Abstract
Eps15 (EGFR pathway substrate clone 15) is well known for its role in clathrin-coated vesicle formation at the plasma membrane through interactions with other clathrin adaptor proteins such as AP-2. Interestingly, we observed that in addition to its plasma membrane localization, Eps15 is also present at the trans-Golgi network (TGN). Therefore, we predicted that Eps15 might associate with clathrin adaptor proteins at the TGN and thereby mediate the formation of Golgi-derived vesicles. Indeed, we have found that Eps15 and the TGN clathrin adaptor AP-1 coimmunoprecipitate from rat liver Golgi fractions. Furthermore, we have identified a 14-amino acid motif near the AP-2–binding domain of Eps15 that is required for binding to AP-1, but not AP-2. Disruption of the Eps15–AP-1 interaction via siRNA knockdown of AP-1 or expression of mutant Eps15 protein, which lacks a 14-amino acid motif representing the AP-1 binding site of Eps15, significantly reduced the exit of secretory proteins from the TGN. Together, these findings indicate that Eps15 plays an important role in clathrin-coated vesicle formation not only at the plasma membrane but also at the TGN during the secretory process.
INTRODUCTION
Clathrin-coated vesicles and associated adaptor proteins play an important role in the cyclical pattern of membrane trafficking throughout the endocytic and secretory pathways (Traub, 2005; McNiven and Thompson, 2006). Conventional clathrin adaptors, such as the adaptor protein (AP) complexes AP-1, -2, -3, and -4, are known to sequester and link membrane cargoes to the clathrin lattice while recruiting other accessory proteins that aid in the formation of the clathrin basket (Robinson and Bonifacino, 2001; Owen et al., 2004; Robinson, 2004; Ungewickell and Hinrichsen, 2007). Although dozens of these additional adaptor proteins, including Eps15 (EGFR pathway substrate clone 15), epsin, AP180, and amphiphysin to name just a few, are known to interact with AP-2 and mediate vesicle formation from the plasma membrane (Schmid and McMahon, 2007), information regarding clathrin-based adaptors at the trans-Golgi network (TGN) is less extensive (Traub, 2003, 2005; Sorkin, 2004; McNiven and Thompson, 2006). The endocytic adaptor Eps15 was originally identified as a substrate of the EGFR (Fazioli et al., 1993) and is well known to participate in clathrin-mediated endocytosis. As examples, Eps15 localizes to plasma membrane clathrin-coated pits and vesicles (Tebar et al., 1996; van Delft et al., 1997) and is recruited to the plasma membrane upon epidermal growth factor receptor (EGFR) activation (Torrisi et al., 1999). In addition, disruption of Eps15 function through injection of cells with antibodies against Eps15 or expression of Eps15 mutants inhibits the internalization of both EGF and transferrin (Carbone et al., 1997; Benmerah et al., 1998, 1999, 2000).
The multiple interaction domains of Eps15 make it well suited to function as an endocytic adaptor. Its N-terminal domain contains three Eps15 homology (EH) domains, a protein–protein interacting module that recognizes NPF (asparagine-proline-phenylalanine) motifs. Through these EH domains, Eps15 interacts with other endocytic accessory proteins such as epsin and synaptojanin (Haffner et al., 1997; Chen et al., 1998). A central coiled-coiled domain mediates Eps15 homo-oligomerization as well as hetero-oligomerization with Eps15R (Tebar et al., 1997; Coda et al., 1998). Slightly more C-terminal is an ∼120-amino acid region containing multiple DPF (aspartate-proline-phenylalanine) repeats, thus facilitating interactions with AP-2 (Benmerah et al., 1996; Iannolo et al., 1997), whereas at the very C-terminus are two ubiquitin-interacting motifs (UIMs), which are necessary for intra- and intermolecular interactions with ubiquitin and/or monoubiquitination (Klapisz et al., 2002; Polo et al., 2002; Hoeller et al., 2006). Many of these structural domains appear to be essential for the participation of Eps15 in endocytosis. For example, cells expressing the AP-2–binding region of Eps15 alone or, alternatively, an Eps15 truncation mutant lacking the EH domains no longer exhibit a punctate distribution of clathrin and/or AP-2 at the plasma membrane and have a reduced capacity for endocytosis (Benmerah et al., 1999, 2000). Furthermore, addition of glutathione S-transferase (GST) fusion proteins of the C-terminal domain of Eps15 containing the AP-2–binding region to perforated cells attenuates the endocytosis of both transferrin and EGF (Benmerah et al., 1998). More recently, the UIMs of Eps15 have been implicated in mediating either a direct or indirect interaction with ubiquitinated EGFRs, and additionally, appear to be important for receptor internalization (de Melker et al., 2004; Sigismund et al., 2005).
In addition to the association of Eps15 with plasma membrane–generated clathrin-coated pits, Eps15 has also been observed to localize to a perinuclear compartment (Tebar et al., 1996; Torrisi et al., 1999; Kent et al., 2002). Moreover, detailed structural studies have shown that the appendage domain of γ-adaptin, a subunit of the TGN- and endosome-localized AP-1 complex, binds to Eps15 (Kent et al., 2002). However, the specific region of Eps15 that mediates the interaction with γ-adaptin is unknown as is whether the association between Eps15 and AP-1 serves a functional role at the TGN or rather at an endosomal compartment.
In this study, we present morphological and biochemical evidence indicating that Eps15 directly associates with the TGN, and furthermore, that this Golgi localization is supported by an interaction with AP-1. Indeed, we have identified a novel 14-amino acid motif in the DPF repeats domain of Eps15 that is required for the interaction between Eps15 and the γ-adaptin appendage domain. Importantly, functional studies demonstrated that the Eps15–AP-1 complex mediates the formation of TGN-derived vesicles containing various cargo proteins, including late endosomal as well as constitutively secreted proteins.
MATERIALS AND METHODS
Plasmid Construction and Small Interfering RNA
Constructs encoding for wild-type Eps15 or a dominant-negative version of Eps15 lacking the second and third EH domains (Eps15 ΔEH2/EH3) have been previously described (Cao et al., 2005) and were used for subcloning of the respective Eps15 inserts into the pCDNA3.1/Myc-His vector (Invitrogen, Carlsbad, CA). Similarly, the construct encoding for His-tagged Eps15 was generated by subcloning of a wild-type Eps15 insert into the pQE-80L vector (Qiagen, Chatsworth, CA). Myc-tagged Eps15 Δ14aa, depicted in Figure 3, was generated by using PCR-based site-directed mutagenesis and verified by sequencing. Constructs encoding for GST-tagged Eps15 EH domains (amino acids 1–320), coiled-coil domain (amino acids 321–540), DPF repeats + proline-rich motif (amino acids 541–790), UIMs (amino acids 791–897), DPF repeats alone (amino acids 630–733), or DPF repeats lacking the C-terminal 14 amino acids (amino acids 630–719) were generated by using PCR to amplify the respective domain from full-length Eps15 cDNA and subsequently cloning the fragments into the pGEX-4T-1 vector (Life Sciences, St. Petersburg, FL). A construct encoding for temperature-sensitive vesicular stomatitis virus glycoprotein (VSVG) was a gift from J. Lippincott-Schwartz (National Institutes of Health) and was subcloned into the pEGFP-N1 vector (Clontech, Palo Alto, CA; Cao et al., 2000). Amplification of mouse M6PR cDNA to generate the M6PR-GFP construct has been previously described (Cao et al., 2005). Full-length rat γ-adaptin (accession number XM_214107) was amplified by PCR using rat liver cDNA as a template and the following primers: 5′-ACTCCCGGGCTCTGTCCCCAGGATGGTGCCTT-3′ (forward); 5′-CAGTAAGTTACTGCCACGTCTCCACAGGCAAG-3′ (reverse). A construct encoding for GST-tagged γ-adaptin appendage domain (amino acids 704–786) was generated by amplifying the relevant region from full-length γ-adaptin cDNA using the following primers: 5′-CCGGAATTCTCAGAGGAGGCTGTT-3′ (forward); 5′-CCGCTCGAGCTGCCACGTCTCCAC-3′ (reverse). The resulting PCR product was subsequently cloned into the pGEX-4T-1 vector.
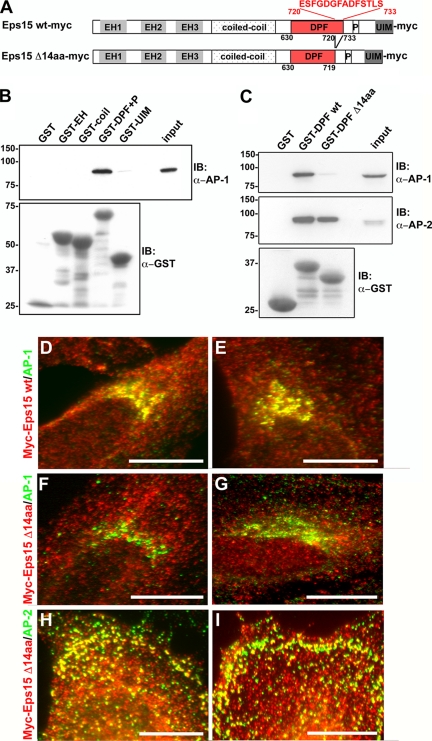
Figure 3.
The interactions between Eps15 and the clathrin adaptors AP-1 and AP-2 are mediated by distinct domains of Eps15. (A) Schematic representation of the Myc-tagged wild-type and 14-amino acid deletion mutant Eps15 proteins used in this study. The indicated Eps15 protein domains include: three Eps15 homology (EH) domains, a coiled-coil domain, a region rich in aspartate-proline-phenylalanine (DPF) repeats, a proline-rich region (P), and two ubiquitin-interacting motifs (UIM). A 14-amino acid region in the C-terminal region of the DPF repeats domain has been predicted to bind to AP-1 (Duncan and Payne, 2003) and was deleted as shown. (B and C) GST fusion proteins of the different Eps15 domains, as indicated, were immobilized on glutathione-Sepharose beads and incubated with HeLa cell lysates. The proteins bound by the different Eps15 domains were then analyzed by immunoblotting with antibodies against AP-1 (B and C) and AP-2 (C). As a control, samples were also immunoblotted for GST to detect the amount of GST-tagged protein loaded (B and C). The GST fusion proteins containing the full-length DPF repeats domain of Eps15, either in combination with the proline-rich region (GST-DPF+P, B) or alone (GST-DPF wt, C), were able to pull down AP-1 and -2. In contrast, GST fusion proteins of the DPF repeats domain deletion mutant lacking the C-terminal 14 amino acids (GST-DPF Δ14aa) no longer pulled down AP-1, whereas the association with AP-2 was maintained (C). The input lanes represent 5% of the total cell lysates used for the pulldown. (D–I) The 14-amino acid motif at the C-terminus of the DPF repeats domain is necessary for the Eps15–AP-1 interaction at the trans-Golgi network within cells. HeLa cells expressing either Eps15 wt-myc (D and E) or Eps15 Δ14aa-myc (F–I) were immunostained for Myc (D–I, red) and AP-1 (D–G, green) or AP-2 (H and I, green). In agreement with the pulldown assays, significant overlap was not detected between the Eps15 deletion mutant, Eps15 Δ14aa-myc, and AP-1 at the Golgi (F and G; compare with Eps15 wt-myc and AP-1 shown in D and E), whereas the colocalization with AP-2 at the plasma membrane was maintained (H and I). Scale bars, 20 μm.
Human AP-1γ1 small interfering RNA (siRNA) pool and nontargeting siRNA pool were purchased from Dharmacon Research (Boulder, CO).
Antibodies
The anti-Eps15 polyclonal antibodies Eps15(I) and Eps15(II) were raised against the peptide sequences EWAKRESEREEEQRLARLNQQEQED (amino acids 855–882) and SQQEISSMQMRLAMKDLETDNNQSN (amino acids 485–510), respectively, and affinity-purified as previously described (Henley and McNiven, 1996). A polyclonal anti-Eps15 antibody was also purchased from Santa Cruz Biotechnology (Santa Cruz, CA) The monoclonal anti-Myc antibody was obtained from Zymed Laboratories (South San Francisco, CA), and the polyclonal anti-Myc antibody was obtained from Cell Signaling Technology (Beverly, MA). Generation of the polyclonal anti-TGN38 antibody has been previously described (Cao et al., 2000). The monoclonal anti-clathrin antibody (X22) was collected from the supernatant of the X22 hybridoma cell line (ATCC, Manassas, VA). The monoclonal anti-TGN38 antibody was kind gift from K. E. Howell (University of Colorado School of Medicine). The monoclonal anti-AP-2 (α) and polyclonal anti-AP-1 (γ) antibodies used for immunoblotting were kindly provided by L. M. Traub (University of Pittsburg School of Medicine). The monoclonal anti-AP-1 (γ) antibody used for immunofluorescence was purchased from Sigma (St. Louis, MO). The anti-p230 antibody and anti-GM130 was obtained from BD Transduction Laboratories (Lexington, KY). The monoclonal anti-GST antibody was obtained from Santa Cruz Biotechnology, and the monoclonal α-tubulin antibody was purchased form Amersham Biosciences (Piscataway, NJ).
Cell Culture and Transfection
BHK-21 cells (ATCC, CCL-10), HeLa cells (ATCC, CCL-2), and HuH7 cells (provided by Dr. G. J. Gores, Mayo Clinic College of Medicine) were maintained in Eagle's minimum essential medium containing Earle's salts and l-glutamine (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 1.5 g/l sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Rat fibroblasts (ATCC, CRL-1213) were maintained in Ham's F-12K medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were kept in a 5% CO2/95% air incubator at 37°C, except for during the M6PR-GFP and VSVG-ts-green fluorescent protein (GFP) trafficking assays. Cells were transiently transfected using the Lipofectamine Plus Reagent kit according to the manufacturer's protocol (Invitrogen). Transfection of HeLa cells with siRNA was performed using Oligofectamine as specified by the manufacturer's protocol (Invitrogen).
Peptide Injection and Fluorescence Microscopy
HeLa cells were injected with 100 nM peptide (amino acids 720–733 of Eps15) diluted in injection buffer (10 mM KH2PO4, pH 7.2, 75 mM KCl). After 6 h, cells were fixed and processed for immunofluorescence as previously described (Cao et al., 1998). For colocalization experiments, cells were grown on coverslips for 1–2 d before being processed for immunofluorescence. Cells were viewed with an Axiovert 35 or 200M microscope (Carl Zeiss, Thornwood, NY) using a 63×, 1.4 NA, oil-immersion lens, and images were acquired with an Orca II or Orca III ERG camera (Hamamatsu, Bridgewater, NJ) using IPLab (Scanalytics, Billerica, MA). Images were subsequently adjusted in Adobe Photoshop (San Jose, CA).
Golgi Fractionation and Coimmunoprecipitation
An enriched stacked Golgi fraction (SGFII) was isolated from rat liver following previously published methods (Taylor et al., 1997). For coimmunoprecipitation assays, 1 mg of fractionated Golgi samples was incubated with anti-Eps15(I) or anti-Eps15(II) antibodies and combined with protein A-Sepharose beads in immunoprecipitation buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 15 mM NaF, 2 mM Na3VO4, and complete protease inhibitors; Roche, Indianapolis, IN) for 2 h at 4°C. The beads were then washed four times with immunoprecipitation buffer and boiled in reducing Laemmli sample buffer. Samples were separated on 7.5% SDS-polyacrylamide gels using electrophoresis and transferred onto PVDF membranes. Immunoblotting was performed using anti-Eps15(II), anti-clathrin, or anti-AP-1 (polyclonal) antibodies.
GST Pulldown Assays
HeLa cells were lysed by sonication in lysis buffer (25 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM DTT, 1% NP-40, 15 mM NaF, 2 mM Na3VO4, and complete protease inhibitors) and cleared by centrifugation at 14,000 rpm for 10 min. The cleared supernatants were used as cell lysates for the pulldown assay. Cell lysates (1 mg) were incubated for 2 h with GST or GST-fusion proteins (5 μg) coupled to glutathione-agarose beads at 4°C. For the in vitro binding assay, 5 μg of GST or GST-γ-adaptin appendage domain coupled to glutathione-agarose beads was incubated with 5 μg of His-Eps15, purified according to the manufacturer's protocol (Qiagen), for 2 h at 4°C. When indicated, peptide (amino acids 720–733 of Eps15) was added directly to the assay mixture at a final concentration of 1 or 10 μM. After incubation with either cell lysates or purified His-Eps15, beads were washed four times with wash buffer (25 mM Tris-HCl, pH 7.4, 300 mM NaCl, 1 mM DTT, 1% NP-40, 15 mM NaF, 2 mM Na3VO4, and complete protease inhibitors), and the bound proteins were eluted from the beads with reducing Laemmli sample buffer. Samples were separated on 7.5% SDS-polyacrylamide gels using electrophoresis, transferred onto PVDF membranes, and immunoblotted using anti-AP-1 (monoclonal), anti-AP-2, anti-Eps15, or anti-GST antibodies.
M6PR-GFP and VSVG-ts-GFP Trafficking Assays
Transport of M6PR-GFP and VSVG-ts-GFP was assayed essentially as previously described (Cao et al., 2000, 2005). For assaying transport of M6PR-GFP to dextran-labeled late endosomes, cells expressing M6PR-GFP were first incubated for 2 h at 20°C to block transport from the Golgi, followed by a 1-h incubation with rhodamine-labeled dextran at 37°C. For assaying transport of VSVG-ts-GFP in AP-1 (γ)-depleted cells, HeLa cells were treated with control siRNA or siRNA probes to AP-1 (γ) for 48 h before VSVG-ts-GFP transfection. Cells were then either homogenized and subjected to Western blot analysis to assess AP1-(γ) levels or viewed by IF microscopy to assess the distribution of nascent VSVG. After the various trafficking assays, cells were processed for fluorescence microscopy and imaged as described above. For quantitation of VSVG-ts-GFP or M6PR-GFP at the Golgi region, the fluorescence intensity within a standardized perinuclear region was measured in IPLab using images taken at the same acquisition settings (exposure time, 5 s). Representative images for each set of trafficking assays were adjusted in Adobe Photoshop using identical “Levels” settings.
RESULTS
Eps15 Localizes to the trans-Golgi Network via an Interaction with the AP-1 Complex
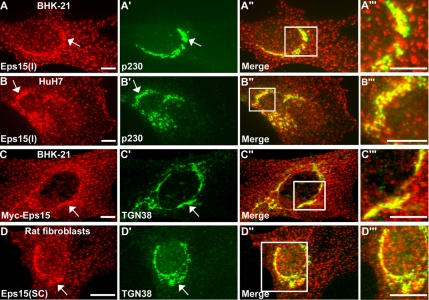
A role for Eps15 in the formation of clathrin-coated vesicles at the plasma membrane and subsequent internalization of receptors is well known (Benmerah et al., 1998, 1999, 2000). In addition, Eps15 has been noted to localize to intracellular organelles (Tebar et al., 1996; Torrisi et al., 1999; Kent et al., 2002); however, its function at these sites is less clear. Here we have applied a variety of different molecular and immunological reagents to epithelial cells (BHK-21 cells and human hepatocellular carcinoma-derived HuH7 cells) and rat fibroblasts to help better define the cytoplasmic localization and function of Eps15. These Eps15 reagents include a polyclonal antibody made against a peptide representing a portion of the UIM of rat Eps15 [Eps15(I)], a purchased polyclonal antibody, and a construct encoding for Myc-tagged full-length Eps15. Immunofluorescence staining of cultured cells for either endogenous Eps15 or exogenously expressed Myc-tagged Eps15 (Eps15-Myc) indicated two distinct distribution patterns: first, a punctate staining along the plasma membrane similar to that observed for clathrin-coated pits, and second, a bright, punctate, perinuclear localization reminiscent of the Golgi apparatus (Figure 1). Indeed, costaining with antibodies against the TGN proteins p230 and TGN38 revealed a considerable level of colocalization between these Golgi proteins and Eps15, as displayed in the color overlay images (Figure 1, A″–D″ and A‴–D‴). Thus, these observations of endogenous as well as Myc-tagged Eps15 using multiple antibodies and cell types strongly suggest that Eps15 associates with the TGN.
Figure 1.
The endocytic adaptor protein Eps15 localizes to the trans-Golgi network. (A–B‴) The localization of endogenous Eps15 to the Golgi was detected after immunofluorescence staining of BHK-21 (A–A‴) and HuH7 (B–B‴) cells using a purified polyclonal anti-Eps15 antibody, Eps15(I), and the trans-Golgi network marker p230. (C–C‴) To support the immunological localization of endogenous Eps15, BHK-21 cells were transfected with a construct encoding for Myc-tagged Eps15 and immunostained for Myc and the trans-Golgi network marker TGN38. (D–D‴) As an additional cell model and using a commercially available antibody against Eps15 from Santa Cruz Biotechnology, Eps15(SC), rat fibroblasts were immunostained for endogenous Eps15 and TGN38. Arrows indicate colocalization of Eps15 staining (A, B, C, and D) with Golgi markers (A′, B′, C′, and D′). Higher magnification images (A‴, B‴, C‴, D‴) of regions boxed in white in the respective merged images to the left (A″, B″, C″, and D″) show substantial overlap (yellow) between the staining patterns of Eps15 (red) and Golgi markers (green) in the perinuclear region. Scale bars, 10 μm.
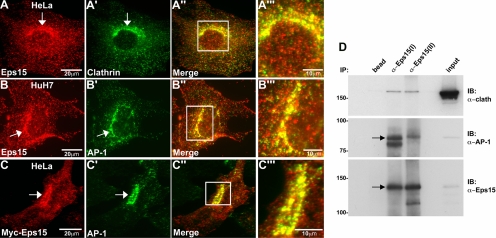
Eps15 participates in the formation of clathrin-coated vesicles from the plasma membrane via an interaction with the AP-2 complex. As a similar AP complex exists at the TGN, AP-1, we predicted that the Golgi-associated Eps15 might perform a similar function at this organelle through interactions with AP-1. Using immunofluorescence staining of cultured epithelial cells (HeLa and HuH7), we first tested whether endogenous Eps15 colocalizes with clathrin and AP-1 at the TGN. The anti-Eps15 distribution pattern indicated a near complete colocalization with clathrin (Figure 2, A–A‴) and AP-1 (Figure 2, B–B‴) in a perinuclear region, consistent with previous observations (Kent et al., 2002), as well as a more peripheral punctate localization that overlapped with the plasma membrane clathrin adaptor AP-2 (data not shown). To extend these observations of endogenous Eps15 distribution, we also analyzed the localization of Myc-tagged Eps15. Here also we found that Eps15-Myc targeted to both the plasma membrane and TGN, as indicated by colocalization with AP-1 (Figure 2, C–C‴).
Figure 2.
Eps15 colocalizes with clathrin and the adaptor protein complex AP-1 at the Golgi. (A–B‴) HeLa cells were immunostained with antibodies against Eps15 and clathrin (A–A‴), whereas HuH7 cells were immunostained with antibodies against Eps15 and AP-1 (B–B‴). (C–C‴) Additionally, HeLa cells expressing Myc-tagged Eps15 were immunostained for Myc and AP-1. Colocalization of Eps15 (A, B, and C) with clathrin (A′) or AP-1 (B′ and C′) at the Golgi region is marked by arrows. Higher magnification images (A‴, B‴, and C‴) of regions boxed in white in the respective merged images shown to the left (A″, B″, and C″) show substantial overlap (yellow) of Eps15 (red) with clathrin (A‴, green) or AP-1 (B‴ and C‴, green) at the perinuclear Golgi region. (D) Protein extracts of fractionated Golgi from rat liver were subjected to immunoprecipitation using two different anti-Eps15 antibodies [anti-Eps15(I) or anti-Eps15(II)] and subsequently analyzed by Western blot, probing with antibodies against clathrin, AP-1, and Eps15. The last lane (input) represents 5% of the total protein extract used for immunoprecipitation. A substantial amount of AP-1 as well as a modest amount of clathrin heavy chain coimmunoprecipitate with either of the anti-Eps15 antibodies. Scale bars, 20 μm (A, B, and C); 10 μm (A‴, B‴, and C‴).
The results from costaining of cells with antibodies against Eps15 and Golgi marker proteins such as p230, AP-1, and TGN38 are consistent with a Golgi localization for Eps15. However, we wanted to further confirm this specific association by determining whether the association of Eps15 with the Golgi was altered upon disruption of Golgi structure. The recruitment of AP-1 to the Golgi is sensitive to brefeldin A (BFA) treatment; therefore, we examined the effects of BFA treatment on the colocalization of Eps15 with AP-1 at the TGN. As shown in Supplementary Figure S1, the perinuclear codistribution of Eps15 and AP-1 was significantly disrupted in BFA-treated cells, supporting the concept that these vesicle coat proteins reside together on the TGN.
We next wanted to confirm and extend the morphological observations made above indicating that Eps15 localizes to the TGN in association with AP-1 using a biochemical approach. Therefore, coimmunoprecipitation assays were performed using fractionated Golgi membranes from rat liver followed by Western blot analysis. Indeed, two different purified anti-Eps15 antibodies [Eps15(I) and Eps15(II)] coimmunoprecipitated both clathrin and AP-1 from Golgi membranes (Figure 2D). Thus, together these findings using both morphological and biochemical methods strongly suggest that these three coat proteins form a complex at the TGN.
A Specific Motif in the DPF Repeat Domain of Eps15 Is Required for Direct Binding between Eps15 and AP-1 But Not AP-2
A C-terminal domain of Eps15 rich in DPF repeats has been demonstrated to directly interact with the appendage domain of α-adaptin (Benmerah et al., 1996; Iannolo et al., 1997), one of the large subunits of the AP-2 complex, and thus participates in the targeting of Eps15 to clathrin-coated vesicles at the plasma membrane (Benmerah et al., 2000). Similarly, a GST fusion protein of the appendage domain of γ-adaptin, a large subunit of the AP-1 complex analogous to α-adaptin, can pull down Eps15 from brain cytosol (Kent et al., 2002). However, the domains of Eps15 responsible for this interaction with AP-1 have not been determined. Moreover, although the AP complexes are thought to exhibit a similar domain structure (Kirchhausen, 1999; Edeling et al., 2006), the γ-adaptin appendage domain appears to lack the protein-binding platform domain present in the α-adaptin appendage domain (Kent et al., 2002) and thus might interact with Eps15 through distinct motifs. Therefore, to identify the specific domains of Eps15 that might mediate the interaction with AP-1, GST pulldown assays were performed using four distinct functional domains of Eps15 tagged to GST. These domains, as illustrated in Figure 3A, represent the three N-terminal EH domains, which interact with proteins such as epsin (Chen et al., 1998) and synaptojanin (Haffner et al., 1997); a coiled-coiled domain that is thought to mediate Eps15 homo-oligomerization as well as hetero-oligomerization with Eps15R (Tebar et al., 1997; Coda et al., 1998); an AP-2–binding domain comprising the DPF repeats + a proline-rich region (DPF + P; Benmerah et al., 1996; Iannolo et al., 1997; Benmerah et al., 2000); and the two C-terminal UIMs involved in self -ubiquitination as well as intra- and intermolecular interactions with ubiquitinated residues (Klapisz et al., 2002; Polo et al., 2002; Hoeller et al., 2006). The different GST fusion proteins of specific Eps15 domains were incubated with HeLa cell lysates, and the bound proteins were analyzed by Western blot, probing with an anti-AP-1 antibody. As shown in Figure 3B, only the DPF+P domain pulled down AP-1, indicating that this domain of Eps15 rich in DPF repeats might contain motifs that mediate not only interactions with AP-2, but also with AP-1. Indeed, a 14-amino acid motif at the C-terminal end of this DPF repeats domain, distinct from the known AP-2–binding region of this domain, has been predicted to bind to AP-1, although never definitively confirmed (Duncan and Payne, 2003). Therefore, constructs encoding for GST fusion proteins containing either the entire DPF repeats domain (amino acids 630–733; GST-DPF wt) or the DPF repeats domain lacking these 14 amino acids (amino acids 630–719; GST-DPF Δ14aa) were generated, and the ability of these mutant fusion proteins to isolate AP-1 versus AP-2 from HeLa cell lysates was tested. Interestingly, although the full-length Eps15 DPF repeats domain bound to both AP-1 and AP-2, binding of the truncated Eps15 DPF repeats domain to AP-1 was significantly reduced, whereas the interaction with AP-2 was maintained (Figure 3C). Thus, these 14 amino acids at the C-terminal end of the DPF repeats domain appear to be particularly important for the Eps15–AP-1 interaction; however, they are dispensable for the binding of Eps15 to AP-2.
The above results from the GST pulldown assays suggested that a 14-amino acid motif at the C-terminus of the DPF repeats domain of Eps15 mediates binding to AP-1; therefore, we next wanted to determine whether this in vitro observation translated to cultured cells. Toward this end, HeLa cells were transiently transfected with constructs encoding for Myc-tagged full-length wild-type Eps15 (Eps15 wt-myc) or a Myc-tagged Eps15 deletion mutant lacking the C-terminal 14 amino acids of the DPF repeats domain (Eps15 Δ14aa-myc), and the colocalization of these Eps15 proteins with AP-1 at the TGN was observed using fluorescence microscopy. Indeed, the full-length Myc-tagged Eps15 protein was localized at the TGN along with AP-1 (Figures 2, C–C‴, and 3, D and E), whereas the Eps15 Δ14aa-myc deletion mutant was no longer targeted to the TGN (Figure 3, F and G). However, the Eps15 Δ14aa-myc deletion mutant did continue to associate with AP-2–positive clathrin-coated pits at the cell surface (Figure 3, H and I; data not shown), indicating that this mutant Eps15 is still capable of binding to AP-2 in cells. Thus, these observations in cultured cells further support our in vitro results suggesting that the AP-1– and AP-2–binding motifs of Eps15 are distinct.
To further test the significance of the identified 14 amino acids within the Eps15 DPF repeats domain as an AP-1–binding motif, we aimed to interfere with or block this interaction both in vitro and in living cells through use of a synthetic peptide encompassing the putative AP-1–binding motif (amino acids 720–733 of Eps15). We first performed in vitro binding assays to characterize the ability of full-length His-tagged Eps15 to bind to GST-tagged γ-adaptin appendage domain (amino acids 704–786) in the presence of increasing concentrations of the 14-amino acid peptide. As shown in Figure 4A, GST-γ-adaptin appendage domain was able to pull down His-Eps15 in the absence of peptide, indicating a direct interaction; however, in the presence of peptide, this association between Eps15 and the γ-adaptin appendage domain was reduced (1 μM peptide) or completely prevented (10 μM peptide). Thus, these results suggest that the 14-amino acid motif at the C-terminus of the Eps15 DPF repeats domain is essential for the direct interaction between the γ-adaptin subunit of AP-1 and Eps15.
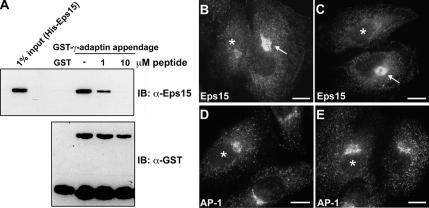
Figure 4.
Disruption of the Eps15–AP-1 interaction reduces recruitment of Eps15 to the trans-Golgi network. The association between Eps15 and AP-1 is reduced by the competitive action of a 14-amino acid peptide representing the C-terminal residues of the DPF repeats domain both in vitro and in vivo. (A) GST or GST-tagged γ-adaptin appendage domain was immobilized on glutathione-Sepharose beads and incubated with full-length His-Eps15 in the absence or presence (1 or 10 μM) of the 14-amino acid Eps15 peptide (720ESFGDGFADFSTLS733). The pulldowns were immunoblotted with anti-Eps15 and anti-GST antibodies. The input lane represents 1% of total His-Eps15 used for the pulldown. The interaction between the γ-adaptin appendage domain and Eps15 was significantly reduced as the peptide concentration increased. (B–E) To test whether the Eps15 peptide might also reduce the Eps15–AP-1 interaction within the confines of living cells, HeLa cells were microinjected with 100 nM peptide then allowed to recover for 6 h before immunostaining for Eps15 and AP-1. Peptide-injected cells (*) showed a dramatic reduction in Eps15 staining (B and C) in the Golgi region, compared with uninjected cells (arrows) or cells injected with buffer alone (not shown). In contrast, the Golgi localization pattern of AP-1 was not altered by peptide injection (D and E). Scale bars, 20 μm.
After characterization of the 14-amino acid Eps15 peptide using the above in vitro assays, the effects of this peptide on interactions between Eps15 and AP-1 in cultured cells was tested. Here, HeLa cells were injected with buffer alone, as a control, or the 14-amino acid Eps15 peptide, allowed to recover for 6 h, and then processed for immunofluorescence, staining with antibodies against either Eps15 (Figure 4, B and C) or AP-1 (Figure 4, D and E). In support of the 14-amino acid motif of the Eps15 DPF repeats domain mediating a physical interaction between AP-1 and Eps15, thus aiding in the targeting of Eps15 to the TGN, peptide-injected cells, but not control-injected cells, showed a marked decrease in Eps15 staining at the TGN (Figure 4, B and C). In contrast, little, if any, reduction in AP-1 staining in a similar region was observed (Figure 4, D and E). Thus, these observations indicate that in vitro and also in cultured cells, this 14-amino acid motif in the Eps15 DPF repeats domain plays an important role in mediating a direct Eps15–AP-1 interaction. Additionally, as injection of the Eps15 peptide representing the AP-1–binding site reduced the association of Eps15 with the TGN but not AP-1, this suggests AP-1 may act to recruit Eps15.
Eps15 Plays a Functional Role in the Trafficking of Nascent Secretory Proteins from the TGN
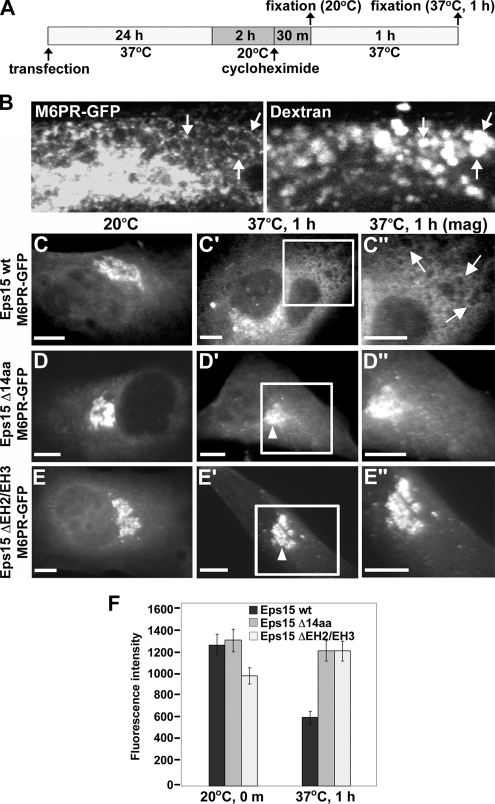
Our observations support the premise that Eps15 localizes to the TGN through a 14-amino acid AP-1–binding motif; however, whether this interaction with AP-1 serves a functional role during specific cellular processes remained to be determined. We hypothesized that, similar to the role of the Eps15–AP-2 complex in cargo sorting and clathrin-coated vesicle formation at the cell surface during endocytosis (Benmerah et al., 1998, 2000), an Eps15–AP-1 complex might perform an analogous function at the TGN. Trafficking of the mannose 6-phosphate receptor (M6PR) between the TGN and late endosomes is known to occur via an AP-1–dependent pathway (Waguri et al., 2003). Therefore, we used GFP-tagged M6PR (M6PR-GFP) as a marker to test whether disruption of the Eps15–AP-1 interaction affects the transit of Golgi cargo proteins destined for endosomes from the TGN. As depicted by the schematic in Figure 5A, BHK cells were cotransfected with constructs encoding for M6PR-GFP and Myc-tagged versions of either Eps15 wt, Eps15 Δ14aa, or an Eps15 deletion mutant lacking the second and third N-terminal EH domains (Eps15 ΔEH2/EH3), a known inhibitor of clathrin-mediated endocytosis (Benmerah et al., 1999), and allowed to recover for 24 h. Subsequently, cells were incubated at 20°C for 2 h to block the exit of M6PR-GFP from the Golgi. In addition, cells were treated with cycloheximide to prevent the synthesis of new M6PR-GFP, which could interfere with tracking the effects of Eps15 wild-type and deletion mutants on the trafficking of M6PR-GFP from the TGN to endosomes. The temperature-induced block in Golgi trafficking was then released by shifting cells from 20 to 37°C for 1 h, and simultaneously, cells were incubated with rhodamine-labeled dextran to identify endosomes. Indeed, control BHK cells expressing M6PR-GFP and incubated with rhodamine-labeled dextran contained brightly labeled endosomes that had a red dextran-filled lumen surrounded by a ring of M6PR-GFP (Figure 5B).
Figure 5.
Eps15 is required for the transport of nascent proteins from the trans-Golgi network to late endosomes. (A) Schematic depicting the experimental procedure used to assay M6PR-GFP trafficking from the trans-Golgi network in BHK-21 cells. (B) To identify the late endosome and confirm the transport of nascent M6PR-GFP to this compartment, BHK-21 cells that had been transfected with M6PR-GFP and allowed to recover for 24 h were incubated at 20°C for 2 h and then warmed to 37°C for 1 h in the presence of rhodamine-labeled dextran to mark endosomal compartments. Bright rings of M6PR-GFP can be seen to delineate the periphery of dextran-containing endosomes (arrows). (C–E″) Cells expressing Myc-tagged versions of wild-type Eps15 (Eps15 wt, C–C″), the Eps15 deletion mutant lacking the 14-amino acid AP-1–binding motif (Eps15 Δ14aa, D–D″) or an Eps15 deletion mutant lacking the second and third EH domains (Eps15 ΔEH2/EH3, E–E″) were assayed for exit of M6PR-GFP from the Golgi as depicted in A. After the 20°C block, M6PR-GFP accumulates in the trans-Golgi network to similar extent in cells expressing either wild-type Eps15 (C) or the Eps15 deletion mutants (D and E). However, after warming to 37°C for 1 h, M6PR-GFP localizes to endosomal membranes in cells expressing Eps15 wt (arrows in C″), whereas M6PR-GFP is blocked in the Golgi in cells expressing Eps15 Δ14aa (arrowhead in D′; D″) or Eps15 ΔEH2/EH3 (arrowhead in E′; E″). Areas boxed in white in C′ and D′ and in E′ are shown at a higher magnification in the respective images to the right (C″, D″, and E″). Expression of Eps15 wild-type and deletion mutants was confirmed by immunostaining with anti-Myc antibody (data not shown). (F) Graph representing quantitation of the average fluorescence intensity of M6PR-GFP in a standardized area covering the Golgi compartment directly after the 20°C block or after 1 h at 37°C in cells expressing either wild-type Eps15 or the Eps15 deletion mutants. Forty cells were measured for each condition. Error bars, SE. Scale bars, 10 μm (C–E″).
Using this assay, we observed that the localization and trafficking of M6PR-GFP was markedly altered in cells expressing either of the two Myc-tagged Eps15 deletion mutants, Eps15 Δ14aa or Eps15 ΔEH2/EH3, when compared with Myc-tagged Eps15 wt–expressing cells. As shown in Figure 5C, cells expressing Eps15 wt displayed substantial levels of nascent M6PR-GFP at the TGN when maintained at 20°C; however, upon shifting of cells to 37°C, thereby releasing the block in Golgi exit, M6PR-GFP was efficiently trafficked to peripherally localized endosomes within 1 h. In contrast, this TGN-to-endosome transport was significantly reduced in cells expressing either Eps15 Δ14aa (Figure 5, D–D″) or Eps15 ΔEH2/EH3 (Figure 5, E–E″). In support of these morphological observations, quantitation of the fluorescence intensity of Golgi-localized M6PR-GFP (see Materials and Methods) revealed about a twofold increase in Golgi-retained M6PR-GFP in cells expressing either of the Eps15 deletion mutants compared with cells expressing wild-type Eps15 (Figure 5F, 37°C, 1 h). Thus, these results suggest that Eps15 is involved in the trafficking of nascent proteins from the TGN to endosomes and furthermore, that an interaction between Eps15 and AP-1 is important for this process.
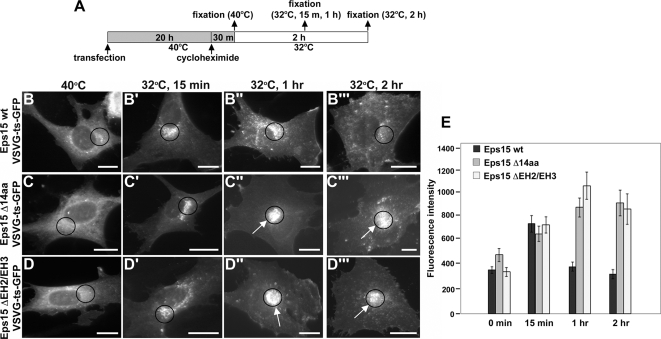
The dependence of M6PR sequestration and trafficking on Eps15 is in agreement with these processes being mediated by clathrin-coated vesicles and associated adaptors (Bonifacino and Traub, 2003; Ghosh et al., 2003; Hinners and Tooze, 2003). However, we next wanted to determine whether Eps15 might also contribute to the exit of additional types of secretory proteins from the TGN. For these assays, we monitored the trafficking of a GFP-tagged, temperature-sensitive variant of the vesicular stomatitis virus G protein (VSVG-ts-GFP), which undergoes constitutive transport from the TGN to the cell surface where it is inserted into the plasma membrane. This tagged protein has been widely used to study the exit of nascent proteins from the TGN (for a few examples, see Toomre et al., 1999; Cao et al., 2000; Folsch et al., 2003; Polishchuk et al., 2003) and possesses a point mutation (F204S) that causes the protein to misfold at 40°C, resulting in its retention in the endoplasmic reticulum (ER; Gallione and Rose, 1985; Presley et al., 1997; Scales et al., 1997). Similar to the M6PR-GFP–trafficking assays and as depicted by the schematic shown in Figure 6A, BHK cells were cotransfected with constructs encoding for VSVG-ts-GFP and Myc-tagged versions of either Eps15 wt or one of the Eps15 deletion mutants (Eps15 Δ14aa or Eps15 ΔEH2/EH3). After 20 h of expression at the restrictive temperature of 40°C, cells were treated with cycloheximide and then shifted to the permissive temperature of 32°C, allowing for transit to the Golgi and subsequently to the plasma membrane. In cells expressing Eps15 wt, within 15 min of shifting to the permissive temperature, VSVG-ts-GFP had exited the ER and accumulated in the ER–Golgi intermediate compartment and Golgi (Figure 6, B and B′). By 1 h, the cargo had been transported from the TGN, and by 2 h most of the VSVG-ts-GFP was present at the plasma membrane (Figure 6, B″ and B‴). Surprisingly, although ER-to-Golgi trafficking appeared normal (Figure 6, C and C′), expression of the Eps15 deletion mutant defective in AP-1 binding, Eps15 Δ14aa, impaired the exit of VSVG-ts-GFP from the TGN. Indeed, even at the 1- and 2-h time points, a significant delay in the transport of VSVG-ts-GFP from the TGN to the cell surface could be detected (Figure 6, C″ and C‴). A similar phenotype was observed upon expression of the Eps15 dominant-negative Eps15 ΔEH2/EH3, with transport of VSVG-ts-GFP out of the ER being unaffected (Figure 6, D and D′), whereas after 1 and 2 h at the permissive temperature, VSVG-ts-GFP was still largely retained in the Golgi (Figure 6, D″ and D‴). Quantitation of VSVG-ts-GFP transport based on the fluorescence intensity within a standardized area covering the Golgi region (Figure 6, B–D‴, circles) revealed that expression of either of the Eps15 deletion mutants, Eps15 Δ14aa or Eps15 ΔEH2/EH3, induced a 2–2.5-fold retention of VSVG-ts-GFP in the Golgi in comparison to Eps15 wt–expressing cells (Figure 6E). Because neither of the Eps15 deletion mutants appeared to affect ER-to-Golgi transport of VSVG-ts-GFP, this suggests a specific step in trafficking from the TGN to the cell surface is defective in cells expressing the Eps15 deletion mutants.
Figure 6.
Eps15 function is required for the cell surface transport of a constitutively secreted protein. (A) Schematic depicting the experimental procedure used to assay VSVG-ts-GFP transport from the trans-Golgi network to the plasma membrane in BHK-21 cells. (B–D‴) Cells expressing Myc-tagged versions of wild-type Eps15 (Eps15 wt, B–B‴), the Eps15 deletion mutant lacking the 14-amino acid AP-1–binding motif (Eps15 Δ14aa, C–C‴) or an Eps15 deletion mutant lacking the second and third EH domains (Eps15 ΔEH2/EH3, D–D‴) were assayed for VSVG-ts-GFP transport as depicted in A. VSVG-ts-GFP was retained in the ER at the restrictive temperature (40°C) and transported to the Golgi after a 15-min incubation at the permissive temperature (32°C) in cells expressing wild-type Eps15 (B and B′) or the Eps15 deletion mutants (C, C′, D, and D′). On longer incubation at the permissive temperature (1 h and 2 h), VSVG-ts-GFP was subsequently transported to the plasma membrane in cells expressing Eps15 wt (B″ and B‴). In contrast, at similar time points, VSVG-ts-GFP was retained in a perinuclear region in cells expressing either of the Eps15 deletion mutants, Eps15 Δ14aa (arrows in C″ and C‴) or Eps15 ΔEH2/EH3 (arrows in D″ and D‴). Expression of Eps15 wild-type and deletion mutants was confirmed by immunostaining with anti-Myc antibody (data not shown). (E) Graph representing quantitation of the average fluorescence intensity of VSVG-ts-GFP in a standardized area covering the Golgi compartment at the indicated time points after release from the restrictive temperature. Circles in images (B–D‴) denote standardized areas in which the fluorescence intensity of VSVG-ts-GFP was measured for each time point and provide a representative example of how the effects of expressing wild-type Eps15 or the Eps15 deletion mutants on VSVG-ts-GFP transport were quantitated. Forty cells were measured for each condition. Error bars, SE. Scale bars, 20 μm (B–D‴).
The appendage domain of the α-adaptin subunit of AP-2 is known to interact with Eps15 (Benmerah et al., 1996; Iannolo et al., 1997), and expression of the Eps15-binding domain of α-adaptin in cultured cells attenuates transferrin internalization, a marker for clathrin-mediated endocytosis (Owen et al., 1999). Therefore, we used a similar approach to further test the role of the Eps15–AP-1 interaction in the transport of Golgi cargo proteins to the cell surface. Here, expression of Myc-tagged γ-adaptin appendage domain, the analogous Eps15-binding domain of the AP-1 complex (Figure 4A and Kent et al., 2002), was used to inhibit the function of endogenous AP-1, and the effects on VSVG-ts-GFP trafficking were observed as above. In support of a role for Eps15 and AP-1 in mediating vesicle trafficking from the TGN, the transport of VSVG-ts-GFP from the Golgi to the cell surface, but not ER-to-Golgi, was significantly reduced in cells expressing the γ-adaptin appendage domain (Supplementary Figure S2, A–A‴) when compared with control cells expressing a γ-adaptin appendage domain mutant (A716D) that no longer interacts with Eps15 (Kent et al., 2002; Supplementary Figure S2, B–B‴). Indeed, the fluorescence intensity of VSVG-ts-GFP localized in the Golgi region after 2 h at the permissive temperature was ∼2.5-fold greater in cells expressing wild-type γ-adaptin appendage domain thanin cells expressing the γ-adaptin appendage domain A716D mutant (Supplementary Figure S2C). Thus, these results together with those above provide strong support to the concept that a specific Eps15–AP-1 interaction is essential for the efficient transport of nascent cargo from the TGN to both endosomes and the plasma membrane.
To further test if VSVG-ts-GFP accumulates at the TGN upon overexpression of the Eps15 deletion mutants, we examined the localization of VSVG-ts-GFP retained as a result of expression of Eps15 deletion mutants at 2 h of permissive temperature using the TGN marker, p230. As expected, VSVG-ts-GFP showed substantial colocalization with p230 (Supplementary Figure S3, A–A″) but not with transferrin recycling endosomes (Supplementary Figure S3, B–B″), in cells expressing Eps15 Δ14aa and Eps15 ΔEH2/EH3 (data not shown), indicating that VSVG-ts-GFP failed to exit the TGN in these mutant cells.
To ensure that the observed reduction of VSVG-ts-GFP transport from the TGN to the cell surface in Eps15 mutant cells is a direct effect on the Golgi and does not result from disruption of endocytosis, we tested for effects on internalization of transferrin. Importantly, no difference of transferrin uptake was observed between control cells and Eps15 Δ14aa–expressing cells (Supplementary Figure S4, A and B). As shown in the graph of Supplementary Figure S4C, 97% of cells expressing Eps15 Δ14aa showed normal transferrin uptake, indicating that inhibition of Eps15–AP-1 interaction directly affects the exit of the secretory protein from the TGN and the inhibitory effect is not a result of an endocytic disruption.
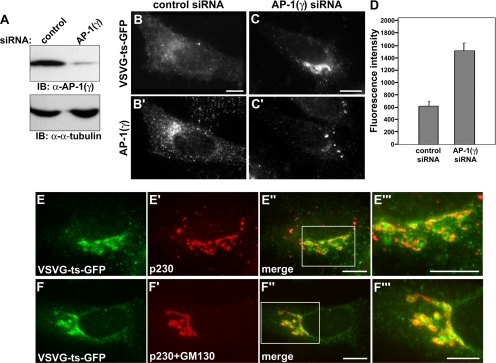
Although a role for AP-1 in trafficking of VSVG has been shown by others in nonpolarized cells (Folsch et al., 2003; Cancino et al., 2007; Gravotta et al., 2007), we studied the effect of AP-1 depletion on trafficking of VSVG in HeLa cells. Western blot analysis showed a ∼90% knockdown of AP-1 (γ) by treatment of AP-1 (γ) siRNA (Figure 7A). AP-1 (γ) depletion significantly disrupted the transport of VSVG-ts-GFP from the Golgi to the cell surface at 1 h (Figure 7, C and C′) and 2 h (data not shown) after a shift to the permissive temperature. Substantial levels of VSVG-ts-GFP were observed at the Golgi region of the AP-1–knocked down cells in comparison to control-treated cells (Figure 7, B and B′). Quantitation of the fluorescence intensity of VSVG-ts-GFP localized at the Golgi region revealed that the AP-1 (γ) knockdown induced a ∼2.5-fold retention of VSVG-ts-GFP at the Golgi compared with control siRNA-treated cells (Figure 7D). To test whether this peri-nuclear accumulation of VSVG-ts-GFP in AP-1 (γ)-depleted cells represented the TGN, we compared the localization of retained VSVG-ts-GFP to the TGN marker, p230 (Figure 7, E–E‴). We observed a marked colocalization between the two proteins with the VSVG protein directly overlapping with significant portions of the TGN in AP-1–depleted cells. As the accumulated VSVG extended out beyond the TGN into adjacent compartments, we predicted that this represented a backlog of nascent protein into the cis and medial cisternae. To test this, AP-1–depleted cells were costained with markers to both the TGN (p230) and the cis-Golgi (GM130). This dual staining significantly increased the overlap between the retained VSVG-ts-GFP and the Golgi (Figure 7, F–F‴). In comparison, a more modest localization of the viral coat cargo with the transferrin-sorting compartment was observed (data not shown). Collectively, these data provide additional support for the premise that AP-1 is required for the transport of VSVG from the TGN to the plasma membrane.
Figure 7.
AP-1 is required for the transport of VSVG from the TGN to the cell surface. (A) Western blot analysis of HeLa cells that were transfected with either a nontargeted control siRNA or a specific AP-1 (γ) siRNA as described in Materials and Methods. Cell homogenates were separated by SDS-PAGE and probed with an anti-AP-1 (γ) and anti-α-tubulin antibodies. Expression levels of AP-1 were reduced by more than 90%. (B and C′) Immunofluorescence images of VSVG-ts-GFP trafficking in control siRNA-treated (B and B′) or AP-1 (γ) siRNA-treated (C and C′) HeLa cells 1 h after shift to the permissive temperature. To confirm a knockdown of AP-1 (γ), cells were immunostained with anti-AP-1 (γ) antibody (B′ and C′). Note the substantial retention of VSVG-ts-GFP at the Golgi region in AP-1 (γ)-depleted cells (C and C′). (D) Graph representing quantitation of the average fluorescence intensity of VSVG-ts-GFP in a standardized area covering the Golgi compartment at 1 h of permissive temperature. AP-1 (γ)-depleted cells retained ∼2.5-fold VSVG-ts-GFP in the Golgi area compared with control siRNA-treated cells. Forty cells were measured for each condition. Error bars, SE. (E–F‴) VSVG-ts-GFP is retained at the TGN by depletion of AP-1. The localization of retained VSVG-ts-GFP in AP-1 (γ)-depleted cells at 1 h of permissive temperature was examined using the TGN marker, p230 (E–E‴) or mixture of Golgi markers: the TGN marker p230 and the cis-Golgi marker GM130 (F–F‴). Areas boxed in white in (E″ and F″) are shown at a higher magnification in the respective images to the right (E‴ and F‴). Note that retained VSVG-ts-GFP shows a marked colocalization with the TGN marker (E‴) or the mixture of Golgi markers (F‴). Scale bars, 20 μm.
DISCUSSION
Eps15 is well known to participate in clathrin-mediated endocytosis, in part through its association with the clathrin adaptor AP-2 (Carbone et al., 1997; Benmerah et al., 1998, 2000). In contrast, although Eps15 has also been observed to localize to a perinuclear region (Tebar et al., 1996; Torrisi et al., 1999; Kent et al., 2002), its function at this site is undefined. Here, we demonstrate that the perinuclear pool of Eps15 is associated with the TGN through an interaction with the appendage domain of γ-adaptin, a subunit of the AP-1 complex. Moreover, a 14-amino acid sequence at the C-terminus of the DPF repeats domain of Eps15 is specifically required for the interaction between Eps15 and AP-1, but not AP-2. Importantly, using M6PR-GFP and VSVG-ts-GFP trafficking assays, we show functional evidence supporting that Eps15 and AP-1 act together to mediate the transport of Golgi cargo proteins targeted to either endosomes or the plasma membrane, respectively. Thus, these results reveal an additional site of action for Eps15 in clathrin-mediated vesicle formation and protein sorting, namely the TGN, and provide insights into how Eps15 might differentially interact with the various AP complexes through specific motifs located in a common AP-binding domain.
A New Adaptor at the TGN
Although Eps15 has been observed at a perinuclear region by several different groups (Tebar et al., 1996; Torrisi et al., 1999; Kent et al., 2002), it has been unclear whether this localization pattern represents the Golgi, a population of endosomes or an accumulation of surface clathrin-coated pits at a thicker region of the cell. Here using multiple reagents, including two distinct antibodies against Eps15 as well as Myc-tagged Eps15, we show that Eps15 localizes to the Golgi in cultured epithelial cells and fibroblasts, as indicated by the significant overlap in cells costained for Eps15 and classical Golgi markers such as p230 or TGN38 (Figure 1). Moreover, treatment of cells with the Golgi-disrupting agent BFA lead to a complete dispersal of Eps15 from the perinuclear region, further supporting that this localization pattern represents an association between Eps15 and the Golgi (Supplementary Figure S1). In addition to these morphological observations, coimmunoprecipitation experiments using Golgi membrane fractions isolated from rat liver provided biochemical evidence in support of the association between Eps15 and the Golgi (Figure 2D). Thus, although our observations do not exclude a potential association of Eps15 with a perinuclear endosomal compartment, they do suggest the presence of a Golgi-localized pool of Eps15 in multiple cell types.
Many of the accessory clathrin adaptor proteins that bind to AP-2, including Eps15, interact with the appendage or “ear” domain of α-adaptin. At the TGN, the γ-adaptin appendage domain appears to function in an analogous manner and serves as a binding site for proteins that interact with AP-1 (Owen et al., 2004; Edeling et al., 2006). Interestingly, a potential γ-adaptin–binding motif was previously identified in Eps15 based on the consensus sequence [D/E][G/A](0–1)F[G/A][D/E]Φ (Duncan and Payne, 2003); however, whether this motif supported an interaction between Eps15 and AP-1 was not tested. Here we provide biochemical and morphological evidence supporting that this predicted site of interaction is a bona fide AP-1–binding motif. The γ-adaptin–binding motif found in Eps15 was also reported to be present in other proteins such as EpsinR and γ-synergin, which function in vesicle trafficking at the Golgi by interaction with AP-1 (Page et al., 1999; Mills et al., 2003), showing that the γ-adaptin–binding motif in adaptor proteins is conserved one for interaction with AP-1. This domain appears to be particularly important for the Eps15–AP-1 interaction, whereas it is not required for the interaction between Eps15 and AP-2 (Figure 3). Indeed, the Eps15 deletion mutant lacking the C-terminal 14 amino acids of the DPF repeats domain (Eps15 Δ14aa) colocalized with AP-2 in cells but not AP-1 (Figure 3, F–I). Accordingly, cells expressing this mutant exhibited normal levels of transferrin uptake, a marker for clathrin-mediated endocytosis (Supplementary Figure S4), whereas vesicle transport from the Golgi was reduced (Figures 5 and 6). Moreover, in contrast to the specific effects of the Eps15 Δ14aa deletion mutant on the trafficking of Golgi cargo proteins, cells expressing the Eps15 dominant-negative Eps15 ΔEH2/EH3 exhibited a reduction in both clathrin-mediated endocytosis, as previously reported (Benmerah et al., 1999), as well as secretion (Figures 5 and 6; data not shown).
A Novel Function for Eps15 in the Transport of Nascent Proteins from the TGN
The inhibition of M6PR-GFP transport from the TGN in cells expressing the Eps15 deletion mutant defective in AP-1 binding, Eps15 Δ14aa, is consistent with previous reports implicating AP-1 in the trafficking of M6PR between the TGN and endosomes (Bonifacino and Traub, 2003; Ghosh et al., 2003; Hinners and Tooze, 2003). Another family of adaptor-related proteins, GGAs (Golgi-localized, γ-ear–containing, Arf-binding proteins), are also present at the Golgi and are involved in M6PR sorting and trafficking (Ghosh et al., 2003; Hinners and Tooze, 2003; Bonifacino, 2004). As the GAE (γ-adaptin ear) domain of GGAs has high homology to the ear, or appendage, domain of the γ-adaptin subunit of AP-1 (Hirst et al., 2000; Takatsu et al., 2000), there is a possibility that Eps15 may interact with GGAs to mediate proper TGN-to-endosome transport of M6PR. In support of our observations suggesting a role for an Eps15–AP-1 complex in M6PR trafficking, various γ-adaptin appendage domain–binding proteins identified so far have shown a preference for AP-1 over GGAs (Lui et al., 2003; Hirst et al., 2005; Neubrand et al., 2005; Kametaka et al., 2007). Moreover, although not the focus of the study by Hirst et al. (2005), a preferential interaction between Eps15 and the γ-adaptin appendage domain versus the GAE domain of GGA1 was detected using GST pulldown assays. Although we have not ruled out a role for GGAs in Eps15-mediated Golgi vesicle trafficking, our current study highlights the importance of AP-1 binding by Eps15 in the efficient transport of M6PR from the TGN.
Perhaps most surprising was finding that expression of the Eps15 deletion mutants impaired the transport of nascent VSVG-ts-GFP from the TGN to the cell surface (Figure 6). We observed that expression of the γ-adaptin appendage domain lead to a dramatic accumulation of VSVG-ts-GFP in the Golgi (Supplementary Figure S2, A–A‴ and C), but importantly, the transport of viral protein to the cell surface was normal in cells expressing the γ-adaptin appendage domain A716D mutant defective in Eps15 binding (Supplementary Figure S2, B–B‴ and C), indicating that the trafficking defects observed in cells expressing the wild-type γ-adaptin appendage domain were a result of disrupting the interaction between Eps15 and AP-1. Finally, we found that reducing the levels of AP-1 (γ) protein in cells via siRNA knockdown resulted in a 2.5-fold accumulation of nascent VSVG in a TGN (p230 positive) compartment (Figure 7).
The packaging and transport of constitutively secreted proteins such as VSVG-ts-GFP has traditionally been viewed as a vesicle coat–independent process mediated by large tubular carriers that are “pulled out” and subsequently “cut” from subdomains of the TGN (Hirschberg et al., 1998; Polishchuk et al., 2000; Polishchuk et al., 2003), although additional models have also been proposed (Luini et al., 2005). As an example, clathrin-coated structures containing AP-1B and polarized epithelial-specific AP-1 complex (Ohno et al., 1999) have been implicated in the transport of VSVG from the TGN to the basolateral domain of polarized epithelial cells (Folsch et al., 2003). Disruption of AP-1B function by microinjection of AP-1B antibody (Cancino et al., 2007) or knockdown of AP-1B (Gravotta et al., 2007) has been reported to block the sorting of VSVG to the basolateral surface in polarized epithelial cells. In nonpolarized cells, the interaction of AP-1 and Crn7 was shown to be required for the export of VSVG from the Golgi (Rybakin et al., 2006). The cell types used in this current study are nonpolarized and, therefore, most likely express exclusively AP-1A. Very recently, it has been shown that transport of VSVG from the TGN in MDCK cells is indeed dependent upon a clathrin coat (Deborde et al., 2008) providing additional detailed evidence that nascent VSVG-containing vesicles forming at the TGN would require a clathrin-based, AP1-Eps15 adaptor complex. Such a process would represent a specific modification of the machinery utilized at the endocytic pit from the plasma membrane (McNiven and Thompson, 2006).
The identification here of a functional role for Eps15 in the secretory pathway through an interaction with AP-1 provides new information regarding the mechanisms of protein sorting and trafficking at the TGN, while also raising exciting new questions. Particularly interesting will be determining how the different domains of Eps15 contribute to regulating protein interactions, cargo sequestration, and vesicle formation at the plasma membrane versus the TGN, thus providing further insights into the similarities and differences in clathrin-mediated processes at distinct cellular sites (Duncan and Payne, 2003; Robinson, 2004; Traub, 2005; McNiven and Thompson, 2006).
Supplementary Material
ACKNOWLEDGMENTS
We thank H. M. Thompson (Mayo Clinic College of Medicine) for help in preparing the manuscript. This study was supported by National Institutes of Health Grant DK 44650 (M.A.M.).
Abbreviations used:
- AP
adaptor protein
- BFA
brefeldin A
- EH
Eps15 homology
- ER
endoplasmic reticulum
- Eps15
EGFR pathway substrate clone 15
- GAE
γ-adaptin ear
- GGA
Golgi-localized, γ-ear–containing, Arf-binding protein
- M6PR
mannose 6-phosphate receptor
- TGN
trans-Golgi network
- UIM
ubiquitin-interacting motif
- VSVG
vesicular stomatitis virus G protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-0997) on June 4, 2008.
REFERENCES
- Benmerah A., Bayrou M., Cerf-Bensussan N., Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Begue B., Dautry-Varsat A., Cerf-Bensussan N. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Benmerah A., Lamaze C., Begue B., Schmid S. L., Dautry-Varsat A., Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A., Poupon V., Cerf-Bensussan N., Dautry-Varsat A. Mapping of Eps15 domains involved in its targeting to clathrin-coated pits. J. Biol. Chem. 2000;275:3288–3295. doi: 10.1074/jbc.275.5.3288. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Cancino J., Torrealba C., Soza A., Yuseff M. I., Gravotta D., Henklein P., Rodriguez-Boulan E., Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol. Biol. Cell. 2007;18:4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Garcia F., McNiven M. A. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Thompson H. M., Krueger E. W., McNiven M. A. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J. Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- Cao H., Weller S., Orth J. D., Chen J., Huang B., Chen J. L., Stamnes M., McNiven M. A. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat. Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- Carbone R., Fre S., Iannolo G., Belleudi F., Mancini P., Pelicci P. G., Torrisi M. R., Di Fiore P. P. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57:5498–5504. [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V. I., Capua M. R., Takei K., Butler M. H., Di Fiore P. P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Coda L., Salcini A. E., Confalonieri S., Pelicci G., Sorkina T., Sorkin A., Pelicci P. G., Di Fiore P. P. Eps15R is a tyrosine kinase substrate with characteristics of a docking protein possibly involved in coated pits-mediated internalization. J. Biol. Chem. 1998;273:3003–3012. doi: 10.1074/jbc.273.5.3003. [DOI] [PubMed] [Google Scholar]
- de Melker A. A., van der Horst G., Borst J. c-Cbl directs EGF receptors into an endocytic pathway that involves the ubiquitin-interacting motif of Eps15. J. Cell Sci. 2004;117:5001–5012. doi: 10.1242/jcs.01354. [DOI] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. C., Payne G. S. ENTH/ANTH domains expand to the Golgi. Trends Cell Biol. 2003;13:211–215. doi: 10.1016/s0962-8924(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Edeling M. A., Smith C., Owen D. J. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Minichiello L., Matoskova B., Wong W. T., Di Fiore P. P. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Maday S., Pelletier L., Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J. Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Dahms N. M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- Gravotta D., Deora A., Perret E., Oyanadel C., Soza A., Schreiner R., Gonzalez A., Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc. Natl. Acad. Sci. USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C., Takei K., Chen H., Ringstad N., Hudson A., Butler M. H., Salcini A. E., Di Fiore P. P., De Camilli P. Synaptojanin 1, localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Henley J. R., McNiven M. A. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J. Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinners I., Tooze S. A. Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- Hirschberg K., Miller C. M., Ellenberg J., Presley J. F., Siggia E. D., Phair R. D., Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Borner G. H., Harbour M., Robinson M. S. The aftiphilin/p200/gamma-synergin complex. Mol. Biol. Cell. 2005;16:2554–2565. doi: 10.1091/mbc.E04-12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Lui W. W., Bright N. A., Totty N., Seaman M. N., Robinson M. S. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller D., Crosetto N., Blagoev B., Raiborg C., Tikkanen R., Wagner S., Kowanetz K., Breitling R., Mann M., Stenmark H., Dikic I. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- Iannolo G., Salcini A. E., Gaidarov I., Goodman O. B., Jr., Baulida J., Carpenter G., Pelicci P. G., Di Fiore P. P., Keen J. H. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Kametaka S., Moriyama K., Burgos P. V., Eisenberg E., Greene L. E., Mattera R., Bonifacino J. S. Canonical interaction of cyclin G associated kinase with adaptor protein 1 regulates lysosomal enzyme sorting. Mol. Biol. Cell. 2007;18:2991–3001. doi: 10.1091/mbc.E06-12-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent H. M., McMahon H. T., Evans P. R., Benmerah A., Owen D. J. Gamma-adaptin appendage domain: structure and binding site for Eps15 and gamma-synergin. Structure. 2002;10:1139–1148. doi: 10.1016/s0969-2126(02)00801-8. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Klapisz E., Sorokina I., Lemeer S., Pijnenburg M., Verkleij A. J., van Bergen en Henegouwen P.M. A ubiquitin-interacting motif (UIM) is essential for Eps15 and Eps15R ubiquitination. J. Biol. Chem. 2002;277:30746–30753. doi: 10.1074/jbc.M203004200. [DOI] [PubMed] [Google Scholar]
- Lui W. W., Collins B. M., Hirst J., Motley A., Millar C., Schu P., Owen D. J., Robinson M. S. Binding partners for the COOH-terminal appendage domains of the GGAs and gamma-adaptin. Mol. Biol. Cell. 2003;14:2385–2398. doi: 10.1091/mbc.E02-11-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., Ragnini-Wilson A., Polishchuck R. S., De Matteis M. A. Large pleiomorphic traffic intermediates in the secretory pathway. Curr. Opin. Cell Biol. 2005;17:353–361. doi: 10.1016/j.ceb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- McNiven M. A., Thompson H. M. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- Mills I. G., Praefcke G. J., Vallis Y., Peter B. J., Olesen L. E., Gallop J. L., Butler P. J., Evans P. R., McMahon H. T. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 2003;160:213–222. doi: 10.1083/jcb.200208023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubrand V. E., Will R. D., Mobius W., Poustka A., Wiemann S., Schu P., Dotti C. G., Pepperkok R., Simpson J. C. Gamma-BAR, a novel AP-1-interacting protein involved in post-Golgi trafficking. EMBO J. 2005;24:1122–1133. doi: 10.1038/sj.emboj.7600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R. C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J. S. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Collins B. M., Evans P. R. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- Page L. J., Sowerby P. J., Lui W. W., Robinson M. S. Gamma-synergin: an EH domain-containing protein that interacts with gamma-adaptin. J. Cell Biol. 1999;146:993–1004. doi: 10.1083/jcb.146.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk E. V., Di Pentima A., Luini A., Polishchuk R. S. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol. Biol. Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R. S., Polishchuk E. V., Marra P., Alberti S., Buccione R., Luini A., Mironov A. A. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J. Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Sigismund S., Faretta M., Guidi M., Capua M. R., Bossi G., Chen H., De Camilli P., Di Fiore P. P. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Bonifacino J. S. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Rybakin V., Gounko N. V., Spate K., Honing S., Majoul I. V., Duden R., Noegel A. A. Crn7 interacts with AP-1 and is required for the maintenance of Golgi morphology and protein export from the Golgi. J. Biol. Chem. 2006;281:31070–31078. doi: 10.1074/jbc.M604680200. [DOI] [PubMed] [Google Scholar]
- Scales S. J., Pepperkok R., Kreis T. E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schmid E. M., McMahon H. T. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Takatsu H., Yoshino K., Nakayama K. Adaptor gamma ear homology domain conserved in gamma-adaptin and GGA proteins that interact with gamma-synergin. Biochem. Biophys. Res. Commun. 2000;271:719–725. doi: 10.1006/bbrc.2000.2700. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Jones S. M., Dahl R. H., Nordeen M. H., Howell K. E. Characterization of the Golgi complex cleared of proteins in transit and examination of calcium uptake activities. Mol. Biol. Cell. 1997;8:1911–1931. doi: 10.1091/mbc.8.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F., Confalonieri S., Carter R. E., Di Fiore P. P., Sorkin A. Eps15 is constitutively oligomerized due to homophilic interaction of its coiled-coil region. J. Biol. Chem. 1997;272:15413–15418. doi: 10.1074/jbc.272.24.15413. [DOI] [PubMed] [Google Scholar]
- Tebar F., Sorkina T., Sorkin A., Ericsson M., Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- Toomre D., Keller P., White J., Olivo J. C., Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 1999;112:21–33. doi: 10.1242/jcs.112.1.21. [DOI] [PubMed] [Google Scholar]
- Torrisi M. R., Lotti L. V., Belleudi F., Gradini R., Salcini A. E., Confalonieri S., Pelicci P. G., Di Fiore P. P. Eps15 is recruited to the plasma membrane upon epidermal growth factor receptor activation and localizes to components of the endocytic pathway during receptor internalization. Mol. Biol. Cell. 1999;10:417–434. doi: 10.1091/mbc.10.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Ungewickell E. J., Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr. Opin. Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- van Delft S., Schumacher C., Hage W., Verkleij A. J., van Bergen en Henegouwen P. M. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J. Cell Biol. 1997;136:811–821. doi: 10.1083/jcb.136.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguri S., Dewitte F., Le Borgne R., Rouille Y., Uchiyama Y., Dubremetz J. F., Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.