Abstract
Cell surface hemichannels (HCs) composed of different connexin (Cx) types are present in diverse cells and their possible role on FGF-1–induced cellular responses remains unknown. Here, we show that FGF-1 transiently (4–14 h, maximal at 7 h) increases the membrane permeability through HCs in HeLa cells expressing Cx43 or Cx45 under physiological extracellular Ca2+/Mg2+ concentrations. The effect does not occur in HeLa cells expressing HCs constituted of Cx26 or Cx43 with its C-terminus truncated at aa 257, or in parental nontransfected HeLa cells. The increase in membrane permeability is associated with a rise in HC levels at the cell surface and a proportional increase in HC unitary events. The response requires an early intracellular free Ca2+ concentration increase, activation of a p38 MAP kinase-dependent pathway, and a regulatory site of Cx subunit C-terminus. The FGF-1–induced rise in membrane permeability is also associated with a late increase in intracellular free Ca2+ concentration, suggesting that responsive HCs allow Ca2+ influx. The cell density of Cx26 and Cx43 HeLa transfectants cultured in serum-free medium was differentially affected by FGF-1. Thus, the FGF-1–induced cell permeabilization and derived consequences depend on the Cx composition of HCs.
INTRODUCTION
Fibroblast growth factors (FGFs) are expressed in almost every tissue of a variety of multicellular organisms (Coumoul and Deng, 2003). Prototypes of this family are the acidic and basic FGFs (FGF-1 and -2, respectively), which participate in diverse cellular processes such as chemotaxis, cell migration, differentiation, motility, and cell survival (Böttcher and Niehrs, 2005). The efficacy of the FGF–FGF receptor (FGFR) complexes activation is determined by the receptor's affinity constant and is regulated by heparan sulfate proteoglycans (Itoh and Ornitz, 2004). Moreover, many FGF induced effects are cell type–dependent and can be explained, at least in part, by differences in the engaged signal transduction pathways (Dailey et al., 2005). Differences in cellular responses to FGFs might also be explained by transactivation of other receptors or ion channels (Mergler et al., 2003; Dailey et al., 2005; Fiorio et al., 2005). The functional consequences of a membrane receptor transactivation will depend on the extracellular concentration of its ligand and efficiency of the coupled transduction pathway. But, if transactivation occurs at a membrane channel level the effects will depend to a great extent on the channel permeability properties. Channel types affected by a member of the FGFs family (FGF-2) include connexin (Cx) hemichannels (HCs) (De Vuyst et al., 2007).
Cx HCs are hexameric pores permeable to ions and small molecules located at unopposed plasma membrane of most vertebrate cells (Sáez et al., 2003). Under resting conditions, Cx43 HCs present a low open probability believed to result from the blocking effect of extracellular Ca2+ and Mg2+ (Contreras et al., 2003). Thus, reducing the extracellular divalent cations concentration has been useful to detect changes in cell membrane permeability elicited by extracellular ligands, such as sphingosine 1-phosphate (Squecco et al., 2006) and FGF-2 (De Vuyst et al., 2007). Nonetheless, elevated HC activity induced by an endogenous ligand in the presence of physiological concentrations of divalent cations remains unknown.
Because enhanced membrane permeability to small molecules could result from opening of other channel types beside Cx HCs (Meyers et al., 2003; Bao et al., 2004a; Pelegrin and Surprenant, 2006), demonstration of the channel molecular identity is relevant. This difficulty can be overcome using complementary experimental paradigms including 1) cells deficient in a particular channel type, 2) reconstituted systems, 3) pharmacological approaches, and 4) electrophysiological characterization of the membrane currents.
Here, we show that FGF-1 induces a transient increase in plasma membrane permeability via Cx HCs in Cx43 and Cx45 but not Cx26 expressing HeLa cells under physiological extracellular Ca2+/Mg2+ concentrations. The mechanism involves an increase in HC activity and surface HCs levels that require an early free intracellular Ca2+ concentration ([Ca2+]i) increase, activation of a p38 MAP kinase–dependent pathway, and a regulatory site located in the C-terminus of Cx subunits. In FGF-1–responsive cells a late increase in [Ca2+]i also occurred and was likely due to HC-mediated Ca2+ influx. The cell density of Cx26 and Cx43 HeLa transfectants cultured in serum-free medium was differentially affected by FGF-1. Thus, the Cx HC composition determines the FGF-1 effects on the cell membrane permeability and contributes to downstream cellular responses.
MATERIALS AND METHODS
Reagents
Previously characterized polyclonal Cx43 and Cx45 antibodies were used (González et al., 2002; Corvalán et al., 2007). An mAb directed against the cytoplasmic loop of Cx26 was used (13.8100: Zymed Laboratories, South San Francisco, CA). Monoclonal anti-phosphorylated ERK antibodies (pErK) were from Santa Cruz Biotechnology (E4: Santa Cruz, CA). A rabbit polyclonal antibody directed to the hemagglutinin sequence conjugated to the C-terminus of Cx43Δ257 was used (71-5500: Zymed Laboratories). SuperSignal kit for ECL detection, polyclonal goat anti-rabbit antibody conjugated to horseradish peroxidase, Sulfo-NHS-SS-biotin, and NeutrAvidin immobilized on agarose beads were from Pierce (Rockford, IL). Ethidium bromide and Lucifer Yellow (LY) were obtained from Sigma-Aldrich (St. Louis, MO). Human recombinant acidic fibroblast growth factor (FGF-1), basic fibroblast growth factor (FGF-2), 4-bromo-A23187 (4-Br-A23187) was purchased from Sigma. BAPTA-AM and Fura 2-AM were from Molecular Probes (Eugene, OR).
Drugs and Stock Solutions
The composition of the Hanks' balanced salt solution (HBSS) contained (in mM) 137 NaCl, 5 KCl, 0.95 CaCl2, 0.5 MgCl2, 0.4 KH2PO4, 0.4 MgSO4, 4 NaHCO3, 0.3 NaH2PO4, and 5 glucose, pH 7.4. The recording solution contained (in mM) 154 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES, pH 7.4.
The ethidium (Etd) used for dye uptake experiments was prepared as 25 mM stock solution in water and diluted to 5 μM final concentration in recording solution before applying it to cells. A 500× 18β-glycyrrhetinic acid stock solution was prepared in ethanol. LaCl3, oxidized ATP (oATP), and capsazepine (Czp) were dissolved in recording solution at 100× of the final concentration. BAPTA-AM and Fura 2-AM were prepared in DMSO as 1000× stock solution. 4-Br-A23187 was prepared as 10,000× stock solution in ethanol. All stock solutions, dilutions to final concentrations and recording solutions were prepared in sterile, filtered water (W3500; Sigma-Aldrich).
HeLa Cell Cultures
Previously described parental HeLa cells (CCL-2, ATCC, Rockville, MD) or HeLa cells stably transfected with mouse Cx26 (HeLa-Cx26), Cx43 (HeLa-Cx43), or Cx45 (HeLa-Cx45) cDNA (Elfgang et al., 1995) were kindly provided by Dr. Klaus Willecke (Bonn University, Germany). Experiments were also performed on previously described HeLa cells transfected with cDNAs encoding for mouse Cx43 with a enhanced green fluorescent protein (EGFP) attached to its C-terminus (Cx43EGFP). Cells expressing Cx43-EGFP were selected with neomycin (300 μg/ml) and identified by their fluorescence emission at 530 nm as described (Contreras et al., 2003; Retamal et al., 2007a). PCR was used to generate a variant of rat Cx43 truncated at amino acid 257 (Cx43Δ257) with the nine-amino acid influenza hemagglutinin (HA) tag appended to the carboxy terminus of the rat Cx43 cDNA. Forward primer: 5′ccaggatccccaccatgggtgactggagtgccttggggaag-3′ and reverse primer: 5′-catgggcccttaagcgtagtctgggacgtcgtatgggtatgatgggctcagtgggccagt-3′ were used. Cx43Δ257 cDNA was cloned between BamHI and ApaI restriction sites into pcDNA3.1 Hygromycin vector (Invitrogen, Carlsbad, CA). Parental HeLa cells (HeLa-parental) were transfected with lineal Cx43Δ257 vector and stable clones (HeLa-Cx43Δ257) were selected with hygromycin (100 μg/ml) in culture medium. The expression of Cx43Δ257 was confirmed by indirect immunofluorescence using rabbit polyclonal antibody directed against the HA epitope tag. Because HeLa-parental and HeLa transfected with plasmid used for Cx transfections showed similar responses, the former were used as controls in most experiments. Cells were seeded onto plastic Petri dishes of 60- or 100-mm diameter (Nunclon, Roskilde, Denmark) or onto no. 1 sterile glass coverslips, placed on the bottom of plastic culture dishes (MarTek, Ashland, MA) and cultured in DMEM supplemented with 10% fetal bovine serum and kept at 37°C in a 5% CO2/95% air atmosphere at nearly 100% relative humidity.
The expression of Cxs in different HeLa cell transfectants was tested by indirect immunofluorescence. Cell cultures showing staining in >95% of the cells were used in all experiments.
Treatments with FGF-1
Cells treated with FGF-1 conjugated to heparin will be referred to treatment with FGF-1. Each FGF-1 aliquot was prepared 6–8 h before the experiment. Final concentrations of FGF-1 and heparin were 10–100 ng/ml and 5–50 IU/ml, respectively, to keep a constant ratio of 10 ng FGF-1:5 IU heparin per milliliter independent of the FGF concentration. For example, 1 μl of FGF-1 stock solution (450 μg/ml) was diluted to 10 μg/ml by adding 44 μl of 5000 IU/ml sodium heparin; this mixture represented a 1000× FGF-1 solution. Subconfluent (<60%) 48 h serum-starved HeLa cell cultures were washed twice with recording solution and incubated in serum-free DMEM. In this medium, transfected or parental HeLa cells were treated for different time periods with FGF-1, heparin alone, or 1 μl/ml PBS (Control condition) and incubated at 37°C within a cell culture incubator.
Time-Lapse Fluorescence Imaging and [Ca2+]i
For time-lapse experiments, cells plated onto glass coverslips were washed twice with recording solution and incubated in 5 μM Etd, and fluorescence was recorded in regions of interest of different cells with a water immersion Olympus 51W1I upright microscope (Melville, NY). Images were captured with a Q Imaging model Retiga 13001 fast cooled monochromatic digital camera (12-bit; Qimaging, Burnaby, BC, Canada) every 20 s (exposure time = 30 ms, gain = 0.5) and Metafluor software (version 6.2R5; Universal Imaging, Downingtown, PA) was used for image analysis and fluorescence quantification. For data representation and Etd uptake slopes calculation, the average of two independent background intensity measurements at each time (FB, expressed as arbitrary units or AU) was subtracted to each of the cells fluorescence intensity at each time interval (F1). Results of this calculation (F1 − FB) at each time interval for each of the 20 cells were averaged and plotted against time (expressed in minutes) during 18 min. Slopes were calculated using Microsoft Excel software (Redmond, WA) and expressed, as AU/min. Microscope and camera settings remained the same in all experiments.
[Ca2+]i changes were monitored in cells plated on glass coverslips. Cells were ester-loaded for 45 min with Fura-2 (5 μM) at 37°C, washed three times in recording solution, and then cells were left to stabilize at 37°C for 5 min before any fluorescence recording was performed. The experimental protocol for Ca2+ imaging involved the acquisition every 20 s (emission at 510 nm), of an image pair of 340- and 380-nm excitation wavelengths using a 20× water immersion objective and a filter switch. Offline analysis involved determination of pixels allocated to each cell. The average pixel value allocated to each cell obtained with excitation at each wavelength was corrected for background. Because of low excitation intensity, no bleaching was observed even when illuminating the cells over a few minutes. The ratio was obtained after dividing the 340-nm image by the 380-nm image on a pixel-by-pixel base (R = F340 nm/F380 nm). All measurements and data analyses were performed using the same microscope and software used for dye uptake experiments (see above).
Electrophysiology
Cells cultured on glass coverslips were placed onto an experimental chamber mounted on the stage of an inverted Olympus IX-51 microscope. For whole cell experiments bath solution contained (in mM) 140 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 2 BaCl2, and 10 HEPES, pH 7.4. The pipette solution contained (in mM) 130 CsCl, 10 AspNa, 0.26 CaCl2, 1 MgCl2, 2 EGTA, 7 TEA-Cl, and 5 HEPES, pH 7.2. Whole cell currents were recorded as described (Contreras et al., 2003). Briefly, patch electrodes were fabricated from borosilicate glass capillaries using a Flaming/Brown micropipette puller (P-87, Sutter Instruments, Union City, CA). The tip resistance was 5–10 MΩ when filled with pipette solution. Currents were filtered at 1 kHz and sampled at 5 kHz. Then, records were filtered with a digital low pass filter of 0.5 kHz. Data acquisition and analysis were performed using pClamp 9 (Axon Instruments, Novato, CA).
Dye Transfer
The intercellular communication via gap junctions between HeLa cells was evaluated in subconfluent cultures (∼85%) by iontophoretic injection into one cell of 5% wt/vol LY (MW = 457.24, −1) in 150 mM LiCl through glass microelectrodes as described previously (Corvalán et al., 2007). Briefly, coverslips containing cells were placed in a perfusion chamber and visualized in an inverted microscope (TE 200; Nikon, Melville, NY) equipped with xenon arc lamp and filters for LY (excitation wavelength 450–490 nm; emission wavelength above 520 nm). After 1 min of dye injection, surrounding cells were examined to determine whether dye transfer occurred. The incidence of dye coupling was calculated as the percentage of cases in which the dye transferred to at least one adjacent cell, and the number of cells to which dye spread was determined and expressed as the index of dye coupling. In all experiments the incidence of dye coupling was evaluated by injecting a minimum of 10 cells.
Immunoblots
Cell cultures were rinsed twice with HBSS (see above), harvested by scraping with a rubber policeman, and sonicated with a Microson Sonicator ultrasonic homogenizer (Misonix, Farmingdale, NY) on ice in 50 μl lysis buffer containing protease (200 μg/ml soybean trypsin inhibitor, 1 mg/ml benzamidine, 1 mg/ml ε-aminocaproic acid, and 2 mM PMSF) and phosphatase (20 mM Na4P2O7 and 100 mM NaF) Inhibitors. Protein levels of cell lysates were measured with the Bio-Rad protein assay (Bio-Rad Laboratories, Richmond, CA). Afterward, samples were analyzed by immunoblotting. Briefly, aliquots of cell lysates (80 μg of protein) or total biotinylated surface membrane proteins were resuspended in 1× Laemmli sample buffer, separated on 8–12% SDS-PAGE, and electro-transferred to nitrocellulose sheets. Nonspecific protein binding was blocked by incubation of nitrocellulose sheets in 5% nonfat milk in PBS for 60 min, and then blots were incubated with primary polyclonal anti-Cx43, anti-Cx45, or anti-HA antibody or with monoclonal anti-pERK antibody overnight at 4°C, followed by six 20-min phosphate-buffered saline (PBS) washes. Each primary antibody was diluted in 5% nonfat milk in PBS. Depending on the primary antibody used, blots were incubated with goat anti-rabbit or -mouse secondary antibody conjugated to horseradish peroxidase (1:5000 in 5% nonfat milk in PBS). Antigen-antibody complexes were detected by ECL using the SuperSignal kit according to the manufacturer's instructions. Resulting immunoblot signals were scanned and densitometric analyses were performed using the Scion Image software (Scion, Frederick, MD).
Surface Protein Biotinylation
Cell cultures seeded in 100-mm culture dishes were washed three times with HBSS containing 1 mM CaCl2 (HBSS-Ca2+). Then, 3 ml of Sulfo-NHS-SS-biotin (0.5 mg/ml HBSS-Ca2+) was added to each dish and incubated for 30 min at 4°C. Cells were then washed three times with HBSS-Ca2+ solution containing 15 mM glycine, pH 8.0, to quench unreacted biotin. Afterward, cells were harvested by scraping with a rubber policeman in the presence of protease and phosphatase inhibitors (as for immunoblots) and centrifuged at 14,000 rpm for 2 min at 4°C. Pellets were resuspended in 50 μl lysis buffer, placed on ice, and lysed by sonication as described above. The immobilized NeutrAvidin was added to each sample (1 μl of NeutrAvidin per 3 μg of biotinylated protein, assuming that 40% of total membrane protein was biotinylated), and the mixture was maintained for 1 h at 4°C. Then, 1 ml of binding buffer (HBSS-Ca2+, pH 7.2, plus 0.1% SDS and 1% NP-40) was added, mixed by soft vortex, and centrifuged for 2 min at 14,000 rpm at 4°C, and the supernatant was discarded. The wash procedure described before was repeated three times. In the last wash, the supernatant was removed and 40 μl of HBSS-Ca2+, pH 2.8, plus 0.1 M glycine was added, mixed gently, and centrifuged at 14,000 rpm for 2 min at 4°C. The supernatant was removed and placed in a 1.5-ml Eppendorf tube, and pH was adjusted to 7.4 immediately by adding 10 μl of 1 M Tris, pH 7.4. Finally, samples were mixed with 4× Laemmli buffer, resolved by SDS-PAGE, and subjected to Western blot analysis. Relative levels of Cxs were determined by densitometry as described above for immunoblots.
Statistical Analysis
Data are presented as means ± SEM; n expresses the number of independent experiments. Means for each group were compared using a nonparametric Mann-Whitney test for continuous variables and a U-test for nonpaired variables. Differences between proportions were assessed using descriptive statistics. Differences were considered significant at p < 0.05. Statistics were performed using Microsoft Excel and the Graph Pad Prism 4.0 (2003, San Diego, CA) software.
RESULTS
FGF-1 Increases the Etd Uptake Rate of HeLa-Cx43 Cells in the Presence of Physiological Concentrations of Extracellular Divalent Cations
Recently, FGF-2 was demonstrated to affect the ATP release through HCs triggered by the removal of extracellular Ca2+/Mg2+ in a cell- and Cx-dependent manner (De Vuyst et al., 2007). Nonetheless, the possible effect of other members of the FGF family on the HCs mediated membrane permeability, both in the presence and absence of extracellular divalent cations remains unknown.
In the present work, we studied the effect of FGF-1 on the functional state of Cx43 HCs by evaluating the La3+-sensitive Etd uptake, as described (Contreras et al., 2002; 2003). Consistent with a very low or lack of HC expression, the Etd uptake rate of parental HeLa cells (HeLa-parental) in the absence or presence of extracellular divalent cations was similarly low and was insensitive to La3+ (Figure 1), a Cx HC blocker (Retamal et al., 2007b). In agreement with the inhibitory effect of extracellular divalent cations, HeLa cells transfected with Cx43 (HeLa-Cx43) bathed with a solution containing Ca2+/Mg2+ showed an Etd uptake rate similar to HeLa-parental (Figure 1). Although in HeLa-Cx43 immersed in a Ca2+/Mg2+-free solution, the dye uptake rate was ∼2.5-fold higher than in the presence of extracellular divalent cations and was sensitive to La3+ (Figure 1).
Figure 1.
HeLa-Cx43 cells present functional Cx HCs. Control HeLa-Cx43 cells were placed in a recording solution (with 1.8 mM Ca2+ and 1.0 mM Mg2+) containing 5 μM Etd (Control), and dye uptake of 20 individual cells was recorded every 20 s as fluorescence emission of Etd binding to nucleic acids (518 nm AU of intensity) and was plotted against time expressed in minutes (min). Average dye uptake rate of parental and Cx43-transfected HeLa cells (HeLa-parental and HeLa-Cx43, respectively) in the presence or absence of extracellular divalent cations (Ca2+/Mg2+) in the recording solution and after addition of 200 μM La3+. Each value represents the average ± SEM of several experiments including 20 cells, and the number of independent experiments is indicated in each bar, ***p < 0.001.
Because FGF-1 requires the presence of heparan sulfate proteoglycans to interact with its receptor (Itoh and Ornitz, 2004), a heparin-containing solution was used to dilute and deliver FGF-1 to cells. In the presence of normal extracellular Ca2+/Mg2+ 10 IU/ml heparin (Figure 2A) did not affect the Etd uptake rate in HeLa-Cx43 or -parental and the basal Etd uptake rate was comparable to that of HeLa-Cx43 under control conditions (Figure 1). Addition of 200 μM La3+ did not affect the dye uptake rate both in parental and Cx43-transfected HeLa cells treated with heparin (Figure 2A). Nonetheless, FGF-1 conjugated to heparin (termed from now and on as FGF-1) increased the Etd uptake rate in Hela-Cx43 but not in parental cells, and the increase was prominently inhibited with La3+ (Figure 2A). Thus, the FGF-1–induced cell permeabilization depends on Cx43 expression.
Figure 2.
FGF-1 increases the membrane permeability of HeLa-Cx43 cells in a concentration- and time-dependent manner. (A) Dye uptake rates of parental and HeLa-Cx43 cells treated for 7 h with heparin alone (vehicle) or FGF-1 and after addition of 200 μM La3+. Each value represents the average ± SEM of several independent experiments including 20 cells. The number of experiments is indicated in each bar. (B) HeLa-Cx43 cells were incubated for different time periods (1–24 h) in serum-free medium containing FGF-1 (FGF-1, ■) or heparin alone (H, □) and Etd (5 μM) uptake rate was measured in the presence of normal concentrations of divalent cations. Each bar represents average ± SEM of three experiments including 20 cells/each. (C) HeLa-Cx43 cells were incubated for 7 h with different FGF-1 concentrations (1–100 ng/ml), and the Etd uptake rate was measured. Each bar represents average ± SEM of three independent experiments including 20 cells/each. *p < 0.05.
The FGF-1–induced increase in the Etd uptake rate of HeLa-Cx43 was time- (Figure 2B) and concentration-dependent (Figure 2C). In cells incubated with 20 ng/ml FGF-1, the effect was evident between 4 and 14 h, being maximal at 7 h (Figure 2B). No significant differences with control cells were found in <4 h or at 24 h of FGF-1 incubation, indicating that the effect is transient. In cells treated for 7 h with different FGF-1 concentrations (1–100 ng/ml), changes in dye uptake rate resulted in a bell-shaped concentration–response curve (Figure 2C). After 7 h of treatment with 10 or 20 ng/ml FGF-1, HeLa-Cx43 showed a significant increase in Etd uptake rate (Figure 2C), whereas higher concentrations (50 or 100 ng/ml) were ineffective (p > 0.05). The decline of the concentration-response curve may be due to a ligand-induced desensitization as described for other tyrosine kinase dimeric receptors (Matveev and Smart, 2002), and the transitory effect of FGF-1 (4–14 h) might be related to the half-life of FGF-1 conjugated with heparin in culture (Zakrzewska et al., 2005) as well as to receptor down-regulation (Zhan et al., 1993).
Permeabilization of spinal astrocytes induced by FGF-1 has been communicated (Garré et al., 2006) and might be related to the above results.
The FGF-1–induced Increase in Etd Uptake Is Sensitive to HC Blockers
To further identify the pathway through which FGF-1 increases the membrane permeability, a pharmacological criterion was applied. The rise in Etd uptake rate observed 7 h after FGF-1 application was significantly inhibited with 200 μM La3+ (Figure 3, A and B) or 50 μM 18-β-glycyrrhetinic acid (Figure 3B). Both HC blockers rapidly reduced the Etd uptake rate (<40 s) to a level similar to that of control HeLa-Cx43 (Figure 3B). On the contrary, the addition of La3+ to HeLa-Cx43 treated for 7 h with PBS or heparin did not significantly affect the Etd uptake rate (Figure 3, A and B).
Figure 3.
The Etd uptake induced by FGF-1 in HeLa-Cx43 cells is sensitive to Cx HC blockers. (A) Time-lapse experiment of Etd uptake in HeLa-Cx43 cells incubated for 7 h with heparin (○) or 20 ng/ml FGF-1 (●). Each value expresses the average ± SEM of the fluorescence intensity in 20 cells. (B) Etd uptake rates of HeLa-Cx43 cells incubated for 7 h with 20 ng/ml FGF-1 and treated after 7 min recording with La3+ (200 μM), 18-β-glycyrrhetinic acid (18β-GA, 50 μM), oxidized ATP (oATP, 150 μM), or capsazepine (Czp, 10 μM). The dotted line denotes the control Etd uptake level. Each bar represents average ± SEM of several experiments including 20 cells. *p < 0.05. The number of independent experiments is indicated in each bar.
The Increase in Membrane Permeability to Etd Induced by FGF-1 Is Not Mediated by P2X7 Receptors, TRPV1 Channels, or Pannexin1 HCs
Because other membrane channels, such as P2X7 purinergic receptors (Cario-Toumaniantz et al., 1998) and the vainilloid transient receptor potential channel 1 (TRPV1; Meyers et al., 2003), can mediate the transmembrane passage of small molecules such as Etd, it was important to study their possible involvement in the FGF-1–induced membrane permeability response. Thus, we studied the effect of oATP, a P2X receptor antagonist (North, 2002) or Czp, a TRPV1 inhibitor (Karai et al., 2004), on the Etd uptake rate of HeLa-Cx43 treated for 7 h with 20 ng/ml FGF-1. Neither oATP (Figure 3B) nor Czp (Figure 3B) significantly affected the Etd uptake rate in FGF-1–treated HeLa-Cx43.
Because pannexin1 (Px1) HCs are permeable to Etd (Thompson et al., 2006; Pelegrin and Surprenant, 2006), we searched for the presence of Px1 in HeLa cells. In contrast to a recent report that Px1 is not present in untransfected HeLa cells (Huang et al., 2007), Px1 was detected in both HeLa-parental and -Cx43 either by immunofluorescence and Western blot analysis (Supplemental Material). Nevertheless, the possible involvement of functional Px1 HCs was unlikely because the FGF-1–induced permeabilization response was Cx expression-dependent and was La3+ sensitive (Figure 3B), a feature not shared by Px1 HCs (Pelegrin and Surprenant, 2006). To further rule out the involvement of functional Px HCs in the FGF-1–induced cell permeabilization, we used whole cell patch clamp recording to search for characteristic unitary current events (see below).
The Etd Uptake Correlates with Cx43 Expression in FGF-1–treated HeLa-Cx43EGFP
To further show that the Etd uptake induced by FGF-1 depends on Cx43 expression, we performed dye uptake experiments in HeLa-Cx43EGFP, previously shown to form functional HCs with properties similar to those composed of wild-type Cx43 (Contreras et al., 2003). In these cells, a direct correlation between levels of Cx43EGFP protein (green fluorescent intensity) versus dye uptake (Etd) has been demonstrated (Contreras et al., 2003; Retamal et al., 2007a). After 7-h treatment with 20 ng/ml FGF-1 the r2 was 0.84, suggesting that the FGF-1–induced dye uptake increase might be limited by the levels of Cx expression (Figure 4).
Figure 4.
The Etd uptake correlates with Cx43 expression in FGF-1–treated HeLa-Cx43EGFP. (A) Photomicrography showing the Etd (red) and Cx43EGFP (green) fluorescence in HeLa-Cx43EGFP. Numbers 1–4 denote different cells measured independently (bar, 20 μM). (B) Time-lapse measurements showing the Etd uptake rates of independent HeLa-Cx43EGFP cells illustrated in A with different Cx43EGFP intensity levels incubated for 7 h with 20 ng/ml FGF-1. Each bar represents average ± SEM of 20 cells. (C) Lineal regression of the Etd and Cx43EGFP fluorescence intensity of HeLa-Cx43EGFP treated for 7 h with 20 ng/ml FGF-1. Fluorescence intensity values were acquired at 17 min of recording; r2 = 0.84, p < 0.001, n = 21 cells.
FGF-1 Increases the Etd Uptake and HC Unitary Current Events in HeLa-Cx43EGFP
To demonstrate the identity of the membrane pathway through which FGF-1 increases the membrane permeability of HeLa transfectants, we used the whole cell voltage-clamp technique.
In cells expressing similar Cx43EGFP fluorescence intensity levels, rectangular voltage pulses from 0 mV to +85 mV (5-s duration) evoked unitary current events that were more numerous and presented a much shorter (about ninefold) “on time” (latency) in FGF-1–treated than in control cells (Figure 5A, upward arrow heads, and B, left graph). Then, the membrane was brought to −20 mV, evoking a tail current with unitary events with longer (about threefold) “off time” (proportional to the open time) in FGF-1–treated than control cells (Figure 5A, downward arrow heads, and 5B, left graph). In both control (Contreras et al., 2003) and FGF-1–treated cells (Figure 5A, bottom trace) the application of La3+ completely abrogated the total current recorded at +85 mV as well as the tail current evoked by the transition from +85 to −20 mV. Moreover, voltage ramps from −110 mV to +110 mV evoked brief transitions likely to be unitary events at negative potentials and large increases in current with numerous discrete transitions at positive voltages (Figure 5C). At V = 0 mV the current value was zero (Figure 5C), characteristic of a nonselective membrane channel such as Cx or Px HCs (Contreras et al., 2003; Bao et al., 2004a).
Figure 5.
FGF-1 does not affect the single channel conductance but increases the total unitary current events and favors the open state of Cx43EGFP HCs. Unitary events were recorded by voltage clamp using the whole cell configuration in HeLa cells transfected with Cx43EGFP. (A) A step from 0 mV to +85 mV elicited multiple events in control cells (top current record) and markedly increased after 7-h treatment with FGF-1 (middle current trace). The application of 200 μM La3+ before the step from 0 mV to +85 mV blocked completely the unitary events in FGF-1–treated cells (bottom current trace, FGF-1/La3+). A tail current with evident unitary events was elicited in control and FGF-1–treated cells when the holding potential was rapidly changed from +85 mV to −20 mV upward (▴) and downward (▾) arrowheads indicate the “on time” and “off time” of unitary events at +85 and −20 mV, respectively. (B) Total current recorded at +85 mV was significantly higher in FGF-1–treated than in control cells. The unitary current “on time” (▴) or latency was much longer in control cells than in FGF-1–treated cells. On the contrary, the unitary current “off time” (▾) was much shorter in control than in FGF-1–treated cells. The number of independent experiments is indicated in each bar. (C) Voltage ramp from −120 to +120 mV, 8 s in duration, was applied. The current value was zero at V = 0 mV. (D) Unitary currents events recorded during the tail current elicited by rapid transition from +85 mV to −20 mV in control and FGF-1–treated cells. Dotted lines indicate transitions between open and close state of HCs.
The tail current generated with a step from + 85 mV to −20 mV presented unitary events with similar single-channel conductances (Figure 5D). Amplification of the tail current traces revealed transitions corresponding to unitary conductances of ∼220 pS or multiples (Figure 5D), previously demonstrated as Cx43 HCs (Contreras et al., 2003) and different from Px1 HCs (475–550 pS; Bao et al., 2004a). The conductance values of the unitary current events recorded in FGF-1–treated cells did not differ from those measured in control cells (Figure 5D). In 10 independent experiments only unitary events of ∼220 pS or multiples were recorded both at negative and positive potentials. Similar values were recorded in control or FGF-1–treated HeLa-Cx43 (Figure 5D), indicating that Cx43 HCs were the only pathway for Etd uptake.
The effect of FGF-1 on gap junctions expressed in HeLa transfectants was unknown. Hence, we tested its effect on the intercellular diffusion of LY in HeLa-Cx43. Dye transfer occurred in ∼100% of the LY-injected HeLa-Cx43 and was completely and rapidly (<5 min) abolished with 1 mM octanol and absent in HeLa-parental (Supplemental Material). Treatment with 20 ng/ml FGF-1 for 7 h significantly reduced the incidence (41 ± 15% reduction of control, p < 0.01, n = 5, 50 cells) and index of dye coupling (57 ± 19% reduction of control, p < 0.01, n = 5, 50 cells; Supplemental Material). Therefore, Cx43 HCs and gap junction channels expressed in HeLa cells are inversely affected by FGF-1. The effect of FGF-1 on intercellular communication might be cell type- and/or Cx type–dependent because it reduces dye coupling between rat Schwann cells (Reimers et al., 2000) known to express Cxs 29 and 32 (Nagy et al., 2004) and in HeLa-Cx43 as shown herein, but increases it in chicken DCDML lens cells expressing Cxs 43, 46, and 50 (Le and Musil, 2001).
The opposite effects of FGF-1 on dye uptake and intercellular dye transfer might be a consequence of differences in type and levels and/or activity of regulatory molecules present at the intracellular compartment of each membrane channel. Alternatively, different spatial conformation of Cx forming HCs and gap junction channels might influence their interactions with specific cytoplasm regulatory molecules. Similar mechanisms might be involved in the opposite regulation of HCs and gap junction channels by increases in [Ca2+]i and the effects of proinflammatory mediators and metabolic inhibition (Contreras et al., 2002; Peracchia, 2004; De Vuyst et al., 2006, 2007; Retamal et al., 2007c). But, the above is not common to all Cx channel regulatory mechanisms, ,since the activity of both channel types is reduced by PKC- or MAP kinase–dependent phosphorylation (Kim et al., 1999; Bao et al., 2004b; Warn-Cramer and Lau, 2004).
The Effect of FGF-1 on Etd Uptake Is Not Restricted to Cx43 HCs and Requires a Regulatory Site Located in the Cx C-Terminal Tail
The carboxy terminus is the most variable domain of Cxs in terms of length and primary sequence and is predicted to be involved in regulatory mechanisms of Cx-based channels (Sáez et al., 2003). Cx43 presents several phosphorylation sites believed to be important in controlling diverse events including channel assembly, degradation, insertion in the plasma membrane, interaction with scaffolding proteins, and gating (Sáez et al., 2003; Solan and Lampe, 2005). To elucidate the possible involvement of Cx43 carboxy terminus in the FGF-1–induced cellular permeabilization, the effect of FGF-1 on the Etd uptake rate of HeLa cells transfected with Cx43 truncated at aa 257 (HeLa-Cx43Δ257) was studied. Either FGF-1 or heparin alone did not increase the Etd uptake rate at 7-h treatment (Figure 6A). Dye uptake in HeLa-Cx43Δ257 under all conditions, including control, was slightly reduced after the addition of La3+, suggesting the presence of few functional HCs (Figure 6A).
Figure 6.
FGF-1 increases the Etd uptake in a Cx-dependent manner, and the lack of response is not due to deficient FGF-1 sensitivity. (A–C) Etd uptake rate after incubation for 7 h with PBS, heparin alone, or FGF-1 in HeLa-Cx43Δ257 (A), HeLa-Cx26 (B), and HeLa-Cx45 (C) cells. Each bar represents average ± SEM of the number of experiments indicated in each bar; 20 cells were recorded in each experiment. Insets in A and B show increased Etd uptake in HeLa-Cx43Δ257 and -Cx26, respectively, after exposure to a medium without Ca2+ and Mg2 (Ca2+/Mg2+-free) that was sensitive to La3. Each value expresses the average ± SEM of 20 cells, m = average slope, *p < 0.05. The number of independent experiments is indicated in each bar plotted in A–C. (D) Representative immunoblot showing levels of phosphorylated ERK (pErk) in parental cells or HeLa cells transfected with Cxs 43, 43Δ257, 26, or 45 in control condition and after incubation for 30 min or 7 h with FGF-1 (FGF-1). The bands correspond to the 42- and 44-kDa isoforms of pERK as indicated in the right.
Cx43 and Cx45 present a long carboxy terminus (155 and 150 aa, respectively) as compared with that of Cx26, which is only 18 aa long (Sáez et al., 2003). HeLa-Cx45 but not HeLa-Cx26 treated with FGF-1 for 7 h showed a significant increase in Etd uptake rate (Figure 6, C and B, respectively). The effect of FGF-1 on HeLa-Cx45 was inhibited with 200 μM La3+ (Figure 6C) or 50 μM 18β-GA (p < 0.05, n = 3, 60 cells), but not with 300 μM oATP or 10 μM Czp (p > 0.05, n = 3, 60 cells, respectively; not shown). Thus, the FGF-1–induced membrane permeabilization is not restricted to cells expressing Cx43 HCs and requires a regulatory site located in the C-terminus of Cx43 and 45 but absent in Cx26.
To rule out that the lack of FGF-1 effect on HeLa-Cx26 and -Cx43Δ257 was due to the absence of surface HCs, cells were exposed to a divalent cation–free solution to induce HC opening (Evans et al., 2006). The exposure to a Ca2+/Mg2+-free solution rapidly (<1 min) increased the Etd uptake in cells transfected with either Cx43Δ257 or Cx26, and the response was sensitive to La3+ (insets in Figure 6, A and B, respectively), indicating that both transfectants presented HCs at their surfaces.
The FGF-1 Insensitivity of HeLa-Cx43Δ257 and -Cx26 Is Not Due to Lack of Receptor-dependent Responses
The retroviral delivery of Cx43 or Cx26 reduces the expression of the FGFR3 in human breast cancer cells (Qin et al., 2002), and this receptor is expressed in HeLa cells and transduces FGF-1–evoked responses (Scotet and Houssaint, 1995; Itoh and Ornitz, 2004). Thus, the possibility that differences in dye uptake rate responses to FGF-1 observed in HeLa cells transfected with a Cx type carrying a long or a short C-terminus were due to changes in FGF-1 responsiveness required to be discarded. To this end, HeLa-parental or transfected with Cxs 26, 43, 43Δ257, and 45 were treated for 30 min or 7 h with either FGF-1 or heparin, and levels of phosphorylated ERK (pERK), a known downstream target of the activated FGFRs (Klint and Claesson-Welsh, 1999), were measured. At 30 min, levels of pERK were increased to a similar extent in all HeLa transfectants and at 7 h had returned to those of control cells (Figure 6D). After treatment with heparin for 30 min or 7 h the relative pERK levels were as in control cells (not shown). Hence, the lack of cellular permeabilization through HCs induced by FGF-1 in Cx26 or Cx43Δ257 transfectants was not due to altered FGF-1 responsiveness.
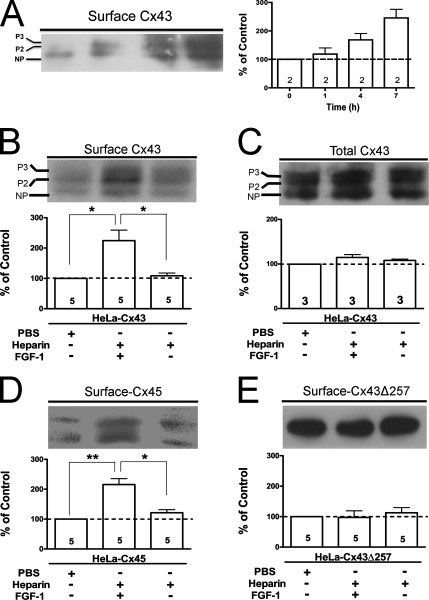
FGF-1 Increases the Cell Surface Levels of Cxs 43 and 45
The half-life of Cx43 ranges from 1.3–3.5 h (Sáez et al., 2003), suggesting that changes in membrane permeability mediated by HCs might result from changes in Cx levels. An increase in surface Cx43 occurs in kidney cells under cytosolic stress (VanSlyke and Musil, 2005) and in cortical astrocytes under metabolic inhibition (Retamal et al., 2006). In the latter the surface Cx43 levels are directly related to the increase in Etd uptake. In HeLa-Cx43 incubation with FGF-1 for different time periods during the development of the maximal dye uptake response (0–7 h) induced a progressive increase in surface Cx43 levels (Figure 7A). At 7-h treatment FGF-1 but not heparin significantly increased the surface Cx43 levels (Figure 7B). Since the relatively long latency of the rise in dye uptake rate might be due to increased synthesis or reduced degradation of Cx, total Cx43 levels were measured. We found no significant increase (∼10%, p > 0.05) in total Cx43 levels (Figure 7C), suggesting the involvement of a different cellular mechanism. Surface and total Cx43 phosphorylation state, evidenced by the electrophoretic mobility changes, were not altered by FGF-1 treatment (Figure 7, B and C).
Figure 7.
FGF-1 increases the levels of surface HCs in HeLa cells expressing Cx43 or Cx45, but not Cx43 with a truncated C-terminus (Cx43Δ257). (A) Left, representative immunoblot showing the relative levels of surface Cx43 in HeLa-Cx43 treated for 1–7 h with 20 ng/ml FGF-1. (A) Right, normalized densitometric values of two independent experiments as shown in the left. The band intensity is represented as relative value to the surface Cx43 levels detected under control condition taken as 100%. (B–E) Top panel, representative immunoblots showing the relative levels of biotinylated Cx43 (B), Cx45 (D), Cx43Δ257 (E), or total Cx43 (C) in cells treated for 7 h with PBS, 20 ng/ml FGF-1, or heparin. (B–E) Bottom panel, normalized densitometric values; the band intensity detected in the surface protein fraction relative to surface Cx43 levels under control condition taken as 100%. The graph illustrates levels of surface Cx43 (B), Cx45 (D), Cx43Δ257 (E), or total Cx43 (C) in cells treated for 7 h with PBS, FGF-1, or heparin alone. Each bar represents mean ± SEM. The digit within each bar expresses the number of independent experiments. **p < 0.01, *p < 0.05. NP and P2-P3 denote the unphosphorylated and phosphorylated forms of Cx43, respectively.
Surface Cx45 levels were also significantly increased by FGF-1 in HeLa-Cx45 transfectants (Figure 7D). Nevertheless, surface levels of truncated Cx43 were not affected by FGF-1 or heparin (Figure 7E) in HeLa-Cx43Δ257 transfectants. Surface detection of Cx43 and Cx43Δ257 with an antibody directed to a common epitope in the N-terminal domain showed no prominent differences in the basal surface Cx levels between the two transfectants (Supplemental Material), indicating that both cell types express comparable HCs levels. Therefore, the FGF-1–induced rise in Etd uptake occurs with a simultaneous increase in levels of surface HCs constituted of Cx types bearing a long carboxyl terminal tail. Biotinylation of surface proteins in HeLa-Cx26 (n = 3) did not yield reproducible results due to variable formation of aggregates of different sizes.
Because activation of FGFRs is followed by a sustained elevation of the [Ca2+]i in different cell types, including Cx43-expressing cells (Munaron, 2002) and elevated [Ca2+]i might affect the distribution of Cxs located in intracellular compartments, we then evaluated the effect of FGF-1 on the [Ca2+]i.
FGF-1 Induces a Late Increase in the [Ca2+]i, Which Depends on HC Cx Composition
FGFs are known to induce rapid (from seconds to minutes) elevation of the [Ca2+]i through intracellular InsP3-activated receptors and membrane Ca2+ channels (Munaron, 2002). Nevertheless, levels of [Ca2+]i at time periods in which the membrane permeability was increased by FGF-1 were unknown. Thus, we decided to study if changes in the FGF-1–induced increase in Etd uptake were associated with changes in [Ca2+]i, HeLa-Cx43 were loaded with Fura-2AM and the 340/380-nm emission intensity ratio was measured every hour after treatment with the growth factor. The emission intensity ratio of FGF-1–treated cells was elevated 1 h after FGF-1 addition, but decayed to a value similar to that of control cells at 2 h (Figure 8A). After 4 h of FGF-1 incubation, the Fura-2 signal increased progressively, indicating that FGF-1 induced a rise in [Ca2+]i over time (Figure 8A). After 7-h treatment with FGF-1 the 340/380-nm emission ratio of FGF-1–treated cells was close to twice that of control cells (dotted line in Figure 8, A and B). FGF-1 did not affect the [Ca2+]i in HeLa-Cx43 preloaded with 5 μM BAPTA-AM (Figure 8B).
Figure 8.
FGF-1 increases the free intracellular Ca2+ only in HeLa cells expressing Cx43 or Cx45, and BAPTA-AM prevents the FGF-1–induced Etd uptake. (A) Fura-2 fluorescence intensity ratio at 340/380 nm in HeLa-Cx43 cells incubated for 1–7 h with 20 ng/ml FGF-1. Dotted line indicates the average value of control cells. (B) Fura-2 intensity levels in HeLa-Cx43 treated for 7 h with heparin or FGF-1 or incubated for 45 min with BAPTA-AM (5 μM) and then treated with FGF-1. Each bar represents mean ± SEM of Fura-2 fluorescence intensity ratio at 340/380 nm in several experiments including measurements of 20 cells. (C) Etd uptake rates of HeLa-Cx43 and HeLa-Cx45 treated for 7 h with heparin, FGF-1, or incubated for 45 min with BAPTA-AM and treated with FGF-1 with or without addition of La3+. Each bar represents average Etd uptake rates ± SEM. (D) Fura-2 fluorescence intensity of parental or HeLa cells transfected with Cx45, 43Δ257, or 26 treated for 7 h with heparin or FGF-1. Each bar represents mean ± SEM of the fluorescence intensity ratio of Fura-2 at 340/380 nm. *p < 0.05, **p < 0.01, ***p < 0.001. In A–D the number of independent experiments are indicated within each bar.
The Etd uptake rate increase of HeLa-Cx43 (cf. Figures 2A, 3B, and 8C) or HeLa-Cx45 (cf. Figures 6C and 8C) unloaded or loaded with Fura-2 and treated with FGF-1 was similar, but it was almost completely abrogated with BAPTA-AM (Figure 8C). Under the latter condition, La3+ did not further affect the Etd uptake rate in both HeLa-Cx43 and -Cx45 (Figure 8C). Thus, the rise in [Ca2+]i and amino acid residues located in the C-terminus of Cxs 43 and 45 are essential for the FGF-1–induced membrane permeability increase mediated by HCs.
To elucidate whether the effect of FGF-1 on the late [Ca2+]i in HeLa transfectants was Cx specific, the [Ca2+]i was evaluated in the other HeLa transfectants. A significant increase in [Ca2+]i occurred 7 h after FGF-1 application in HeLa-Cx45 (Figure 8D), although HeLa-Cx43Δ257 were unaffected and HeLa-Cx26 showed a small reduction in [Ca2+]i after FGF-1 stimulation (Figure 8D).
The Ca2+ Ionophore 4-Br-A23187 Mimics the FGF-1 Effects Only on HeLa-Cx43 and -Cx45
Rises in [Ca2+]i are known to enhance the Cx32 HC activity evaluated through the release of ATP (De Vuyst et al., 2006). To determine if Cx43 HCs are affected in a similar way, we studied the effect of a Ca2+ ionophore on the membrane permeability to Etd. The application of 2.5 μM 4-Br-A23187 induced a progressive and similar rise in [Ca2+]i in parental and Cx-transfected HeLa cells (Figure 9A). The 4-Br-A23187 application prominently increased the La3+-sensitive dye uptake rate in HeLa-Cx43 (Figure 9B) and HeLa-Cx45 (Figure 9B). Nevertheless, the Etd uptake rate was not affected by 4-Br-A23187 and remained insensitive to La3+ in HeLa-parental, Cx43Δ257 and -Cx26 (Figure 9B).
Figure 9.
The Ca2+ ionophore 4-Br-A23187 increases the Etd uptake rate and levels of surface HCs only in HeLa-Cx43 and HeLa-Cx45 cells. (A) Time-lapse experiment showing the Fura-2 intensity ratio of fluorescence emission at 340/380 nm of parental HeLa cells (○), HeLa-Cx43 (●), HeLa-Cx43Δ257 (gray triangles), HeLa-Cx26 (□) or HeLa-Cx45 (♦) cells under control conditions (0–10 min) or after addition of 2.5 μM 4-Br-A23187 (10–70 min). Each point represents the average ± SEM of 20 cells, n = 3. (B) Etd uptake rates of parental, HeLa-Cx43, HeLa-Cx43Δ257, HeLa-26, or HeLa-Cx45 cells in control conditions or (C) or treated for 30–60 min with 4-Br-A23187 with or without acute addition of La3+ at the end of the experiment. Each bar represents average ± SEM, and 20 cells were recorded in each experiment. (C) Top panels, immunoblots showing levels of biotinylated Cx43, Cx45, or Cx43Δ257 under control conditions and after treatment with 4-Br-A23187 (2.5 μM, 40 min). The graph represents normalized densitometric values of the band intensity of biotinylated proteins normalized to levels of surface Cx43, Cx45, or Cx43Δ257 in cells under control condition. (D) Top panel, immunoblot showing levels of biotinylated Cx43, in HeLa-Cx43 treated for 7 h with heparin or FGF-1 or preincubated with BAPTA (5 μM, 45 min) and then treated for 7 h with FGF-1. The graph shows normalized densitometric values as in C. Each bar represents the mean ± SEM. In B–D the digit within each bar denotes the number of independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Treatment with 4-Br-A23187 for 40 min also increased the Cx43 and Cx45 but not Cx43Δ257 HC levels (Figure 9C). The increase in surface levels of Cxs occurred without changes in phosphorylation state detectable as electrophoretic mobility shifts of either Cx43 or Cx45. Consequently, the 4-Br-A23187 induced rise in [Ca2+]i and cellular permeabilization through Cx43 or Cx45 HCs are directly related to increased levels of surface HCs. In addition, incubation of HeLa-Cx43 with BAPTA fully prevented the increase in surface Cx43 levels induced by FGF-1 (Figure 9D), indicating that FGF-1 acts through increased [Ca2+]i to augment the HC levels in responsive cells.
Activation of a p38 MAP Kinase–dependent Pathway Mediates the FGF-1 and Ca2+ Ionophore–induced Cell Permeabilization
Rises in [Ca2+]i through different sources are known to activate p38 MAP kinase in diverse cell types (Sakai et al., 2002; Hsu et al., 2007). Moreover, treatment with 4-Br-A23187 activates p38 MAP kinase (Tokuda et al., 2000), which has been recently shown to be involved in cell permeabilization mediated by Cx43 HCs (Retamal et al., 2007c). Therefore, the effect of SB202190, a p38 MAP kinase inhibitor (Lee et al., 1994), on the FGF-1– or Ca2+ ionophore–induced cell permeabilization responses were studied. Incubation of HeLa-Cx43 with 10 μM SB202190 for 30 min prominently reduced the induced increase in Etd uptake rate (Supplemental Material), indicating that p38 MAP kinase pathway activation is required for cellular permeabilization mediated by Cx43 HCs in cells treated either with FGF-1 or Ca2+ ionophore.
The Expression of Cx26 and Cx43 Differentially Affect the Cell Density in Response to Serum-Free Medium or FGF-1
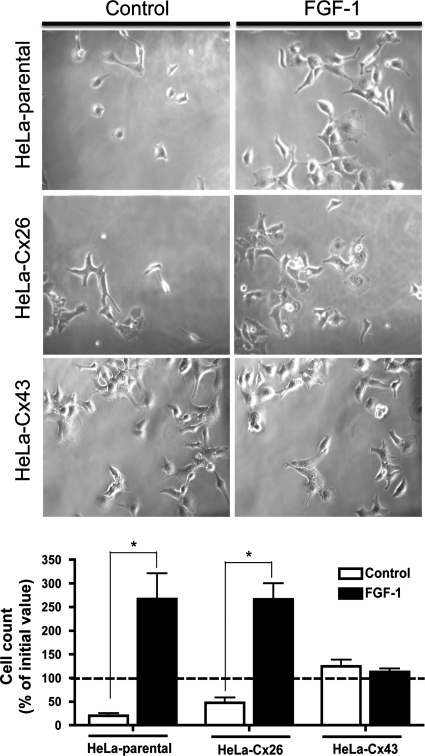
To study whether the expression of different Cxs affects the cell density, subconfluent parental, Cx26- or Cx43-transfected HeLa cells were cultured in serum-free medium for 96 h or in serum-free medium for 48 h and then treated with FGF-1 or heparin in a serum-free medium for additional 48 h. After each treatment, cells were counted, and the number was normalized against the initial value. Parental and Cx26-transfected HeLa cells cultured in serum-free medium with or without heparin showed a reduction in cell density (Figure 10). In contrast, both cell types treated with FGF-1 showed a prominent increase in cell density (Figure 10). However, both conditions did not affect the cell density of HeLa-Cx43 (Figure 10).
Figure 10.
The expression of Cx26 and Cx43 differentially affect the cell density in response to serum-free medium or FGF-1. Low density (2 × 104 cell/well) parental, Cx26-, or Cx43-transfected HeLa cells were cultured for 48 h in serum-free medium and then treated either with FGF-1 or heparin (control) for additional 48 h. Top panels are representative fields of cell cultures under the conditions indicated in the figure. The graph shows the cell count as percentage of the initial value evaluated at the end of the experiment. Each value corresponds to the mean ± SEM (n = 3). *p < 0.05.
DISCUSSION
Here, we show that FGF-1 increases in membrane permeability of HeLa cells through HCs composed of specific Cx types in the presence of physiological extracellular Ca2+/Mg2+ concentrations, because 1) the effect occurs in cells transfected with Cxs 43 or 45, but not in cells transfected with Cxs 26 or 43Δ257; 2) the increase in membrane permeability is sensitive to HC blockers but not to inhibitors of other possible Etd uptake pathways; 3) the increase in Etd uptake correlates directly with elevated activity of HC unitary current events and increased levels of Cxs at the cell surface; and 4) the rise in membrane permeability is also associated with a late increase in [Ca2+]i, in a Cx type–dependent manner.
The involvement of Cx HCs as the only pathway mediating the Etd uptake in response to FGF-1 in our model was supported by the lack of effect of blockers of other Etd uptake pathways. In addition, the FGF-1–induced permeabilization response was rapidly and completely inhibited by two Cx HC blockers, La3+ and 18β-GA. Moreover, HeLa-Cx43EGFP presented a single type of unitary current events corresponding to Cx HCs that was also completely inhibited by La3+ as described previously (Contreras et al., 2003).
The low Etd uptake rate and insensitivity to HC blockers of HeLa-Cx43 under resting conditions can be explained by the low HC open probability described previously (Contreras et al., 2003). A similar explanation might also apply to the other studied HeLa transfectants. On the contrary, a Cx-dependent increase in membrane permeability occurred in HeLa cells treated for several hours with FGF-1. These findings are different from the effect of FGF-2 on HC-mediated ATP release to the extracellular medium (De Vuyst et al., 2007). Although the effect of FGF-2 on the ATP release via Cx43 and Cx26 or Cx43 with a truncated C-terminus is opposite (De Vuyst et al., 2007), FGF-1 enhanced the membrane permeability in Cx43 and Cx45 transfectants but was without effect on Cx26 or Cx43 with a truncated C-terminus. Differences in FGF-1– and -2–induced effects on HC-dependent responses might be explained by differences in FGFRs and downstream pathways (Dailey et al., 2005).
The FGF-1–induced cellular permeabilization through Cx43 HCs can be fully explained by the rise in surface HC levels, because both the average dye uptake and levels of surface HCs increased about twofold. This observation suggests that HCs of FGF-1–treated cells presented permeability properties comparable to those of control HeLa-Cx43. In support of this interpretation, the HC unitary conductance of control and FGF-1–treated cells was the same. Consistent with the increase in HC levels, a total current increase of about threefold was observed, which was also reflected in higher open probability (shorter “on time”) and the longer open time (longer “off time”) of HCs recorded in FGF-1–treated cells. This increase in HC activity explains the FGF-1–induced cellular permeabilization. The increase Cx43 HC levels occurred without changes in total Cx43 protein, suggesting redistribution of HCs rather than changes in Cx43 turnover as possible mechanism associated with the increase in dye uptake response.
Because an approximately twofold increase of both dye uptake rate and surface HC levels also occurred in FGF-1–treated HeLa-Cx45, the above mechanisms might also explain the permeabilization response in these cells. In HeLa-Cx43 and -Cx45 the permeabilization response was related to regulatory sequences located in their C-terminal domain, as indicated by the lack of response to FGF-1 in HeLa cells transfected with Cx types carrying a short C-terminal tail, Cx26 and Cx43Δ257. The lack of response of these two transfectants was not due to the absence of HCs at the surface because similar levels of Cx43 and Cx43Δ257 were detected at the cells surface, and HeLa-Cx26 and -Cx43Δ257 showed a rapid increase in membrane permeability when exposed to a Ca2+/Mg2+-free solution. Moreover, the absence of FGF-1–induced responses of HeLa-Cx26 and -Cx43Δ257 was unrelated to a deficient FGF-1 transduction because the 42/44 kDa MAP kinases were phosphorylated to a similar level as that in FGF-1–sensitive HeLa transfectants. Although several other cytoplasmic proteins (e.g., scaffolding proteins and calmodulin) interact with the carboxy terminus of Cxs 43 and 45 (Singh and Lampe, 2003; Laing et al., 2005), it is unknown whether they interact with Cx HCs. Therefore, the FGF-1–induced cellular permeabilization was Cx specific and requires the interaction with cytoplasmic regulatory sites in the Cx C-terminal domain.
A rise in [Ca2+]i was required for the cellular permeabilization, and the HC levels increase induced by FGF-1. In support to this notion, the intracellular Ca2+ chelator abrogated both responses and they were mimicked with the Ca2+ ionophore. Moreover, the Ca2+ ionophore increased the Etd uptake rate only on HeLa transfected with Cxs 43 or 45, demonstrating a direct relationship between the Cx composition of HCs and the increase in [Ca2+]i. The Ca2+ ionophore effect is most likely related to the early rise in [Ca2+]i after activation of FGFRs in different cell types (Munaron, 2002) and might be linked to the high level of Fura-2 signal detected 1 h after FGF-1 treatment. The involvement of intracellular regulatory mechanism in the FGF-1–elicited response on HeLa-Cx43 is supported by the prominent reduction in dye uptake rate increase induced with the p38 MAP kinase inhibitor. Moreover, the reduction in Ca2+ ionophore–induced dye uptake with the p38 MAP kinase inhibitor suggests that activation of this kinase is upstream the rise in [Ca2+]i.
The stimulation of HeLa-Cx43 or -Cx45 with 4-Br-A23187 for only 30 min induced a faster and more pronounced rise in Etd uptake and levels of surface Cx than FGF-1. Both parameters also maintained a close association (Figure 9, B and C), suggesting the involvement of the same mechanism underlying the cellular responses induced by FGF-1 and the Ca2+ ionophore. These findings also indicate that the cellular permeabilization response can be elicited with a much faster time course than that induced by FGF-1. The response is possibly limited by the kinetic of the rise in [Ca2+]i, and mechanisms engaged in enhancing the surface levels of Cx including interactions of cytoplasmic molecules and specific regulatory sites located in the Cx C-terminus.
HCs composed of Cxs capable of sensing changes in [Ca2+]i might also serve as pathway for Ca2+ influx. This notion is consistent with the higher [Ca2+]i detected in HeLa-Cx43 and -Cx45 treated with FGF-1 for 7 h because their membranes were also more permeable to the cationic molecule Etd (+1). Controlled increase in membrane permeability through HCs as that induced by FGF-1 may not drastically alter the intracellular Ca2+ homeostasis possibly due to a proper Ca2+ buffering.
Previous studies have demonstrated that stable overexpression of different Cxs induce distinct cell phenotypes (Bradshaw et al., 1993; Koffler et al., 2000; Qin et al., 2002). In agreement, the cell density of subconfluent HeLa-Cx26 but not HeLa-Cx43 was reduced in serum-free medium. Moreover, these two transfectants responded differentially to FGF-1, indicating that the outcome of the growth factor stimulation on HeLa cells might be influenced by the Cx HC composition. Nevertheless, final demonstration of this conclusion will require rapid and selective manipulation of Cx expression and the availability of specific HC or gap junction channel blockers to attribute the results to one or the other channel type.
Supplementary Material
ACKNOWLEDGMENTS
The data of this work was presented by Dr. Kurt A. Schalper as partial fulfillment of the requirements to obtain the degree of Ph.D. in Medical Sciences at the Pontificia Universidad Católica de Chile. This work was partially supported by Fondo Nacional de Desarrollo Cientifico y Technológico Grants 1070591 (to J.C.S. and Núcleo Milenio P04/030-f to J.C.S. Dr. K. A. Schalper received a Comisión Nacional de Investigación Cientifico y Technológico, PUC fellowships for graduate studies, and Proyecto Anillo de Ciencia Y Tecnología ACT-46.
Abbreviations used:
- Cx
connexin
- Px
pannexin
- Etd
ethidium
- FGF-1
acidic fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- 18β-GA
18β-glycerrithinic acid.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-12-1240) on May 21, 2008.
REFERENCES
- Bao L., Locovei S., Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004a;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bao X., Reuss L., Altenberg G. A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004b;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- Böttcher R. T., Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Bradshaw S. L., Naus C. C, Zhu D., Kidder G. M., D'Ercole A. J., Han V. K. Alterations in the synthesis of insulin-like growth factor binding proteins and insulin-like growth factors in rat C6 glioma cells transfected with a gap junction connexin43 cDNA. Regul. Pept. 1993;48:99–112. doi: 10.1016/0167-0115(93)90339-a. [DOI] [PubMed] [Google Scholar]
- Cario-Toumaniantz C., Loirand G., Ladoux A., Pacaud P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ. Res. 1998;83:196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- Contreras J. E., Sánchez H. A., Eugenín E. A., Speidel D., Theis M., Willecke K., Bukauskas F. F., Bennett M. V., Sáez J. C. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J. E., Sáez J. C., Bukauskas F. F., Bennett M. V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvalán L. A., Araya R., Brañes M. C., Sáez P. J., Kalergis A. M., Tobar J. A., Theis M., Willecke K., Sáez J. C. Injury of skeletal muscle and specific cytokines induce the expression of gap junction channels in mouse dendritic cells. J. Cell Physiol. 2007;211:649–660. doi: 10.1002/jcp.20971. [DOI] [PubMed] [Google Scholar]
- Coumoul X., Deng C. X. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res. C. Embryo. Today. 2003;69:286–304. doi: 10.1002/bdrc.10025. [DOI] [PubMed] [Google Scholar]
- Dailey L., Ambrosetti D., Mansukhani A., Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- De Vuyst E., Decrock E., Cabooter L., Dubyak G. R., Naus C. C., Evans W. H., Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E., Decrock E., De Bock M., Yamasaki H., Naus C. C., Evans W. H., Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Frate H., Butterweck A., Traub O., Klein R. A., Hulser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., De Vuyst E., Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio Pla A., Maric D., Brazer S. C., Giacobini P., Liu X., Chang Y. H., Ambudkar I. S., Barker J. L. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garré J. M., Retamal M. A., Schalper K., Barbeito L., Bennett M. V., Cassina P., Sáez J. C., Abdura V. FGF-1 and ATP increase permeability of spinal cord astrocytes in culture via P2X receptors and connexin-based hemichannels. Society for Neuroscience 2006 Abstract Viewer Program/Poster 136.8/D26. 2006 [Google Scholar]
- González H. E., Eugenín E. A., Garcés G., Solis N., Pizarro M., Accatino L., Sáez J. C. Regulation of hepatic connexins in cholestasis: possible involvement of Kupffer cells and inflammatory mediators. Am. J. Physiol. Gastrointest Liver Physiol. 2002;282:G991–G1001. doi: 10.1152/ajpgi.00298.2001. [DOI] [PubMed] [Google Scholar]
- Hsu S. S., et al. Anandamide-induced Ca2+ elevation leading to p38 MAPK phosphorylation and subsequent cell death via apoptosis in human osteosarcoma cells. Toxicology. 2007;231:21–29. doi: 10.1016/j.tox.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Huang Y., Grinspan J. B., Abrams C. K., Scherer S. S. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- Itoh N., Ornitz D. M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Karai L. J., Russell J. T., Iadarola M. J., Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 2004;279:16377–16387. doi: 10.1074/jbc.M310891200. [DOI] [PubMed] [Google Scholar]
- Kim D. Y., Kam Y., Koo S. K., Joe C. O. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J. Biol. Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- Klint P., Claesson-Welsh L. Signal transduction by fibroblast growth factor receptors. Front. Biosci. 1999;4:D165–D177. doi: 10.2741/klint. [DOI] [PubMed] [Google Scholar]
- Koffler L., Roshong S., Kyu Park I., Cesen-Cummings K., Thompson D. C., Dwyer-Nield L. D., Rice P., Mamay C., Malkinson A. M., Ruch R. J. Growth inhibition in G(1) and altered expression of cyclin D1 and p27(kip-1) after forced connexin expression in lung and liver carcinoma cells. J. Cell Biochem. 2000;79:347–354. doi: 10.1002/1097-4644(20001201)79:3<347::aid-jcb10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Laing J. G., Koval M., Steinberg T. H. Association with ZO-1 correlates with plasma membrane partitioning in truncated connexin45 mutants. J. Membr. Biol. 2005;207:45–53. doi: 10.1007/s00232-005-0803-2. [DOI] [PubMed] [Google Scholar]
- Le A. C., Musil L. S. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Matveev S. V., Smart E. J. Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am. J. Physiol. Cell Physiol. 2002;282:935–946. doi: 10.1152/ajpcell.00349.2001. [DOI] [PubMed] [Google Scholar]
- Mergler S., Dannowski H., Bednarz J., Engelmann K., Hartmann C., Pleyer U. Calcium influx induced by activation of receptor tyrosine kinases in SV40-transfected human corneal endothelial cells. Exp. Eye Res. 2003;77:485–495. doi: 10.1016/s0014-4835(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Meyers J. R., MacDonald R. B., Duggan A., Lenzi D., Standaert D. G., Corwin J. T., Corey D. P. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munaron L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review) Int. J. Mol. Med. 2002;10:671–686. [PubMed] [Google Scholar]
- Nagy J. I., Dudek F. E., Rash J. E. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Brain Res. Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- North R. A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pelegrin P., Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Qin H., Shao Q., Curtis H., Galipeau J., Belliveau D. J., Wang T., Alaoui-Jamali M. A., Laird D. W. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002;277:29132–29138. doi: 10.1074/jbc.M200797200. [DOI] [PubMed] [Google Scholar]
- Reimers D., Prieto R., Gimenez-Gallego G., Cuevas P., Barrio L.C. Acidic fibroblast growth factor inhibits junctional communication of Schwann cells in culture. Neurol. Res. 2000;22:685–691. doi: 10.1080/01616412.2000.11740740. [DOI] [PubMed] [Google Scholar]
- Retamal M. A., Cortes C. J., Reuss L., Bennett M. V., Saez J. C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Schalper K. A., Shoji K. F., Bennett M. V., Sáez J. C. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. USA. 2007a;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal M. A., Schalper K. A., Shoji K. F., Orellana J. A., Bennett M. V., Sáez J. C. Possible involvement of different connexin43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J. Membr. Biol. 2007b;218:49–63. doi: 10.1007/s00232-007-9043-y. [DOI] [PubMed] [Google Scholar]
- Retamal M. A., Froger N., Palacios-Prado N., Ezan P., Sáez P. J., Sáez J. C., Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are oppositely regulated by pro-inflammatory cytokines released from activated microglia. J. Neurosci. 2007c;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez J. C., Berthoud V. M., Brañes M. C., Martínez A. D., Beyer E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Hashimoto H., Shintani N., Ichibori A., Tomimoto S., Tanaka K., Hirose M., Baba A. Involvement of intracellular Ca2+ elevation but not cyclic AMP in PACAP-induced p38 MAP kinase activation in PC12 cells. Regul. Pept. 2002;109:149–153. doi: 10.1016/s0167-0115(02)00198-2. [DOI] [PubMed] [Google Scholar]
- Scotet E., Houssaint E. The choice between alternative IIIb and IIIc exons of the FGFR-3 gene is not strictly tissue-specific. Biochim. Biophys. Acta. 1995;1264:238–242. doi: 10.1016/0167-4781(95)00156-b. [DOI] [PubMed] [Google Scholar]
- Singh D., Lampe P. D. Identification of connexin-43 interacting proteins. Cell Commun. Adhes. 2003;10:215–220. doi: 10.1080/cac.10.4-6.215.220. [DOI] [PubMed] [Google Scholar]
- Solan J. L., Lampe P. D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Squecco R., Sassoli C., Nuti F., Martinesi M., Chellini F., Nosi D., Zecchi-Orlandini S., Francini F., Formigli L., Meacci E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol. Biol. Cell. 2006;17:4896–4910. doi: 10.1091/mbc.E06-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Zhou N., MacVicar B. A. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Kozawa O., Uematsu T. Basic fibroblast growth factor stimulates vascular endothelial growth factor release in osteoblasts: divergent regulation by p42/p44 mitogen-activated protein kinase and p38 mitogen-activated protein kinase. J. Bone Miner Res. 2000;15:2371–2379. doi: 10.1359/jbmr.2000.15.12.2371. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Musil L. S. Cytosolic stress reduces degradation of connexin43 internalized from the cell surface and enhances gap junction formation and function. Mol. Biol. Cell. 2005;16:5247–5257. doi: 10.1091/mbc.E05-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer B. J., Lau A. F. Regulation of gap junctions by tyrosine protein kinases. Biochim. Biophys. Acta. 2004;1662:81–95. doi: 10.1016/j.bbamem.2003.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska M., Krowarsch D., Wiedlocha A., Olsnes S., Otlewski J. Highly stable mutants of human fibroblast growth factor-1 exhibit prolonged biological action. J. Mol. Biol. 2005;352:860–875. doi: 10.1016/j.jmb.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Zhan X., Hu X., Friesel R., Maciag T. Long term growth factor exposure and differential tyrosine phosphorylation are required for DNA synthesis in BALB/c 3T3 cells. J. Biol. Chem. 1993;268:9611–9620. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.