during recycling; Maquoi et al., 2000; Zucker et al., 2004) (Figure 5C).
Figure 5.
MT1-MMP tethering to the cell surface and regulation of proteolytic activity. (A) Cell surface MT1-MMP or MT1ΔCT were labeled with biotin at 4°C, and COS cells warmed to 37°C for 40 min to allow for internalization. An aliquot of cells from each sample was lysed, immunoprecipitated with streptavidin-agarose, and immunoblotted with anti-MT1-MMP to determine the total pool size of biotinylated MT1-MMP (TP). A second aliquot of cells was washed with reducing buffer, immunoprecipitated and immunoblotted to determine the intracellular pool of MT1-MMP (IP). To monitor the recycling of internalized MT1-MMP to the cell surface, the remaining cell samples were washed in reducing buffer (i.e., all residual MT1-MMP confined to the intracellular pool), and then they were incubated for an additional 60 min at 37°C. Samples were then treated described as above to determine TP and IP. Relative protein levels of each sample were quantified using ImageQuant5.2, and they are expressed as arbitrary density units. In the right-hand panel, MT1-MMP or MT1ΔCT trafficking was visualized by confocal laser microscopy (bar, 10 μm). Cell surface MT1-MMP (HA epitope tagged) was labeled with fluorescently tagged anti-HA antibody for 60 min at 4°C, followed by a 40-min incubation at 37°C in the presence of TX Red-labeled Tf. Cells were fixed, and separate, or merged, laser confocal images of MT1-MMP (green) and transferrin (red) were recorded. Whereas almost all wild-type MT1-MMP is internalized in transferrin-positive compartments, the bulk of MT1ΔCT is confined to the cell surface. (B) HA-tagged MT1-MMP, MT1-MMP E/A, or MT1ΔCT was expressed in COS cells and cultured atop a Fluor-594–labeled gelatin film. In the top panels, HA epitope-tagged MT1-MMP localized to the cell–substratum interface was visualized by confocal laser microscopy with fluorescein-labeled anti-HA antibodies (green; bar, 10 μm). In the bottom panel, images taken from within the boxed field were enlarged and merged with areas of focal degradation. Green-colored foci represent MT1-MMP localized over black holes of degraded gelatin, whereas areas that are yellow display sites where MT1-MMP overlies an intact gelatin substratum. Bar, 10 μm. (C) The relative cell surface expression levels of the MT1-MMP deletion mutant and chimera as assessed after surface biotinylation are 140 and 94% of wt levels, respectively. The doublet occurring for the MT1-MT6 · GPI chimera is presumed to represent pro- and mature forms of the protease. Invasive activity was quantified for COS cells expressing wild-type MT1-MMP, the tethering domain mutants (MT1ΔCT, MT1-MT6 · GPI), and soluble, transmembrane-deleted MT1-MMP (MT1ΔTM) as well as soluble MT1/MMP-1CAT (MT1/MMP-1CATΔTM) cultured atop type I collagen gels for a 3-d culture period. The inhibitory effect of 25 and 2000 ng/ml TIMP-2 on invasive activity is shown for COS cells expressing wild-type MT1-MMP or MT1ΔCT. Results are expressed as mean ± SEM of a representative single experiment of three or more performed. *p < 0.01. (D) Representative laser confocal images of subjacent collagen degradation mediated by MT1-MMP-, MT1ΔCT-, or MT1-MT6 · GPI-transfected COS cells (bar, 50 μm). In vivo invasive activity as assessed in the CAM system after a 3-d culture period (bottom row; bar, 100 μm).
The ability of MT1-MMP to confer pericellular proteolytic activity to COS cells in the absence of both its hemopexin domain and cytosolic tail is consistent with a new model wherein the proteinase operates directly as a membrane-tethered, pericellular collagenase. Because the MT1-MMP transmembrane domain could potentially embed regulatory motifs that modulate proteolytic and/or invasive activity, chimeric constructs were next designed to anchor the extracellular domain of wt MT1-MMP or MT1ΔPEX to the cell surface via the MT6-MMP GPI anchor (Figure 6 and Supplemental Figure 3). Despite the facts that 1) the cell surface levels of GPI-anchored MT1-MMP (i.e., MT1-MT6 · GPI) are consistently lower than those observed with other tethered constructs (presumably due to surface shedding; Sohail et al., 2008) and 2) GPI-anchored proteins are preferentially sorted to apical compartments in epithelial cells (Schuck and Simons, 2006), transfected COS cells express collagenolytic activity, invade 3-D type I collagen gels, and traverse the CAM (Figure 5, C and D). Similar results are obtained when the hemopexin domain of the MT1-MT6 · GPI chimera is deleted (Supplemental Figure 3). If, however, membrane tethering is eliminated by deleting the transmembrane domain of the wt enzyme (i.e., MT1-MMPΔTM) or the MT1/MMP-1CAT chimera (i.e., MT1/MMP-1CATΔTM), transfected COS cells lose all degradative or tissue-invasive activity (Figure 5C). Hence, significant pericellular collagenolytic as well as tissue-invasive activity are retained within the hemopexin-free extracellular domains of the membrane-tethered proteinase.
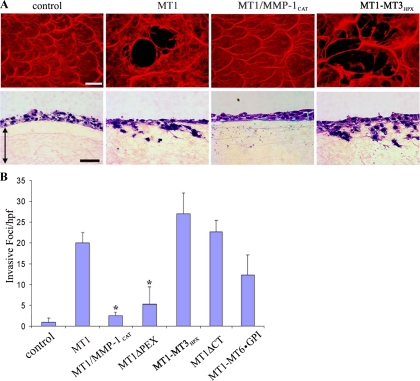
Figure 6.
Regulation of MT1-MMP–dependent fibrinolytic and fibrin-invasive activity. (A) COS cells transfected with control, MT1-MMP, MT1/MMP-1CAT, or MT1-MT3HPX expression vectors were cultured atop fibrin films labeled with Alexa Fluor-594 (red) to assess subjacent proteolysis (representative laser confocal micrographs are shown in the top row) or atop fibrin gels to monitor invasive activity (H&E-stained cross sections shown in the bottom row with double-headed arrows demarcating the upper and lower boundaries of the fibrin gels). (B) Fibrin invasive activities expressed by COS cells transfected with control, MT1-MMP, MT1/MMP-1CAT, MT1ΔPEX, MT1-MT3HPX, MT1ΔCT, and MT1-MT6 · GPI expression vectors were quantified as invasive foci/hpf (mean ± SEM of 5 randomly selected fields in a single representative experiment of 5 performed). Bar, 50 μm. *p < 0.01.
Differential Regulation of MT1-MMP-dependent Fibrin-invasive Activity
Independently of the ability of MT1-MMP to endow expressing cells with the capacity to traverse collagenous matrices, the membrane-anchored proteinase also supports fibrin-invasive activity (Hiraoka et al., 1998; Hotary et al., 2002). Although fibrin, the dominant component of the provisional matrix assembled at wound or neoplastic sites (Hiraoka et al., 1998; Hotary et al., 2002), is structurally distinct from type I collagen, MT1-MMP–transfected COS cells readily degrade and invade 3-D fibrin gels (Figure 6, A and B). However, whereas type I collagen-invasive activity is maintained in the presence of either the MT1-MMP or MMP-1 catalytic domains (Figure 3), the ability to degrade or invade fibrin gels is confined to wild-type MT1-MMP and cannot be supported by the MMP-1 catalytic domain swap (Figure 6, A and B). Furthermore, although hemopexin-deleted MT1-MMP retains significant collagen-invasive activity (Figure 4), the MT1ΔPEX mutant is devoid of almost all fibrin-invasive activity (Figure 6B). Interestingly, the requirement for an intact hemopexin domain to confer fibrin-invasive activity is not confined to the MT1-MMP hemopexin domain per se. Despite the fact that the MT3-MMP hemopexin domain cannot support fully the collagen-invasive activity of MT1-MMP (Figure 4), the MT1-MT3HPX chimera displays full fibrinolytic and proinvasive activities (Figure 6, A and B). Finally, as observed for collagenous substrates, fibrin-invasive activity is maintained when the transmembrane and cytosolic domains of MT1-MMP are replaced with a GPI anchor (Figure 6B). Hence, in contrast with the more relaxed structural requirements underlying collagenolytic activity, MT1-MMP–dependent fibrinolysis displays a strict reliance on both MT-MMP–derived catalytic and hemopexin domains.
DISCUSSION
Normal as well as neoplastic cells mobilize MT1-MMP to negotiate the 3-D connective tissue barriers presented by cross-linked networks of type I collagen or fibrin (Hiraoka et al., 1998; Chun et al., 2004; Sabeh et al., 2004; Filippov et al., 2005; Hotary et al., 2006; Itoh and Seiki, 2006). Given this unusual activity, an extensive body of structure–function analyses support a complex model wherein the broad substrate specificity of MT1-MMP is complemented by a multidomain structure that regulates the translocation, surface oligomerization, proteolytic activity, endocytosis, and recycling of the active enzyme (Sato et al., 2005; Itoh and Seiki, 2006; Nie et al., 2007; Nyalendo et al., 2007; D'Alessio et al., 2008). Based on these data, the ability of MT1-MMP to promote cell trafficking through ECM barriers is most often considered to be the product of a combination of intracellular, intramembrane, and extracellular proteinase–protein interactions (Sato et al., 2005; Itoh and Seiki, 2006; Nie et al., 2007; Nyalendo et al., 2007; D'Alessio et al., 2008). Instead, we demonstrate that parsimonious protease constructs can selectively mediate collagen and/or fibrin proinvasive programs while bypassing the multiple structural requirements heretofore assumed to function as irreplaceable components of MT1-MMP activity.
At first glance, the manner in which cellular behavior as complex as 3-D invasion can be incorporated into a relatively simple structural design wherein the appropriate proteolytic activity is passively tethered to the cell surface is not easily reconciled with existing paradigms in the literature (Itoh and Seiki, 2006; Nyalendo et al., 2007; D'Alessio et al., 2008). Until recently, many of the specialized properties of MT1-MMP activity were attributed to the proteinase's ability to activate the MMP-2 or MMP-13 zymogens (Sato et al., 2005; Itoh and Seiki, 2006). MT1-MMP can, however, directly exert potent proteolytic effects against a variety of ECM substrates, including type I collagen and fibrin (Pei and Weiss, 1996; d'Ortho et al., 1997; Ohuchi et al., 1997; Hiraoka et al., 1998; Hotary et al., 2000; Koshikawa et al., 2005; Itoh and Seiki, 2006). Consistent with earlier reports that neither MMP-2−/− nor MMP-13−/− fibroblasts, endothelial cells or vascular smooth muscle cells display defects in matrix degradation or invasion (Chun et al., 2004; Sabeh et al., 2004; Filippov et al., 2005), the invasive potential of HT-1080 cells can be reconstituted in COS cells by “simply” expressing MT1-MMP in the absence of these accessory MMPs.
In an effort to better rationalize the ability of MT1-MMP to mediate its proinvasive effects in a stand-alone manner, our attention alternatively focused on the unusually broad substrate specificity of its catalytic domain (Tam et al., 2004b; Itoh and Seiki, 2006). Independent of potential ECM substrates, MT1-MMP has been reported to modify cell function by processing integrins, adhesion molecules, cell surface proteoglycans, or surface receptors (Sato et al., 2005; Itoh and Seiki, 2006; Abd El-Aziz et al., 2007; Basile et al., 2007; Freudenberg and Chen, 2007). In keeping with these observations, we considered the possibility that by exchanging the MT1-MMP catalytic domain with that of the structurally distinct collagenase MMP-1, pericellular collagenolytic activity might be retained, whereas the higher order, functional activity associated with 3-D invasive behavior would be lost. Instead, the ability to confer collagenolytic as well as tissue-invasive activity was not necessarily restricted to the MT1-MMP catalytic domain. Conceivably, the substrate specificity of the inserted MMP-1 catalytic domain could have been redirected toward that of wild-type MT1-MMP by the MT1-MMP hemopexin domain itself. Indeed, the MT1-MMP hemopexin domain has previously been assigned critical roles in regulating collagenolytic activity by 1) supporting MT1-MMP dimerization at the cell surface, 2) mediating the local unwinding of the type I collagen triple-helix, and 3) acting as collagen-binding moiety that anchors the protease to its target substrate (Lehti et al., 2002; Tam et al., 2004a; Itoh et al., 2006; Itoh and Seiki, 2006; Lafleur et al., 2006). As such, the ability of hemopexin-deleted MT1-MMP or the hemopexin-deleted MT1/MMP-1CAT chimera to confer COS cells with the ability to degrade type I collagen, invade collagen gels, or traverse interstitial tissues in the in vivo setting contradicts a series of recent studies. With regard to the ability of hemopexin-deleted MT1-MMP constructs to display collagenolytic activity, Itoh and colleagues reported that COS cells expressing the hemopexin deletion mutant are incapable of degrading a subjacent bed of type I collagen fibrils (Itoh et al., 2006). Collagenolysis was, however, monitored in a qualitative manner via a colorimetric technique that lacks the sensitivity of our assay system, and invasive activity was not assessed. Because hemopexin-deleted MT1-MMP expresses ∼50% of the collagenolytic activity of the wt enzyme, we posit that this degree of activity was likely overlooked in the absence of quantifiable endpoints. Similarly, whereas MT1ΔPEX was reported to be incapable of supporting collagen-invasive activity (Wang et al., 2004), the type I collagenolytic activity of the construct was not examined. Furthermore, invasion was monitored in Madin-Darby canine kidney (MDCK) cells, a well-differentiated epithelial cell line that already expresses a full complement of proinvasive MMPs (Kadono et al., 1998; Hotary et al., 2000). In our hands, MDCK cells engineered to overexpress wt MT1-MMP fail to express heightened invasive activity unless stimulated with motogens that transiently disrupt the cadherin-rich adherens junctions that normally serve as powerful repressors of motility in the 3-D setting (Kadono et al., 1998; Hotary et al., 2000).
The above-mentioned issues notwithstanding, the ability of MT1ΔPEX to mediate collagenolysis and invasion through collagen-rich barriers is puzzling because these results further demonstrate that neither the triple-helicase nor collagen-binding activities of the hemopexin domain play required roles in supporting MT1-MMP function in an intact cell system. These results are, however, consistent with recent studies demonstrating that MT1-MMP–mediated triple-helicase activity may reside within the catalytic domain itself and that the collagenolytic activity of secreted MMPs, including MMP-8 and MMP-2, are retained in the absence of their respective hemopexin domains (Gioia et al., 2002; Minond et al., 2006; Gioia et al., 2007). The fact that the MT1-MMP hemopexin domain is dispensable for collagen remodeling contrasts with the observation that both fibrinolytic and fibrin-invasive activity proved to be hemopexin dependent, a finding similar to that recently reported for MMP-2–dependent fibrinogenolysis where the hemopexin domain participates directly in fibrin binding (Monaco et al., 2007). It should be stressed, however, that our results do not support a conclusion that the hemopexin domain is functionless with regard to collagen turnover. Clearly, MT1ΔPEX is a less efficient collagenolysin and proinvasive factor than is the wild-type proteinase. Furthermore, the hemopexin domain may regulate MT1-MMP binding interactions with other accessory molecules that modulate cell functions other than those required for collagenolysis or invasion per se (e.g., collagen internalization) (Itoh and Seiki, 2006; Lee et al., 2006). Nevertheless, the ability of hemopexin-deleted MT1-MMP constructs to retain significant collagenolytic activity in tandem with an almost complete loss of fibrinolytic activity indicates that current paradigms regarding MT1-MMP function in the context of intact cell systems need be revisited and reconsidered.
Having established a required role for collagenolytic activity during invasion that cannot be recapitulated by secreted collagenases, we confirmed the fact that transmembrane-deleted, secreted forms of MT1-MMP are unable to confer tissue-invasive activity. These findings might seem to support reports documenting important roles for the MT1-MMP transmembrane domain and/or cytosolic tail in regulating MT1-MMP oligomerization, proteolytic activity, endocytosis, and recycling (Wu et al., 2005; Itoh and Seiki, 2006; D'Alessio et al., 2008). We find, however, that tail-deleted MT1-MMP as well as the tail-deleted MT1/MMP-1CAT chimera is able to focus proteolytic activity at the membrane surface to drive pericellular collagenolysis and invasion. Although we considered the possibility that the MT1-MMP transmembrane domain itself might embed trafficking or membrane localization signals critical to proteolytic function, MT1-MMP mutants retain wild-type-like activity when the transmembrane domain is replaced with the GPI anchor of MT6-MMP. GPI-anchored MT1-MMP has recently been shown to support cell growth in 3-D collagen gels, but its ability to confer invasive activity was not examined (Nie et al., 2007). Hence, despite earlier suggestions that higher order functions for the MT1-MMP C-terminus might be restricted to cells engaged in traversing ECM barriers (Uekita et al., 2001; Itoh and Seiki, 2006), our results demonstrate that tissue-invasive activity is retained when either the MT1-MMP transmembrane domain or cytosolic tail is deleted.
Despite the fact that MT1-MMP can effectively mediate subjacent proteolysis and proinvasive activity when shorn of its transmembrane and cytoplasmic domains, these findings should not be construed to suggest that these structures are devoid of functional activity. By controlling MT1-MMP recycling, the cytosolic tail can serve as a means to regenerate the active proteinase from inactive MT1-MMP–TIMP-2 complexes formed at the cell surface (Maquoi et al., 2000; Zucker et al., 2004). Indeed, tail-deleted MT1-MMP rendered cells hypersensitive to the inhibitory effects of TIMP-2 during collagen invasion. We should also stress that MT1-MMP–mediated effects on cell function need not be confined to motility or invasion. For example, the MT1-MMP cytosolic tail has been reported to control vascular endothelial growth factor expression via a src-dependent pathway and may induce other signaling pathways as well (Labrecque et al., 2004; Sounni et al., 2004; Nyalendo et al., 2007; D'Alessio et al., 2008). Furthermore, in preliminary studies, we have found that the MT1-MMP tail plays a critical role in regulating basolateral sorting and branching morphogenesis in polarized epithelial cells (unpublished observation). From these perspectives, a narrow focus on tissue-invasive properties alone may oversimplify the complex functions required of MT1-MMP during growth and development in vivo. Even so, our data demonstrate that a remarkable amount of functional information can be conveyed by a relatively simple proteinase construct and that predicted requirements for the MT1-MMP, catalytic, hemopexin, transmembrane, or cytoplasmic domains during invasion can be bypassed in vitro as well as in vivo. Of course, complex functions assigned to a “simple” protease in intact cell systems are, in part, illusory. By initiating ECM remodeling, cell function is likely modulated by a litany of associated events, including the release of bioactive matrix fragments, alterations in pericellular matrix rigidity, and the consequent effects on cell shape as well as gene expression (Chun et al., 2006; Itoh and Seiki, 2006; Freudenberg and Chen, 2007; Cao et al., 2008). Nevertheless, our studies demonstrate that by purposefully restricting the collagenolytic or fibrinolytic activity of MT1-MMP to the pericellular milieu, the cell can remodel multicomponent tissue barriers while maintaining the necessary adhesive interactions with the surrounding ECM to support propulsive 3-D movement.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by National Institutes of Health grants CA-88308 and CA-71699 as well as funds from the Breast Cancer Research Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0016) on May 21, 2008.
REFERENCES
- Abd El-Aziz S. H., Endo Y., Miyamaori H., Takino T., Sato H. Cleavage of growth differentiation factor 15 (GDF15) by membrane type 1-matrix metalloproteinase abrogates GDF15-mediated suppression of tumor cell growth. Cancer Sci. 2007;98:1330–1335. doi: 10.1111/j.1349-7006.2007.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar N., Uekita T., Couchman J. R., Nagase H., Seiki M., Itoh Y. Palmitoylation at Cys574 is essential for MT1-MMP to promote cell migration. FASEB J. 2005;19:1326–1328. doi: 10.1096/fj.04-3651fje. [DOI] [PubMed] [Google Scholar]
- Atkinson S. J., Roghi C., Murphy G. MT1-MMP hemopexin domain exchange with MT4-MMP blocks enzyme maturation and trafficking to the plasma membrane in MCF7 cells. Biochem. J. 2006;398:15–22. doi: 10.1042/BJ20060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile J. R., Holmbeck K., Bugge T. H., Gutkind J. S. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J. Biol. Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Matrisian L. M. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Cao J., Chiarelli C., Richman O., Zarrabi K., Kozarekar P., Zucker S. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J. Biol. Chem. 2008;283:6232–6240. doi: 10.1074/jbc.M705759200. [DOI] [PubMed] [Google Scholar]
- Cao J., Kozarekar P., Pavlaki M., Chiarelli C., Bahou W. F., Zucker S. Distinct roles for the catalytic and hemopexin domains of membrane type 1-matrix metalloproteinase in substrate degradation and cell migration. J. Biol. Chem. 2004;279:14129–14139. doi: 10.1074/jbc.M312120200. [DOI] [PubMed] [Google Scholar]
- Chun T. H., Hotary K. B., Sabeh F., Saltiel A. R., Allen E. D., Weiss S. J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Chun T. H., Sabeh F., Ota I., Murphy H., McDonagh K. T., Holmbeck K., Birkedal-Hansen H., Allen E. D., Weiss S. J. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J. Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio S., et al. Tissue inhibitor of metalloproteinases-2 binding to membrane-type 1 matrix metalloproteinase induces MAPK activation and cell growth by a non-proteolytic mechanism. J. Biol. Chem. 2008;283:87–99. doi: 10.1074/jbc.M705492200. [DOI] [PubMed] [Google Scholar]
- d'Ortho M. P., Will H., Atkinson S., Butler G., Messent A., Gavrilovic J., Smith B., Timpl R., Zardi L., Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur. J. Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- English W. R., Holtz B., Vogt G., Knauper V., Murphy G. Characterization of the role of the “MT-loop”: an eight-amino acid insertion specific to progelatinase A (MMP2) activating membrane-type matrix metalloproteinases. J. Biol. Chem. 2001;276:42018–42026. doi: 10.1074/jbc.M107783200. [DOI] [PubMed] [Google Scholar]
- Fabbri M., Fumagalli L., Bossi G., Bianchi E., Bender J. R., Pardi R. A tyrosine-based sorting signal in the beta2 integrin cytoplasmic domain mediates its recycling to the plasma membrane and is required for ligand-supported migration. EMBO J. 1999;18:4915–4925. doi: 10.1093/emboj/18.18.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov S., et al. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J. Exp. Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg J. A., Chen W. T. Induction of Smad1 by MT1-MMP contributes to tumor growth. Int. J. Cancer. 2007;121:966–977. doi: 10.1002/ijc.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia M., Fasciglione G. F., Marini S., D'Alessio S., De Sanctis G., Diekmann O., Pieper M., Politi V., Tschesche H., Coletta M. Modulation of the catalytic activity of neutrophil collagenase MMP-8 on bovine collagen I. Role of the activation cleavage and of the hemopexin-like domain. J. Biol. Chem. 2002;277:23123–23130. doi: 10.1074/jbc.M110873200. [DOI] [PubMed] [Google Scholar]
- Gioia M., Monaco S., Fasciglione G. F., Coletti A., Modesti A., Marini S., Coletta M. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. J. Mol. Biol. 2007;368:1101–1113. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- Hiraoka N., Allen E., Apel I. J., Gyetko M. R., Weiss S. J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Hotary K., Allen E., Punturieri A., Yana I., Weiss S. J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K., Li X. Y., Allen E., Stevens S. L., Weiss S. J. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- Hotary K. B., Yana I., Sabeh F., Li X. Y., Holmbeck K., Birkedal-Hansen H., Allen E. D., Hiraoka N., Weiss S. J. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J. Exp. Med. 2002;195:295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst D. R., Schwartz M. A., Ghaffari M. A., Jin Y., Tschesche H., Fields G. B., Sang Q. X. Catalytic- and ecto-domains of membrane type 1-matrix metalloproteinase have similar inhibition profiles but distinct endopeptidase activities. Biochem. J. 2004;377:775–779. doi: 10.1042/BJ20031067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Ito N., Nagase H., Evans R. D., Bird S. A., Seiki M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol. Biol. Cell. 2006;17:5390–5399. doi: 10.1091/mbc.E06-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- Jiang A., Pei D. Distinct roles of catalytic and pexin-like domains in membrane-type matrix metalloproteinase (MMP)-mediated pro-MMP-2 activation and collagenolysis. J. Biol. Chem. 2003;278:38765–38771. doi: 10.1074/jbc.M306618200. [DOI] [PubMed] [Google Scholar]
- Jiang X., Dutton C. M., Qi W. N., Block J. A., Garamszegi N., Scully S. P. siRNA mediated inhibition of MMP-1 reduces invasive potential of a human chondrosarcoma cell line. J. Cell. Physiol. 2005;202:723–730. doi: 10.1002/jcp.20162. [DOI] [PubMed] [Google Scholar]
- Kadono Y., Shibahara K., Namiki M., Watanabe Y., Seiki M., Sato H. Membrane type 1-matrix metalloproteinase is involved in the formation of hepatocyte growth factor/scatter factor-induced branching tubules in Madin-Darby canine kidney epithelial cells. Biochem. Biophys. Res. Commun. 1998;251:681–687. doi: 10.1006/bbrc.1998.9531. [DOI] [PubMed] [Google Scholar]
- Kojima S., Itoh Y., Matsumoto S., Masuho Y., Seiki M. Membrane-type 6 matrix metalloproteinase (MT6-MMP, MMP-25) is the second glycosyl-phosphatidyl inositol (GPI)-anchored MMP. FEBS Lett. 2000;480:142–146. doi: 10.1016/s0014-5793(00)01919-0. [DOI] [PubMed] [Google Scholar]
- Koshikawa N., Minegishi T., Sharabi A., Quaranta V., Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. J. Biol. Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- Kridel S. J., Sawai H., Ratnikov B. I., Chen E. I., Li W., Godzik A., Strongin A. Y., Smith J. W. A unique substrate binding mode discriminates membrane type-1 matrix metalloproteinase from other matrix metalloproteinases. J. Biol. Chem. 2002;277:23788–23793. doi: 10.1074/jbc.M111574200. [DOI] [PubMed] [Google Scholar]
- Labrecque L., Nyalendo C., Langlois S., Durocher Y., Roghi C., Murphy G., Gingras D., Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J. Biol. Chem. 2004;279:52132–52140. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- Lafleur M. A., Mercuri F. A., Ruangpanit N., Seiki M., Sato H., Thompson E. W. Type I collagen abrogates the clathrin-mediated internalization of membrane type 1 matrix metalloproteinase (MT1-MMP) via the MT1-MMP hemopexin domain. J. Biol. Chem. 2006;281:6826–6840. doi: 10.1074/jbc.M513084200. [DOI] [PubMed] [Google Scholar]
- Lee H., Overall C. M., McCulloch C. A., Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol. Biol. Cell. 2006;17:4812–4826. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K., Lohi J., Juntunen M. M., Pei D., Keski-Oja J. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 2002;277:8440–8448. doi: 10.1074/jbc.M109128200. [DOI] [PubMed] [Google Scholar]
- Lehti K., Valtanen H., Wickstrom S. A., Lohi J., Keski-Oja J. Regulation of membrane-type-1 matrix metalloproteinase activity by its cytoplasmic domain. J. Biol. Chem. 2000;275:15006–15013. doi: 10.1074/jbc.M910220199. [DOI] [PubMed] [Google Scholar]
- Maquoi E., Frankenne F., Baramova E., Munaut C., Sounni N. E., Remacle A., Noel A., Murphy G., Foidart J. M. Membrane type 1 matrix metalloproteinase-associated degradation of tissue inhibitor of metalloproteinase 2 in human tumor cell lines. J. Biol. Chem. 2000;275:11368–11378. doi: 10.1074/jbc.275.15.11368. [DOI] [PubMed] [Google Scholar]
- Minond D., Lauer-Fields J. L., Cudic M., Overall C. M., Pei D., Brew K., Visse R., Nagase H., Fields G. B. The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J. Biol. Chem. 2006;281:38302–38313. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- Monaco S., Gioia M., Rodriguez J., Fasciglione G. F., Di Pierro D., Lupidi G., Krippahl L., Marini S., Coletta M. Modulation of the proteolytic activity of matrix metalloproteinase-2 (gelatinase A) on fibrinogen. Biochem. J. 2007;402:503–513. doi: 10.1042/BJ20061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Tomari T., Koshikawa N., Kajita M., Itoh Y., Sato H., Tojo H., Yana I., Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21:3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Howard L., Thompson E. W., Sato H., Seiki M., Yeh Y., Chen W. T. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl. Acad. Sci. USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Pei J., Blumenthal M., Pei D. Complete restoration of cell surface activity of transmembrane-truncated MT1-MMP by a glycosylphosphatidylinositol anchor. Implications for MT1-MMP-mediated prommp2 activation and collagenolysis in three-dimensions. J. Biol. Chem. 2007;282:6438–6443. doi: 10.1074/jbc.M607337200. [DOI] [PubMed] [Google Scholar]
- Nyalendo C., Michaud M., Beaulieu E., Roghi C., Murphy G., Gingras D., Beliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573, role in endothelial and tumor cell migration. J. Biol. Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- Ohuchi E., Imai K., Fujii Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Osenkowski P., Meroueh S. O., Pavel D., Mobashery S., Fridman R. Mutational and structural analyses of the hinge region of membrane type 1-matrix metalloproteinase and enzyme processing. J. Biol. Chem. 2005;280:26160–26168. doi: 10.1074/jbc.M414379200. [DOI] [PubMed] [Google Scholar]
- Pei D., Weiss S. J. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- Pei D., Weiss S. J. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J. Biol. Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Puente X. S., Sanchez L. M., Overall C. M., Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- Sabeh F., et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S., Simons K. Controversy fuels trafficking of GPI-anchored proteins. J. Cell Biol. 2006;172:963–965. doi: 10.1083/jcb.200603015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Son M. Y., Yamada S., Szabova L., Kahan S., Chrysovergis K., Wolf L., Surmak A., Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev. Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail A., Sun Q., Zhao H., Bernardo M. M., Cho J.-A., Fridman R. MT4-(MMP17) and MT6-MMP (MMP25), a unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev. 2008;27:289–302. doi: 10.1007/s10555-008-9129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni N. E., et al. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J. Biol. Chem. 2004;279:13564–13574. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- Tam E. M., Moore T. R., Butler G. S., Overall C. M. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J. Biol. Chem. 2004a;279:43336–43344. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- Tam E. M., Morrison C. J., Wu Y. I., Stack M. S., Overall C. M. Membrane protease proteomics: isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc. Natl. Acad. Sci. USA. 2004b;101:6917–6922. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam E. M., Wu Y. I., Butler G. S., Stack M. S., Overall C. M. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J. Biol. Chem. 2002;277:39005–39014. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- Uekita T., Itoh Y., Yana I., Ohno H., Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J. Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nie J., Pei D. The hemopexin domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) Is not required for its activation of proMMP2 on cell surface but is essential for MT1-MMP-mediated invasion in three-dimensional type I collagen. J. Biol. Chem. 2004;279:51148–51155. doi: 10.1074/jbc.M409074200. [DOI] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., von Andrian U. H., Deryugina E. I., Strongin A. Y., Brocker E. B., Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Wu X., Gan B., Yoo Y., Guan J. L. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev. Cell. 2005;9:185–196. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Wyatt C. A., Geoghegan J. C., Brinckerhoff C. E. Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth. Cancer Res. 2005;65:11101–11108. doi: 10.1158/0008-5472.CAN-05-2446. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yana I., Weiss S. J. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S., Hymowitz M., Conner C., DeClerck Y., Cao J. TIMP-2 is released as an intact molecule following binding to MT1-MMP on the cell surface. Exp. Cell Res. 2004;293:164–174. doi: 10.1016/j.yexcr.2003.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.