Abstract
Although regions of the parietal cortex have been consistently implicated in episodic memory retrieval, the functional roles of these regions remain poorly understood. The present review presents a meta-analysis of findings from event-related fMRI studies reporting the loci of retrieval effects associated with familiarity- and recollection-related recognition judgments. The results of this analysis support previous suggestions that retrieval-related activity in lateral parietal cortex dissociates between superior regions, where activity likely reflects the task relevance of different classes of recognition test items, and more inferior regions where retrieval-related activity appears closely linked to successful recollection. It is proposed that inferior lateral parietal cortex forms part of a neural network supporting the ‘episodic buffer’ [Baddeley, A.D. (2000). The episodic buffer: a new component of working memory? Trends in Cognitive Sciences, 4, 417–423.].
Keywords: recollection, familiarity, episodic memory, recognition memory, meta-analysis
The advent of functional neuroimaging, first with PET and subsequently with fMRI, has led to a heightened appreciation of the extent of cortical involvement in long-term memory encoding and retrieval. Among the regions identified most consistently in early studies of memory retrieval were medial and lateral posterior parietal cortex (Fletcher et al., 1997). These findings were (and remain) unexpected, given the paucity of neuropsychological evidence implicating these regions in long-term memory.
The blocked designs employed in early PET and fMRI studies present serious obstacles to the interpretation of retrieval-related neural activity. These arise from the inability to characterize item-related activity according to the study history of the item or the nature of the associated memory judgment (although see Kapur et al., 1995; Rugg et al., 1996 for attempts to overcome this problem). With the development of event-related fMRI it became possible to directly contrast the activity elicited by different classes of recognition memory test item, and it soon became evident that, as had been suspected on the basis of earlier studies (Kapur et al., 1995; Lepage et al., 2000; Rugg et al., 1998a), both lateral and medial parietal cortex demonstrate ‘retrieval success’ effects (also referred to as ‘old/new’ effects). The effects take the form of greater activity for recognized studied items than for correctly rejected new items (e.g., Henson et al., 1999; Konishi et al., 2000; see Rugg & Henson, 2002 for a review of these and other early studies).
The purpose of the present paper is to review findings from event-related fMRI studies reporting retrieval-related modulation of activity in posterior parietal cortex (see also Wagner et al., 2005). Almost invariably, these studies have tested memory using some variant of yes/no recognition memory, and we therefore begin with a brief overview of current models of recognition memory. We then present a meta-analysis of findings from studies in which recognition memory judgments were segregated according to whether recognition was associated with retrieval of episodic detail (recollection), or was instead based on an undifferentiated sense of familiarity. We go on to review findings from individual studies that we believe to be especially relevant for understanding the functional significance of retrieval-related activity in the parietal cortex, relating these findings where appropriate to those obtained in analogous event-related potential (ERP) studies. We discuss the findings in relation to current ideas about the functions supported by different parietal regions, and what might be done to further elucidate the role of the parietal cortex in memory retrieval.
Recognition memory
There is substantial evidence that recognition memory judgments are not ‘process-pure’, but rather are supported by at least two sources of information about past occurrence (Rugg & Yonelinas, 2003). This evidence has motivated a number of ‘dual-process’ accounts of recognition memory (e.g., Atkinson & Juola, 1974; Mandler, 1980; Yonelinas & Jacoby, 1995; for review see Yonelinas, 2002). Common to all of these accounts is the proposal that recognition can be supported by both an undifferentiated, strength-like memory signal (usually referred to as familiarity), and by the retrieval of qualitative information about the study episode such as contextual details. This second process, which is assumed to rely on a sub-set of the processes that support recall, is referred to as recollection. Hereafter, we use the terms ‘recollection’ and ‘episodic retrieval’ interchangeably. From the dual-process perspective, familiarity-driven memory is conceived as non-episodic, since it provides no information specific to a given study episode.
If dual-process accounts of recognition memory are valid, the neural activity elicited by correctly recognized items in tests of recognition memory will be a mixture of the neural correlates of familiarity and recollection. Therefore findings from such tests are of limited utility in elucidating the functional significance of retrieval-related neural activity, whether the activity is located in the parietal cortex or elsewhere. More useful are findings from studies that have employed variants of the recognition test procedure that allow recognized items to be segregated on the basis of whether or not recognition was accompanied by recollection. In one such procedure, recollection is operationalized as recognition accompanied by accurate source memory – memory for a specific feature of the study context, such as the location or color in which an item was presented. A second popular method for segregating recollection- and familiarity-driven recognition – the ‘Remember/Know’ procedure (Tulving, 1985) – requires the subject to make an introspective judgment as to whether recognition is accompanied by retrieval of details of the study episode.
Neither of these procedures for separating recollection- and familiarity-driven recognition judgments is free from criticism. For example, whereas an accurate source judgment can be assumed to depend on recollection of specific details of the study episode, it cannot be assumed that an inaccurate judgment is indicative of an absence of recollection because details other than those required for an accurate source judgment may still have been recollected. This problem of ‘non-criterial’ recollection is less acute for ‘Remember/Know’ judgments, but there is evidence that these judgments may be influenced by such factors as recognition confidence and criterion placement, leading to an imperfect mapping between Remember vs. Know judgments and recollection vs. familiarity (e.g., Rotello et al., 2005). Given their respective difficulties in interpretation, it is reassuring that there is substantial convergence between the two procedures with respect to the psychological characteristics of recollection and familiarity (Yonelinas, 2002). The majority of fMRI studies attempting to selectively characterize the neural correlates of recollection and familiarity have however employed the Remember/Know procedure, and there is a need for additional fMRI studies in which source memory procedures are used to directly contrast the neural correlates of recollection and familiarity.
Implicit in the foregoing discussion are two assumptions about recognition judgments that have recently been challenged (Wixted, 2007; Squire et al., 2007), namely, that familiarity and recollection provide independent bases for recognition decisions, and that unlike familiarity, recollection is a thresholded rather than a continuously-varying memory signal. We discuss the implications of these and related challenges for the interpretation of retrieval-related parietal activity in a later section. We begin, however, by discussing relevant findings from the perspective of the ‘standard’ dual-process framework outlined above.
Meta-analysis of fMRI studies of recollection and familiarity
We conducted a review of published event-related fMRI studies where contrasts were employed that allowed retrieval-related activity associated with recollection- and familiarity-driven recognition judgments to be separately identified. We restricted our analysis to studies requiring judgments on individually presented test items that differed in their study histories (that is, tests employing judgments on ‘old’ vs. ‘new’ items). Among these studies, we further narrowed our selection to those reporting contrasts between classes of test items associated with memory judgments indicative of successful recollection versus familiarity, or where ‘parametric’ analyses were conducted across a set of items in order to identify regions responding in a manner concordant with a recollection or familiarity signal (Montaldi et al., 2006; Yonelinas et al., 2005; Daselaar et al., 2006). We also included two studies (Henson et al., 2005; Iidaka et al., 2006) where probability of recollection was modulated by a ‘depth of processing’ manipulation during study, under the assumption that the benefit to recognition accruing from deep as opposed to shallow study is predominantly (albeit not exclusively) due to increased probability of recollection (Yonelinas, 2002). We did not include data points derived from the interrogation of regions of interest (ROIs) rather than through voxel-wise analyses (e.g., Wheeler & Buckner, 2003, 2004; Kahn et al., 2004), although we do discuss findings from such studies in later sections. This is because ROI analyses i) do not provide an unbiased estimate of the locus of the peak of an experimental effect, and ii) run the risk of aggregating activity from adjacent but functionally dissociable regions. In addition, we only include data points derived from contrasts performed between items belonging to a common retrieval task, omitting between-task contrasts on the grounds that these potentially confound retrieval-related effects with effects due to differential task demands.
We identified the loci (in MNI co-ordinates1; Cocosco et al., 1997) of significant contrasts identifying recollection- and familiarity-related effects falling within, or adjacent to, the borders of posterior parietal cortex. Since these borders are somewhat arbitrary, and cannot be precisely demarcated at the spatial scale afforded by fMRI, we adopted a fairly liberal definition. Using Caret visualization software (Van Essen et al., 2001), we include loci that fell within, or just posterior to, the borders of Brodmann Areas (BA) 7, 39 and 40, as demarcated on the PALS B-12 human brain atlas (Van Essen, 2002, 2005). In the case of the medial cortical surface, we report loci inferior to BA 7 (the precuneus) that fall between the parieto-occipital fissure and the splenium, since these are frequently described as falling within medial parietal cortex.
The studies fulfilling the above criteria are listed in Table 1. Also included in the table are a description of the test materials, the contrasts employed to identify familiarity and recollection effects, and a listing of the MNI co-ordinates of the peaks of effects localized to lateral and medial parietal cortex. The co-ordinates are projected onto an inflated fiducial brain in Figure 1, separated according to whether they were associated with familiarity or recollection. The majority of the reported effects for both familiarity and recollection are localized to left lateral cortex, where they appear to be spatially separated: familiarity effects tend to cluster around the intra-parietal sulcus (IPS) with a center of mass at −38, −62, 46, whereas effects associated with recollection are mainly found in cortex lateral and inferior to the IPS, center of mass −43, −66, 38. Separate pairwise contrasts (Mann-Whitney U) of the x, y and z co-ordinates corresponding to the two classes of retrieval effect confirm this impression. Whereas co-ordinates on the y axis did not differ significantly, x co-ordinates were significantly more lateral (p < 0.0025) and z co-ordinates more inferior (p < 0.025) for recollection than familiarity.
Table 1.
Coordinates of Parietal Peak Voxels for Recollection vs. Familiarity
| Reference | Study Material | Test cue | Test task | Study task | Contrast | Class | Left Parietal coordinates | Medial Parietal coordinates | Right Parietal coordinates | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cansino et al. (2002) | Po | Po | SourceL/N | Art | SC > SI | R | −14 | −87 | 43 | 63 | −36 | 24 | |||
| 61 | −45 | 26 | |||||||||||||
| Daaselar et al. (2006) | W/NW | W | O/N, c1/c2/c3/c4 | Lex | O4 > O3=O2=N2=N3=N4 | R | −45 | −65 | 31 | ||||||
| N4 → O4 | F | −38 | −80 | 32* | −15 | −68 | 34 | ||||||||
| 15 | −58 | 17 | |||||||||||||
| Eldridge et al. (2000) | W | W | O/N, R/K if O | Int | Rh > Kh | R | −43 | −60 | 40 | 54 | −62 | 35 | |||
| −44 | −66 | 18 | |||||||||||||
| Fenker et al. (2005) | Pfe-W/Pfn-W | W | R/K/N | Int | Rhe > Khe | R | −39 | −69 | 36 | 6 | −66 | 21 | 51 | −66 | 21 |
| −39 | −72 | 51 | |||||||||||||
| Henson et al. (1999) | W/NW | W | R/K/N | Lex | Rh > Kh | R | −58 | −55 | 40 | ||||||
| −42 | −76 | 38 | |||||||||||||
| Kh > CR | F | −24 | −67 | 42 | |||||||||||
| Henson et al. (2005) | W | W | O/N | Deep/Shal | Dh > Sh | R | −51 | −57 | 45 | 51 | −51 | 21 | |||
| −51 | −66 | 42 | |||||||||||||
| −54 | −66 | 15 | |||||||||||||
| Sh > SM | F | −42 | −54 | 60 | −15 | −60 | 21 | 30 | −69 | 36 | |||||
| −30 | −69 | 36 | 12 | −66 | 36 | ||||||||||
| −30 | −72 | 51 | |||||||||||||
| Iidaka et al. (2006) | LDo | LDo | O/N | Art/LR | H vs. CR × Art vs. LR | R | −32 | −50 | 38 | ||||||
| LRh > CR | F | −40 | −62 | 46 | 42 | −64 | 50 | ||||||||
| Johnson & Rugg (2007) | Wsc/W | W | R/K/N | Vis/Sen | Rh > Kh ex cse | R | −42 | −78 | 30 | 54 | −72 | 18 | |||
| 36 | −81 | 33 | |||||||||||||
| Kahn et al. (2004) | W | W | SourceT/N | Read/Image | SC > SI | R | 36 | −48 | 42 | ||||||
| Kensinger & Schacter (2006) | W-Po/W | W | SourceP | Big | SC(W-Po) > SI(W-Po) | R | −48 | −51 | 45 | ||||||
| −35 | −42 | 54 | |||||||||||||
| Montaldi et al. (2006) | Psc | Psc | N/R/F1/F2/F3 | PMTS | R > F3 | R | −42 | −72 | 30 | ||||||
| M → F3h | F | −39 | −51 | 48 | 9 | −75 | 48 | ||||||||
| −42 | −66 | 42 | |||||||||||||
| Ragland et al. (2006) | W | W | SourceT/N | UL/Con | SC > SI | R | −41 | −60 | 51 | ||||||
| SI > CR | F | −34 | −56 | 48 | |||||||||||
| Vilberg & Rugg (2007) | PPo | Po | R2/R1/K/N | Vis | Rh > Kh ex Kh > M | R | −36 | −78 | 33 | −9 | −78 | 33 | 33 | −60 | 57 |
| Kh > Rh ex Rh > Kh | F | −30 | −63 | 45 | 15 | −72 | 60 | 42 | −42 | 36 | |||||
| 6 | −66 | 30 | 33 | −54 | 45 | ||||||||||
| Wheeler & Buckner (2004) | W-Po/W-S | W | R/K/N/G | Int | Rh > Kh | R | −52 | −54 | 38 | ||||||
| −43 | −71 | 40 | |||||||||||||
| Woodruff et al. (2005) | Po/W | W | R/K/N | Deep | Rh > Kh | R | −46 | −80 | 30 | 0 | −68 | 30 | |||
| Kh > CR | F | −40 | −62 | 50 | 52 | −50 | 48 | ||||||||
| −48 | −50 | 50 | |||||||||||||
| Yonelinas et al. (2005) | W | W | R/4/3/2/1 | Con | R > 4 | R | −42 | −54 | 15 | 54 | −69 | 9 | |||
| 1 → 4 | F | −33 | −60 | 36 | 9 | −72 | 36 | 39 | −51 | 36 | |||||
| 36 | −66 | 48 | |||||||||||||
Note: All coordinates are reported in MNI space. Study/Test material: Po = pictures of objects, W = words, NW = non-words, Pfe-W = picture of a face with a fearful expression followed by a word, Pfn-W = picture of a face with a neutral expression followed by a word, LDo = line drawings of objects, Wsc = word overlaid on a scene, Psc = pictures of scenes, PPo = pairs of pictures of objects, W-Po = word followed by a picture of an object, W-S = word followed by a sound. Test tasks: SourceL = location source, SourceP = picture at study/new, N = new, O = old, c1/c2/c3/c4 = confidence scale (where 4 indicates greatest confidence), R = remember, K = know, F1 = low familiarity, F2 = moderate familiarity, F3 = high familiarity, SourceT = task source, R2 = remember associated picture, R1 = remember some detail but not associated picture, G = guess, 4/3/2/1 = high confidence old/low confidence old/low confidence new/high confidence new. Study tasks: Art = artificial/natural discrimination, Lex = lexical decision, Int = intentional encoding, Deep = living/non-living, Shal = alphabetical task, LR = left/right location, Vis = visualize an integration of the stimuli, Sen = create a sentence, Read/Image = read/imagine, Big = bigger/smaller than a shoebox, PMTS = perceptual match to sample, UL = uppercase/lowercase, Con = concrete/abstract judgment. Contrast: SC = source correct, SI = source incorrect, “O4 > O3 = O2 = N2 = N3 = N4” = parametric analysis where high confidence old > all other confidence levels for old and new responses, N4 → O4 = monotonic increases in activity across familiarity from high confidence new (N4) to high confidence old (O4), R = remember, K = know, h = hit, Rhe = remember hit for an item associated with a fearful face stimulus at study, CR = correct rejection, D = deep, S = shallow, M = miss, Art = artificial/natural task, LR = left/right task, ex = exclusively masked by, cse = content-specific Rh > Kh, Miss → F3h = monotonic increases in activity with increasing familiarity, 1 → 4 = monotonic increases in activity across confidence responses from 1 (least) to 4 (greatest).
This coordinate was reported by Daaselar et al. (2006) as −38, 80, 32, falling within the “parieto-occipital cortex.” We assume the authors intended the y coordinate to read −80.
Figure 1.
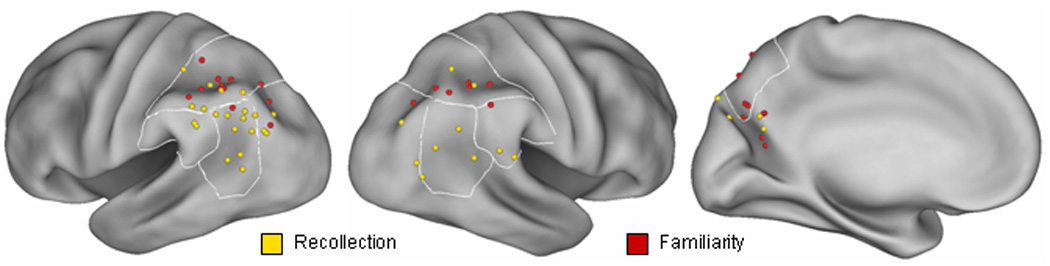
Parietal loci sensitive to recollection vs. familiarity. These loci, which are reported in Table 1, have been mapped onto inflated fiducial brains (see text). Displayed from left to right are the left lateral, right lateral, and left medial surfaces of the inflated brain. The borders of Brodmann areas 7, 39, and 40 are demarcated by white lines. Note that for illustrative purposes, medial foci have been rendered onto the surface of the left hemisphere by reversing the sign of all positive x coordinates.
Figure 1 also depicts the loci of recollection and familiarity effects in right lateral and medial parietal cortex. Although there are fewer data points than in the left hemisphere, effects in right lateral cortex again appear to be spatially segregated, with more superior loci in the case of familiarity (centers of mass 39, −54, 45, and 53, −61, 25 for familiarity and recollection, respectively). Consistent with this impression, there was a significant difference in the z co-ordinates of the two effects (p < 0.025). By contrast, the few effects localized to the medial parietal surface show little sign of segregation according to the recollection/familiarity distinction.
The data presented in Table 1 and Figure 1 indicate, across studies that vary widely in their designs, analysis methods, stimulus materials and test procedures, that there is a consistent tendency for familiarity- and recollection-related effects to be localized to different regions of lateral parietal cortex. Whereas familiarity-related effects are concentrated around the IPS (center of mass in BA 7/40), recollection-related effects are more likely to be localized to the posterior part of inferior parietal cortex (center of mass in BA 39). There is little sign that the loci of either effect vary according to any of the experimental characteristics mentioned above, although the small number of data points leaves this possibility open.
We defer a detailed discussion of the functional significance of this dissociation between superior and inferior lateral parietal cortex to the sections that follow. Suffice it to say for now that these findings are strongly indicative of functional heterogeneity within the lateral parietal regions which demonstrate fMRI retrieval effects. Thus, attempts to explain these effects in terms of a single process or function are unlikely to succeed.
Findings from specific studies
In this section, we discuss specific findings that we consider especially relevant to the understanding of the functional significance of retrieval-related activity in the parietal cortex. In light of the findings of the above meta-analysis, we focus on lateral, and especially left lateral, cortex. We consider results from some of the studies listed in Table 1 along with findings from additional studies where relevant.
Superior Lateral Cortex
We turn first to the possible significance of the ‘familiarity’ effects localized to superior lateral parietal cortex in the vicinity of the IPS. Although the findings reported in Table 1 suggest that activity in this region co-varies with familiarity strength, this does not necessarily lead to the conclusion that the region specifically supports processes underlying familiarity-driven recognition. Indeed, the evidence suggests otherwise. In the first place, not only does this region demonstrate greater activity for familiar than for new items (Table 1), but it does so also for the contrast between recollected and new items (e.g., Henson et al., 1999; Woodruff et al., 2005). Although this finding can be explained on the assumption that a high proportion of recollected items are also familiar (Montaldi et al., 2006), it is equally compatible with the possibility that activity in this region is modulated not by the nature of the memory signal elicited by a test item, but by some more general distinction between correctly classified old and new test items. This possibility is consistent with the wealth of evidence pointing to a role for parietal cortex in the vicinity of the IPS in top-down attentional control and in the detection of behaviorally relevant stimulus events (Corbetta & Shulman, 2002; Kastner & Ungerleider, 2000). It is also consistent with the findings of fMRI studies of the ‘oddball task’, in which subjects must detect an infrequent class of stimuli (the oddballs, or targets) embedded in a series of more frequently occurring non-target stimuli. Relative to non-targets, oddball stimuli are frequently reported to elicit enhanced activity in superior parietal cortex close to those seemingly sensitive to recognition familiarity (e.g., Fichtenholtz et al., 2004; Kiehl & Liddle, 2003; Brazdil et al., 2007). For example, in one recent study investigating fMRI responses to visual oddballs (Brazdil et al., 2007), the peak loci of the superior parietal responses were at co-ordinates −30, −57, 42 and 33, −57, 45 in the left and right hemispheres respectively, close to the centers of mass of the ‘familiarity-responsive’ regions identified in our meta-analysis (−36, −62, 45 and 39, −57, 43).
Direct evidence that recognition memory effects in superior parietal cortex do not reflect processes supporting memory retrieval comes from a study in which the ratio of old and new items was varied across different test blocks (Herron et al., 2004a). When old items were relatively infrequent (25:75 ratio) robust old/new effects were evident in two left superior parietal regions (peaks at −39, −30, 54, and −33, −57, 60). These effects were absent however, and showed a non-significant tendency to reverse direction, when the old/new ratio was reversed. The sensitivity of these regions to old/new ratio is difficult to reconcile with a direct role in memory retrieval. Rather, as was argued by Herron et al. (2004a), it appears that ‘old/new’ effects in superior parietal cortex reflect processes downstream from those responsible for distinguishing between studied and unstudied items. One possibility is that the effects are a reflection of the role played by this region in responding to task-relevant events (Corbetta & Shulman, 2002), as discussed above. By this argument, subjects in recognition memory tasks typically approach the task as one of target detection, in which ‘targets’ (old items) must be singled out against a background of ‘non-targets’ (new items) (see Neville et al., 1986 for a similar argument). The relative salience of old and new items can be modulated however by such factors as the relative probabilities of the two stimulus classes (Herron et al., 2004a), or the confidence with which they are classified as old or new (Yonelinas et al., 2005).
In summary, retrieval-related effects localized to superior parietal cortex in the vicinity of the IPS do not appear to reflect processes directly supporting either familiarity- or recollection-driven recognition. Rather, the effects appear to be reflections of processes downstream from retrieval, the engagement of which depends on the salience or task-relevance of the eliciting item. Additional evidence that could be construed as favoring this proposal comes from two studies that assessed retrieval-related parietal activity using an ROI-based approach (Kahn et al., 2004; 6mm radius centered on −33,−51,45; Wheeler & Buckner, 2003; unspecified volume centered on −39, −52, 36). These ROIs likely span the superior and inferior parietal regions identified in our meta-analysis (although that from Kahn et al. appears to be centered on the intra-parietal sulcus). In both studies, relative to correctly rejected new items, activity in the respective ROIs was elevated for both correctly recognized old items and incorrectly recognized new items (false alarms). To the extent that these findings were driven by superior rather than inferior parietal activity, they add further weight to the proposal that retrieval-related activity in the more superior region reflects not a memory signal, but something akin to the salience or ‘target-value’ of the eliciting stimulus event.
Inferior Parietal Cortex
The meta-analysis of the findings listed in Table 1 shows that retrieval-related effects in parietal cortex inferior to the IPS arise mainly from contrasts aimed at identifying neural correlates of recollection. The question arises whether these inferior effects are indicative of a specific role for lateral parietal cortex in recollective processing, or whether, as appears to be the case for superior parietal cortex, they index the engagement of processes confounded with, but not directly involved in, successful memory retrieval.
Two findings in particular suggest that, unlike the superior parietal region, retrieval effects in inferior parietal cortex do not merely reflect the behavioral salience of the eliciting item in any simple way. First, in the study of Herron et al. (2004a) described above, old/new effects in two inferior parietal regions (−36, −72, 30 and 36, −66, 36) were insensitive to old/new ratio, a pattern different (and statistically dissociable) from that evident in superior parietal cortex (see above). The second finding comes from a study reported by Shannon and Buckner (2004), who contrasted old/new effects in a lateral parietal ROI according to whether a behavioral response was required to both old and new recognition test items, to old items only, or to new items only (12 mm radius centered on −44, −61, 42; Note that this ROI spans both the superior and inferior regions identified by the meta-analysis described previously.). No differences were found in the magnitude of the effects across the three response contingencies. [In two companion experiments, Shannon and Buckner (2004) also reported that old/new effects in the same region were unaffected by the modality of the recognition test items (visual vs. auditory), and were larger for words subjected to ‘deep’ rather than ‘shallow’ encoding (cf. Table 1).]
Although caution in required when drawing conclusions on the basis of null effects, the findings of Herron et al. (2004a) and Shannon and Buckner (2004) suggest that retrieval-related effects in inferior parietal cortex do not simply reflect differences in the behavioral relevance or salience of different classes of test item (in the case of the latter study, further caution is warranted in view of the fact that the ROI sampled both inferior and superior parietal activity, see above). Two further possibilities that can likely be ruled out are that the effects are reflections of either the confidence with which items are endorsed as old, or the ‘strength’ of a common memory signal supporting both familiarity and recollection. These possibilities need to be taken seriously in light of the fact that most of the studies listed in Table 1 that report inferior parietal recollection effects employed the Remember/Know procedure to separate recollection- and familiarity-driven recognition. Several critiques of this procedure have argued that Remember responses do not necessarily reflect retrieval of information qualitatively different from the information supporting Know judgments (Donaldson, 1996; Dunn, 2004; Wixted, 2007). According to these critiques, Remember judgments are simply recognition judgments based on relatively strong memories, and consequently are associated with higher levels of response confidence than are Know judgments. The same general argument can be applied to other procedures that have been employed to segregate recollection and familiarity; other things being equal, it is likely that recollected items will on average attract more confident endorsements than will items judged old on the basis of familiarity (Yonelinas, 2001; indeed, this assumption is the basis of the analytic rationale employed by Daselaar et al., 2006). In two of the studies listed in Table 1 however, a modified Remember/Know procedure was employed in which test items that were not endorsed as ‘Remembered’ were rated either in terms of confidence (‘confident new’ to ‘confident old’; Yonelinas et al., 2005), or familiarity strength (‘highly unfamiliar’ to highly familiar’; Montaldi et al., 2006). In both cases, inferior parietal regions were identified where activity failed to vary according to the rating given to non-recollected items, but nonetheless was greater for Remembered items than for unrecollected items recognized with high confidence or familiarity (see Table 1). These findings suggest that inferior parietal recollection effects are not a consequence of differential response confidence or memory strength. If this were the case, then the differences in activity elicited by items receiving Remember and high confidence/familiarity judgments should have been mirrored in the neural activity elicited by unrecollected items attracting different confident ratings.
Together, the findings discussed in the two preceding paragraphs suggest that retrieval-related activity in inferior parietal cortex is closely tied to successful recollection rather than to some extraneous confounding variable. This raises the obvious question: what specific component or components of recollective processing are supported by this cortical region?
Timing of Recollection-Related Neural Activity – Evidence from Event-Related Potentials
Information about the time-course of the recollection effects in inferior parietal cortex would be very helpful in constraining their functional interpretation. For example, if it turned out that the onset latency of the effects exceeded the latency of recollection-based behavioral responses, this would invalidate any interpretation that linked the effects to processes directly supporting successful retrieval. Unfortunately, the low temporal resolution of the BOLD signal means the precise timing of inferior parietal recollection effects cannot be determined from fMRI evidence alone.
Another source of evidence is available, however, from studies of recognition memory that have employed scalp-recorded event-related potentials (ERPs). Importantly, while lacking the spatial resolution of fMRI, ERPs allow neural correlates of cognitive processing to be identified with a temporal resolution on the order of a few tens of milliseconds. Beginning in the early 1980s, numerous studies have reported that ERPs elicited in recognition memory tasks demonstrate old/new effects. These effects take the form of more positive-going waveforms for recognized old items than for items correctly classified as new. Among the most prominent is the so-called ‘left parietal old/new effect.’ As its label suggests, this effect is typically maximal over the left posterior scalp (see Figure 2), consistent with a generator in or near left lateral parietal cortex. As is evident from Figure 2, the effect takes the form of a phasic positivity that typically onsets around 400–450 ms post-stimulus, early enough to reflect processes that contribute to recollection-based behavioral responses (which typically average around 800 ms or more, implying a latency for response selection of at least 600 ms; Hackley et al., 2007). Crucially, the findings from a substantial literature (for reviews see Friedman & Johnson, 2000; Rugg & Curran, 2007) converge to suggest that, like inferior parietal fMRI effects, the left parietal ERP effect is a neural correlate of successful recollection (but see Finnigan et al., 2002 for an alternative perspective). For example, the effect is larger when elicited by items endorsed as ‘Remembered’ rather than ‘Known’ (e.g., Smith, 1993), by items attracting correct rather than incorrect source memory judgments (e.g., Wilding & Rugg, 1996), and by items subjected to deep as opposed to shallow encoding (e.g., Rugg et al., 1998b). Other parallels with inferior parietal fMRI effects include a lack of sensitivity to old/new ratio (Herron et al., 2004b), and a magnitude that varies according to the amount of information recollected (Wilding, 2000; Vilberg et al., 2006; see below).
Figure 2.
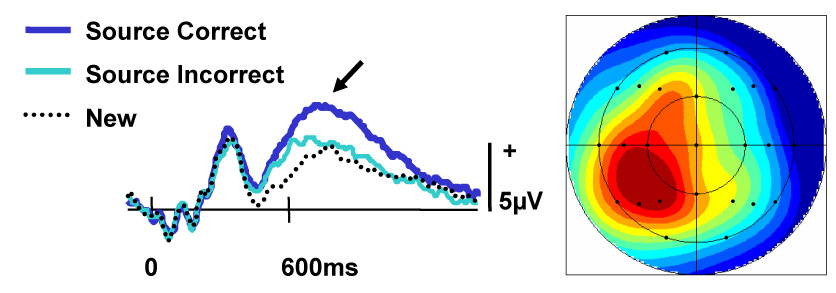
Example of the left parietal ERP old/new effect (Rugg, unpublished data). Waveforms elicited by three classes of test item in a source memory experiment are displayed at a left parietal scalp site (left panel). The arrow indicates the effect’s peak. The topography of the effect (right panel) is shown as a subtraction of the source incorrect waveforms from the source correct waveforms from 500 to 800ms poststimulus onset.
Whereas it is not possible to definitively localize the generators of the left parietal old/new effect to the same inferior parietal regions identified by fMRI studies of recollection, the functional parallels between the two classes of effect are striking and, in our opinion, persuasive. We therefore think it highly likely that the ERP effects are the electrophysiological correlate of the hemodynamic effects identified by fMRI. To the extent this conjecture is valid, it can be concluded that inferior parietal recollection effects onset sufficiently early to play a direct role in recollection-based memory judgments.
Recollection and the Functions of Inferior Parietal Cortex
Before proposing a specific role for inferior parietal cortex in recollection, it would seem sensible to ask whether any of the functions already proposed for this region might accommodate its involvement in recollective processing. There is no shortage of potential functions to choose from (for reviews see Culham & Valyear, 2006; Hussain & Nachev, 2006; Jackson & Husain, 2006). Among the more prominent are control of visuospatial attention, attention switching, the temporary storage of phonological and visual information, organization of action sequences, and top-down control of working memory. As was noted by Shannon and Buckner (2004), the finding that inferior parietal recollection effects are equally evident for visual and auditory material makes it unlikely that the effects reflect processes specifically supporting visuospatial functions. By the same token, the apparent insensitivity of the effects to whether study or test materials are verbal or pictorial (see Table 1) is difficult to reconcile with the idea that the effects reflect the engagement of material-specific short-term stores.
Among other functions held to be supported by inferior parietal cortex, we believe that two stand out as possible explanations for the involvement of this region in recollection. The first of these functions is that of a modality-independent ‘attentional circuit-breaker’ (Astafiev et al., 2006; Corbetta & Shulman, 2002), responsible for re-orienting attention toward a potentially relevant stimulus event. By this account (see Rugg & Henson, 2002; and Wagner et al., 2005 for similar proposals) successful recollection is an ‘attention-grabbing’ internal event that causes attention to be disengaged from the environment so as to focus on the contents of retrieval. Thus, inferior parietal recollection effects may be a reflection of the engagement of the aforementioned circuit breaker.
A related account that may also be able accommodate the involvement of this region in recollection proposes that inferior parietal cortex supports the sustained focusing of attention on the contents of working memory (Ravizza et al., 2004). According to this account, recollected information is maintained in working memory in much the same way as is information derived from the environment, therefore requiring engagement of the same control processes that maintain working memory representations of external stimulus events.
Of these two possible accounts of the role of inferior parietal cortex in recollection we consider the second to be preferable for two reasons. First, the attentional circuit breaker is held to be localized to cortex in the vicinity of the right temporo-parietal junction (Astafiev et al., 2006; Corbetta & Shulman, 2002), ventral and contralateral to the loci where recollection effects are most commonly reported (see Table 1). By contrast, the region identified as participating in attentional control of working memory by Ravizza et al. (2004; labeled by these authors as ‘dorsal inferior parietal cortex’) is much closer to the center of mass of recollection effects (left hemisphere peak co-ordinates from Ravizza et al. (2004), averaged across all conditions where activations in this region were reported, were −35, −50, 37). In addition to these anatomical considerations, the ‘circuit breaker’ account also appears to be incompatible with one of functional characteristics of inferior parietal recollection effects. In a recent fMRI study (Vilberg & Rugg, 2007; see Wilding, 2000 and Vilberg et al., 2006 for parallel ERP findings) we identified a left inferior parietal region that was sensitive both to whether recognition was accompanied by recollection (as operationalized by the Remember/Know procedure) and to the amount of information that subjects reported they had recollected. Whereas ‘load effects’ would be expected in a system supporting working memory maintenance (and indeed, were reported for dorsal inferior parietal cortex by Ravizza et al. (2004)), it is less clear why they would be manifest in a system responsible simply for the redirection of attention.
The above discussion suggests that what we have referred to previously (Vilberg et al., 2006; Vilberg & Rugg, 2007) as the ‘orienting’ account of the role of inferior parietal cortex in recollection fits rather poorly with current anatomical and functional evidence. By contrast, the proposal that recollection effects in this region reflect its role in maintaining attention on representations of recollected information held in working memory fares well. This proposal is not without it problems, however. These arise out of the widely held assumption that working memory is comprised of a set of modality- or material-dependent buffers, each linked to a central executive control system (the ‘central executive’ in the terminology of Baddeley & Hitch, 1974). As discussed by Baddeley (2000), it is not obvious how a working memory system organized along these lines could represent retrieved episodic information in an integrated manner: the contents of retrieval are often multi-modal, and the amount of information retrieved seemingly well in excess of the apparent storage capacities of modality- or material-specific buffers. In consideration of these and related issues, Baddeley (2000) proposed that in addition to a set of limited capacity, informationally-specific stores, working memory also comprises a multi-modal ‘episodic buffer’, a temporary store that acts as an interface between episodic memory and the central executive. He argued that it is through the episodic buffer that recollected information is integrated into an episodic representation that can inform on-line cognition.
We propose that inferior parietal recollection effects reflect the role played by this region in supporting something akin to the episodic buffer described by Baddeley (2000). According to this proposal, by contributing to the generation and maintenance of an integrated representation of retrieved information, inferior parietal cortex acts as an interface between episodic memory and the executive systems that monitor and control on-line processing. This proposal accommodates both the fMRI (and ERP) findings pointing to a specific role for inferior parietal cortex in recollection-based recognition memory, and Baddeley’s arguments for the need to expand the functional architecture of working memory to include a system able to temporarily represent complex, multi-modal information. In addition, the proposal is consistent with what is known about the anatomical connectivity of the region, which includes both direct and indirect connections with the hippocampal formation and medial temporal cortex (reviewed in Vincent et al., 2006). It is also consistent with the recent finding (Vincent et al., 2006) that, as indexed by correlational analyses of spontaneous fluctuations in the fMRI BOLD signal, the hippocampus and recollection-sensitive inferior parietal regions are strongly interconnected functionally as well as anatomically (see Figure 3).
Figure 3.
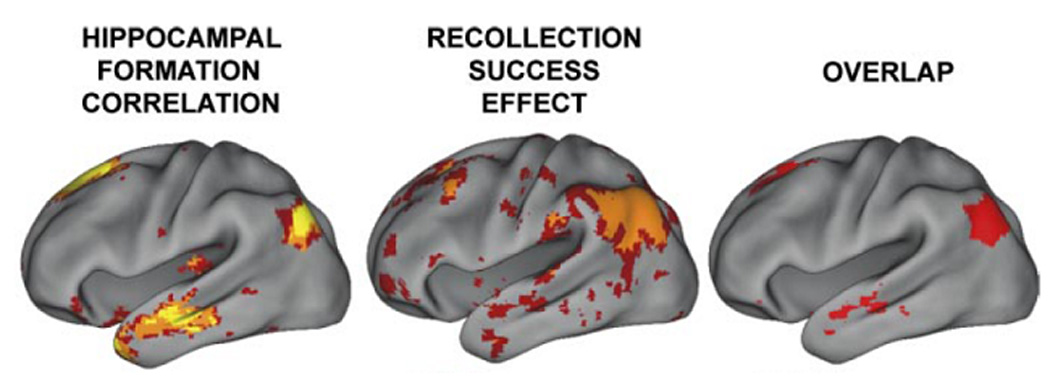
Left hemisphere cortical regions showing (left) spontaneous BOLD signal correlations with the hippocampus during rest, (middle) recollection effects (from Wagner et al., 2005), and (right) their overlap. Adapted from Figure 8 of Vincent et al. (2006) with permission of the authors and publishers.
It is important to emphasize that in proposing a link between inferior lateral parietal cortex and the episodic buffer we do not mean to suggest that the buffer is strictly localized to this region. Rather, as in the case of parietal contributions to modality-dependent working memory, we assume that the left inferior parietal region operates in concert with other regions, in this case to support the representation and manipulation of episodic information. Among these other regions, right anterior prefrontal cortex seems likely to play a prominent role (Prabhakaran et al., 2000; Zhang et al., 2004).2
Alternatives to Dual-Process Interpretations of Retrieval-Related Parietal Activity
As was noted in the Introduction, dual-process interpretations of retrieval-related neural activity (whether in the parietal cortex or elsewhere) depend on the twin assumptions that recollection and familiarity provide independent bases for recognition decisions, and that recollection has a thresholded (‘all-or-none’) character. These assumptions are challenged by models proposing that recognition decisions are based on a continuous, unidimensional memory signal (e.g., Dunn, 2004). For example, in one recent model (Wixted, 2007), recollection and familiarity are conceived as two separate signals that are combined prior to a memory judgment, rather than serving as independent sources of evidence about the study status of a test item. As already noted, in these strength-based models, it is assumed that recollection simply reflects a stronger memory signal than does familiarity. Thus, a correct source judgment or a ‘remember’ response reflects a stronger memory than an incorrect source judgment or a ‘know’ response.
The ability of strength-based models to account for the fMRI, ERP and neuropsychological evidence held to support dual-process accounts of recognition memory has been discussed elsewhere (e.g., Rugg & Yonelinas, 2003; Rugg & Curran, 2007; Squire et al., 2007; Eichenbaum et al., 2007). We have already noted that the idea that inferior parietal activity scales with memory strength is difficult to reconcile with the finding that the activity appears to be largely insensitive to ratings of the strength of test items that are not endorsed as recollected (Yonelinas et al., 2005; Montaldi et al., 2006). From a single-process perspective, this finding would seem to imply that the region responds only to especially strong memories, which is tantamount to proposing a threshold-like, rather than a continuously-varying, response profile. One recourse to avoiding this conclusion is to posit that the profile reflects non-linearity of the hemodynamic transfer function, such that graded changes in left inferior parietal neural activity lead to a discontinuous change in the corresponding fMRI BOLD response (see Squire et al., 2007 for an example of this argument applied to the medial temporal lobe). Not only would such a proposal be entirely ad hoc, in the present case it runs foul of the finding that the left parietal old/new ERP effect – a direct measure of neural activity – is also insensitive to the memory strength of unrecollected test items (Woodruff et al., 2006). To the extent that the hypothesis linking this ERP effect to left inferior parietal activity is correct (see above), it would appear that this region does indeed respond selectively to items whose ‘strength’ is sufficiently high to support recollection-based memory judgments. In our view, this conclusion is indistinguishable from the proposal that left inferior parietal activity is a neural correlate of recollection.
A second alternative to the idea that left inferior parietal activity is a direct neural correlate of recollection is to propose that the activity reflects processes downstream of retrieval, such as those engaged during memory-guided decision making. Of course, this is essentially the interpretation we endorse with respect to retrieval-related effects in the more superior lateral parietal region identified in our meta-analysis. The question thus arises as to the identity of the additional decision-related processes associated specifically with the inferior region. As already discussed, fMRI and ERP findings converge to suggest that these processes are unlikely to reflect the confidence of recognition decisions (Woodruff et al., 2006; Yonelinas et al., 2005), and the range of retrieval tasks in which recollection-related inferior parietal activity has been elicited (including Remember/Know, Source memory, and yes/no recognition; see Table 1) suggests that this activity is unlikely to be idiosyncratic to the decisional demands of any one method of segregating recollection- and familiarity-based responding. Thus, whereas an account of left inferior parietal recollection-related activity framed in terms of general decisional or other downstream processes cannot be ruled out, it is not obvious (to us) what form such an account would take.
Hemispheric Asymmetry of Lateral Parietal Contributions to Retrieval
Both neuropsychological and functional neuroimaging evidence suggest that parietal cortex is functionally lateralized, with particularly striking asymmetries of function in the cases of visuospatial attention, organization of action, and language (Husain & Nachev, 2006). The fact that retrieval-related parietal activity is more frequently observed in the left than the right hemisphere, and the predominant finding of a left-sided distribution of recollection-related parietal ERP effects (see Figure 2), suggest that memory retrieval might represent another example of lateralized parietal function. An alternative possibility however is that this lateral asymmetry owes more to the limited range of materials that have been employed in studies to date than to an asymmetry at the level of retrieval processing. All of the studies listed in Table 1, for example, employed meaningful material - either words or pictures – as test items, raising the possibility that the left-sided predominance of retrieval effects reflects a left hemisphere bias for processing verbally- or conceptually-encodable material. To the extent that our proposal that recollection-related inferior parietal effects reflect engagement of a multi-modal buffer is correct, however, the strength of the lateralization of these effects should be minimally affected by the nature of the experimental materials. Resolution of this issue will require studies that directly contrast retrieval-related activity as a function of different stimulus materials. We are not aware of any studies where asymmetries in lateral parietal activity associated specifically with recollection have been investigated according to test material, although there are hints that material effects may indeed exist (Simons et al., in press). Regardless of how this issue plays out, it is noteworthy that the meta-analysis we described previously suggests that superior and inferior parietal retrieval effects dissociate along similar lines in the two hemispheres. This raises the possibility that there is some degree of redundancy between the two hemispheres in their support of retrieval processes (see below).
Medial Parietal Cortex
Medial parietal cortex (BA 7) – often referred to as the precuneus – has been implicated in retrieval since the earliest functional imaging studies, and is frequently held to be a key component of a cortical network subserving episodic retrieval (e.g., Cavanna & Trimble, 2006). Consistent with this view, the precuneus, along with inferior parietal cortex, was identified as a region demonstrating strong functional interconnectivity with the hippocampal formation in the study of Vincent et al. (2006). The results of our meta-analysis were therefore somewhat surprising: of the relatively few precuneus effects that were identified, the majority were associated with familiarity-driven recognition rather than recollection. From the perspective of studies of recognition memory there is therefore little evidence to suggest that medial parietal cortex is selectively involved in episodic retrieval. This does not, of course, imply that the region plays no role in recollective processing, although what this role might be is currently obscure.
Retrieval Tests other than Recognition
As already noted, the overriding majority of studies investigating the neural correlates of retrieval success have employed variants of recognition memory to test memory. This raises the question whether the findings reported in such studies generalize to other kinds of memory test. If recollection-driven recognition does indeed rely on the same core retrieval operations that support episodic retrieval more generally, then the recollection-sensitive inferior parietal regions identified with recognition tasks should also demonstrate retrieval-success effects during tests of recall. While tests of free recall do not easily lend themselves to event-related designs, this is not the case for tests of cued recall. To our knowledge, however, only two studies have reported contrasts between cues eliciting successful versus unsuccessful recall (de Zubicaray et al., 2007; Schott et al., 2005; but see also Meltzer & Constable, 2004), in both cases reporting that successful recall was associated with enhanced left inferior (and medial) parietal activity. These findings suggest that the results obtained for recollection-driven recognition do indeed extend to episodic retrieval more generally. That said, much more work is required to characterize the involvement of parietal cortex across the full range of memory tests that can be employed to elicit episodic memory retrieval.
Effects of Parietal Lesions on Memory
As we noted in the Introduction, functional neuroimaging findings suggesting a role for parietal cortex in memory were unanticipated given the lack of neuropsychological evidence for long-term memory deficits following parietal damage. However, while clinical observation unquestionably suggests that long-term memory is largely unaffected following parietal lesions, there are only a few published studies in which memory performance of parietal patients has been studied formally. In one such study (Simons et al., in press), source memory for words and faces was assessed in six patients with unilateral lateral parietal lesions (three left and three right), and revealed no evidence of impairment. Another study on a larger sample of unilaterally lesioned patients (6 left, 11 right; Haramati et al., in press) tested yes/no recognition memory for words, pictures, and sounds. Left-lesioned patients demonstrated no impairment in recognition for any class of material, whereas right-lesioned patients showed a modest deficit for pictures and sounds that, on further analysis, was potentially attributable to extra-parietal damage. As was noted by the authors, the employment of a simple recognition memory test may have led to an underestimation of recollective impairments in these patients, although the findings of Simons et al. (in press) weigh against this possibility. In a final recent study (Berryhill et al., in press), two patients with extensive bilateral lesions, involving both inferior and superior regions extending into the IPS, were investigated. Both patients demonstrated deficits in spontaneous (but not cued) recall of autobiographical memories. The patient with the more extensive lesions also demonstrated a severe (3 SDs below the norm) impairment on the delayed auditory recognition memory subtest of the Wechsler Memory Scale. Whereas these findings are clearly consistent with a role in memory for parietal cortex, their interpretation is arguably difficult because of the severe attentional and visuomotor impairments of the two patients (both were described as suffering from Balint’s syndrome).
In a different approach to the investigation of lateral parietal dysfunction, Rossi et al. (2006) assessed the effects of reversible ‘functional’ lesions induced by repetitive transcranial magnetic stimulation (rTMS). Stimulation was targeted at the middle/posterior IPS, superior to the inferior lateral region argued above to be implicated in episodic retrieval. This was administered during either the encoding or retrieval phase of a yes/no recognition memory task, a task in which performance can be supported by both recollection and familiarity. In light of the fMRI evidence reviewed above, both of these aspects of the study design (location of stimulation and the nature of the test task) might be expected to mitigate against a positive finding. Nonetheless, and contrary to the conclusions drawn by the authors, the results suggest that interference with lateral parietal function may have resulted in impairment of memory performance. At the higher of the two stimulation intensities employed in the study, performance (assessed by the discriminability metric pHit-pFalse Alarm) was reduced to 70% of the level associated with sham stimulation when rTMS was applied to the left hemisphere during recognition test trials, and to 82% of the sham level under right-sided stimulation (albeit, in this case with high inter-subject variability). Intriguingly, performance was impaired to an even greater extent when stimulation was applied during encoding, with performance reduced to 44% and 56% of the sham level under left and right TMS respectively. Unfortunately, the statistical significance of these effects is not reported.
The results of Rossi et al. (2006) suggest that rTMS may be prove a fruitful method for investigating the impact of parietal dysfunction on memory performance. The fact remains however that there is little clinical evidence for memory impairment after structural lesions of lateral parietal cortex, and two out of the three formal studies currently available in the literature reported null findings (Haramati et al., in press; Simons et al., in press). Although future studies of patients with unilateral parietal lesions may identify circumstances in which an impairment is evident, there are grounds for thinking such a result might prove elusive. As we noted in a previous section, fMRI data suggest that retrieval-related processing is supported by the lateral parietal cortex of both hemispheres. To the extent that this implies a significant level of functional redundancy, impairment following a unilateral lesion may be difficult to demonstrate, especially if functional compensation and reorganization have had an opportunity to further mitigate the effects of the lesion. Some support for this proposal comes from the findings of Berryhill et al. (in press) although, as already noted, the severe attentional deficits associated with bilateral parietal lesions may compromise interpretation of any deficits in memory performance. This is not to say that impairments will not be found following unilateral lesions. For example, unilateral prefrontal lesions are associated with impairment of familiarity-driven recognition memory, but only when test items are presented in the visual field contralateral to the lesion (Duarte et al., 2005). It will be of interest to see whether deficits following unilateral parietal lesions can also be identified by lateralized stimulus presentation.
Concluding Comments
Event-related fMRI findings have amply confirmed earlier suspicions that neural activity in lateral and medial parietal cortex is sensitive to retrieval success. The findings further point to a functional dissociation between superior and inferior lateral parietal regions, with the latter showing strong evidence of a direct role in episodic memory retrieval. We conjecture that this role involves the maintenance or representation of retrieved information in something like the episodic buffer proposed by Baddeley (2000). Obviously, further fMRI studies are required to directly assess these and other ideas about parietal involvement in episodic memory. In addition, considerably more work is needed to characterize the role of parietal cortex in tests other than recognition memory, and to assess the effects of disrupted parietal function on episodic memory performance.
Acknowledgements
K. Vilberg was supported by NSF Graduate Research Fellowship Award D/DGE-0234621. Preparation of this article, and some of the research reported within it, were supported by NIMH grant 5R01MH072966.
Footnotes
All coordinates not originally reported in MNI space were transformed using the tal2mni function within Matlab (see Brett, 1999). These MNI coordinates were then used to plot the individual foci displayed in Figure 1 and are reported in Table 1.
The findings from these two studies converge in suggesting that right anterior prefrontal cortex is more active when multi- rather uni-modal information is maintained in working memory (an operationalization of the engagement of the episodic buffer according to Baddeley, 2000), but the findings are divergent with respect to activity in posterior cortex. Whereas Prabhakaran et al. reported a relative decrease in the engagement of posterior regions, including inferior parietal cortex, Zhang et al. reported increased posterior activity. As noted by Zhang et al. (2004), these discordant findings likely reflect a reversal in the relative difficulty of the multi- and uni-modal tasks in the two studies. The findings thus offer little insight into the role (if any) of parietal cortex in supporting the episodic buffer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. European Journal of Neuroscience. 2006;23:591–596. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Luce RD, Suppes P, editors. Contemporary developments in mathematical psychology. Vol. 1. San Francisco: Freeman; 1974. pp. 243–293. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working Memory. In: Bower GA, editor. The psychology of learning and motivation: advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. The Journal of Neuroscience. doi: 10.1523/JNEUROSCI.4163-07.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazdil M, Mikl M, Marecek R, Krupa P, Rektor I. Effective connectivity in target stimulus processing: a dynamic causal modeling study of visual oddball task. NeuroImage. 2007;35:827–835. doi: 10.1016/j.neuroimage.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 1999 Retrieved Aug 30, 2007, from the CBU Imaging Wiki at the Cambridge MRC Cognition and Brain Sciences Unit website: http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan RS, Evans A. Brainweb: Online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:S425. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Daaselar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- de Zubicaray G, McMahon K, Eastburn M, Pringle AJ, Lorenz L, Humphreys MS. Support for an auto-associative model of spoken cued recall: evidence from fMRI. Neuropsychologia. 2007;45:824–835. doi: 10.1016/j.neuropsychologia.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Memory & Cognition. 1996;24:523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. The Journal of Neuroscience. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JC. Remember-know: A matter of confidence. Psychological Review. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Reviews Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson-Klavehn A, Heinze HJ, Düzel E. Recapitualting emotional context: activity of amygdala, hippocampus and fusiform cortex during recollection and familiarity. European Journal of Neuroscience. 2005;21:1993–1999. doi: 10.1111/j.1460-9568.2005.04033.x. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS. Emotion-attention network interactions during a visual oddball task. Brain Research Cognitive Brain Research. 2004;20:67–80. doi: 10.1016/j.cogbrainres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Finnigan S, Humphreys MS, Dennis S, Geffen G. ERP ‘old/new’ effects: memory strength and decisional factor(s) Neuropsychologia. 2002;40:2288–2304. doi: 10.1016/s0028-3932(02)00113-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends in Neurosciences. 1997;20:213–218. doi: 10.1016/s0166-2236(96)01013-2. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson J., Jr Event-Related Potential (ERP) Studies of Memory Encoding and Retrieval: A Selective Review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Schankin A, Wohlschlaeger A, Wascher E. Localization of temporal preparation effects via trisected reaction time. Psychophysiology. 2007;44:334–338. doi: 10.1111/j.1469-8986.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: A neuropsychological study. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2007.11.015. in press. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: An fMRI study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JE, Henson RNA, Rugg MD. Probability effects on the neural correlates of retrieval success: an fMRI study. NeuroImage. 2004a;21:302–310. doi: 10.1016/j.neuroimage.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Herron JE, Quayle AH, Rugg MD. Probability effects on event-related potential correlates of recognition memory. Cognitive Brain Research. 2004b;16:66–73. doi: 10.1016/s0926-6410(02)00220-3. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends in Cognitive Sciences. 2006;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. Frontoparietal network involved in successful retrieval from episodic memory. Spatial and temporal analyses using fMRI and ERP. Cerebral Cortex. 2006;16:1349–1360. doi: 10.1093/cercor/bhl040. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Husain M. Visuomotor functions of the posterior parietal cortex. Neuropsychologia. 2006;44:2589–2593. doi: 10.1016/j.neuropsychologia.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cerebral Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. The Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Reviews of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes underlying memory attribution on a reality-monitoring task. Cerebral Cortex. 2006;16:1126–1133. doi: 10.1093/cercor/bhj054. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. Reproducibility of the hemodynamic response to auditory oddball stimuli: a six-week test-retest study. Human Brain Mapping. 2003;18:42–52. doi: 10.1002/hbm.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. NeuroImage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. PNAS. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Meltzer JA, Constable RT. Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. NeuroImage. 2004;24:384–397. doi: 10.1016/j.neuroimage.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Kutas M, Chesney G, Schmidt AL. Event-related brain potentials during initial encoding and recognition memory of congruous and incongruous words. Journal of Memory and Language. 1986;25:75–92. [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JDE. Integration of diverse information in working memory within the frontal lobe. Nature Neuroscience. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Valdez JN, Loughead J, Gur RC, Gur RE. Functional magnetic resonance imaging of internal source monitoring in schizophrenia: recognition with and without recollection. Schizophrenia Research. 2006;87:160–171. doi: 10.1016/j.schres.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, Babiloni C, Rossini PM. Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation. European Journal of Neuroscience. 2006;23:793–800. doi: 10.1111/j.1460-9568.2006.04600.x. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA, Wong M. The remember response: Subject to bias, graded, and not a process-pure indicator of recollection. Psychonomic Bulletin & Review. 2005;12:865–873. doi: 10.3758/bf03196778. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Allan K, Frith CD, Frackowiak RS, Dolan RJ. Neural correlates of memory retrieval during recognition memory and cued recall. NeuroImage. 1998a;8:262–273. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Psychology Press; 2002. [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998b;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. Human recognition memory: a cognitive neuroscience perspective. Trends in Cognitive Sciences. 2003;7:313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, Duzel E. Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. PNAS. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. The Journal of Neuroscience. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. doi: 10.1016/j.neuropsychologia.2007.07.024. in press. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgements. Journal of Cognitive Neuroscience. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Van Essen DC. Windows on the brain. The emerging role of atlases and databases in neuroscience. Current Opinions in Neurobiology. 2002;12:574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An Integrated Software System for Surface-based Analyses of Cerebral Cortex. JAMIA. 2001;41:1359–1378. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal mnemonic network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. The Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. Erratum on 1416. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. Journal of Experimental Psychology: General. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. Journal of Memory and Language. 1995;34:622–643. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Sun X, Li Z, Wang Z, He S, Hu X. Cross-modal temporal order memory for auditory digits and visual locations: An fMRI study. Human Brain Mapping. 2004;22:280–289. doi: 10.1002/hbm.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]


