Abstract
In basal adipocytes, glucose transporter 4 (GLUT4) is sequestered intracellularly by an insulin-reversible retention mechanism. Here, we analyze the roles of three GLUT4 trafficking motifs (FQQI, TELEY, and LL), providing molecular links between insulin signaling, cellular trafficking machinery, and the motifs in the specialized trafficking of GLUT4. Our results support a GLUT4 retention model that involves two linked intracellular cycles: one between endosomes and a retention compartment, and the other between endosomes and specialized GLUT4 transport vesicles. Targeting of GLUT4 to the former is dependent on the FQQI motif and its targeting to the latter is dependent on the TELEY motif. These two motifs act independently in retention, with the TELEY-dependent step being under the control of signaling downstream of the AS160 rab GTPase activating protein. Segregation of GLUT4 from endosomes, although positively correlated with the degree of basal retention, does not completely account for GLUT4 retention or insulin-responsiveness. Mutation of the LL motif slows return to basal intracellular retention after insulin withdrawal. Knockdown of clathrin adaptin protein complex-1 (AP-1) causes a delay in the return to intracellular retention after insulin withdrawal. The effects of mutating the LL motif and knockdown of AP-1 were not additive, establishing that AP-1 regulation of GLUT4 trafficking requires the LL motif.
INTRODUCTION
Insulin regulation of glucose uptake into adipose and muscle is key for the disposal of dietary glucose. The facilitative glucose transporter 4 (GLUT4) mediates the effect of insulin on glucose transport. GLUT4 is constitutively active for hexose transport, and glucose uptake is regulated by insulin controlling the amount of GLUT4 in the plasma membrane (PM) (Huang and Czech, 2007). In basal adipocytes, GLUT4 is slowly exocytosed and rapidly endocytosed, and <5% of the total amount of GLUT4 is in the PM. Insulin signaling results in changes in GLUT4 trafficking parameters: GLUT4 exocytosis is accelerated, whereas GLUT4 endocytosis is inhibited, resulting in a net 10- to 15-fold increase of surface GLUT4 and glucose uptake.
The effect of insulin on the fraction of GLUT4 in the PM of adipose and muscle cells reflects a specific regulation of GLUT4 trafficking rather than a general perturbation of membrane trafficking. Besides GLUT4, the only other protein whose amount in the PM is comparably regulated by insulin signaling is the insulin-responsive amino peptidase (IRAP) (Kandror et al., 1994; Keller et al., 1995; Garza and Birnbaum, 2000). The amounts of other proteins in the PM are only slightly affected by insulin. For example, the PM levels of the ubiquitously expressed GLUT1 and the transferrin receptor (TR) are only increased by approximately twofold upon insulin stimulation (Piper et al., 1991; Yang et al., 1992; Zeigerer et al., 2002).
The effects of insulin on GLUT4 are cell–type-specific since insulin has a minor effect on the trafficking of GLUT4 ectopically expressed in fibroblast-like cell types (Lampson et al., 2001). The robust regulation of GLUT4 trafficking in adipocytes develops around day 3 of differentiation (El-Jack et al., 1999; Govers et al., 2004). The main difference in GLUT4 trafficking between fibroblasts/preadipocytes and adipocytes is in basal conditions. As noted above, in basal adipocytes GLUT4 is predominantly excluded from the cell surface (<5%), whereas in fibroblasts and preadipocytes, ∼30% of GLUT4 is in the PM (Govers et al., 2004). In all cell types ∼50% of GLUT4 is in the PM in insulin-stimulated steady-state conditions. Therefore, the more pronounced effect of insulin on GLUT4 distribution in adipocytes is mostly due to an efficient, insulin-reversible basal retention mechanism. Consequently, adipocytes must express factors that promote GLUT4 retention in basal conditions. One of these factors, AS160 (TBC1D4), is a rab GTPase activating protein (GAP) whose activity is inhibited by insulin-mediated activation of Akt (Sano et al., 2003; Eguez et al., 2005; Larance et al., 2005). Active AS160 promotes GLUT4 retention by maintaining its target rab(s), one of which is rab10, in the inactive GDP-bound form (Sano et al., 2007). AS160 knockdown results in a partial release of GLUT4 basal retention, indicating that other steps independently of AS160 also participate to full intracellular GLUT4 sequestration (Eguez et al., 2005; Larance et al., 2005).
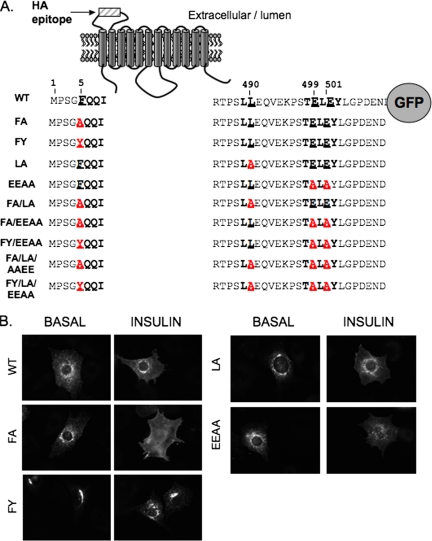
GLUT4 trafficking in adipocytes differs greatly from that of other recycling proteins in the same cells, indicating that GLUT4 contains specific sequences that control its specialized behavior. Three cytoplasmic motifs have been described to have roles in the specialized GLUT4 trafficking (Figure 1A): 1) F5QQI sequence located in the N terminus (Piper et al., 1993; Garippa et al., 1994; Marsh et al., 1995a) and 2) LL490 (Czech et al., 1993; Corvera et al., 1994; Marsh et al., 1995a; Verhey et al., 1995), and 3) TE499LE501Y sequences located in the C terminus (Shewan et al., 2000). The FQQI and LL motifs belong to the aromatic-based and dileucine-based family of trafficking motifs, respectively. Both families of motifs regulate intracellular trafficking by interacting with adaptor proteins involved in vesicle formation and clathrin recruitment (reviewed in Bonifacino and Traub, 2003), although the proteins that interact with the GLUT4 motifs have not been identified. The GLUT4 FQQI motif is unusual because it contains a phenylalanine instead of the tyrosine typically found in this class of motifs. The TELEY motif is conserved in IRAP, and the protein that binds this motif is not known (Shewan et al., 2000).
Figure 1.
GLUT4 mutants used in this study. (A) Schematic representation of HA-GLUT4-GFP. The positions of the amino acids from the N terminus (position 1) are indicated. The mutated amino acids are underlined and the changes in red type. (B) HA-GLUT4-GFP mutants in the basal or the insulin-stimulated adipocytes detected by GFP fluorescence in epifluorescence microscopy. The images were collected with the same exposure times and scaled identically so that they are comparable.
Although there is general agreement that these motifs are required for GLUT4 trafficking, their functions and relative contributions remain an area of controversy. Some studies indicate that the amino terminus FQQI motif is required for GLUT4 basal intracellular retention (Piper et al., 1993; Marsh et al., 1995a), whereas others emphasize the importance of the carboxy terminus TELEY motif (Shewan et al., 2000; Govers et al., 2004). The function of the LL-based motif is also unclear (Marsh et al., 1995b; Al-Hasani et al., 2002; Govers et al., 2004; Blot and McGraw, 2006). These controversies might be explained by the use of steady-state assays that do not directly measure kinetics of GLUT4 movements, and potentially by the use of different cell systems and reporters that make comparisons between studies challenging (Lalioti et al., 2001; Watson et al., 2004).
We have recently examined the roles of the motifs in GLUT4 endocytosis (Blot and McGraw, 2006). The FQQI motif mediates GLUT4 internalization through a clathrin adaptin protein complex-2 (AP-2)-dependent pathway in insulin-stimulated adipocytes. The FQQI motif is dispensable for GLUT4 internalization in basal adipocytes and the LL-based motif is dispensable for both basal and insulin-stimulated endocytosis. Here, we examine the roles of the FQQI, LL, and TELEY motifs in the specialized intracellular trafficking of GLUT4 in adipocytes.
MATERIALS AND METHODS
Cell Culture and Electroporation
3T3-L1 fibroblasts were differentiated and electroporated as described previously (Zeigerer et al., 2002). Studies were performed 1 d after electroporation. For small interfering RNA (siRNA) experiments, 2 nmol of siRNA was added in each electroporation cuvette, and experiments were done 2 d after electroporation. AS160 knockdown adipocytes are 3T3-L1 in which AS160 was knocked down by 70–90% by using retroviral transfer of AS160-specific short hairpin RNA (shRNA) (Eguez et al., 2005). 3T3-L1 cells stably expressing HA-GLUT4 have been described previously (Martin et al., 2006).
Plasmids, siRNAs, Ligands, and Antibodies
Creation of the FA, LA, and FY HA-GLUT4-green fluorescent protein (GFP) mutants have been described previously (Blot and McGraw, 2006). Alanine substitutions for the glutamic acids of the TELEY motif and the various double mutants were created using Stratagene (La Jolla, CA) QuikChange mutagenesis kit. The mutations were verified by sequencing (Cornell DNA sequencing facility, BioResource Center, Ithaca, NY). pCMV transferrin receptor (TR) and pCMV IRAP-TR containing cDNA coding for the human TR and a fusion protein between the intracellular and transmembrane domains of TR fused the cytosolic tail of IRAP, respectively, have been described previously (Johnson et al., 1998). The Stealth siRNAs used were from Invitrogen (Carlsbad, CA): control (Ctrl) (GAGCUACGAGCAACAUUUCGGAUCA), μ1A371 (GAGAGCAUCCGAGACAACUUUGUCA), μ1A585 (UCCUGGAUGUCAUUGAGGCUGUUAA), and γ-adaptin (AGAUCUUUCAGACAGUCCACAAUUG).
The HA.11 anti-hemagglutinin (HA) epitope mouse monoclonal antibody (mAb) (Covance, Berkley, CA) and horseradish peroxidase (HRP)-conjugated human transferrin were prepared as described previously (Karylowski et al., 2004). Fluorescent secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Mouse anti γ-adaptin was from Transduction Laboratories (BD Biosciences, San Jose, CA), and rabbit anti-μ1A serum was a kind gift from Dr. Rodriguez-Boulan (Weill Cornell Medical College, New York).
Data Acquisition and Processing
Images were collected on a DMIRB inverted Leica microscope (Leica Microsystems, Deerfield, IL) by using a 40 × 1.25 numerical aperture oil-immersion objective. The microscope was coupled to a charge-coupled device 12-bit camera (Princeton Instruments, West Chester, PA). Exposure times for each fluorescence channel were chosen such that >95% of the image pixel intensities were below camera saturation and the exposure times for each fluorophore were kept constant within each experiment. Fluorescence quantifications were done using MetaMorph image processing software (Molecular Devices, Sunnyvale, CA) as described previously (Lampson et al., 2000; Lampson et al., 2001; Zeigerer et al., 2002). Backgrounds were measured on HA-GLUT4-GFP–negative cells and were subtracted from specific signals.
HA-GLUT4-GFP Trafficking Assays
The HA-GLUT4-GFP surface-to-total ratio determination assay was described previously (Lampson et al., 2001). To calculate the percentage of GLUT4 in the plasma membrane, the total HA-GLUT4-GFP was measured by indirect immunofluorescence on saponin-permeabilized adipocytes with HA.11 and in parallel plasma membrane was measured in unpermeabilized cells. The fraction of HA-GLUT4-GFP on the surface is thus (Cy3/GFP)surface/(Cy3/GFP)total. In fibroblasts stably expressing HA-GLUT4, surface HA-GLUT4 was measured by anti-HA staining on intact fixed cells, and total HA-GLUT4 was measured on a separate coverslip by anti-HA staining on saponin-permeabilized fixed cells. The fraction of HA-GLUT4 on the surface is thus (Cy3)surface/(Cy3)total.
The HA-GLUT4-GFP exocytosis assay measures the increase of cell associated HA.11 anti-HA mAb over time (Karylowski et al., 2004). A plot of the cyanine 3 (CY3)/GFP ratio versus incubation times was fit to a single exponential described by the equation: (Cy3/GFP)t = (Cy3/GFP)plateau − (Cy3/GFP)t = 0 × exp(−kext); where (Cy3/GFP)t is the Cy3/GFP ratio measured at time t, (Cy3/GFP)plateau is the Cy3/GFP ratio measured at the plateau, and kex is HA-GLUT4-GFP exocytosis rate constant.
The HA-GLUT4-GFP return to basal levels after insulin withdrawal assay was described in detail previously (Blot and McGraw, 2006).
HA-GLUT4-GFP Fast Recycling Assay
Twenty-four hours after electroporation, the cells are incubated in serum free (DMEM-BB) at 37°C for 2 h. HA.11 antibody (saturating concentration in DMEM-BB supplemented with 1 mg/ml ovalbumin) is pulsed for 5 min at 37°C, and unbound antibody is removed by extensive washes with warm DMEM-BB medium for 1 min. Two coverslips per mutant are immediately transferred onto ice water slurry (time 0 and total pulse) and another coverslip is incubated for 30 min at 37°C in DMEM-BB in the presence of Cy3-goat anti-mouse antibody (GAM). In parallel, cells for time 0 are incubated for 30 min on ice water slurry with a cold solution of Cy3-GAM. At the end of the incubations, cells are washed three times with cold medium II solution and fixed. Cells for total pulse are permeabilized and stained with a saponin containing Cy3-GAM solution. Cells are imaged and GFP and cell-associated Cy3 fluorescence are quantified. The average fraction of pulsed GLUT4 in the cell surface at time 0 is the ratio (Cy3/GFP)t = 0/Cy3/GFP)total and was equal to 15%. The fraction of internalized GLUT4 that recycled to the cell surface after 30 min was ((Cy3/GFP)t = 30/Cy3/GFP)total) − (Cy3/GFP)t = 0/Cy3/GFP)total.
Epitope Ablation Assay
HA-GLUT4-GFP intracellular distribution between TR-containing endosomes and GLUT4 specialized/storage compartments/vesicles (GSVs) is determined by epitope ablation after HRP-mediated 3,3′-diaminobenzidine (DAB) polymerization (Lampson et al., 2001; Zeigerer et al., 2002; Karylowski et al., 2004).
RESULTS
Both FQQI and TELEY Motifs Are Required for Full Basal GLUT4 Retention
We mutated by alanine substitutions the F5QQI, LL490, and TE499LE501Y motifs of HA-GLUT4-GFP, either individually (mutated residues in bold, FA, LA, and EEAA) or together (FA/LA, FA/EEAA, and FA/LA/EEAA) (Figure 1A). The phenylalanine at position 5 was also mutated to a tyrosine (FY, FY/EEAA, and FY/LA/EEAA mutants). Adipocytes were electroporated with the different cDNAs and 8 or 24 h later the expression of the mutants was assessed by GFP fluorescence. At both times, all mutants were expressed to the same level as wild-type (WT) HA-GLUT4-GFP, indicating that the mutations did not markedly alter HA-GLUT4-GFP synthesis or turnover (Supplemental Figure 1). All subsequent experiments were performed 24 h after electroporation.
In the basal state, WT HA-GLUT4-GFP was found in perinuclear compartments as well as in peripheral puncta (Figure 1B). The increased diffuse GFP fluorescence in insulin-stimulated WT GLUT4 reflects the redistribution of HA-GLUT4-GFP to the PM (Figure 1B). The FA, LA, and EEAA mutations did not induce changes in the intracellular distribution of GLUT4 that were evident by qualitative visual inspection of GFP fluorescence (Figure 1B). However, the pattern of the FY mutant GLUT4 was altered compared with WT GLUT4. The FY mutant was more concentrated in the perinuclear region of basal adipocytes, with a reduction in the peripheral punctate structures. Insulin induced a partial redistribution of the FY GLUT4 mutant toward peripheral puncta, whereas no increase in diffuse GFP fluorescence was observed.
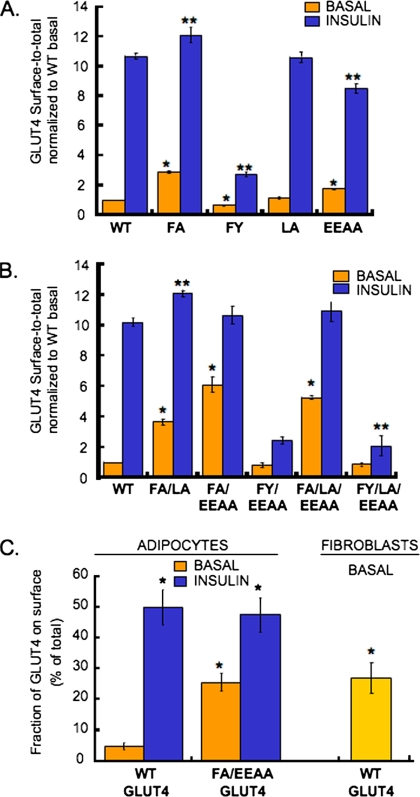
We used quantitative fluorescence microscopy to measure the effects of the mutations on GLUT4 distribution (Figure 2A). PM HA-GLUT4-GFP was measured by indirect immunofluorescence of the HA epitope in intact cells, and the total amount of HA-GLUT4-GFP per cell was determined by the GFP fluorescence (Lampson et al., 2001; Zeigerer et al., 2002). The anti-HA/GFP ratio is proportional to the fraction of GLUT4 in the PM. The WT GLUT4 PM fraction was increased ∼10-fold after insulin stimulation. The LA mutant did not show any significant changes in either basal or insulin-stimulated steady-state surface fraction compared with the WT GLUT4, consistent with previous studies (Verhey et al., 1995; Govers et al., 2004; Blot and McGraw, 2006). The fraction of the EEAA GLUT4 mutant in the PM of basal adipocytes was increased approximately twofold compared with the WT GLUT4, whereas the fraction of EEAA GLUT4 mutant in the PM after insulin-stimulation was reduced by ∼20% (Figure 2A). Thus, mutation of the glutamic acids of the acidic cluster TELEY motif affects GLUT4 behavior in both basal and insulin-stimulated adipocytes. The basal PM fraction of the FA mutant was increased approximately threefold compared with WT GLUT4, and the PM fraction after insulin stimulation was slightly increased. The FY mutation had a slightly reduced fraction in the PM of basal adipocytes and a striking reduction in the fraction of GLUT4 in the PM after insulin stimulation (Figure 2A). Thus, the FA and FY mutations have opposite effects on GLUT4 PM levels: the FA mutation decreased GLUT4 intracellular basal retention, whereas the FY mutation increased GLUT4 retention and significantly blunted insulin-mediated translocation of GLUT4 to the PM.
Figure 2.
The FQQI and TELEY motifs constitute the basal retention signal. (A) Surface-to-total distribution of HA-GLUT4-GFP mutants in basal or insulin-stimulated adipocytes. For insulin stimulation, cells were incubated with 170 nM insulin for 30 min at 37°C. Results are averages ± SEM from 9 to 16 experiments. The data from the individual experiments are normalized to WT GLUT4 basal surface-to-total ratio measured in the individual experiments (*p < 0.001 compared with WT basal, **p < 0.005 compared with WT insulin. Paired Student's t test). (B) The surface-to-total distribution of the HA-GLUT4-GFP double or triple mutants in basal or insulin-stimulated adipocytes. Results are averages ± SD from two to nine independent experiments. The data from the individual experiments are normalized to WT GLUT4 basal surface-to-total ratio measured in the individual experiments (*p < 0.001 compared with WT basal, **p < 0.05 compared with WT insulin, paired Student's t test). (C) Fraction of total WT or FA/EEAA HA-GLUT4-GFP present in the plasma membrane of basal or insulin-stimulated adipocytes, and of HA-GLUT4 in the plasma membrane of 3T3-L1 fibroblasts. Results are average ± SD of three to four independent experiments (*p = 0.006 compared with WT basal, unpaired Student's t test).
Coexpression of the different GLUT4 mutants was without effect on either IRAP-TR, a reporter for IRAP trafficking (Subtil et al., 2000; Zeigerer et al., 2002) or the TR (Supplemental Figure 2). These results demonstrate that GLUT4 motifs specifically control the sorting of GLUT4 and that expression of the mutant GLUT4 do not have a dominant effect on the trafficking of IRAP or the TR.
The behaviors of GLUT4 simultaneously mutated in two or all three motifs were analyzed (Figure 2B). The fraction of the FA/EEAA mutant GLUT4 in the PM of basal adipocytes was increased approximately sixfold compared with WT, indicating that the two motifs have nonredundant roles in determining basal GLUT4 retention. In the presence of insulin, the fraction of the FA/EEAA mutant in the PM was increased to the same level as WT GLUT4, demonstrating that the FA/EEAA mutant, albeit poorly retained in basal adipocytes, was still recruited to the PM upon insulin stimulation.
Quantifying the distribution data as of percentage of total HA-GLUT4-GFP expressed per cell revealed that ∼5% of WT HA-GLUT4-GFP was in the plasma membrane of basal adipocytes, and this fraction increased to 50% upon insulin-stimulation (Figure 2C), consistent with previous reports (Govers et al., 2004). In basal adipocytes, ∼25% of the FA/EEAA-GLUT4 double mutant was in the plasma membrane, which increased to ∼50% with insulin stimulation (Figure 2C). The FA/EEAA-GLUT4 double mutant accumulated in the PM of basal adipocytes to the same degree as WT GLUT4 expressed in undifferentiated 3T3-L1 fibroblasts, demonstrating that the FQQI and TELEY motifs together constitute the information necessary for GLUT4 basal intracellular retention in adipocytes (Figure 2C).
The FY/EEAA double mutant and the FY/LA/EEAA triple mutant were retained in the basal state and failed to efficiently redistribute after insulin stimulation, behaving like the FY mutant. Therefore, the FY mutation-induced retention phenotype was dominant to the EEAA mutation-induced lack of retention. The mutation of the LL motif did not modify GLUT4 surface-to-total distribution when introduced alone (Figure 2A) or when combined with other mutations (Figure 2B).
Mutations in the FQQI and TELEY Motifs Alter GLUT4 Exocytosis
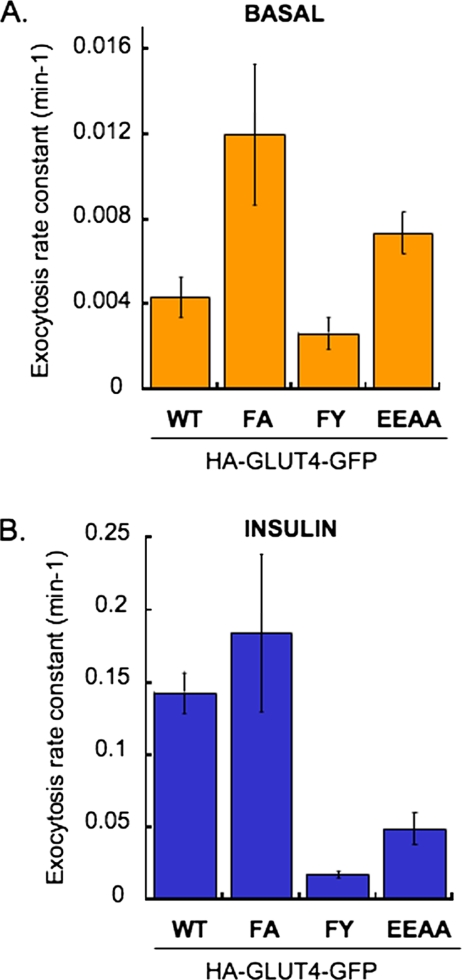
The changes in PM expression of GLUT4 could reflect changes in exocytosis, endocytosis or both. To further understand how mutations of the motifs affect the fraction of GLUT4 in the PM, we measured GLUT4 exocytosis (Figure 3). For all the mutants changes in exocytosis paralleled the changes in steady-state PM GLUT4 (Figure 2). Basal exocytosis of the FA mutant was ∼3 times faster than WT GLUT4, whereas in insulin-stimulated adipocytes the FA mutant and WT GLUT4 were exocytosed at similar rates. Exocytosis of the FY mutant was slightly decreased in the basal state and it was ∼10-fold slower than WT GLUT4 in insulin-stimulated conditions. The exocytosis of the EEAA mutant was increased by approximately twofold compared with WT in the basal state, and although accelerated by insulin, EEAA exocytosis was slower than WT GLUT4. These results support the hypothesis that the FQQI and TELEY motifs control GLUT4 basal intracellular sequestration by regulating exocytosis. The decreased exocytosis of the EEAA mutant and the FY mutant in insulin-stimulated cells is consistent with the decreased net redistribution of these mutants to the PM.
Figure 3.
Effects of different mutations in GLUT4 trafficking motifs on HA-GLUT4-GFP exocytosis rate constant in basal (A) and insulin-stimulated (B) adipocytes. Data from at least three different experiments were averaged and a plot of CY3/GFP ratio versus time were fit to a single exponential described by the equation: (Cy3/GFP)t = (Cy3/GFP)plateau − (Cy3/GFP)t = 0 X exp(−kext), where (Cy3/GFP)t is the Cy3/GFP ratio measured at time t, (Cy3/GFP)plateau is the Cy3/GFP ratio measured (Karylowski et al., 2004). The r value for each fit was equal or greater than 0.98, and the χ2 for the lines were WT, 0.003; FA, 0.02; FY, 0.007; and EEAA, 0.003. The exocytosis rate constants (Kex) are determined from nonlinear curve fit to the above-mentioned equation using Kaleidagraph software (Synergy Software, Reading, PA). The rate constants are presented ± SE.
GLUT4 Motifs Determine GLUT4 Distribution between Endosomes and GSVs
The specific distribution of GLUT4 between endosomes and GSVs can be determined using HRP-catalyzed DAB polymerization-induced epitope ablation (Stoorvogel, 1998). In these studies, HRP is delivered to endosomes by TR uptake of HRP-transferrin (Tf), or to both endosomes and GSV by IRAP-TR uptake of HRP-Tf (Livingstone et al., 1996; Zeigerer et al., 2002). The fraction of GLUT4 in GSV is determined as the difference in HRP-mediated epitope ablation between IRAP-TR and TR-delivered HRP-Tf.
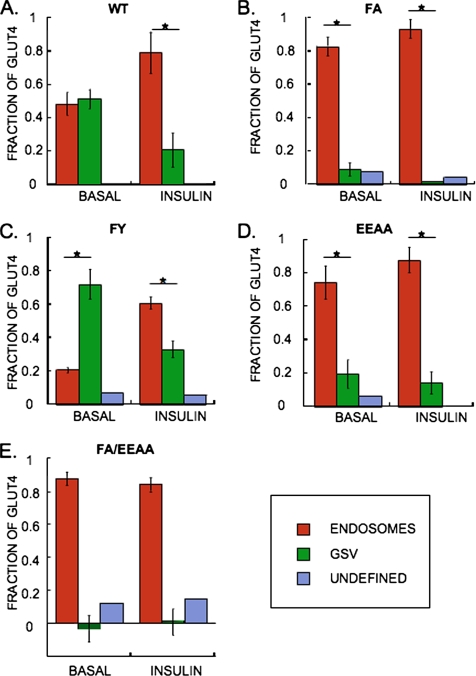
Intracellular WT GLUT4 was evenly distributed between endosomes and GSV in basal adipocytes, and insulin stimulation resulted in a decrease of GLUT4 in the GSV (Figure 4), consistent with previous results (Livingstone et al., 1996; Zeigerer et al., 2002; Karylowski et al., 2004). Both the FA and EEAA mutations caused pronounced, although incomplete redistributions of GLUT4 to TR-containing endosomes in basal adipocytes, whereas the FA/EEAA double mutant was excluded from the GSV (Figure 4). Thus, consistent with the changes in basal retention (Figure 2), the FA and EEAA mutations had partial effects on the intracellular distribution of GLUT4, whereas the effect of the double mutation was additive. The intracellular distributions of the FA and EEAA mutants were further shifted toward endosomes upon insulin stimulation.
Figure 4.
Mutations in GLUT4 motifs alter HA-GLUT4-GFP intracellular distribution between endosomes and GSV. (A) WT GLUT4, (B) FA GLUT4, (C) FY GLUT4, (D) EEAA GLUT4, and (E) FA/EEAA double mutant GLUT4. The distribution of HA-GLUT4-GFP between endosomes and GSV is determined by compartment specific HRP-induced DAB polymerization-mediated ablation of the anti-HA epitopes. HRP taken up by IRAP-TR resulted in 85% ablation of WT GLUT4, which is the maximal ablation expected for two proteins colocalized (Zeigerer et al., 2002). The results presented are corrected for this factor, and nonablated GLUT4 above this 15% threshold are subsequently categorized as being localized in an “undefined” intracellular compartment. Importantly, mutations in GLUT4 resulted in only 0–8% maximum delocalization of HA-GLUT4-GFP in undefined compartments, indicating that the mutations did not result in aberrant delocalization of GLUT4. Results are the average ± SD from three experiments (*p < 0.007, unpaired Student's t test) except for the FA/EEAA mutant, which are the results from a representative experiment plotted as the average ± SEM of at least 20 cells per condition.
The FY mutation had the opposite effect of the FA mutation, resulting in an increased localization of GLUT4 to the GSV in basal adipocytes, and although insulin increased the endosomal localization of the FY mutant, a significant fraction of this mutant remained localized to the GSV (Figure 4). Altogether, these data thus demonstrate a positive correlation between the amount of GLUT4 in the GSV and the efficiency of GLUT4 retention such that mutations that increased basal state localization to endosomes also increased PM levels and vice versa.
Effect of AS160 Knockdown Is Additive to the Effect of FA but Not EEAA Mutation
AS160 is a target of insulin signaling whose activity is required for full GLUT4 retention. AS160 knockdown in adipocytes results in an increase in both GLUT4 basal exocytosis and basal surface-to-total distribution (Sano et al., 2003; Eguez et al., 2005; Larance et al., 2005; Sano et al., 2007). We next examined the behavior the GLUT4 mutants in adipocytes in which AS160 has been knocked down by shRNA (Eguez et al., 2005).
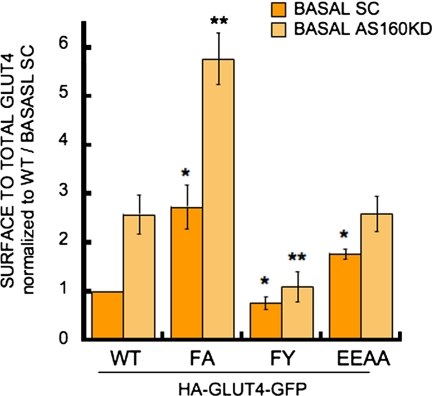
The behavior of the FA mutant was dramatically altered in the AS160 knockdown adipocytes (Figure 5). The threefold increase in basal PM GLUT4 induced by the FA mutation was increased to sixfold by knockdown of AS160. Thus, the effects of the FA mutation and AS160 knockdown were additive, demonstrating that the FA mutant was still under the control of AS160 and therefore indicating that the FQQI motif and AS160 function independently in basal GLUT4 retention. On the contrary, the effect of the EEAA mutation on GLUT4 distribution was not additive to the effect of AS160 knockdown, demonstrating that the EEAA mutant was not under the control of AS160 and therefore indicating that AS160 and the TELEY motif are required at the same “step” of GLUT4 retention The pronounced basal retention of the FY mutant was dominant to the AS160 knockdown phenotype, consistent with the FY mutation being dominant to the effect of the TELEY mutation (Figure 1).
Figure 5.
AS160 knockdown in adipocytes and FA mutation have additive affects on GLUT4 basal levels. AS160 knockdown (KD) adipocytes and control cells expressing a scramble shRNA (SC) were described in Eguez et al. (2005). Results are average ± SD of HA-GLUT4-GFP surface to total distributions in either basal SC or AS160 KD adipocytes from four to seven experiments and are normalized to WT GLUT4 in control SC cells (*p < 0.05 compared with WT GLUT4 in control cells (SC). **p <0.01 compared with WT GLUT4 in AS160 KD adipocytes, paired Student's t test).
AP-1 and GLUT4 LL490 Motif Function in the Same Step Controlling the Return of GLUT4 to Basal Retention
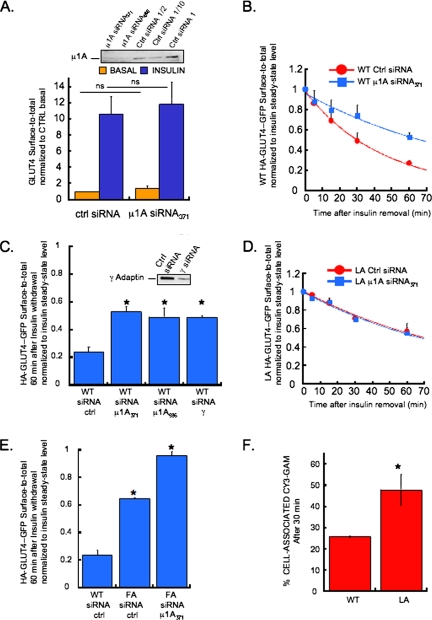
The GLUT4 FQQI motif was shown previously to bind in vitro to the μ chain of the AP clathrin adaptor complex (Al-Hasani et al., 2002). The AP-1 complex has been localized to GLUT4 vesicles (Gillingham et al., 1999). To determine whether AP-1 has a role in GLUT4 traffic, we used siRNA to the μ1A chain to knockdown the AP-1 complex in adipocytes. μ1A knockdown did not result in changes in either basal or insulin-stimulated steady-state GLUT4 distributions, demonstrating that the steady-state amounts of GLUT4 in the PM are not under the control of AP-1 (Figure 6A). These results show that AP-1 does not participate in the trafficking steps regulated by either the FQQI and TELEY motifs, because the basal steady-state PM levels of GLUT4 are under the control of these motifs.
Figure 6.
Clathrin adaptor AP-1 and the LL490 motif work at the same step to control GLUT4 return to basal levels after insulin removal. (A) Knockdown of μ1A using two different siRNA (371 and 585) as determined by western blotting (top inset) and effects of μ1A siRNA371 on GLUT4 basal and insulin-stimulated steady- state surface-to-total distributions. The graph is the average ± SD from six independent experiments. ns, nonsignificant, unpaired Student's t test. (B) Effects of μ1A siRNA371 on WT HA-GLUT4-GFP surface-to-total distribution at increasing times after insulin removal. Plots are average ± SD from two independent experiments fitted to an exponential decrease. (C) Effects of μ1A siRNA371, μ1A siRNA585, or γ-adaptin siRNA on the surface-to-total distribution of WT HA-GLUT4-GFP 60 min after insulin removal. The results are the average ± SD from two to three independent experiments. *p < 0.005 compared with WT siRNA ctrl, unpaired Student's t test. The top inset shows the siRNA-mediated knockdown of γ-adaptin as determined by Western blotting. (D) Effects of μ1A siRNA371 on LA HA-GLUT4-GFP surface-to-total distribution at increasing times after insulin removal. Plots are average ± SD from two independent experiments fitted to an exponential decrease. (E) Effects of μ1A siRNA371 on the surface-to-total distribution of FA HA-GLUT4-GFP 60 min after insulin removal. The results are the average ± SD from two independent experiments. *p ≤ 0.01 compared with WT siRNA ctrl, unpaired Student's t test. (F) The LL490 motif determines the fraction of GLUT4 in the rapid recycling pathway. HA-GLUT4-GFP (WT or LA mutant)-expressing cells were pulsed for 5 min with a saturated concentration of HA.11 antibody. Cy3-conjugated secondary antibodies were then added to the medium for 30 min. The increase in cell-associated Cy3 fluorescence compared with time 0 is a measure of GLUT4 return to the PM during the 30-min incubation. At the beginning of the 30 min incubation, ∼15% of WT and LA mutant HA.11 bound GLUT4 (GLUT4 labeled during the 5-min pulse) were in the PM. The values shown are the total cell-associated at the end of the 30-min incubation with Cy3-GAM and therefore are the initial 15% plus what ever returned to the surface during the incubation. The results are average ± SD from two (WT GLUT4) and three (LA mutant) experiments (*p = 0.077, unpaired Student's t test).
The redistribution of GLUT4 to the PM is reversible, reachieving basal intracellular retention within 2 h of insulin withdrawal (Verhey et al., 1995; Blot and McGraw, 2006). Knockdown of μ1A resulted in a significant delay in GLUT4 return to basal intracellular retention after insulin withdrawal, increasing the half-time by approximately twofold (Figure 6B). Similar results were obtained when μ1A was knocked down using a different siRNA or when the γ-chain was targeted to reduce the AP-1 complex (Figure 6C), both of which rule out nonspecific effects due to siRNA. These data demonstrate that AP-1 has a rate-limiting role in the return to basal retention.
The effect of AP-1 knockdown on return to basal GLUT4 intracellular retention was reminiscent of the previously established phenotype of the L490 to A mutation of GLUT4 LL-based motif (Verhey et al., 1995; Blot and McGraw, 2006). We next determined whether AP-1 knockdown and the LA mutation had additive effects on the return to GLUT4 basal intracellular retention (Figure 6D). Knockdown of μ1A did not further decrease the rate of return to basal of the LA mutant GLUT4, indicating that the LL-based motif and AP-1 complex function at the same step in return to basal retention.
We have previously shown that the mutation of the FA motif also slows return to basal GLUT4 intracellular retention (Blot and McGraw, 2006). However, unlike mutation of the LL-based motif, the F-to-A mutation of the GLUT4 FQQI motif affects the steady-state distributions of GLUT4 in basal and insulin-stimulated adipocytes (Figure 2). Thus, mutation of the FQQI and LL-based motifs likely affect return to basal retention by different mechanisms. To test this hypothesis, we examined the behavior of the FA mutant GLUT4 in AP-1 knockdown adipocytes 60 min after insulin withdrawal (Figure 6E). At this time, WT GLUT4 in the PM of control adipocytes is reduced by ∼80%, the FA GLUT4 mutant is reduced only by ∼40% and the FA mutant expressed in AP-1 knockdown adipocytes was reduced by <10%, demonstrating that knockdown of AP-1 further impairs return to basal retention of the FA mutant GLUT4. Thus, there is additivity in the effects of AP-1 knockdown and FA mutation in the return to basal retention, establishing that the FA and AP-1 are required for different aspects of this process.
A hypothesis for the role of the LL-based motif in the return to basal intracellular retention is that this motif controls the efficiency of the sorting of GLUT4 away from a rapid PM recycling pathway to a compartment where GLUT4 is engaged by the retention machinery. To test this hypothesis, we examined the amount of WT and LA mutant GLUT4 that is rapidly returned to the PM after internalization. Adipocytes expressing WT or LA mutant HA-GLUT4-GFP were pulsed for 5 min with anti-HA antibody (HA.11), washed to remove unbound HA.11, and incubated with a saturating concentration of Cy3-GAM for 30 min at 37°C. During the 5-min pulse with the HA.11, HA-GLUT4-GFP on the PM and any intracellular HA-GLUT4-GFP that cycled to the PM during this time will be bound by HA.11. During the 30-min incubation, HA.11-bound GLUT4 in the PM at the start of this incubation will be immediately bound and the amount of Cy3-GAM will increase over the 30-min incubation as internalized HA.11-bound GLUT4 recycles to the PM.
Approximately 85% of both WT GLUT4 and LA mutant GLUT4 was intracellular at the end of the 5-min pulse. These data are consistent with the LA mutation having no effect on GLUT4 endocytosis (Blot and McGraw, 2006). In cells expressing WT GLUT4, cell-associated Cy3-GAM increased to ∼25% of the total 5-min HA.11 pulse, whereas in cells expressing the LA mutant GLUT4, the cell-associated Cy3-GAM amount increased to nearly 50% (Figure 6F). These data establish that a greater fraction of LA mutant GLUT4 labeled during the 5-min pulse was rapidly recycled to the PM. These data provide direct evidence that the LL490 motif regulates the efficiency of transport of internalized GLUT4 out of a fast recycling pathway toward the retention pathway.
DISCUSSION
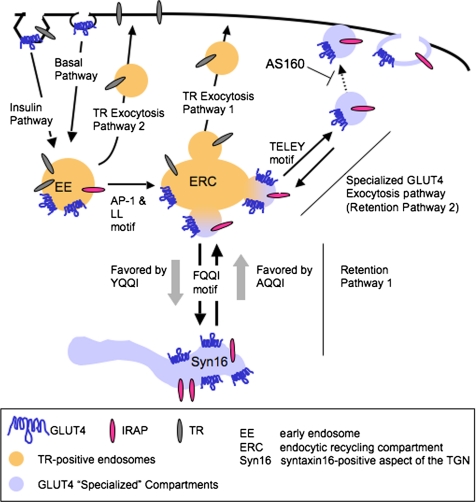
The roles and contributions of GLUT4 sequences in specialized GLUT4 trafficking in adipocytes have not been elucidated. Here we show that 1) the full basal intracellular GLUT4 retention characteristic of adipocytes requires both the amino-terminal FQQI motif and the carboxy-terminal acidic cluster TELEY motif, 2) these motifs regulate different steps of GLUT4 traffic, 3) the step controlled by the TELEY motif is ultimately under the regulation of AS160, and 4) the LL motif and the AP-1 adaptin complex are required for the rapid return to basal GLUT4 intracellular retention after insulin withdrawal. As discussed below, these data support the GLUT4 trafficking model depicted in Figure 7.
Figure 7.
GLUT4 trafficking in adipocytes. For discussion of the intracellular pathways, see the text. We have shown previously, as noted in the figure, that GLUT4 is internalized by different mechanism in basal and insulin-stimulated conditions (Blot and McGraw, 2006). However in both cases GLUT4 is delivered to TR-containing endosomes.
In basal adipocytes, intracellular GLUT4 is equally distributed between TR-containing endosomes and GLUT4-specialized compartments (Livingstone et al., 1996; Martin et al., 1996; Zeigerer et al., 2002; Karylowski et al., 2004). We propose that two distinct intracellular trafficking cycles (Figure 7, retention pathways 1 and 2) are required for segregation of GLUT4 from endosomes. This segregation is necessary to prevent the rapid, unregulated recycling of GLUT4 from endosomes back to the PM via the TR recycling pathway (Figure 7, TR exocytosis pathway 1); therefore, it is essential for the proper basal intracellular retention of GLUT4. One of the GLUT4-specific transport steps is FQQI-mediated targeting from endosomes to a retention compartment (Figure 7, retention pathway 1). We hypothesize that GLUT4 can return to endosomes from this compartment but that GLUT4 cannot move directly to the PM. This retention compartment, which might be a syntaxin-16–positive subcompartment of the trans-Golgi network (TGN) (see discussion below) is similar to that proposed by James and colleagues (Govers et al., 2004). The second retention cycle is TELEY-mediated targeting of GLUT4 from endosomes to specialized transport vesicles. These vesicles can fuse with the PM (Figure 7, specialized GLUT4 exocytosis pathway) or refuse with endosomes (Figure 7, retention pathway 2). We propose that this cycle contributes to the overall basal retention because in basal conditions fusion of these vesicles with endosomes is more frequent than fusion with the PM, whereas in the presence of insulin fusion with the PM is favored. Finally, we propose that the docking of the vesicles of the specialized GLUT4 exocytosis pathway with the PM is under the negative control of AS160 and one effect of insulin is to promote docking of these vesicles to the PM by inactivating the AS160 rab GAP activity. The data supporting this model are as follows.
The results that alanine mutation of the FQQI motif or the TELEY motif increased basal PM GLUT4 by approximately two- to threefold, whereas combining both mutations increased PM GLUT4 by sixfold demonstrate that both sequences are involved in basal retention. The observation that the amount of the FA/EEAA double mutant in the PM of basal adipocytes is similar to the amount of ectopically expressed GLUT4 in the PM of preadipocytes (Figure 2C), suggests that the specialized intracellular retention of GLUT4 characteristic of adipocytes is determined by the FQQI and TELEY motifs. Finally, the increased basal PM accumulation of GLUT4 induced by the FA and EEAA mutations correlated with increased basal exocytosis, supporting the proposal that these motifs target GLUT4 to pathways that determine the characteristic slow basal exocytosis of GLUT4.
A key aspect of the model in Figure 7 is that GLUT4 basal retention involves two intracellular trafficking cycles. The additive effects of alanine mutations of the FQQI and TELEY motifs demonstrate that both motifs are involved in basal retention; however, those results do not distinguish between the motifs functioning independently in retention from the alternative interpretation that the sequences are elements of a single motif that regulates a single trafficking step. Our data that GLUT4 mutated in the individual motifs behaved differently in AS160 knockdown adipocytes strongly support the hypothesis that these motifs regulate distinct GLUT4 trafficking steps.
Considerable data support the proposal that AS160 functions in the regulation of GLUT4 vesicle docking (prefusion step) (Zeigerer et al., 2004; Gonzalez and McGraw, 2006; Jiang et al., 2008). Based on those data, we propose that the TELEY motif functions to target GLUT4 to the specialized transport vesicles that carry GLUT4 to the cell surface (Figure 7, specialized GLUT4 exocytosis pathway). Mutation of the TELEY motif would reduce GLUT4 concentration in these vesicles and thereby reduce the effect of AS160 knockdown on the behavior of EEAA mutant GLUT4. In contrast, the FA mutant GLUT4 would remain under the control of AS160 because the TELEY motif, which is intact in this mutant, would target the FA-mutant GLUT4 to the specialized transport vesicles (Figure 7, specialized GLUT4 exocytosis pathway), whose docking with the PM is under negative regulation of AS160. Finally, the retention of the double FA, EEAA mutant would also be largely independent of AS160 because this mutant would not be efficiently targeted to the specialized GLUT4 transport vesicles. It has recently been reported that the expression of a dominant-interfering AS160 construct (AS160-4P), which inhibits GLUT4 translocation by ∼80%, had a significantly smaller inhibitory effect on the insulin-stimulated translocation of the FA mutant GLUT4 (Capilla et al., 2007). Those results suggest that some of the FA GLUT4 in insulin-stimulated cells moves to the PM by the TR exocytosis pathway, because we have previously shown that AS160-4P does not inhibit insulin-stimulated translocation of TR (Zeigerer et al., 2004).
Consistent with our finding that the FQQI and TELEY individually have roles in determine the basal retention, it was reported previously that the FA mutation and the substitution of the carboxy-terminal 12 amino acids of GLUT4 with GLUT3 sequences, which eliminates the TELEY motif, resulted in an increase in GLUT4 in the PM of basal adipocytes (Govers et al., 2004). Those results are in agreement with our findings that mutation of the individual motifs cause only relatively small changes of GLUT4 in the PM. During preparation of this manuscript, a report was published showing that sequences, in addition to the TELEY motif, within the carboxy terminus of GLUT4 have a role in GLUT4 trafficking because mutation of six of the last nine carboxy-terminal amino acids blocked insulin-stimulated GLUT4 recruitment to the PM (Song et al., 2008).
Mutation of the F to Y of the FQQI motif blunted the insulin-stimulated translocation of GLUT4 to the PM, and it reversed the increase of basal GLUT4 in the PM induced by the EEAA mutation or by knockdown of AS160 (Figures 2 and 5). These data support the proposal that the FQQI motif determines trafficking to a retention cycle, with the Y mutation (YQQI motif) more avidly targeting GLUT4 to this pathway than the WT FQQI sequence (Figure 7, retention pathway 1). The FY mutant GLUT4 is poorly translocated to the surface by insulin-stimulation, suggesting that GLUT4 in this cycle is not directly recruited to the PM by insulin. Furthermore, the FY mutant GLUT4 is also more slowly exocytosed in basal conditions, supporting the proposal that GLUT4 does not efficiently move from this cycle to the PM.
Here, we have focused on the roles of the motifs in regulating basal retention. We have previously shown that the FQQI motif is not required for GLUT4 internalization in basal adipocytes but that it does have a role in GLUT4 endocytosis in insulin-stimulated adipocytes (Blot and McGraw, 2006). The FA mutant is more slowly internalized in insulin-stimulated adipocytes, and this change in internalization will contribute to the increased FA-GLUT4 in the PM of insulin-stimulated adipocytes (Figure 2). The FY mutant is more rapidly internalized than WT GLUT4 in insulin-stimulated adipocytes, and this change will contribute to the reduced amount of the FY GLUT4 mutant on the PM of insulin-stimulated adipocytes. Although, we have not directly analyzed the endocytic behavior of EEAA mutant GLUT4, the changes in exocytosis that we have measured for this mutant largely account for the changes PM expression of the EEAA mutant, suggesting that the major role of the EEAA mutant is to regulate intracellular traffic.
Segregation of GLUT4 from Endosomes
The segregation of GLUT4 from endosomes is a feature of insulin-responsive cells and it is thought to be essential for insulin-responsive GLUT4 trafficking (El-Jack et al., 1999; Shi and Kandror, 2005). Our analysis of the GLUT4 mutants establishes roles for the cytoplasmic motifs in the steady-state intracellular distribution of GLUT4 between endosomes and the specialized compartments (defined as accessible to IRAP but not TR) (Figure 4). Alanine mutation of the FQQI or TELEY motif resulted in partial redistribution of GLUT4 to endosomes and the FA/EEAA double mutant was entirely relocated to endosomes of basal adipocytes. The shift of these mutants toward endosomes corresponded with an increase of GLUT4 in the PM. The distribution of the FY mutant was shifted away from endosomes in both basal and insulin-stimulated conditions and this shift in intracellular distribution correlated with greater intracellular retention of the FY mutant GLUT4. These data indicate that targeting of GLUT4 away from endosomes correlates with retention, possibly by preventing GLUT4 exocytosis via the rapid TR exocytosis pathway (Figure 7, TR exocytosis pathway 1).
Based on the behaviors of the mutants, we propose that the GLUT4 excluded from endosomes is distributed between a retention cycle (Figure 7, retention pathway 1) and regulation of GLUT4 transport vesicles (Figure 7, retention pathway 2). Past studies have shown that GLUT4 continually cycles between endosomes and specialized (non-TR–containing) compartments (Martin et al., 2000a; Bryant et al., 2002; Karylowski et al., 2004); therefore, in basal adipocytes GLUT4 in the retention cycle (pathway 1) is in equilibrium with GLUT4 cycling through the specialized transport vesicles (pathway 2). We propose that GLUT4 is in equilibrium between these two cycles via transport through endosomes.
Our data establish a positive correlation between normal intracellular GLUT4 retention and segregation from endosomes; yet, they also emphasize the important role of transferrin-containing endosomes in the retention of GLUT4. Both the FA and EEAA mutants, despite large relocalizations to endosomes, are still more prominently retained than the FA/EEAA double mutant. These data provide additional support for the concept that the endosomes have an important role in the specialized basal trafficking of GLUT4 (Lampson et al., 2001; Zeigerer et al., 2002; Govers et al., 2004). Furthermore, the twofold redistribution of the FA/EEAA double mutant suggests that a portion of GLUT4 insulin response is not dependent on these motifs. It is possible that the effect of insulin of the double mutant is not specific for GLUT4. For example, the effect of insulin on the FA/EEAA double mutant might be due to the changes in membrane trafficking that are responsible for the twofold translocation of TR to the PM, which would be consistent with the complete localization of the double mutant to endosomes.
There is evidence that GLUT4 retention depends on its localization (or transport through) syntaxin-16 positive subdomains of the TGN (Shewan et al., 2003). Thus, it is possible that the FQQI motif is required for the cycling of GLUT4 between endosomes and the syntaxin-16 positive compartments. In basal adipocytes the FY mutant, compared with WT GLUT4, was concentrated to a greater degree in the peripheral region, with a corresponding reduction in peripheral punctate structures (Figure 1B). The perinuclear region is where the TGN is localized, although we cannot rigorously assign the FY mutant localization to the TGN because in fluorescence microscopy we have been unable to distinguish the TGN from TR-containing endosomes that are also localized in the same region of the cells. Regardless, these findings are consistent with the perinuclear localization being the retention compartment. It is important to note that the perinuclear concentration of FY is accessible to IRAP-TR, suggesting that the FY mutant is redistributed among compartments normally traversed by IRAP and GLUT4. The FY mutant cycles to the PM, albeit more slowly than WT GLUT4, showing that the mutant is not statically stored within the perinuclear compartment (Figure 3).
The data presented here supporting a retention mechanism requiring two specialized GLUT4 intracellular trafficking pathways bring closer two current models for GLUT4 basal intracellular sequestration (reviewed in Dugani and Klip, 2005). Both models agree that there is an intracellular storage pool of GLUT4 in basal adipocytes, with the main difference between the models being whether the storage pool is in constant communication with the plasma membrane. Recently, a detailed analysis of SNARE proteins involved in GLUT4 trafficking also support a model for specialized GLUT4 trafficking involving several distinct intracellular transport steps (Watson and Pessin, 2008). Future studies are required to assign specific SNARE proteins to the steps regulated by the different GLUT4 motifs.
LL490 Motif and AP-1 Are Required for Efficient Return to GLUT4 Basal Retention
Clathrin adaptor AP-1 was previously found on GLUT4-containing vesicles (Gillingham et al., 1999). AP-1 is important for transport between endosomes and the TGN (Bonifacino and Traub, 2003), and the μ1A chain of AP-1 binds GLUT4 FQQI motif in a yeast two-hybrid assay (Al-Hasani et al., 2002). The effect of AP-1 knockdown was similar to the phenotype of the L490A mutant GLUT4 (Verhey et al., 1993; Govers et al., 2004; Blot and McGraw, 2006; Figure 6). Although individually both AP-1 knockdown and mutation of the LL motif slowed by twofold the return of GLUT4 to basal intracellular retention after insulin withdrawal, these effects were not additive, strongly suggesting that the LL motif and AP-1 function at the same transport step. Furthermore, these data suggest that the GLUT4 LL-based motif interacts with AP-1, consistent with previous reports that motifs of the LL-based family bind to AP-1 (Rapoport et al., 1998; Rodionov and Bakke, 1998; Hofmann et al., 1999; Yao et al., 2002). The previous findings that the fungal metabolite brefeldin A causes a delay in GLUT4 return to basal retention after insulin withdrawal (Martin et al., 2000b) and that AP-1 recruitment to GLUT4 vesicles is inhibited by brefeldin A (Gillingham et al., 1999) are also consistent with our results of the effect of AP-1 knockdown on GLUT4 behavior.
How does mutation of the LL motif affect return to basal intracellular retention but not the steady-state distributions of GLUT4? When insulin signaling is attenuated, GLUT4 is internalized and sorted to the retention pathway. We propose that upon insulin withdrawal the LL-based motif efficiently sorts GLUT4 from rapidly recycling pathway (Figure 7, TR exocytosis pathway 2) to an intracellular compartment where the FQQI and TELEY determine the steady-state retention. There are at least two pathways from endosomes back to the PM, one pathway from peripheral endosomes and one pathway from the perinuclear endosome recycling compartment and recycling from the peripheral is much more rapid (Sheff et al., 1999; Hao and Maxfield, 2000). When the LL-motif is mutated, GLUT4 is not efficiently sorted from the peripheral rapidly recycling pathway and consequently a greater fraction of the LL mutant is returned to the PM than WT GLUT4 (Figure 6F). On average, each LL-mutant GLUT4 will cycle through the rapid recycling pathway a greater number of times than WT GLUT4 before moving to later endosomal compartments where it engages the retention pathways controlled by the FQQI and TELEY motifs. Because the steady-state basal retention is based on the functions of the FQQI and TELEY motifs, when the LL-mutant GLUT4 is delivered to a compartment where these motifs function it will be properly retained. It is important to note that we do not know the exact compartment in which the LL motif and AP-1 act, a question to be addressed in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel Chuang and Vadim Meytes for technical assistance, and past and current members of the McGraw lab for discussion and critical reading of the manuscript. We thank Dr. E Rodriguez-Boulan (Weill Cornell, New York) for the gift of the anti-μ1A antibodies. This work was supported by grants DK52852 and DK69982 from the NIH and a gift from the Robert Pollack and Anh-Tuyet Nguyen Charitable Trust.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-03-0236) on June 11, 2006.
REFERENCES
- Al-Hasani H., Kunamneni R. K., Dawson K., Hinck C. S., Muller-Wieland D., Cushman S. W. Roles of the N- and C-termini of GLUT4 in endocytosis. J. Cell Sci. 2002;115:131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- Blot V., McGraw T. E. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J. 2006;25:5648–5658. doi: 10.1038/sj.emboj.7601462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bryant N., Govers R., James D. Regulated transport of the glucose transporter GLUT4. Nat. Rev. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Capilla E., Suzuki N., Pessin J. E., Hou J. C. The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol. Endocrinol. 2007;21:3087–3099. doi: 10.1210/me.2006-0476. [DOI] [PubMed] [Google Scholar]
- Corvera S., Chawla A., Chakrabarti R., Joly M., Buxton J., Czech M. P. A double leucine within the GLUT4 glucose transporter COOH-terminal domain functions as an endocytosis signal. J. Cell Biol. 1994;126:1625. doi: 10.1083/jcb.126.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Chawla A., Woon C. W., Buxton J., Armoni M., Tang W., Joly M., Corvera S. Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J. Cell Biol. 1993;123:127–135. doi: 10.1083/jcb.123.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugani C. B., Klip A. Glucose transporter 4, cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguez L., Lee A., Chavez J. A., Miinea C. P., Kane S., Lienhard G. E., McGraw T. E. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- El-Jack A. K., Kandror K. V., Pilch P. F. The formation of an insulin-responsive vesicular cargo compartment is an early event in 3T3–L1 adipocyte differentiation. Mol. Biol. Cell. 1999;10:1581–1594. doi: 10.1091/mbc.10.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garippa R. J., Judge T. W., James D. E., McGraw T. E. The amino terminus of GLUT4 functions as an internalization motif but not an intracellular retention signal when substituted for the transferrin receptor cytoplasmic domain. J. Cell Biol. 1994;124:705–715. doi: 10.1083/jcb.124.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza L., Birnbaum M. Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J. Biol. Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- Gillingham A. K., Koumanov F., Pryor P. R., Reaves B. J., Holman G. D. Association of AP1 adaptor complexes with GLUT4 vesicles. J. Cell Sci. 1999;112:4793–4800. doi: 10.1242/jcs.112.24.4793. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., McGraw T. E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers R., Coster A. C., James D. E. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol. Cell. Biol. 2004;24:6456–6466. doi: 10.1128/MCB.24.14.6456-6466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M., Maxfield F. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- Hofmann M. W., Honing S., Rodionov D., Dobberstein B., von Figura K., Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J. Biol. Chem. 1999;274:36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- Huang S., Czech M. P. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Jiang L., Fan J., Bai L., Wang Y., Chen Y., Yang L., Chen L., Xu T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J. Biol. Chem. 2008;283:8508–8516. doi: 10.1074/jbc.M708688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. O., Subtil A., Petrush R., Kobylarz K., Keller S. R., McGraw T. E. Identification of an insulin-responsive, slow endocytic recycling mechanism in Chinese hamster ovary cells. J. Biol. Chem. 1998;273:17968–17977. doi: 10.1074/jbc.273.28.17968. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Yu L., Pilch P. F. The major protein of GLUT4-containing vesicles, gp160, has aminopeptidase activity. J. Biol. Chem. 1994;269:30777–30780. [PubMed] [Google Scholar]
- Karylowski O., Zeigerer A., Cohen A., McGraw T. E. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell. 2004;15:870–882. doi: 10.1091/mbc.E03-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. R., Scott H. M., Mastick C. C., Aebersold R., Lienhard G. E. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles [correction published in J. Biol. Chem. (1995). 270, 30236] J. Biol. Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Lalioti V., Vergarajauregui S., Sandoval I. V. Targeting motifs in GLUT4 (review) Mol. Membr. Biol. 2001;18:257–264. doi: 10.1080/09687680110090780. [DOI] [PubMed] [Google Scholar]
- Lampson M. A., Racz A., Cushman S. W., McGraw T. E. Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J. Cell Sci. 2000;113:4065–4076. doi: 10.1242/jcs.113.22.4065. [DOI] [PubMed] [Google Scholar]
- Lampson M. A., Schmoranzer J., Zeigerer A., Simon S. M., McGraw T. E. Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell. 2001;12:3489–3501. doi: 10.1091/mbc.12.11.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larance M., et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- Livingstone C., James D. E., Rice J. E., Hanpeter D., Gould G. W. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3–L1 adipocytes. Biochem. J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B. J., Alm R. A., McIntosh S. R., James D. E. Molecular regulation of GLUT-4 targeting in 3T3–L1 adipocytes. J. Cell Biol. 1995a;130:1081–1091. doi: 10.1083/jcb.130.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E. W., Leopold P. L., Jones N. L., Maxfield F. R. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J. Cell Biol. 1995b;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O. J., Lee A., McGraw T. E. GLUT4 distribution between the plasma membrane and the intracellular compartments is maintained by an insulin-modulated bipartite dynamic mechanism. J. Biol. Chem. 2006;281:484–490. doi: 10.1074/jbc.M505944200. [DOI] [PubMed] [Google Scholar]
- Martin S., Millar C. A., Lyttle C. T., Meerloo T., Marsh B. J., Gould G. W., James D. E. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J. Cell Sci. 2000a;113(19):3427–3438. doi: 10.1242/jcs.113.19.3427. [DOI] [PubMed] [Google Scholar]
- Martin S., Ramm G., Lyttle C. T., Meerloo T., Stoorvogel W., James D. E. Biogenesis of insulin-responsive GLUT4 vesicles is independent of brefeldin A-sensitive trafficking. Traffic. 2000b;1:652–660. doi: 10.1034/j.1600-0854.2000.010809.x. [DOI] [PubMed] [Google Scholar]
- Martin S., Tellam J., Livingstone C., Slot J. W., Gould G. W., James D. E. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 1991;260:C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Tai C., Kulesza P., Pang S., Warnock D., Baenziger J., Slot J. W., Geuze H. J., Puri C., James D. E. GLUT-4 NH2 terminus contains a phenylalanine-based targeting motif that regulates intracellular sequestration. J. Cell Biol. 1993;121:1221–1232. doi: 10.1083/jcb.121.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I., Chen Y. C., Cupers P., Shoelson S. E., Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D. G., Bakke O. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J. Biol. Chem. 1998;273:6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- Sano H., Eguez L., Teruel M., Fukuda M., Chuang T., Chavez J., Lienhard G., McGraw T. Rab10 is a target of the insulin regulated AS160 rabGAP protein required for insulin-stimulated translocation of GLUT4 to the plasma membrane of adipocytes. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sano H., Kane S., Sano E., Miinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sheff D. R., Daro E. A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan A., Marsh B., Melvin D., Martin S., Gould G., James D. The cytosolic C-terminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem. J. 2000;350:99–107. [PMC free article] [PubMed] [Google Scholar]
- Shewan A. M., van Dam E. M., Martin S., Luen T. B., Hong W., Bryant N. J., James D. E. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN 38, involvement of an acidic targeting motif. Mol. Biol. Cell. 2003;14:973–986. doi: 10.1091/mbc.E02-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Kandror K. V. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3–L1 adipocytes. Dev. Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Song X. M., Hresko R. C., Mueckler M. Identification of amino acid residues within the C terminus of the Glut4 glucose transporter that are essential for insulin-stimulated redistribution to the plasma membrane. J. Biol. Chem. 2008;283:12571–12585. doi: 10.1074/jbc.M800838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W. Analysis of the endocytic system by using horseradish peroxidase. Trends Cell Biol. 1998;8:503–505. doi: 10.1016/s0962-8924(98)01380-4. [DOI] [PubMed] [Google Scholar]
- Subtil A., Lampson M. A., Keller S. R., McGraw T. E. Characterization of the insulin-regulated endocytic recycling mechanism in 3T3–L1 adipocytes using a novel reporter molecule. J. Biol. Chem. 2000;275:4787–4795. doi: 10.1074/jbc.275.7.4787. [DOI] [PubMed] [Google Scholar]
- Verhey K. J., Hausdorff S. F., Birnbaum M. J. Identification of the carboxy terminus as important for the isoform-specific subcellular targeting of glucose transporter proteins. J. Cell Biol. 1993;123:137–147. doi: 10.1083/jcb.123.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K. J., Yeh J. I., Birnbaum M. J. Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J. Cell Biol. 1995;130:1071–1079. doi: 10.1083/jcb.130.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Pessin J. E. Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors. J. Cell Sci. 2008;121:1243–1251. doi: 10.1242/jcs.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Clark A. E., Kozka I. J., Cushman S. W., Holman G. D. Development of an intracellular pool of glucose transporters in 3T3–L1 cells. J. Biol. Chem. 1992;267:10393–10399. [PubMed] [Google Scholar]
- Yao D., Ehrlich M., Henis Y. I., Leof E. B. Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol. Biol. Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A., Lampson M., Karylowski O., Sabatini D., Adesnik M., Ren M., McGraw T. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.