Abstract
Members of the transforming acidic coiled coil (TACC) protein family are emerging as important mitotic spindle assembly proteins in a variety of organisms. The molecular details of how TACC proteins function are unknown, but TACC proteins have been proposed to recruit microtubule-stabilizing proteins of the tumor overexpressed gene (TOG) family to the centrosome and to facilitate their loading onto newly emerging microtubules. Using Xenopus egg extracts and in vitro assays, we show that the Xenopus TACC protein maskin is required for centrosome function beyond recruiting the Xenopus TOG protein XMAP215. The conserved C-terminal TACC domain of maskin is both necessary and sufficient to restore centrosome function in maskin-depleted extracts, and we provide evidence that the N terminus of maskin inhibits the function of the TACC domain. Time-lapse video microscopy reveals that microtubule dynamics in Xenopus egg extracts are unaffected by maskin depletion. Our results provide direct experimental evidence of a role for maskin in centrosome function and suggest that maskin is required for microtubule anchoring at the centrosome.
INTRODUCTION
The centrosome is a nonmembrane-bound organelle that serves as the major microtubule-organizing center of animal cells and has crucial functions in cell division (Azimzadeh and Bornens, 2007). Increasing numbers of human diseases have been linked to defects in centrosomal proteins (Badano et al., 2005; Bettencourt-Dias and Glover, 2007). Despite its importance to human disease and more than a century of scrutiny, however, many aspects of centrosome structure, function, and composition remain unknown. For example, little is known to date about how the hundreds of proteins that associate with the centrosome are assembled into functional microtubule-organizing centers (Andersen et al., 2003).
During mitosis, centrosomes organize the poles of the mitotic spindle, an elaborate macromolecular machine designed to equally partition the chromosomes between the daughter cells during cell division (Gadde and Heald, 2004). Centrosomes perform three major microtubule-related functions: they nucleate, anchor, and organize microtubules. How the centrosome carries out these functions is not yet fully understood.
Emerging evidence suggests that mitotic spindle assembly requires protein complexes containing members of the transforming acidic coiled coil (TACC) protein family of centrosomal proteins (Raff, 2002; Wiese and Zheng, 2006). However, the molecular mechanisms of how TACC proteins function during spindle assembly remain obscure. Although there is little or no similarity among members of the TACC family otherwise, all TACC proteins share a conserved ∼200 amino acid carboxy-terminal coiled coil domain (TACC domain) that targets the protein to the centrosome (Lee et al., 2001; Gergely, 2002; Bellanger and Gönczy, 2003; Srayko et al., 2003). Several lines of evidence have implicated TACC proteins in mitotic spindle assembly and centrosome function. For example, Drosophila “D-TACC” mutants show destabilized spindle microtubules (Raff, 2002), TAC-1 mutants in Caenorhabditis elegans have short spindle microtubules (Bellanger and Gönczy, 2003; Le Bot et al., 2003; Srayko et al., 2003), and depletion of the Xenopus TACC protein maskin from mitotic egg extracts results in fewer and smaller microtubule asters (O'Brien et al., 2005; Kinoshita et al., 2005; Peset et al., 2005). These phenotypes (destabilized spindles and small asters) could reflect a role for TACC proteins in regulating overall microtubule stability. Alternatively, these same phenotypes could also arise from defects in centrosome function, with no role for maskin in microtubule stabilization. In the latter scenario, either defects in microtubule nucleation or defects in microtubule anchoring or organization could lead to fewer microtubules being associated with a given centrosome. Previous studies suggested that there was no apparent defect in microtubule nucleation by centrosomes assembled in the absence of maskin (Peset et al., 2005; Kinoshita et al., 2005; also see Srayko et al., 2003). Consistent with this, depletion of maskin had little effect on the levels of γ-tubulin (the major centrosomal microtubule nucleator) associated with centrosomes (O'Brien et al., 2005). Thus, maskin is unlikely to be required for microtubule nucleation. However, the role of maskin in other centrosome functions (notably, microtubule anchoring and organization) has not been tested experimentally. Similarly, the effect on microtubule dynamics of disrupting TACC proteins has not been reported to date.
In all species examined thus far, TACC proteins interact with microtubule stabilizing proteins of the tumor overexpressed (TOG) protein family (Gergely, 2002; Wiese and Zheng, 2006). TOG proteins localize to microtubule-organizing centers and regulate microtubule assembly and organization (Gard et al., 2004). The TACC/TOG complex has been proposed to help stabilize the plus ends of newly formed microtubules as they emerge from the centrosome (Lee et al., 2001; Srayko et al., 2003; Barros et al., 2005; Brittle and Ohkura, 2005). Implicit in this model is that TACC proteins enhance the activity of TOG proteins, and consistent with this idea maskin was reported to increase the affinity of XMAP215 for microtubules (Kinoshita et al., 2005). Because TOG proteins exert their effects mainly on the plus ends of microtubules (Tournebize et al., 2000; Brouhard et al., 2008), this could help to explain why disruption of TACC proteins affects microtubules nucleated from centrosomes. However, it is not clear how increasing the affinity of XMAP215 for microtubules affects its microtubule-stabilizing activity, and why mainly centrosome-nucleated microtubules would be affected, whereas other spindle microtubules are not. It is also worth considering that protein complex formation might influence the activity not just of XMAP215 but also of the TACC protein. Thus, it is important to revisit this question to gain a better understanding of how TACC proteins influence microtubule dynamics and centrosome function.
Previous work implicated maskin in centrosome function; this was proposed to be mediated by a stabilizing effect of maskin on newly formed microtubule plus ends rather than a direct role for maskin in centrosome functions (Kinoshita et al., 2005). One prediction from the model that TACC/TOG complexes stabilize newly formed microtubules at the centrosome is that TACC proteins should have a stabilizing effect on microtubule dynamics. Conversely, depletion of TACC proteins should alter microtubule dynamics to destabilize microtubules. In this study, we depleted maskin from Xenopus egg extract and measured microtubule dynamics. Surprisingly, we found no detectable difference in growth or shrinkage rates, or in the frequencies of transitions between growth and shrinkage (“catastrophe”) or shrinkage and growth (“rescue”). In contrast, using in vitro centrosome assembly assays we found that maskin is required for stable association of microtubules with centrosomes. We further found that the phosphorylation state of maskin regulates the ability of XMAP215 to anchor microtubule minus ends. Together, these results suggest that maskin is required for centrosome function.
MATERIALS AND METHODS
Recombinant Protein Expression and Purification
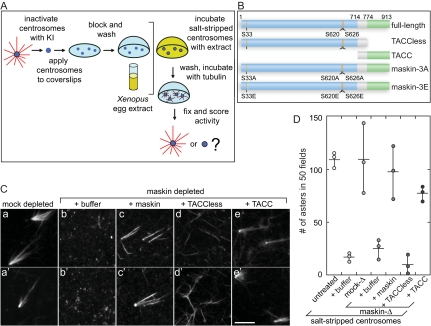
Full-length maskin and the TACC domain (amino acids [aa] 714–913) were described in O'Brien et al. (2005), and the “TACCless” domain (aa 1–774) was described in Albee et al. (2006). The 3A and 3E mutants (Ser33, Ser620, and Ser626 mutated to alanine or to glutamic acid, respectively) were generated by site-directed mutagenesis of the full-length maskin clone. All proteins were expressed in Escherichia. coli as glutathione transferase (GST) fusion proteins and purified by GST-agarose affinity chromatography. The GST portion was cleaved off with PreScission protease according to the manufacturer's instructions, as described previously (O'Brien et al., 2005). All proteins were concentrated to >10 mg/ml, dialyzed against XB (10 mM K-HEPES, pH 7.6, 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 50 mM sucrose, and 5 mM EGTA), flash-frozen in liquid nitrogen in small aliquots, and stored at −80°C.
Egg Extract Preparation and Depletion
Cytostatic factor (CSF)-arrested Xenopus egg extracts were prepared as described previously (Murray, 1991) and supplemented with Δ90 cyclin (1:40) to arrest them in mitosis (Murray et al., 1989). Immunodepletions were performed using either Affi-prep protein A beads (Bio-Rad, Hercules, CA) or protein A Dynabeads (Dynal Biotech, Oslo, Norway) as described in O'Brien et al. (2005).
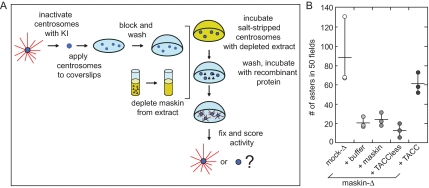
Centrosome Complementation Assay
The assay was performed as described in Moritz et al. (1998), with the following modifications: maskin was depleted from extracts, and full-length maskin or maskin mutants were added back to the depleted extract before incubation with the salt-stripped centrosomes, as indicated in the figures. Complementation was assessed based on the number of asters formed in 50 random microscope fields. For the “sequential” assay, salt-stripped centrosomes were first incubated with maskin-depleted extracts, the extract was washed away, and the “complemented” centrosomes were then incubated with full-length maskin or maskin mutants in BRB80 buffer (80 mM K-PIPES, 1 mM EGTA, and 1 mM MgCl2, pH 6.8) supplemented with 10 mg/ml bovine serum albumin and 10% glycerol; the samples were then processed as described above.
Time-Lapse Analysis of Microtubule Asters
Microtubules were nucleated from demembranated Xenopus sperm chromatin (0.5 μl) or Drosophila centrosomes (1 μl; isolated as described by Moritz et al., 1995) added to Xenopus CSF-arrested egg extracts. In a typical reaction, centrosomes or chromatin, 0.2 μl of rhodamine-tubulin (prepared as described in Hyman et al., 1991), 0.5 μl of saturated hemoglobin, and 0.33 μl of antifade (Tournebize et al., 1997) were added to 10 μl of extract on ice. Two microliters of the mixture was then spotted onto a microscope slide, covered with a 22- × 22-mm coverslip, and the edges were sealed with Valap (equal parts Vaseline, lanolin, and paraffin; McGee-Russel and Allen, 1971). Images were recorded every 1 s for 4 min with a 400-ms exposure (Supplemental Videos 3 and 4) or every 2 s for 5 min with a 250-ms exposure (Supplemental Videos 1, 2, 5, and 6) by using a Photometrics CoolSnap HQ cooled charge-coupled device camera (Roper Scientific, Tucson, AZ) through a 100×/1.4 numerical aperture plan apo objective mounted on a Nikon Eclipse E800 fluorescence microscope equipped with MetaMorph software (Molecular Devices, Sunnyvale, CA). Dynamic parameters were calculated as described previously (Wilde et al., 2001).
γ-Tubulin, XMAP215, and Maskin Recruitment to Centrosomes
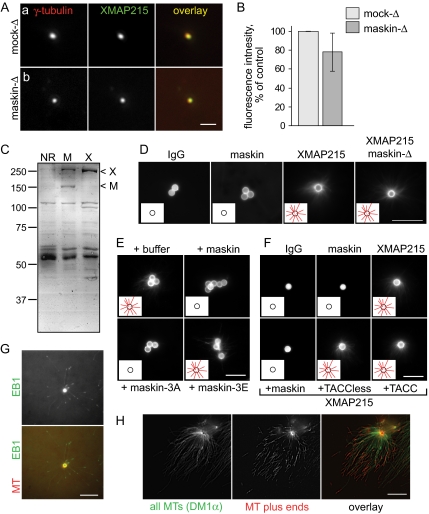
To study γ-tubulin and XMAP215 recruitment, salt-stripped centrosomes were incubated with mock- or maskin-depleted extracts. The extracts were washed away, and the centrosomes were fixed with 1% glutaraldehyde in BRB80 and postfixed in −20°C methanol. Immunofluorescence was performed as described in O'Brien et al. (2005) by using anti-acetylated tubulin (Sigma-Aldrich, St. Louis, MO) to locate the centrosome and either XenC for γ-tubulin or DDL for XMAP215 (a kind gift from Y. Zheng). The amount of protein recruited to the centrosome was quantified by the fluorescence intensity. Alexa-488 and Alexa-594 anti-mouse or anti-rabbit secondary antibodies (for immunofluorescence) were purchased from Invitrogen (Carlsbad, CA).
Microtubule Nucleation from Beads
Protein A Dynabeads (10 μl) were incubated with 10 μl of either anti-maskin, anti-XMAP215, or unspecific rabbit serum immunoglobulin (IgG) according to the manufacturer's instructions. The antibody-bound beads were washed three times with XB and then incubated with 63 μl of CSF extract (or maskin-depleted extract, as indicated in Figure 4) for 1 h at 4°C. The beads were retrieved with a magnet and washed three times with XB. The washed beads (0.5 μl) were added to an in vitro polymerization reaction (3 mg/ml tubulin, 0.3 mg/ml rhodamine-tubulin, and 1 mM guanosine triphosphate [GTP] in BRB80; for the experiments shown in Figure 6G, the polymerization reaction also contained maskin) and incubated at 30°C for 10 min. Samples were fixed with 1% glutaraldehyde in BRB80 for 3 min and diluted with 250 μl of 80% glycerol in BRB80. A 3-μl aliquot of each reaction was spotted onto a microscope slide, covered with a coverslip, sealed with nail polish, and viewed in the microscope. The remaining beads (9.5 μl) were boiled in SDS sample buffer, and proteins were separated on 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels.
Figure 4.
The sequential centrosome complementation assay reveals that full-length maskin needs to be exposed to extract to be functional, whereas the TACC domain of maskin can complement centrosomes independent of extract. (A) Schematic diagram of the sequential complementation assay. Salt-stripped centrosomes are incubated with extract as before. However, the extract is washed away before the centrosomes are incubated with recombinant protein. The recombinant protein is then washed away and the centrosomes are challenged with purified bovine tubulin as before. (B) Quantification of centrosome activity in the sequential assay, expressed as the number of asters found in 50 randomly chosen microscope fields. Centrosomes were incubated with recombinant maskin or truncation mutants as indicated below the graph. The graph represents three independent experiments (indicated by circles).
Figure 6.
Maskin is required for centrosomal microtubule assembly independently of XMAP215 localization to the centrosome. (A) Salt-stripped centrosomes incubated in mock (a) or maskin-depleted (b) extract recruit similar amounts of XMAP215. Centrosomes were double labeled for γ-tubulin (red) and XMAP215 (green). Individual channels and overlays are indicated on the micrographs. Bar, 2 μm. (B) Quantification XMAP215 fluorescence intensity (normalized against γ-tubulin fluorescence and expressed as percent of mock-depleted control) for centrosomes incubated in mock-depleted (light bars) or maskin-depleted (dark bars) extracts. n = 3; values are mean ± SD. (C) Coomassie-stained gel of proteins coimmunoprecipitated with antibodies to maskin (M) or XMAP215 (X). Arrowheads mark the positions of XMAP215 (X) and maskin (M). (D) Microtubule asters are able to form around beads coated with XMAP215, but not maskin. Antibodies against random IgG (left), maskin (middle), or XMAP215 (right) were bound to beads and incubated in normal or maskin-depleted extract, as indicated. The beads were isolated, washed, and then challenged with purified tubulin containing a small amount of rhodamine-labeled tubulin to allow visualization in the microscope. The micrographs show representative beads for each sample. The insets show schematics of the beads (circles) with or without microtubules (red lines), to aid visualization. Bar, 10 μm. (E and F) Full-length maskin and maskin-3A, but not truncation mutants or maskin-3E, suppress XMAP215-mediated aster formation. XMAP215-coated beads were challenged with purified tubulin in the presence of 200 nM maskin, maskin-3A, maskin-3E, TACC domain, or TACC-less domain as indicated for each panel. The beads were processed as in D. Insets as in D. Bar, 10 μm. (G and H). XMAP215 attaches to the minus ends of microtubules. (G) XMAP215-coated beads (generated by incubating XMAP215 antibodies with protein A beads and then incubating the antibody-coated beads in egg extract) were incubated in egg extract supplemented with small amounts of rhodamine tubulin to visualize microtubules, and GFP-EB1 to visualize microtubule plus ends. A single timeframe is shown for microtubules (red in the overlay) and GFP-EB1 (green in the overlay). Bar, 10 μm. (H) XMAP215-coated beads (generated as in G) were washed and incubated in unlabeled tubulin for 9 min, followed by incubation in rhodamine-labeled tubulin for 1 min. The microtubules were then fixed, spun onto coverslips, and processed for immunofluorescence to visualize the microtubules. The rhodamine-labeled microtubule plus ends are distal to the beads. Bar, 10 μm.
To determine the orientation of microtubules associated with XMAP215-coated beads, 0.5 μl of beads was added to an in vitro polymerization reaction (final volume, 5 μl) without rhodamine tubulin (2.5 mg/ml tubulin and 1 mM GTP in BRB80) and incubated at 37°C for 9 min. Then, 15 μl of prewarmed elongation mixture containing rhodamine tubulin (2.5 mg/ml tubulin, 0.25 mg/ml rhodamine-tubulin, and 1 mM GTP in BRB80) was added to the reaction and incubated for 1 min before the reaction was fixed with 200 μl of 1% glutaraldehyde in BRB80 for 3 min and diluted with 800 μl of 30% glycerol in BRB80. The reactions were spun onto coverslips, postfixed in −20°C methanol, and processed for immunofluorescence using an anti-tubulin antibody (DM1α; Sigma-Aldrich, St. Louis, MO). Images were taken as a Z-series and then processed using blind three-dimensional deconvolution software (AutoDeblur, Media Cybernetics, Silver Spring, MD) at the default settings. The images shown in Figure 6, D and E, are Z-projections of the deconvolved images.
Alternatively, 0.5 μl of XMAP215-coated beads was added to 10 μl of a mitotic high-speed supernatant (Sampath et al., 2004) containing 0.2 μl of rhodamine-tubulin and 0.01 mg/ml recombinant green fluorescent protein (GFP)-EB1 and photographed live. Individual frames of the time-lapse series are shown. To enhance visualization, the images were processed using the unsharpmask (16 pixel filter width) and median (3 pixel filter width) filters of MetaMorph.
Online Supplemental Material
Six videos are included, showing the assembly of microtubules by sperm-associated centrioles (Supplemental Videos 1–4) or exogenously added centrosomes (Supplemental Videos 5 and 6) in mock-depleted (Supplemental Videos 1, 3, and 5) or maskin-depleted (Supplemental Videos 2, 4, and 6) Xenopus egg extracts.
RESULTS
Sperm-induced Asters Assembled in Maskin-depleted Extracts Are Highly Disorganized
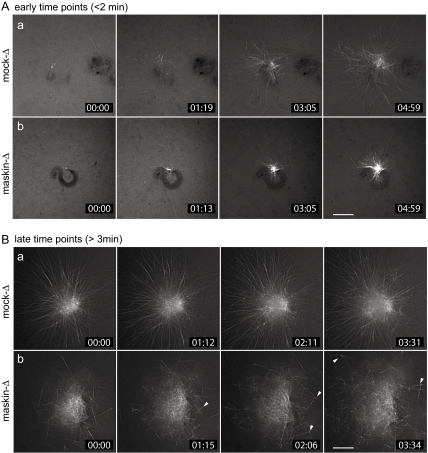
We showed previously that asters assembled in maskin-depleted Xenopus egg extracts supplemented with demembranated sperm chromatin are smaller and contain fewer microtubules than asters assembled in control mock-depleted extracts (O'Brien et al., 2005). To gain insight into the effects of maskin depletion on microtubule assembly, we followed the assembly of microtubule asters induced by addition of sperm chromatin to maskin-depleted or mock-depleted extracts spiked with small amounts of rhodamine-labeled tubulin to allow imaging of microtubules by time-lapse fluorescence microscopy (Figure 1 and Supplemental Videos 1–4). Under conditions in which fixed samples showed at least a threefold reduction in aster size compared with mock-depleted controls (O'Brien et al., 2005; Supplemental Figure S1), sperm chromatin incubated in mock- or maskin-depleted extracts nucleated comparable numbers of microtubules. This supported the notion that microtubule nucleation is not affected by maskin depletion, as reported previously (Peset et al., 2005). Interestingly, both mock- and maskin-depleted extracts exhibited remarkably high rates of microtubule release early during the assembly process (within ∼5 min of the start of the reaction; Figure 1A and Supplemental Videos 1 and 2). With slightly longer incubation times (>5 min after initiating microtubule assembly), asters assembled in mock-depleted extracts underwent a qualitative transition: fewer microtubules seemed to be released, and microtubules began to grow longer (Supplemental Videos 1 and 3). In contrast, asters assembled in maskin-depleted extracts failed to undergo this qualitative transition, because microtubule release seemed to continue to occur (Supplemental Video 2). Concomitantly, maskin-depleted asters became poorly organized and often contained clusters of several microtubules that seemed to be released from the centrosome in groups (arrowheads in Figure 1Bb). This suggested that centrosome function may be compromised in the absence of maskin.
Figure 1.
Maskin depletion from Xenopus egg extracts results in smaller, disorganized asters. (A and B) Time-lapse fluorescence microscopy of microtubules assembled in mock-depleted (a) or maskin-depleted (b) egg extracts induced to assemble centrosomes and microtubule structures by the addition of demembranated sperm chromatin. The extracts were spiked with a small amount of rhodamine-labeled tubulin to allow visualization of microtubules by fluorescence microscopy. Recording was initiated within the first 2 min (A) or after a minimum of 3 min (B) after warming the reaction mixture to initiate microtubule assembly. Elapsed time is given in the lower right-hand corner of each frame in minutes:seconds. These stills correspond to Supplemental Videos 1 (Aa), 2 (Ab), 3 (Ba), and 4 (Bb). Bars, 10 μm.
Maskin Depletion Has No Effect on the Dynamics of Microtubules Nucleated from Purified Centrosomes
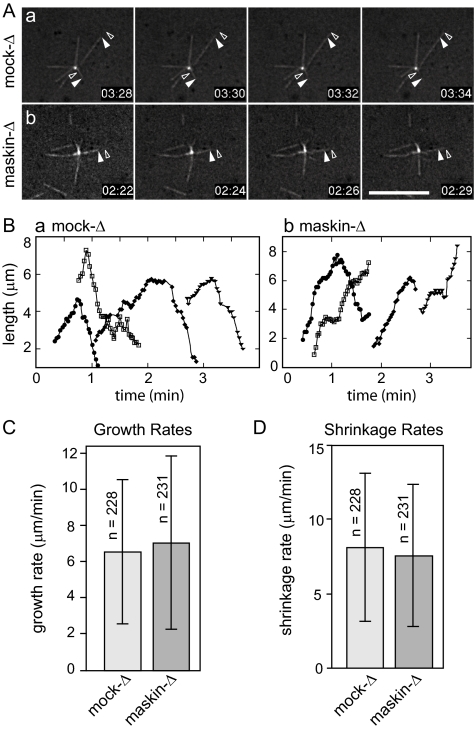
Asters organized by sperm chromatin were too dense to allow us to measure microtubule dynamics by following individual microtubules, or to assay potential defects in centrosome function. To tease apart the contributions to aster assembly of changes in microtubule dynamics and potential defects in centrosome function upon depletion of maskin, we took advantage of in vitro assays to examine centrosome functions and microtubule dynamics separately. We began by examining microtubules nucleated from purified centrosomes, which only nucleate a handful of microtubules under our experimental conditions. This allowed us to follow individual microtubules by time-lapse fluorescence microscopy in mock-depleted or maskin-depleted extracts (Figure 2 and Supplemental Videos 5 and 6) and calculate growth rates, shrinkage rates, and frequencies of catastrophe (transition between growth and shrinkage) or rescue (transition between shrinkage and growth). Analysis of the behavior of >200 individual microtubules per condition (in 9 independent experiments) revealed that maskin depletion had no detectable effect on microtubule dynamics, as the parameters of dynamic instability were indistinguishable for microtubules assembled in mock-depleted or maskin-depleted extracts (Table 1). This suggested that under these experimental conditions, maskin was not required for microtubule stability. We noticed, however, that centrosomes incubated in maskin-depleted extracts seemed to release their microtubules more readily than centrosomes incubated in mock-depleted extract (Supplemental Videos 5 and 6). Although we cannot yet rule out that enough TACC protein was carried in by the exogenously added centrosomes to overcome any potential defects of maskin-depleted extracts in microtubule plus end dynamics, these centrosomes apparently did not carry in enough TACC protein to mask the observed anchoring defects.
Figure 2.
Microtubule dynamics are not affected by maskin depletion. (A) Representative micrographs from time-lapse series showing microtubule dynamics in mock (a) or maskin-depleted (b) extracts supplemented with centrosomes. Closed arrowhead denotes the position of the microtubule end at the start; open arrowhead denotes the position of the microtubule end at the end of the time sequence. Elapsed time in minutes:seconds after the start of the recording is shown in the lower right of each panel. Corresponding videos are provided as Supplemental Videos 5 and 6. Bar, 10 μm. (B) Microtubule lifetime plots for four representative microtubules in mock-depleted (a) or maskin-depleted (b) egg extracts. (C and D) Quantification of microtubule growth (C) and shrinkage rates (D). Dynamics of 228 (mock-depleted) or 231 (maskin-depleted) microtubules in nine independent experiments were measured. These data are summarized in Table 1.
Table 1.
Summary of microtubule dynamics measured in mock- or maskin-depleted extracts
| No. MTs | Growth rate (μm/min) | Shrinkage rate (μm/min) | Catatrophe events/s | Rescue events/s | Pausing while growing events/s | Pausing while shrinking events/s | |
|---|---|---|---|---|---|---|---|
| Mock-depleted | 228 | 6.5 ± 4.0 | 8.0 ± 4.9 | 0.014 | 0.009 | 0.004 | 0.002 |
| Maskin-depleted | 231 | 7.0 ± 4.8 | 7.5 ± 4.7 | 0.013 | 0.008 | 0.004 | 0.003 |
| For comparison: | |||||||
| Wilde et al. 2001 | 229 | 8.3 ± 3.4 | 8.6 ± 3.4 | 0.033 | 0.004 | 0.008 | 0.004 |
| Tournebize et al. 2000 | |||||||
| Control extract | 91 | 11.5 | 16.9 | 0.015 | |||
| XMAP215-depleted extract | 74 | 10.6 | 14.4 | 0.035 | |||
| Belmont et al. 1990 | 96 | 12.3 ± 6 | 15.3 ± 5.6 | 0.117 | 0.021 | ||
The TACC Domain of Maskin Is Necessary and Sufficient for Centrosome Function
To examine the potential effects of maskin depletion on centrosomes in a way that is truly independent of microtubule-stabilizing proteins present in the extract, we turned to an assay that directly tests centrosome function. In this in vitro “complementation assay” for centrosome assembly (Moritz et al., 1998; also see Schnackenberg et al., 1998; Popov et al., 2002), which is diagrammed in Figure 3A, purified centrosomes are treated with a chaotropic agent (in our case, 2 M potassium iodide) to “salt-strip” them of the microtubule-nucleating material. This treatment renders the centrosomes unable to nucleate microtubules when challenged with purified tubulin. As expected, incubation of salt-stripped centrosomes with Xenopus egg extracts (or mock-depleted Xenopus egg extract) restored their ability to nucleate microtubules (Figure 3A). In contrast, incubation of salt-stripped centrosomes with maskin-depleted extracts resulted in centrosomes unable to support the formation of microtubule asters (Figure 3, C and D), suggesting that maskin was required for centrosome function. Importantly, the ability to assemble asters was mostly restored by addition of recombinant full-length maskin to depleted extracts. Aster assembly activity was also mostly restored by the addition of the conserved portion of maskin (Gergely, 2002; Still et al., 2004), i.e., the ∼200 amino acid C-terminal TACC domain (Figure 3, C and D; constructs used in this study are diagramed in Figure 3B). In contrast, a truncation mutant lacking the TACC domain (“TACC-less”) was unable to restore function. We concluded from these experiments that although it was dispensable for microtubule nucleation, maskin was required for full centrosome activity, and that the TACC domain of maskin carried most of the activity necessary for centrosome function.
Figure 3.
Maskin is required for centrosome function. (A) Schematic diagram of the centrosome complementation assay. Salt-stripped centrosomes are applied to a coverslip and incubated with Xenopus egg extract. The extract is washed away and the centrosomes are challenged with purified bovine tubulin. The samples are then fixed and scored for activity. (B) Schematic diagram of the maskin constructs used in this study. The conserved TACC domain (amino acids 714–931) is shown in green, the TACCless domain (amino acids 1–774) is blue. The overlap between the TACC and TACCless domains is gray. The highlighted Aurora A phosphorylation sites (S33, S620, and S626) were mutated to glutamic acids (maskin-3E) or alanines (maskin-3A). (C) Centrosome activity can be reconstituted if salt-stripped centrosomes are incubated with maskin-depleted extracts containing recombinant full-length maskin or TACC domain. Centrosomes were salt-stripped and incubated with extracts supplemented with recombinant proteins (or buffer) as indicated above the micrographs. Two representative micrographs per complemented centrosomes are shown. Bar, 10 mm. (D) Quantification of centrosome activity in the complementation assay, expressed as the number of asters found in 50 randomly selected microscope fields. The results for three independent experiments are shown (circles). The average is represented as a horizontal line. Vertical lines indicate the spread of the data. Conditions are indicated below the graph.
The model in which TACC proteins function at least in part by recruiting TOG proteins to the centrosome and facilitating their loading onto newly born microtubules (Barros et al., 2005; Brittle and Ohkura, 2005) predicts that both proteins would have to be present in the egg extract simultaneously to be recruited. To test this idea, we developed what we named the sequential assay. In this assay, salt-stripped centrosomes were first incubated with maskin-depleted extracts (presumably to rebuild centrosome substructure and recruit γ-tubulin, without which centrosomes are nonfunctional (Felix et al., 1994; Moritz et al., 1998; Schnackenberg et al., 1998). The extract was then removed, and, after a buffer wash step, the centrosomes were incubated with recombinant maskin or maskin truncation mutants in buffer (Figure 4). After another wash step to remove the excess recombinant protein, the centrosomes were then challenged with purified tubulin as described above. As shown in Figure 4B, incubation with full-length maskin was unable to restore function to salt-stripped centrosomes in this assay. This suggested that full-length maskin had to be present in the extract to function. Surprisingly, however, the TACC-domain alone of maskin restored significant levels of activity to salt-stripped centrosomes in the sequential assay (Figure 4B), confirming that the TACC domain of maskin harbors the activity required for centrosome function and ruling out a role for maskin in recruitment of cytosolic proteins.
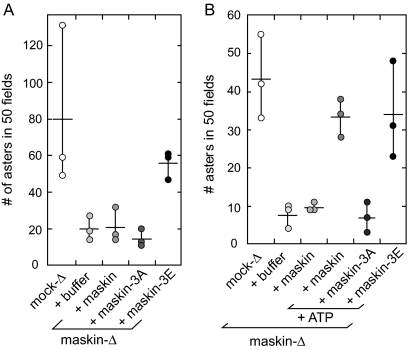
Aurora A Phosphorylation of Maskin Exposes the TACC Domain
Why does full-length maskin need to be present in the extract, whereas the TACC domain by itself can restore function to salt-stripped centrosomes? Association of maskin (or its Drosophila homologue D-TACC) with the centrosome is regulated by phosphorylation of TACC proteins by the mitotic kinase Aurora A (Giet et al., 2002; Barros et al., 2005; Kinoshita et al., 2005; Peset et al., 2005; Albee et al., 2006). It was therefore possible that the need for full-length maskin to be incubated in the extracts represents a need for maskin to be phosphorylated to allow its association with the centrosome. Based in part on the observation that the Aurora A phosphorylation sites on maskin (Peset et al., 2005; Kinoshita et al., 2005) lie outside of the TACC domain (Figure 3B), we reasoned that the TACC domain may not be accessible in the unphosphorylated molecule, as proposed previously (Peset et al., 2005). One prediction from this “inaccessibility” model, namely, that the TACC domain restores function in the sequential assay, whereas the full-length protein does not, is supported by the results shown in Figure 4B. A second prediction from this model is that phosphorylated recombinant maskin, or a phospho-mimetic mutant of maskin in which the three Aurora-A phosphorylation sites (Ser33, Ser620, and Ser626) has been mutated to glutamic acid residues (“maskin-3E”; Peset et al., 2005), should be able to restore function in the sequential assay, whereas a nonphosphorylatable maskin mutant (in which all three serine residues have been mutated to alanines; “maskin-3A”) should be unable to do so. We tested our hypothesis in two ways: 1) we examined maskin-3E for its ability to rescue centrosomes in the sequential assay (Figure 5A), and 2) we added ATP to full-length recombinant maskin in the sequential assay, which presumably would allow maskin to become phosphorylated by centrosome-associated Aurora-A kinase (Figure 5B). Consistent with our hypothesis, maskin-3E, but not the nonphosphorylatable maskin-3A, was able to restore activity to salt-stripped centrosomes in the sequential assay (Figure 5A). Similarly, recombinant maskin was able to restore activity only when ATP was included in the incubation buffer (Figure 5B). These results provide experimental evidence that phosphorylation of maskin is required to allow access to the TACC domain.
Figure 5.
Phosphorylation of maskin restores centrosome function. (A and B) Quantification of centrosome activity in the sequential assay, expressed as the number of asters found in 50 randomly chosen microscope fields. Centrosomes were incubated with recombinant maskin phosphorylation mutants (A), or recombinant maskin in the presence or absence of ATP (B) as indicated below the graphs. Graphs represent three independent experiments (indicated by circles).
Maskin and XMAP215 Can Function Independently of One Another
The results from the sequential complementation assay suggested that the role of maskin in centrosome function was unlikely to involve recruitment of a cytosolic factor to the centrosome. To test more specifically whether maskin's role in centrosome function could be explained by the failure to recruit XMAP215 to centrosomes in our assay, we first estimated the amount of XMAP215 associated with salt-stripped centrosomes complemented in mock- or maskin-depleted extracts by immunofluorescence. For technical reasons, this was an estimate rather than a true measurement: to distinguish centrosomes from random background specks, we measured only those specks that were positive with both γ-tubulin (control) and XMAP215 antibodies. Given these technical limitations, we found that for those centrosomes that we could unambiguously identify, maskin depletion had only minor effects on the levels of XMAP215 recruited to complemented centrosomes (Figure 6, A and B). This finding is consistent with the observation that knockdown of human TACC3 by RNA interference in HeLa cells has little effect on centrosomal levels of ch-TOG, the human XMAP215 homologue (Gergely et al., 2003). However, we and others reported previously that maskin depletion resulted in reduced XMAP215 recruitment to centrosomes assembled in Xenopus egg extracts (O'Brien et al., 2005; Peset et al., 2005; Kinoshita et al., 2005). We speculate that the discrepancy arises from differences in the assembly pathway of centrosomes assembled by sperm centrioles or from salt-stripped centrosome scaffolds. Alternatively, the presence or absence of microtubules might influence XMAP215 recruitment, or our ability to detect XMAP215 by immunofluorescence. Regardless of the reasons for these differing observations, our finding that many salt-stripped centrosomes incubated in maskin-depleted extracts recruited nearly normal levels of XMAP215, yet were unable to organize microtubule asters, strongly suggested that failure to recruit XMAP215 to centrosomes was an unlikely explanation for the observed defects in centrosome function in the experiments presented here.
To further explore the interaction between XMAP215 and maskin, we immunoprecipitated XMAP215 or maskin from Xenopus egg extracts and compared their protein profiles by Coomassie-stained SDS-PAGE (Figure 6C). Although maskin antibodies coimmunoprecipitated XMAP215 from egg extracts, only small amounts of maskin coimmunoprecipitated with XMAP215 (Figure 6C). Together with the observation that the concentration of XMAP215 is ∼25-fold higher in egg extracts than the concentration of maskin (∼500 nM for XMAP215 vs. ∼20 nM for maskin; Gard and Kirschner, 1987; O'Brien et al., 2005), this suggested to us that only a fraction of XMAP215 might be in a complex with maskin in Xenopus egg extracts.
Beads coated with XMAP215 nucleate microtubule asters when incubated in egg extracts, or when incubated with purified tubulin in vitro (Popov et al., 2002). To test whether maskin might be involved in this process, we coated beads with maskin antibodies, incubated the beads in egg extract to recruit maskin (and XMAP215; see above), recovered the beads, washed them, and incubated the washed beads with purified tubulin. To our surprise, maskin-coated beads were unable to form microtubule asters under these conditions (Figure 6D) despite the presence of XMAP215 on maskin-coated beads (Figure 6C). In contrast, XMAP215-coated beads were able to form asters, as reported previously (Popov et al., 2002). The aster-forming activity of XMAP215 was unaffected by depletion of maskin from the egg extract used to isolate XMAP215 (Figure 6D), further underscoring that the microtubule-anchoring activity of XMAP215 did not require maskin. One interpretation of these findings is that maskin might suppress the aster-forming activity of XMAP215. To test this idea, we added purified maskin to in vitro microtubule polymerization assays with XMAP215-coated beads. Surprisingly, under these conditions, maskin suppressed the ability of XMAP215 to generate microtubule asters (Figure 6, E and F). Moreover, this activity of maskin was regulated by its phosphorylation state, because only wild type or maskin-3A suppressed XMAP215-mediated aster formation, whereas maskin-3E had no effect (Figure 6E). Neither the TACC domain alone nor the TACC-less domain was active in this assay (Figure 6F). Together, these observations suggested that full-length maskin was able to regulate the interaction between XMAP215 and microtubules, but phosphorylation of maskin suppressed this regulatory function.
These observations prompted us to ask whether XMAP215- coated beads interacted with the plus or the minus ends of microtubules, and thus, whether maskin was more likely to regulate the interaction of XMAP215 with the microtubule-plus or the microtubule minus ends. We first tested the orientation of microtubules tethered to XMAP215-coated beads in purified tubulin by adding—at the end of the reaction—a small amount of rhodamine labeled tubulin to a polymerization reaction containing only unlabeled tubulin (Figure 6G). We found that most of the rhodamine-labeled tubulin was incorporated distal to the beads, suggesting that microtubules were tethered to XMAP215 beads with their plus ends extending outward. However, we could not determine whether the observed fluorescence signal near the beads arose solely from bead autofluorescence, or whether it could also be attributed to the presence of microtubule plus ends proximal to the beads. To determine what fraction, if any, of microtubules were oriented “plus end in,” we tracked microtubule plus ends by adding a small amount of purified GFP-EB1 to the egg extract. A still image from the experiment is shown in Figure 6H. We found that all microtubule ends we could follow were labeled with GFP-EB1 while they were growing (but, as expected, GFP-EB1 did not associate with shrinking microtubule). Furthermore, the GFP-EB1 always moved away from the beads. This observation supported the notion that all microtubule plus ends were pointing away from the XMAP215-coated beads. Together, these results therefore suggest that maskin regulated the interaction of XMAP215 with microtubule minus ends.
DISCUSSION
An understanding of how TACC proteins participate in normal cellular functions and how they contribute to cancer (Raff, 2002) requires molecular insight into the details of how TACC proteins are involved in mitotic spindle assembly, and what role, if any, they play in centrosome function. A previous model for how TACC proteins contribute to spindle assembly (reviewed by Brittle and Ohkura, 2005) postulates that the role of TACC proteins is to bring TOG proteins to the centrosome to stabilize the plus ends of newly emerging centrosomal microtubules. Support for this “loading” model comes from the observation that TACC proteins interact with TOG proteins in all systems studied to date and that Xenopus TOG (XMAP215) binds more strongly to microtubules in vitro in the presence of Xenopus TACC (maskin) (Kinoshita et al., 2005). Further support for this model is given by the observation that GFP-labeled D-TACC moves away from and toward centrosomes in Drosophila embryos, as if it were tracking microtubule plus ends (Lee et al., 2001). A second model, based on the observation that phosphorylated D-TACC accumulates at the minus ends of spindle microtubules, takes into consideration a more direct centrosomal role for TACCs by postulating that a small fraction of TACC proteins become activated by phosphorylation at the centrosome and thus help to stabilize microtubule minus ends (Barros et al., 2005). Although both models are supported by experimental evidence, several of their features have not yet been tested experimentally. For example, it has not been determined whether D-TACC moves with microtubule plus ends or whether it moves on microtubules powered by molecular motors. In addition, it is not known whether the putative plus end tracking of maskin serves to stabilize microtubule plus ends or serves other functions such as facilitating maskin turnover. Similarly, the microtubule minus end-stabilizing role of TACC proteins has not been explored.
An important prediction from the existing models is that disrupting TACC proteins should affect the microtubule dynamics. Here, we report that maskin depletion from Xenopus egg extracts has no detectable effect on the dynamics of microtubules associated with centrosomes. Thus, we provide experimental evidence that maskin is not required for the microtubule plus end-stabilizing activity of XMAP215. This was surprising for two reasons. First, maskin was proposed to enhance the activity of XMAP215 (Kinoshita et al., 2005), and this presumably would lead to impaired microtubule plus end stabilization by XMAP215 upon maskin depletion from extracts. Our results argue against this possibility, consistent with a recent report by Brouhard et al. (2008) that XMAP215 associates with microtubule plus ends in the absence of maskin. Second, we previously showed that maskin depletion impairs Ran-induced microtubule aster assembly, which suggests that maskin depletion affects microtubule dynamics (O'Brien et al., 2005). One possible explanation for these apparent discrepancies might be that maskin depletion affects microtubule minus end dynamics in systems that lack centrosomes (such as, for example, Ran-induced asters) rather than affecting plus end dynamics. Because we measured the plus end dynamics of microtubules anchored in the centrosome, the experiments described here would not be expected to provide information about the effect of maskin depletion on minus end dynamics.
A second major finding reported here is that maskin is required for the assembly of a functional centrosome. Consistent with several reports that the TACC domain targets TACC proteins to the centrosome (Bellanger and Gönczy, 2003; Le Bot et al., 2003; Srayko et al., 2003; Peset et al., 2005; Srayko et al., 2003; Kinoshita et al., 2005), our experimental evidence supports the idea that the TACC domain is both necessary and sufficient for centrosome function. Previous work has shown that centrosomes do not require maskin to nucleate microtubules (Kinoshita et al., 2005; Peset et al., 2005) and that the centrosomal levels of the microtubule nucleator γ-tubulin are unaffected by the disruption of TACC proteins (Lee et al., 2001; O'Brien et al., 2005). Our time-lapse analysis confirms these results. However, centrosomes assembled in maskin-depleted extracts seem unable to properly anchor the microtubules. This is apparent both for centrosomes assembled around sperm chromatin-associated centrioles (Figure 1) and for purified centrosomes incubated in maskin-depleted extract (Figures 2 and 3). Interestingly, mutants in the Schizosaccharomyces pombe TACC protein Mia1/Alp7 also show microtubule detachment phenotypes (Zheng et al., 2006). Alp14 (TOG) mutants, in contrast, have problems in microtubule-based force production, but not with microtubule detachment (Zheng et al., 2006). These observations are consistent with the finding that distinct phenotypes arise from the depletion of maskin or of XMAP215: XMAP215 depletion results in asters with very short or no microtubules, due to an increase in catastrophe frequency (Tournebize et al., 2000), whereas maskin depletion results in smaller asters with fewer microtubules. Our hypothesis that maskin is required for microtubule anchoring at the centrosome is consistent with the observations that TACC protein disruption selectively affects microtubules associated with centrosomes (Le Bot et al., 2003; Barros et al., 2005). For example, experiments in flies, worms, and frog egg extracts have shown that astral (i.e., centrosome-anchored) microtubules are affected when TACC proteins are mutated or disrupted, whereas microtubules assembled by centrosome-independent mechanisms (i.e., by the chromosome-mediated microtubule assembly pathway; Gadde and Heald, 2004) seemto function properly (Barros et al., 2005; Kinoshita et al., 2005; Peset et al., 2005). Our model is also consistent with the observation that D-TACC localizes to the minus ends of spindle microtubules (Barros et al., 2005).
What are the potential mechanisms by which TACC proteins regulate microtubule anchoring? One possibility is that maskin serves as a connector between centrosomes and microtubules, and in its absence microtubules are released from centrosomes. We do not favor this possibility, because maskin does not have a very high-affinity for microtubule minus ends in vitro (Kinoshita et al., 2005; O'Brien et al., 2005; Peset et al., 2005). A more likely explanation is that maskin might regulate the activity of a microtubule-severing protein such as katanin at the centrosome. In this context it is interesting to note that the tac-1 (worm TACC) and zyg-9 (worm TOG) mutant phenotypes in C. elegans resemble the phenotypes of mutants of the microtubule severing protein mei-1 (katanin) (Srayko et al., 2003). Thus, many of the observed phenotypes (including the lack of astral microtubules and small spindles) can be accounted for by proposing a role for TACC proteins in regulating severing proteins.
Two additional new findings are reported here. First, we provide experimental evidence that Aurora A phosphorylation serves to regulate the accessibility of the TACC domain by moving the non-TACC-domain portion of maskin out of the way. Aurora A phosphorylation of maskin was known to be required for centrosomal targeting of maskin (Kinoshita et al., 2005; Peset et al., 2005). We provide evidence that the TACC domain alone of maskin can restore function to centrosomes assembled in maskin-depleted extracts and that the non-TACC portion of maskin prevents the TACC domain from rescuing the depletion phenotype unless it is phosphorylated. The non-TACC portion of maskin therefore seems to serve an autoinhibitory function. These experiments provide a biochemical explanation for the paradox that the “activating” phosphorylation of maskin lies outside the TACC domain.
Second, we show that maskin suppresses the microtubule–aster-forming activity of XMAP215. Several lines of evidence support this unexpected finding. Popov et al. (2002) showed that recombinant XMAP215 tethered to beads was able to assemble asters in purified tubulin. Together with our results that the microtubules in XMAP215 asters are oriented with their minus ends proximal to the beads, this shows that XMAP215 interacts with microtubule minus ends in the absence of maskin. Consistent with this idea, we found that only small amounts of maskin coimmunoprecipitated with XMAP215 and that XMAP215 attached to beads via antibodies was able to form asters. It was therefore surprising that maskin-coated beads, which were prepared by incubating beads coated with anti-maskin antibodies in egg extract and which also had XMAP215 on them, were unable to form asters. Our biggest surprise, however, was that soluble maskin was able to inhibit aster formation from XMAP215-coated beads. The mechanism of this inhibition is not yet clear, as maskin may affect either XMAP215 or the microtubule minus end, or both. Maskin does not seem to have an effect on the overall stability of microtubules assembled in vitro (O'Brien et al., 2005), which suggests that maskin does not destabilize microtubule minus ends directly. It is nonetheless possible that maskin prevented microtubules from forming on XMAP215-coated beads or that microtubules were formed and were then released. Extant experimental evidence strongly implicates XMAP215 in regulating microtubule plus end dynamics, which is difficult to reconcile with the observation that XMAP215 nucleates and anchors microtubules by their minus ends. It is therefore possible that microtubule minus end dynamics, and/or XMAP215 function, require that XMAP215 be released from microtubule minus ends. We speculate that maskin could be involved in this aspect of XMAP215 function.
In summary, the work presented here provides direct evidence that maskin is required for centrosome function and that this activity is distinct from any role TACC proteins may have in recruiting XMAP215 to centrosomes or loading XMAP215 onto microtubules. We also show that maskin inhibits the interaction of XMAP215 with the minus ends of microtubules. It remains to be determined whether and how the putative role of maskin in regulating microtubule anchoring is related to its role in regulating XMAP215.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lori O'Brien and Lingling Liu for helpful discussions and comments on the manuscript; Y. Zheng for the XMAP215 antibody; Y. Zheng and D. Ducat for the GFP-EB1 construct; and the Weibel lab for use of MetaMorph workstation. This work was supported by National Science Foundation grants MCB-0344723 and MCB-0643879 (to C.W.), and Steenbock Predoctoral and Wisconsin Distinguished Fellowships from the Department of Biochemistry, University of Wisconsin-Madison (to A.J.A.).
Abbreviations used:
- TACC
transforming acidic coiled coil
- TOG
tumor overexpressed gene
- XMAP
Xenopus microtubule-associated protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-11-1204) on May 28, 2008.
REFERENCES
- Albee A. J., Tao W., Wiese C. Phosphorylation of maskin by Aurora-A is regulated by RanGTP and importin β. J. Biol. Chem. 2006;281:38293–38301. doi: 10.1074/jbc.M607203200. [DOI] [PubMed] [Google Scholar]
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Bornens M. Structure and duplication of the centrosome. J. Cell Sci. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- Badano J. L., Teslovich T. M., Katsanis N. The centrosome in human genetic disease. Nat. Rev. Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- Barros T. P., Kinoshita K., Hyman A. A., Raff J. W. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger J. M., Gönczy P. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 2003;13:1488–1498. doi: 10.1016/s0960-9822(03)00582-7. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Glover D. M. Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Brittle A. L., Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr. Biol. 2005;15:R880–R882. doi: 10.1016/j.cub.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Brouhard G. J., Stear J. H., Noetzel T. L., Al-Bassam J., Kinoshita K., Harrison S. C., Howard J., Hyman A. A. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M. A., Antony C., Wright M., Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde S., Heald R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 2004;14:R797–R805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J. Cell Biol. 1987;105:2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Becker B. E., Romney S. J. MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins. Int. Rev. Cytol. 2004;239:179–272. doi: 10.1016/S0074-7696(04)39004-2. [DOI] [PubMed] [Google Scholar]
- Giet R., McLean D., Descamps S., Lee M. J., Raff J. W., Prigent C., Glover D. M. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely F. Centrosomal TACCtics. BioEssays. 2002;24:915–925. doi: 10.1002/bies.10162. [DOI] [PubMed] [Google Scholar]
- Gergely F., Draviam V. M., Raff J. W. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A., Drechsel D., Kellogg D., Salser S., Sawin K., Steffen P., Wordeman L., Mitchison T. J. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Kinoshita K., Noetzel T. L., Pelletier L., Mechtler K., Drechsel D. N., Schwager A., Lee M., Raff J. W., Hyman A. A. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bot N., Tsai M. C., Andrews R. K., Ahringer J. TAC-1, a regulator of microtubule length in the C. elegans embryo. Curr. Biol. 2003;13:1499–1505. doi: 10.1016/s0960-9822(03)00577-3. [DOI] [PubMed] [Google Scholar]
- Lee M. J., Gergely F., Jeffers K., Peak-Chew S. Y., Raff J. W. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behavior. Nat. Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- McGee-Russel S., Allen R. D. Reversible stabilization of labile microtubules in the reticulopodial network of Allogromia. Adv. Cell Mol. Biol. 1971;1:153–184. [Google Scholar]
- Moritz M., Braunfeld M. B., Fung J. C., Sedat J. W., Alberts B. M., Agard D. A. Three-dimensional characterization of centrosomes from early Drosphila embryos. J. Cell Biol. 1995;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Zheng Y., Alberts B. M., Oegema K. Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- O'Brien L. L., Albee A. J., Liu L., Tao W., Dobrzyn P., Lizarraga S. B., Wiese C. The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly. Mol. Biol. Cell. 2005;16:2836–2847. doi: 10.1091/mbc.E04-10-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peset I., Seiler J., Sardon T., Bejarano L. A., Rybina S., Vernos I. Function and regulation of maskin, a TACC family of protein, in microtubule growth during mitosis. J. Cell Biol. 2005;170:1058–1066. doi: 10.1083/jcb.200504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov A. V., Severin F., Karsenti E. XMAP215 regulates microtubule-nucleating activity of centrosomes. Curr. Biol. 2002;12:1326–1330. doi: 10.1016/s0960-9822(02)01033-3. [DOI] [PubMed] [Google Scholar]
- Raff J. W. Centrosomes and cancer: lessons from a TACC. Trends Cell Biol. 2002;12:222–225. doi: 10.1016/s0962-8924(02)02268-7. [DOI] [PubMed] [Google Scholar]
- Sampath S. C., Ohi R., Leismann O., Salic A., Pozniakovski A., Funabiki H. The chromosomal passenger complex is required for chromatin- induced microtubule stablilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Schnackenberg B. J., Khodjakov A., Rieder C. L., Palazzo R. E. The disassembly and reassembly of functional centrosomes in vitro. Proc. Natl. Acad. Sci. USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still I. H., Vettaikkorumakankauv A. K., DiMatteo A., Liang P. Structure-function evolution of the transforming acidic coiled coil genes revealed by analysis of phylogenetically diverse organisms. BMC Evol. Biol. 2004;4:16–32. doi: 10.1186/1471-2148-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srayko M., Quintin S., Schwager A., Hyman A. A. Caenorhabditis elegans TAC-1 and ZYG-9 form a complex that is essential for long astral and spindle microtubules. Curr. Biol. 2003;13:1506–1511. doi: 10.1016/s0960-9822(03)00597-9. [DOI] [PubMed] [Google Scholar]
- Tournebize R., Andersen S. S., Verde F., Dorée M., Karsenti E., Hyman A. A. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize R., Popov A., Kinoshita K., Ashford A. J., Rybina S., Pozniakovsky A., Mayer T. U., Walczak C. E., Karsenti E., Hyman A. A. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2000;2:13–19. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. Microtubule nucleation: γ-tubulin and beyond. J. Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- Wilde A., Lizarraga S. B., Zhang L., Wiese C., Gliksman N. R., Walczak C. E., Zheng Y. Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol. 2001;3:221–227. doi: 10.1038/35060000. [DOI] [PubMed] [Google Scholar]
- Zheng L., Schwartz C., Wee L., Oliferenko S. The fission yeast transforming acidic coiled coil-related protein Mia1p/Alp7p is required for formation and maintenance of persistent microtubule-organizing centers at the nuclear envelope. Mol. Biol. Cell. 2006;17:2212–2222. doi: 10.1091/mbc.E05-08-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.